Interspecific Variation between the American Quercus virginiana and Mediterranean Quercus Species in Terms of Seed Nutritional Composition, Phytochemical Content, and Antioxidant Activity
Abstract
1. Introduction
2. Results
2.1. Morphometric and Chemical Composition Analysis of Seeds
2.2. Fatty Acid Determination of Seeds
2.3. Phytochemical Analysis and Antioxidant Activity of Seeds
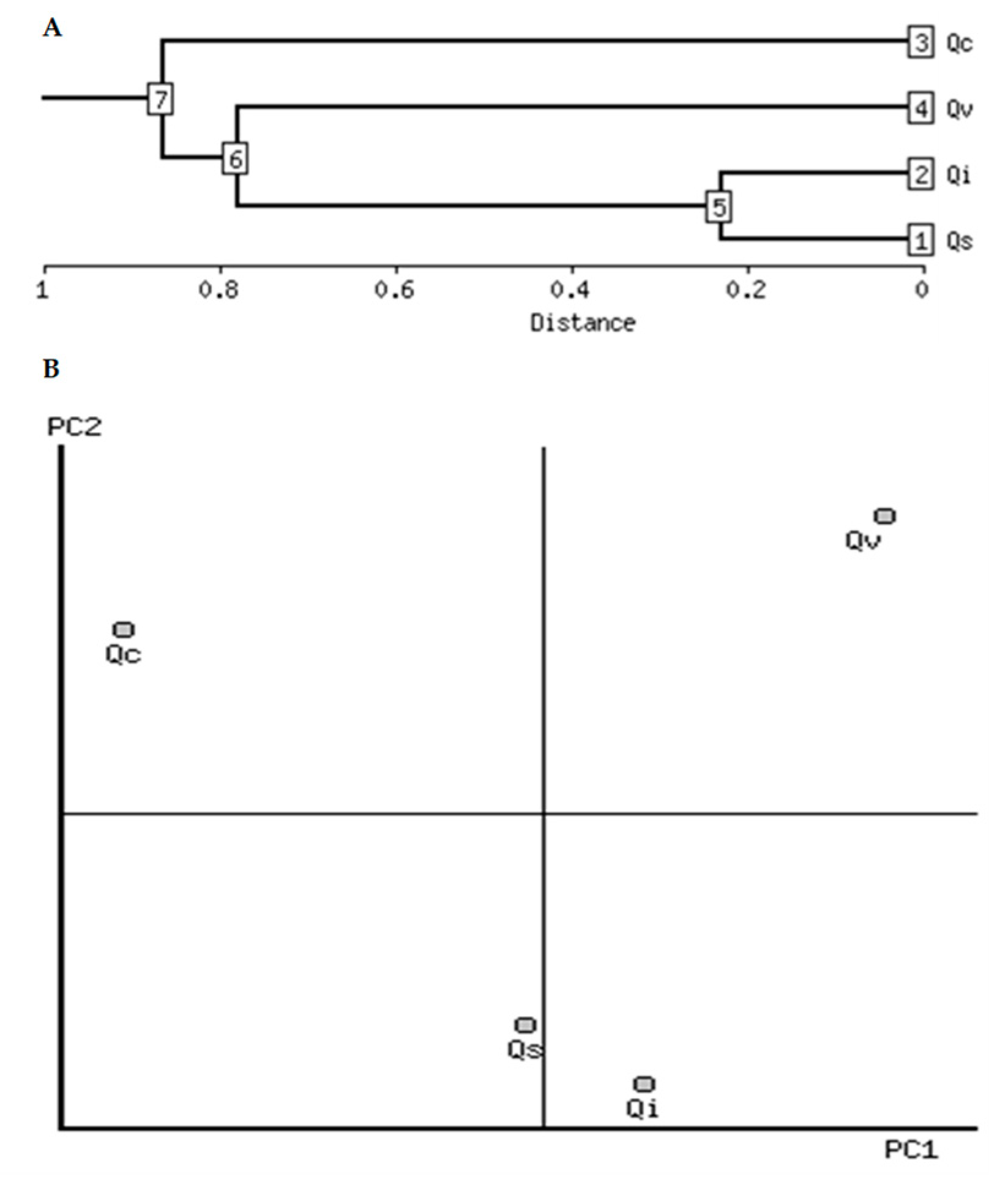
2.4. Clustering Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Acorn Morphometry Study
4.3. Chemical Analysis
4.4. Total Phenols, Total Flavonoids, and Antioxidant Activity
4.4.1. Total Phenolics and Total Flavonoids
4.4.2. Antioxidant Activity Quantification
4.5. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Menezes-Silva, P.E.; Loram-Lourenço, L.; Alves, R.D.F.B.; Sousa, L.F.; Almeida, S.E.D.S.; Farnese, F.S. Different ways to die in a changing world: Consequences of climate change for tree species performance and survival through an ecophysiological perspective. Ecol. Evol. 2019, 9, 11979–11999. [Google Scholar] [CrossRef] [PubMed]
- Amimi, N.; Dussert, S.; Vaissayre, V.; Ghouil, H.; Doulbeau, S.; Costantini, C.; Ammari, Y.; Joët, T. Variation in seed traits among Mediterranean oaks in Tunisia and their ecological significance. Ann. Bot. 2020, 125, 891–904. [Google Scholar] [CrossRef] [PubMed]
- Gea-Izquierdo, G.; Cañellas, I.; Montero, G. Acorn production in Spanish holm oak woodlands. Investig. Agrar. Sist. Recur. For. 2006, 15, 339. [Google Scholar] [CrossRef]
- Tejerina, D.; García-Torres, S.; de Vaca, M.C.; Vázquez, F.; Cava, R. Acorns (Quercus rotundifolia Lam.) and grass as natural sources of antioxidants and fatty acids in the “montanera” feeding of Iberian pig: Intra- and inter-annual variations. Food Chem. 2011, 124, 997–1004. [Google Scholar] [CrossRef]
- Ventanas, S.; Ventanas, J.; Tovar, J.; Garcia, C.; Estévez, M. Extensive feeding versus oleic acid and tocopherol enriched mixed diets for the production of Iberian dry-cured hams: Effect on chemical composition, oxidative status and sensory traits. Meat Sci. 2007, 77, 246–256. [Google Scholar] [CrossRef]
- Soto, E.; Hoz, L.; Ordóñez, J.; Hierro, E.; Herranz, B.; López-Bote, C.; Cambero, M. Impact of feeding and rearing systems of Iberian pigs on volatile profile and sensory characteristics of dry-cured loin. Meat Sci. 2008, 79, 666–676. [Google Scholar] [CrossRef]
- Pasqualone, A.; Makhlouf, F.Z.; Barkat, M.; Difonzo, G.; Summo, C.; Squeo, G.; Caponio, F. Effect of acorn flour on the physico-chemical and sensory properties of biscuits. Heliyon 2019, 5, e02242. [Google Scholar] [CrossRef]
- Vinha, A.F.; Barreira, J.C.M.; Costa, A.S.; Oliveira, M.B.P.P. A New Age for Quercus spp. Fruits: Review on Nutritional and Phytochemical Composition and Related Biological Activities of Acorns. Compr. Rev. Food Sci. Food Saf. 2016, 15, 947–981. [Google Scholar] [CrossRef]
- Sales-Campos, H.; de Souza, P.R.; Peghini, B.C.; da Silva, J.S.; Cardoso, C.R. An overview of the modulatory effects of oleic acid in health and disease. Mini Rev. Med. Chem. 2013, 13, 201–210. [Google Scholar]
- Cantos, E.; Espín, J.C.; López-Bote, C.; De La Hoz, L.; Ordóñez, J.A.; Tomás-Barberán, F.A. Phenolic Compounds and Fatty Acids from Acorns (Quercusspp.), the Main Dietary Constituent of Free-Ranged Iberian Pigs. J. Agric. Food Chem. 2003, 51, 6248–6255. [Google Scholar] [CrossRef]
- Giertych, M.J.; Chmielarz, P. Size variability in embryonic axes, cotyledons, acorns and seedlings in fifteen species of the genus Quercus. Trees 2019, 34, 593–601. [Google Scholar] [CrossRef]
- López-Hidalgo, C.; Trigueros, M.; Menéndez, M.; Jorrin-Novo, J.V. Phytochemical composition and variability in Quercus ilex acorn morphotypes as determined by NIRS and MS-based approaches. Food Chem. 2021, 338, 127803. [Google Scholar] [CrossRef] [PubMed]
- Galván, J.V.; Novo, J.J.J.; Cabrera, A.G.; Ariza, D.; García-Olmo, J.; Cerrillo, R.M.N. Population variability based on the morphometry and chemical composition of the acorn in Holm oak (Quercus ilex subsp. ballota [Desf.] Samp.). Eur. J. For. Res. 2011, 131, 893–904. [Google Scholar] [CrossRef]
- Özcan, T. Characterization of Turkish Quercus L. Taxa Based on Fatty Acid Compositions of the Acorns. J. Am. Oil Chem. Soc. 2007, 84, 653–662. [Google Scholar] [CrossRef]
- Dodd, R.S.; Rafii, Z.A.; Zavarin, E. Chemosystematic variation in acorn fatty acids of Californian live oaks (Quercus agrifolia and Q. wislizenii). Biochem. Syst. Ecol. 1993, 21, 279–285. [Google Scholar] [CrossRef]
- Rafii, Z.A.; Zavarin, E.; Pelleau, Y. Chemosystematic differentiation of Quercus ilex and Q. rotundifolia based on acorn fatty acids. Biochem. Syst. Ecol. 1991, 19, 163–166. [Google Scholar] [CrossRef]
- Akcan, T.; Gökçe, R.; Asensio, M.; Estevez, M.; Morcuende, D. Acorn (Quercus spp.) as a novel source of oleic acid and tocopherols for livestock and humans: Discrimination of selected species from Mediterranean forest. J. Food Sci. Technol. 2017, 54, 3050–3057. [Google Scholar] [CrossRef]
- Bryant, F.C.; Merrill, L.B.; Kothmann, M.M. Nutritive Content of Sheep, Goat, and White-Tailed Deer Diets on Excellent Condition Rangeland in Texas. J. Range Manag. 1980, 33, 410. [Google Scholar] [CrossRef]
- Huston, J.E.; Rector, B.S.; Merrill, L.B.; Engdahl, B.S. Nutritional Value of Range Plants in the Edwards Plateau Region of Texas; B-1357; Agricultural Research and Development Center: Ohio, OH, USA, 1981. [Google Scholar]
- Short, H.L. Composition and Squirrel Use of Acorns of Black and White Oak Groups. J. Wildl. Manag. 1976, 40, 479. [Google Scholar] [CrossRef]
- Ramírez-Valiente, J.; Valladares, F.; Gil, L.; Aranda, I. Population differences in juvenile survival under increasing drought are mediated by seed size in cork oak (Quercus suber L.). For. Ecol. Manag. 2009, 257, 1676–1683. [Google Scholar] [CrossRef]
- Singh, B.; Saklani, K.P.; Bhatt, B.P. Provenance variation in seed and seedlings attributes of Quercus glauca Thunb. Dendrobiology 2010, 63, 59–63. [Google Scholar]
- Vinha, A.; Costa, A.; Barreira, J.C.; Pacheco, R.; Oliveira, M.B.P. Chemical and antioxidant profiles of acorn tissues from Quercus spp.: Potential as new industrial raw materials. Ind. Crop. Prod. 2016, 94, 143–151. [Google Scholar] [CrossRef]
- Gómez-Casero, M.T.; Galán, C.; Domínguez-Vilches, E. Flowering phenology of Mediterranean Quercus species in different locations (Córdoba, SW Iberian Peninsula). Acta Bot. Malacit. 2007, 32, 127–146. [Google Scholar] [CrossRef]
- Bonner, F.; Vozzo, J.A. Seed Biology and Technology of Quercus. Seed Biol. Technol. Quercus 1987, 66. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2000. [Google Scholar]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef]
- Georgé, S.; Brat, P.; Alter, A.P.; Amiot, M.J. Rapid Determination of Polyphenols and Vitamin C in Plant-Derived Products. J. Agric. Food Chem. 2005, 53, 1370–1373. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Moreno-Escamilla, J.O.; Alvarez-Parrilla, E.; De La Rosa, L.A.; Núñez-Gastélum, J.A.; González-Aguilar, G.A.; Rodrigo-Garcia, J. Effect of different elicitors and pre-harvest day application on the content of phytochemicals and antioxidant activity of butterhead lettuce (Lactuca sativa var capitata) produced under hydroponic conditions. J. Agric. Food Chem. 2017, 65, 5244–5254. [Google Scholar] [CrossRef]
- Sharov, A.A.; Dudekula, D.B.; Ko, M.S.H. A web-based tool for principal component and significance analysis of microarray data. Bioinformatics 2005, 21, 2548–2549. [Google Scholar] [CrossRef]
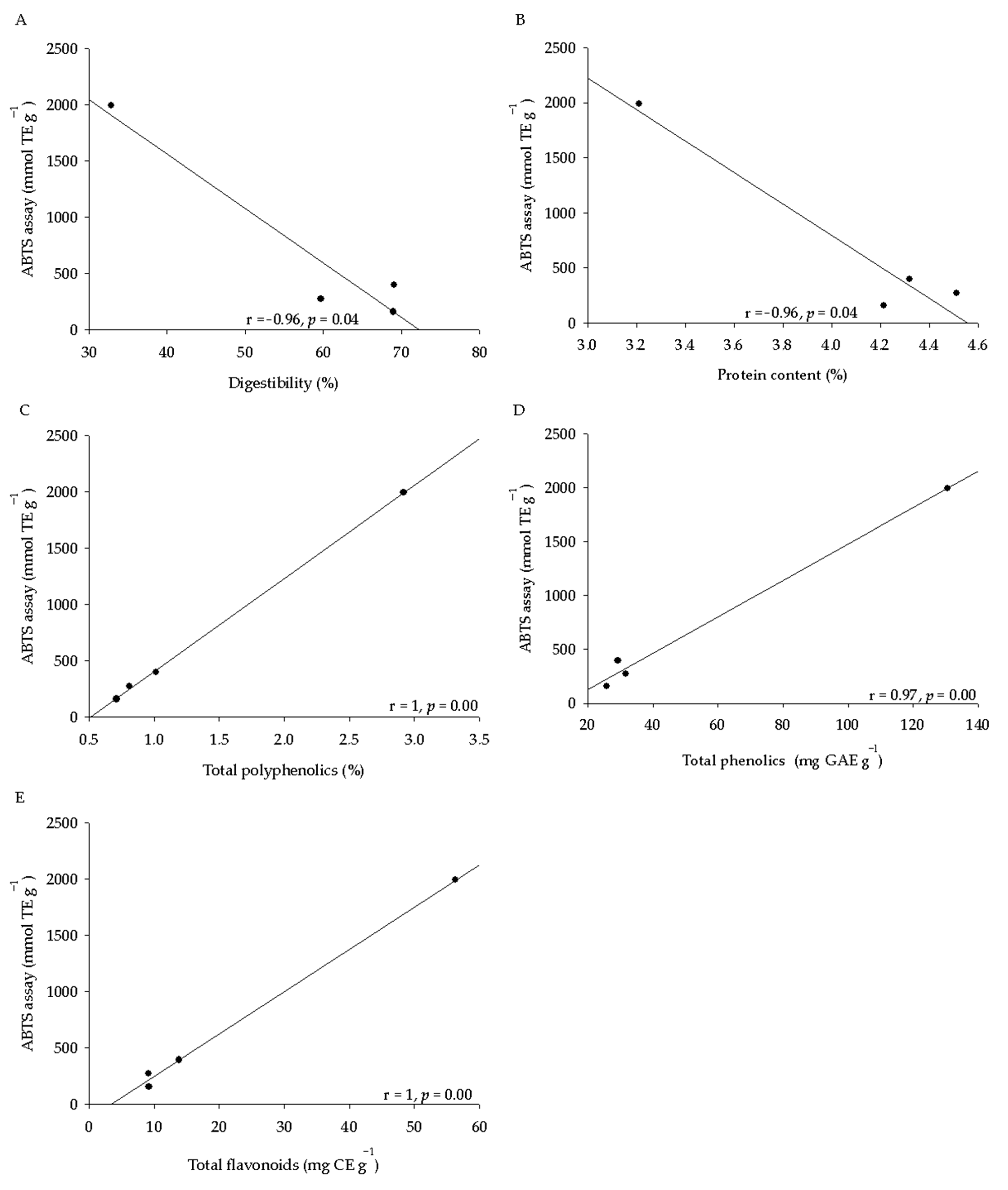
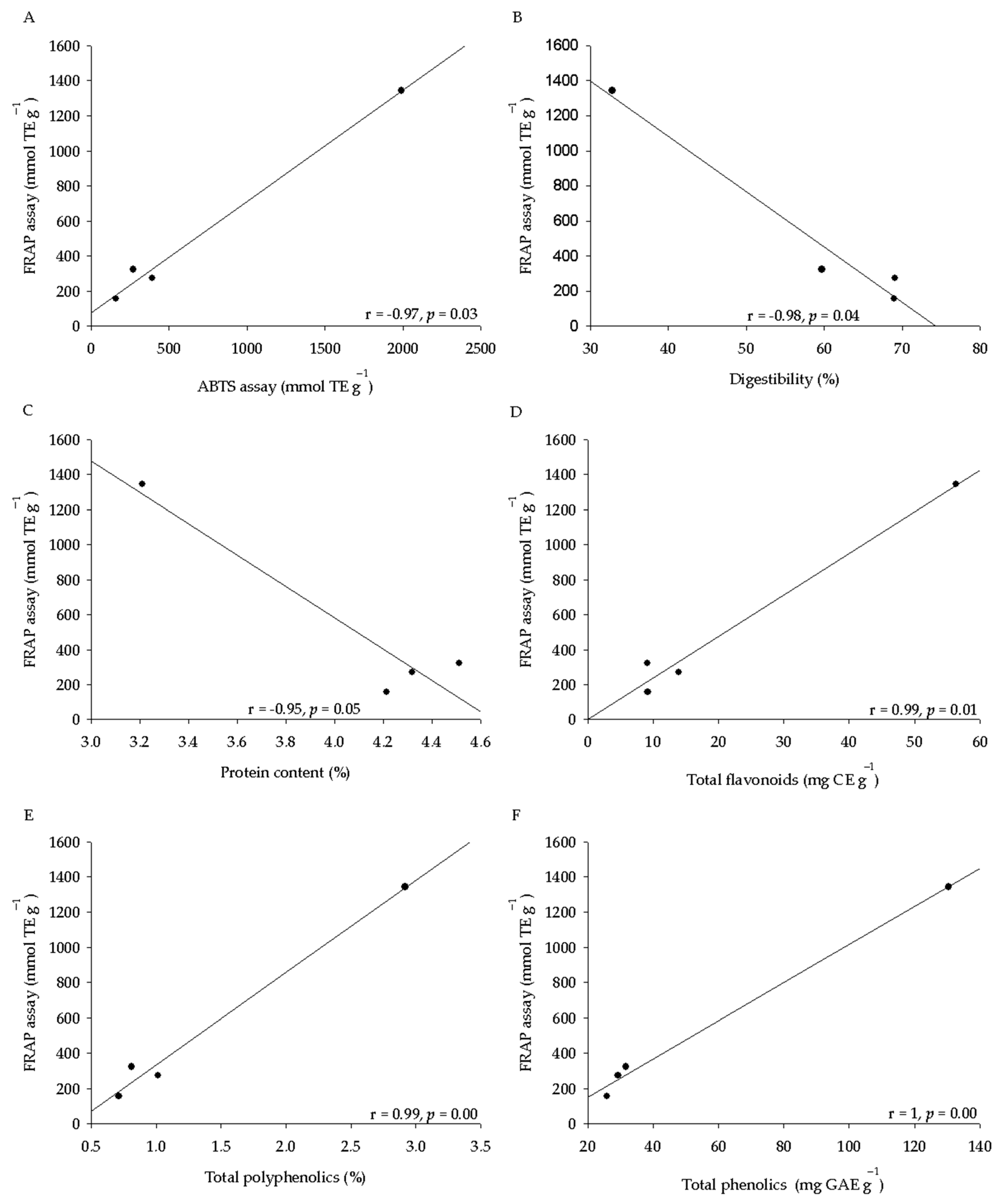

| Seed Characteristics | Q. virginiana | Q. suber | Q. ilex | Q. coccifera | Anova (p ≤ 0.05) |
|---|---|---|---|---|---|
| Weight (g) | 2.1 ± 0.2 a | 6.5 ± 1.4 c | 4.5 ± 1.0 b | 4.6 ± 1.6 b | 1.64 × 10−11 |
| Diameter (cm) | 1.3 ± 0.1 a | 1.6 ± 0.1 d | 1.4 ± 0.1 b | 1.5 ± 0.2 c | 1.54 × 10−10 |
| Length (cm) | 2.3 ± 0.1 a | 3.5 ± 0.3 c | 3.8 ± 0.4 d | 3.2 ± 0.5 b | 6.41 × 10−17 |
| Coat weight (g) | 0.6 ± 0.1 a | 1.3 ± 0.3 b | 1.0 ± 0.2 b | 1.3 ± 0.4 b | 3.05 × 10−7 |
| Megagametophyte weight (g) | 1.5 ± 0.2 a | 4.3 ± 0.8 c | 3.6 ± 0.7 b | 4.1 ± 0.8 bc | 5.83 × 10−11 |
| Chemical Composition | Q. virginiana | Q. suber | Q. ilex | Q. coccifera | Anova (p ≤ 0.05) |
|---|---|---|---|---|---|
| Water content (%) | 73.5 ± 0.61 a | 71.6 ± 1.8 a | 74.3 ± 2.2 a | 72.3 ± 3.6 a | 0.15 |
| Ash content (%) | 1.6 ± 0.10 ab | 1.8 ± 0.0 c | 1.7 ± 0.1 bc | 1.6 ± 0.1 a | 0.01 |
| Protein content (%) | 4.3 ± 0.51 b | 4.5 ± 0.3 b | 4.2 ± 0.2 b | 3.2 ± 0.5 a | 0.01 |
| Total lipids (%) | 12.0 ± 1.62 c | 8.0 ± 1.6 b | 12.9 ± 0.7 c | 2.9 ± 0.4 a | 0.01 |
| Carbohydrates (%) | 82.1 ± 1.0 a | 85.7 ± 1.0 b | 81.2 ± 1.0 a | 92 ± 1.0 c | 0.01 |
| Sugar content (%) | 11.3 ± 0.91 c | 6.9 ± 0.4 ab | 6.0 ± 0.5 a | 7.5 ± 1.4 b | 0.01 |
| Fiber content (%) | 2.3 ± 0.03 a | 2.3 ± 0.0 a | 2.3 ± 0.0 a | 2.3 ± 0.0 a | 0.61 |
| Starch content (%) | 57.6 ± 1.57 a | 61.9 ± 1.3 b | 62.0 ± 1.5 b | 63.9 ± 1.7 b | 0.01 |
| Digestibility (%) | 69.1 ± 02.15 c | 59.7 ± 01.0 b | 69.0 ± 03.1 c | 32.8 ± 1.1 a | 0.01 |
| Energy (kcal/100 g) | 490.1 ± 8.6 c | 466.3 ± 8.2 b | 488.6 ± 4.6 c | 444.5 ± 3.9 a | 0.01 |
| Palmitic acid (%) | 16.4 ± 0.4 c | 12.5 ± 0.4 a | 15.3 ± 0.7 b | 16.9 ± 0.7 c | 0.01 |
| Stearic acid (%) | 4.3 ± 0.2 b | 3.1 ± 0.0 a | 3.1 ± 0.2 a | 2.8 ± 0.3 a | 0.01 |
| Oleic acid (%) | 66.0 ± 1.6 a | 67.9 ± 1.2 a | 66.5 ± 1.7 a | 66.5 ± 1.9 a | 0.01 |
| Linoleic acid (%) | 13.4 ± 2.6 a | 15.8 ± 1.1 b | 14.8 ± 1.2 ab | 19.8 ± 2.0 c | 0.01 |
| Total polyphenolics (NIRS) (%) | 1.03 ± 0.05 b | 0.80 ± 0.05 a | 0.73 ± 0.10 a | 2.98 ± 0.36 c | 0.01 |
| Total phenolics (Folin Ciocalteau) * | 29.4 ± 1.4 a | 31.8 ± 1.6 a | 25.9 ± 0.9 a | 130.5 ± 10.0 b | 0.01 |
| Total flavonoids ** | 13.9 ± 1.9 a | 9.2 ± 1.0 a | 9.2 ± 1.1 a | 56.3 ± 3.4 b | 0.01 |
| ABTS *** | 394.1 ± 32.2 b | 271.9 ± 14.5 ab | 157.6 ± 19.7 a | 1990.0 ± 167.1 c | 0.01 |
| FRAP *** | 272.1 ± 29.0 b | 323.1 ± 24.4 b | 157.9 ± 18.1 a | 1343.4 ± 68.9 c | 0.01 |
| Seed Characteristic | Log10 Change | Correlation | PCA Number | Direction | Qs | Qi | Qc | Qv |
|---|---|---|---|---|---|---|---|---|
| Total phenolics | −0.70 | −0.88 | 1 | - | 1.5 | 1.4 | 2.1 | 1.4 |
| Digestibility | 0.34 | 0.93 | 1 | + | 1.7 | 1.8 | 1.5 | 1.8 |
| Total flavonoids | −0.67 | −0.75 | 1 | - | 0.96 | 0.96 | 1.75 | 1.14 |
| FRAP assay | −0.79 | −0.83 | 1 | - | 2.5 | 2.1 | 3.1 | 2.4 |
| Total lipids | 0.66 | 0.93 | 1 | + | 0.90 | 1.10 | 0.46 | 1.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valero-Galván, J.; González-Fernández, R.; Jorrin-Novo, J.V. Interspecific Variation between the American Quercus virginiana and Mediterranean Quercus Species in Terms of Seed Nutritional Composition, Phytochemical Content, and Antioxidant Activity. Molecules 2021, 26, 2351. https://doi.org/10.3390/molecules26082351
Valero-Galván J, González-Fernández R, Jorrin-Novo JV. Interspecific Variation between the American Quercus virginiana and Mediterranean Quercus Species in Terms of Seed Nutritional Composition, Phytochemical Content, and Antioxidant Activity. Molecules. 2021; 26(8):2351. https://doi.org/10.3390/molecules26082351
Chicago/Turabian StyleValero-Galván, José, Raquel González-Fernández, and Jesús V. Jorrin-Novo. 2021. "Interspecific Variation between the American Quercus virginiana and Mediterranean Quercus Species in Terms of Seed Nutritional Composition, Phytochemical Content, and Antioxidant Activity" Molecules 26, no. 8: 2351. https://doi.org/10.3390/molecules26082351
APA StyleValero-Galván, J., González-Fernández, R., & Jorrin-Novo, J. V. (2021). Interspecific Variation between the American Quercus virginiana and Mediterranean Quercus Species in Terms of Seed Nutritional Composition, Phytochemical Content, and Antioxidant Activity. Molecules, 26(8), 2351. https://doi.org/10.3390/molecules26082351






