Hydrogel Films Based on Chitosan and Oxidized Carboxymethylcellulose Optimized for the Controlled Release of Curcumin with Applications in Treating Dermatological Conditions
Abstract
1. Introduction
2. Results and Discussions
2.1. Preparation and Characterization of CMCOx
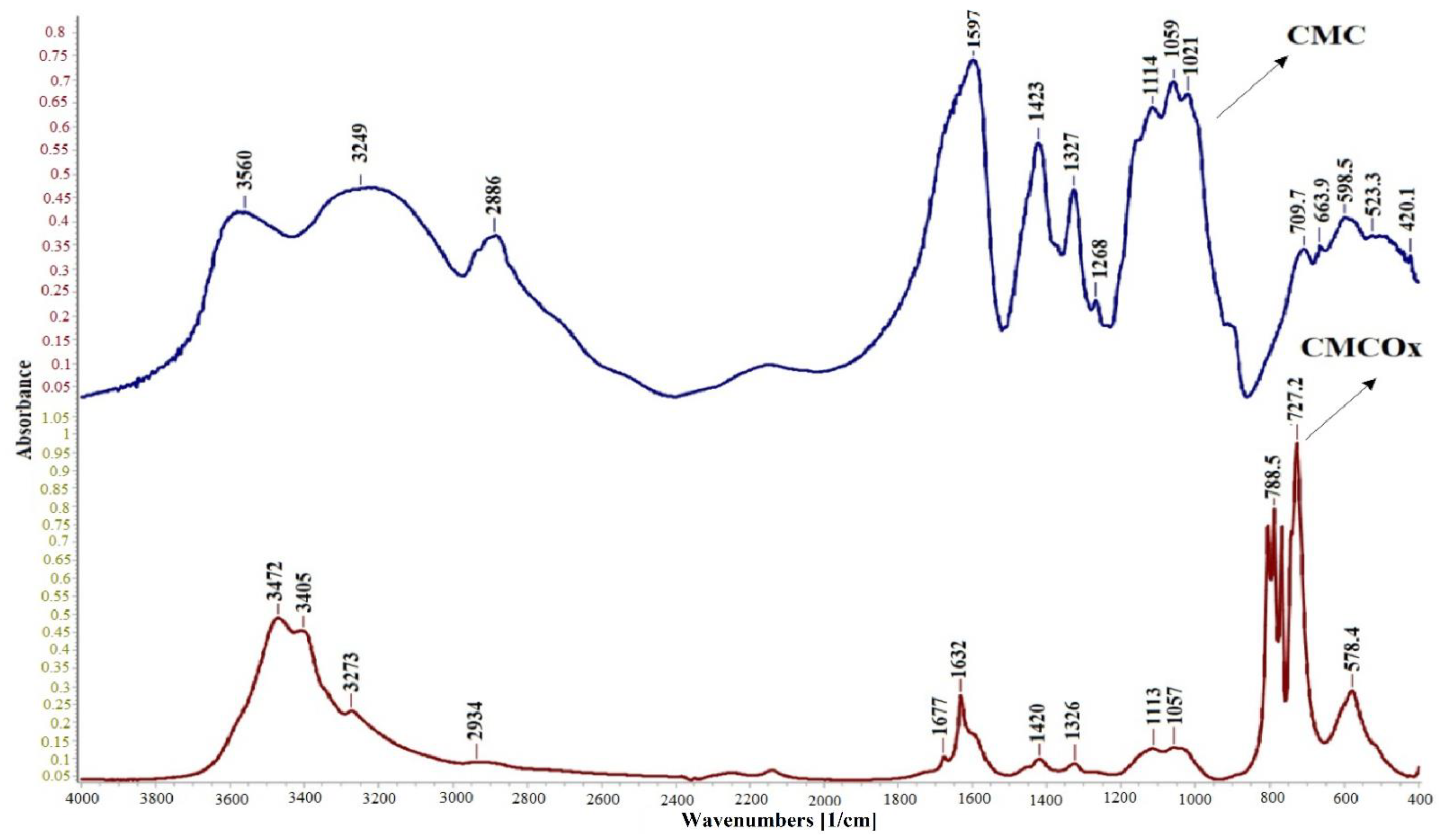
2.1.1. FTIR Spectroscopy of CMC and CMCOx
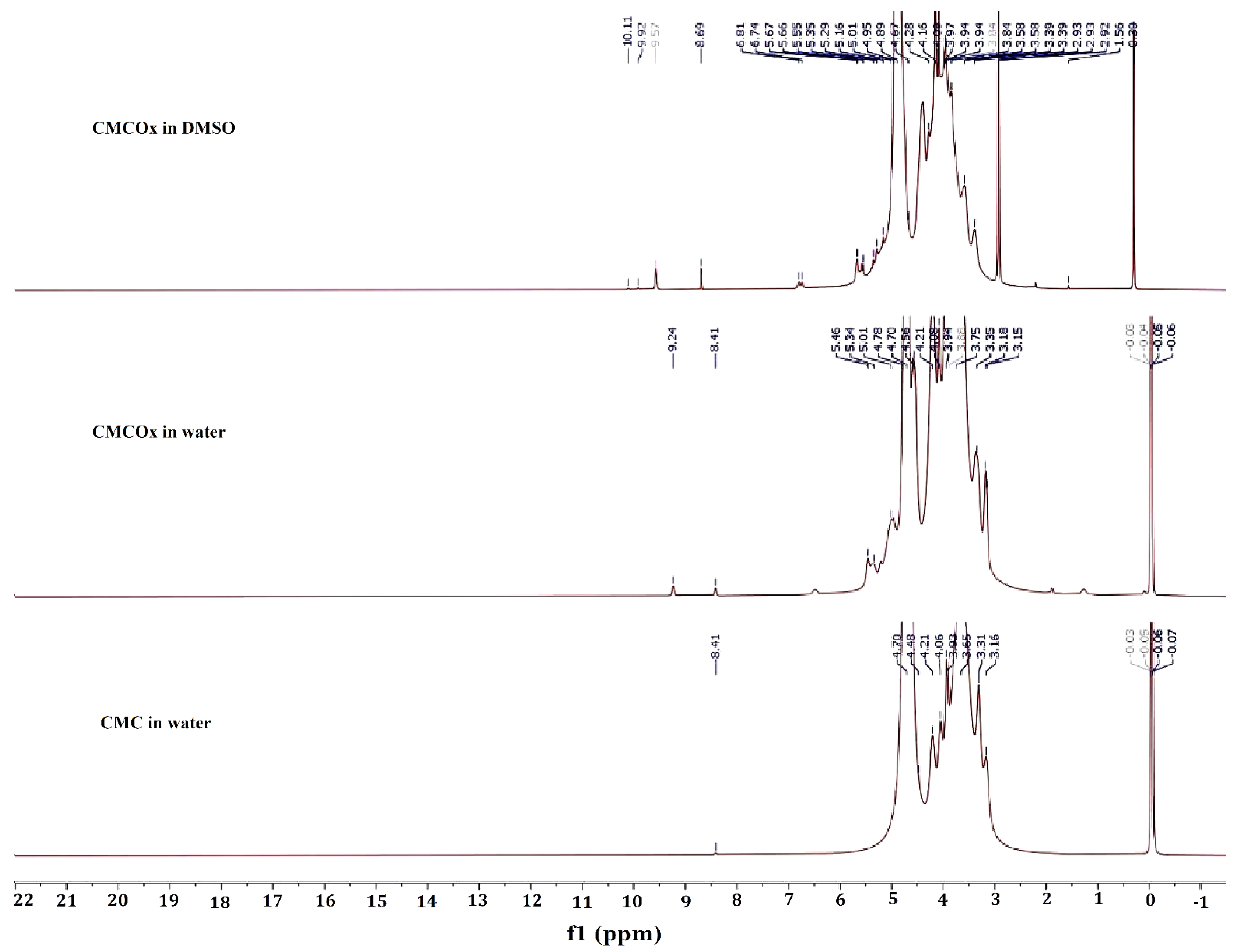
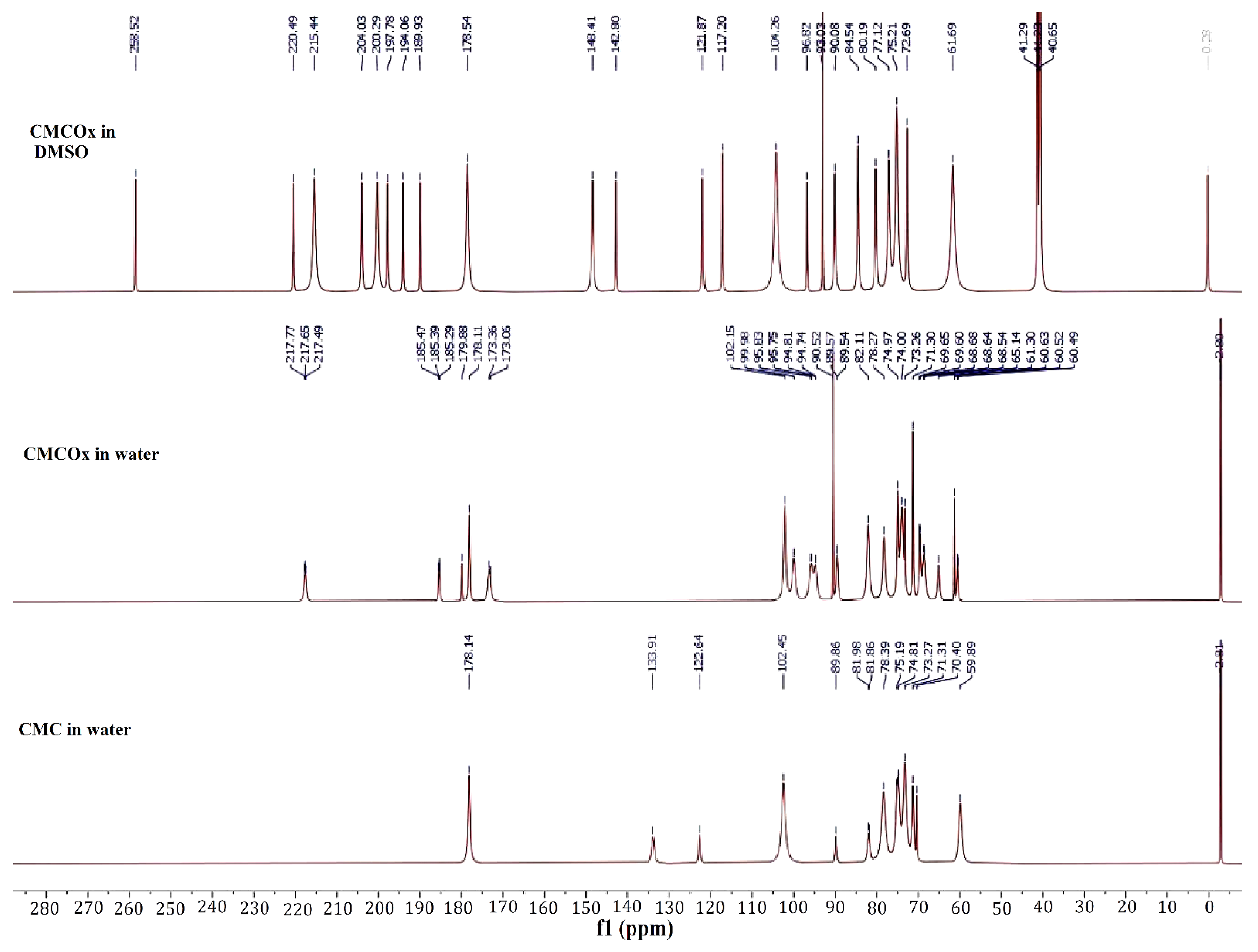
2.1.2. NMR Spectra of CMC and CMCOx
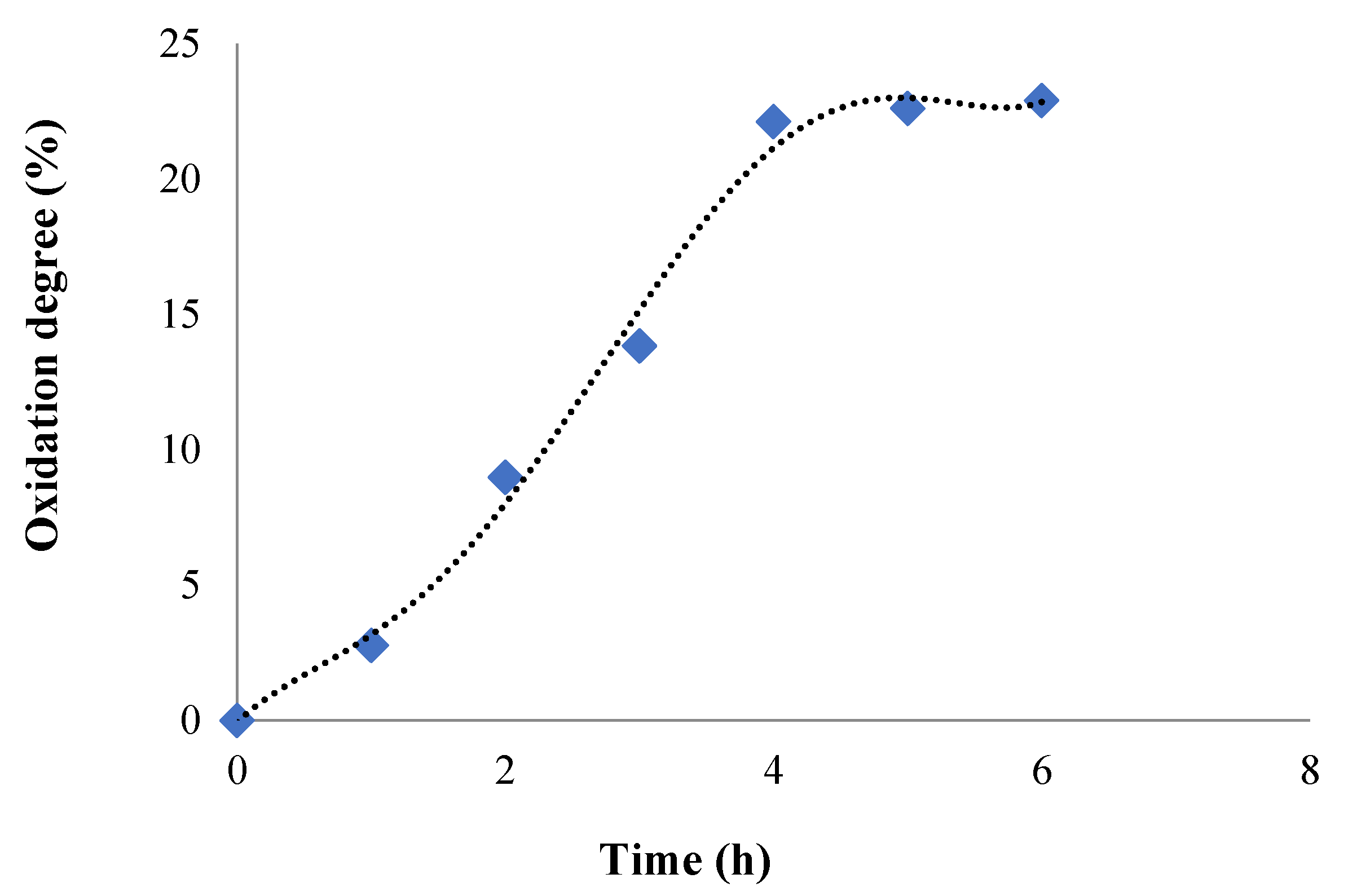
2.1.3. Oxidation Reaction Kinetic and Quantitative Determination of the Aldehyde Groups Obtained in CMCOx
2.1.4. Molecular Weight Determination
2.2. Obtaining and Characterization of the CS Films with CMCOx
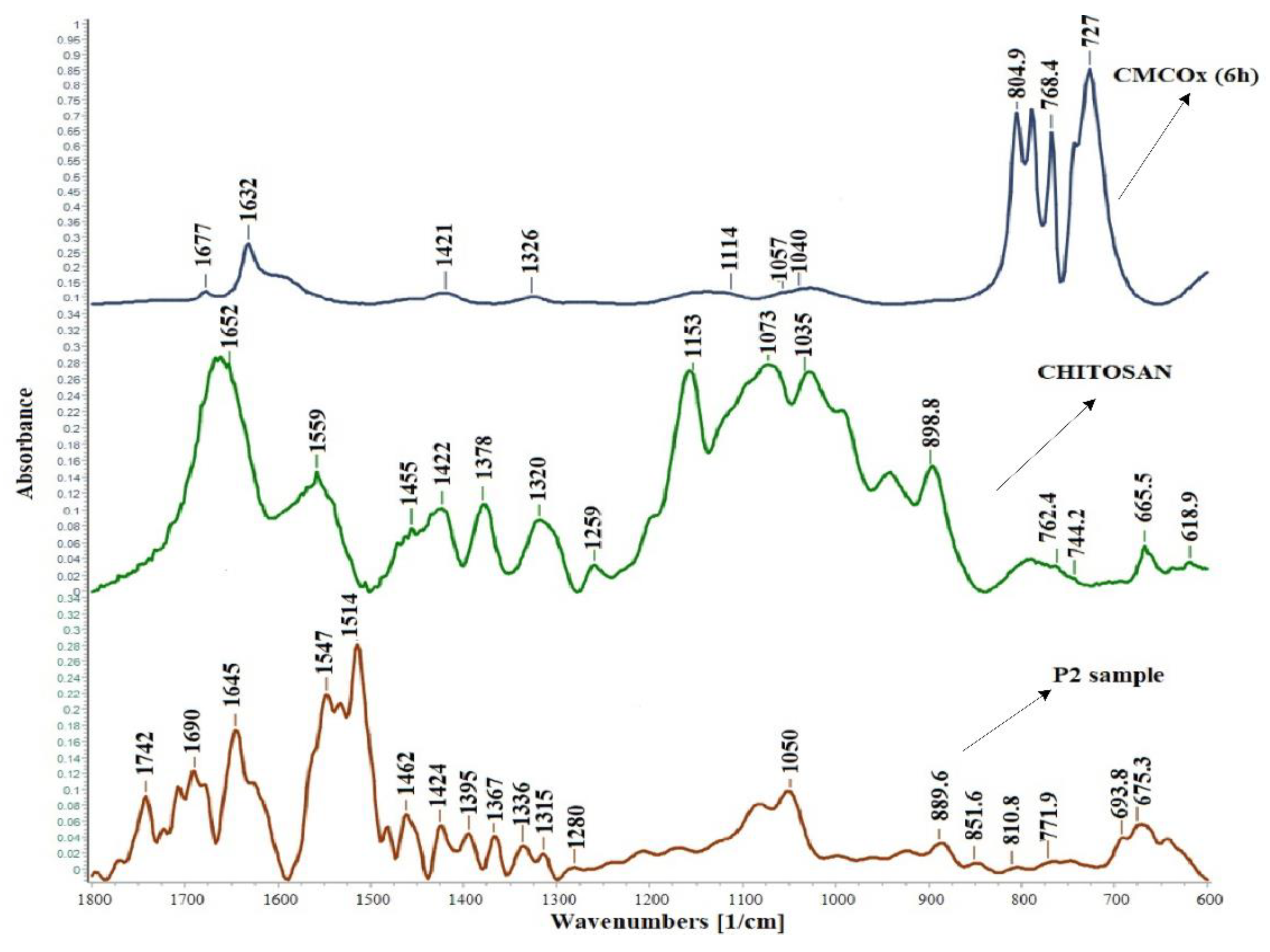
2.2.1. FTIR Spectroscopy of the Hydrogels Obtained
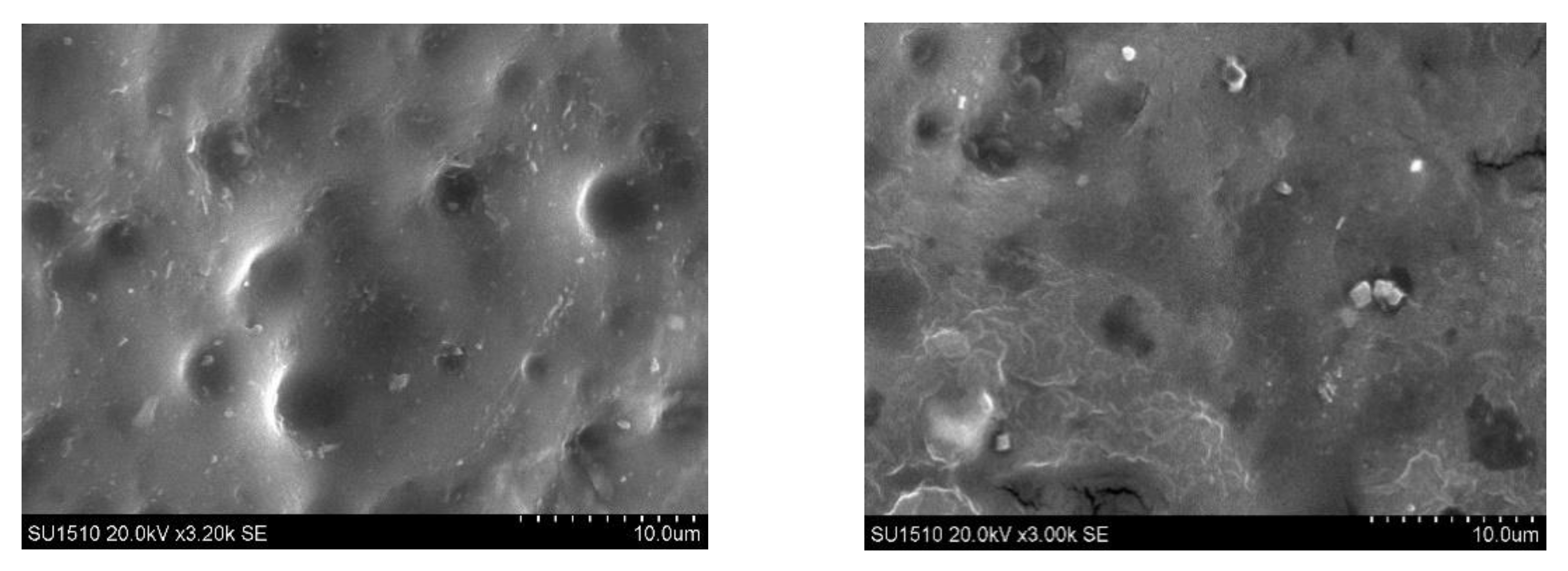
2.2.2. Scanning Electron Microscopy
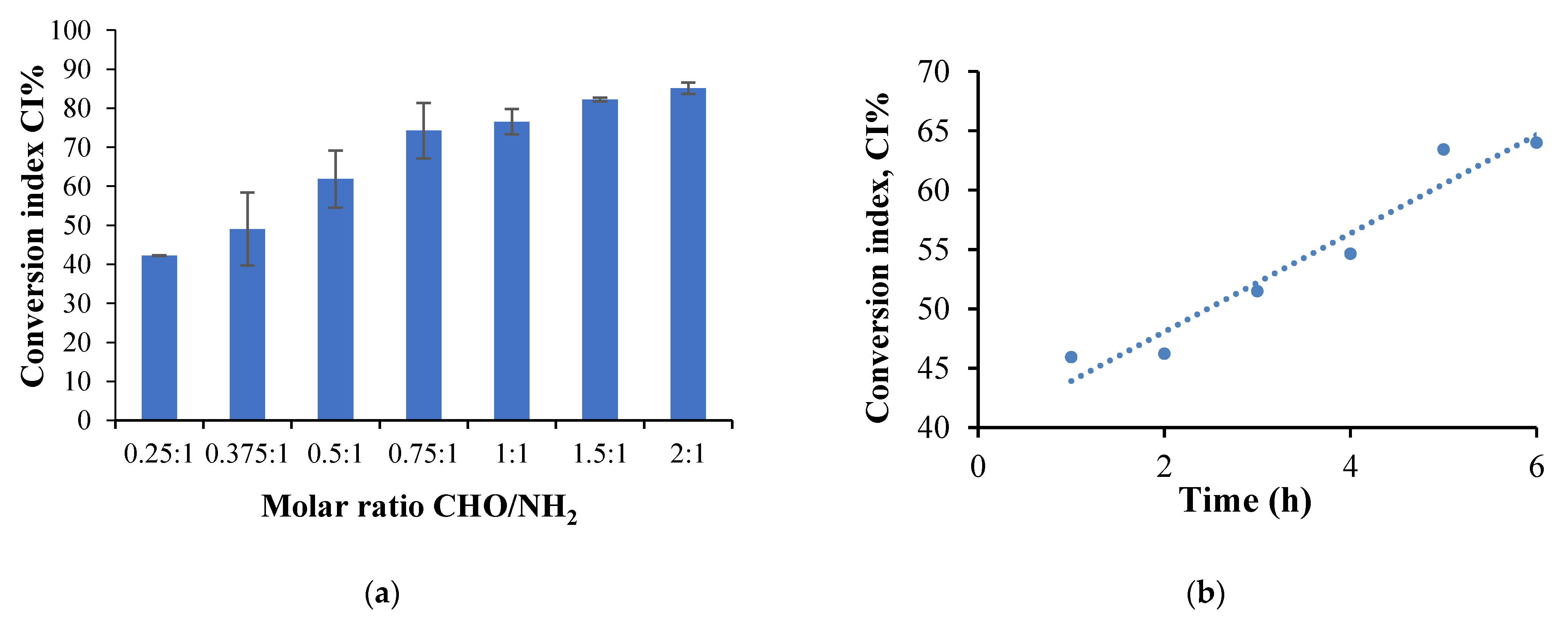
2.2.3. Amino Groups Conversion Index Determination
- The molar ratio CHO/NH2 influence the CI values
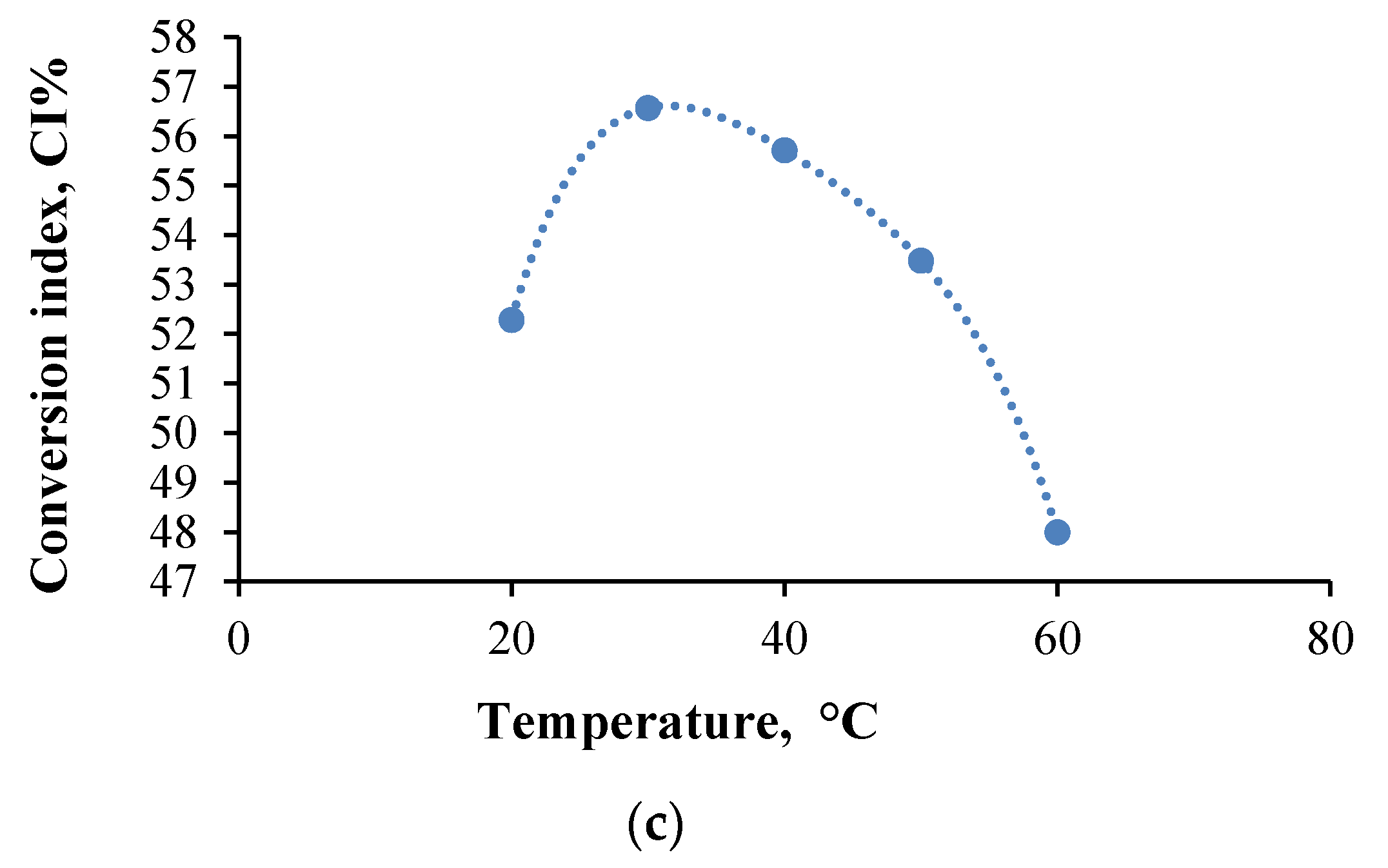
- The cross-linking time and temperature influence on the amine group CI
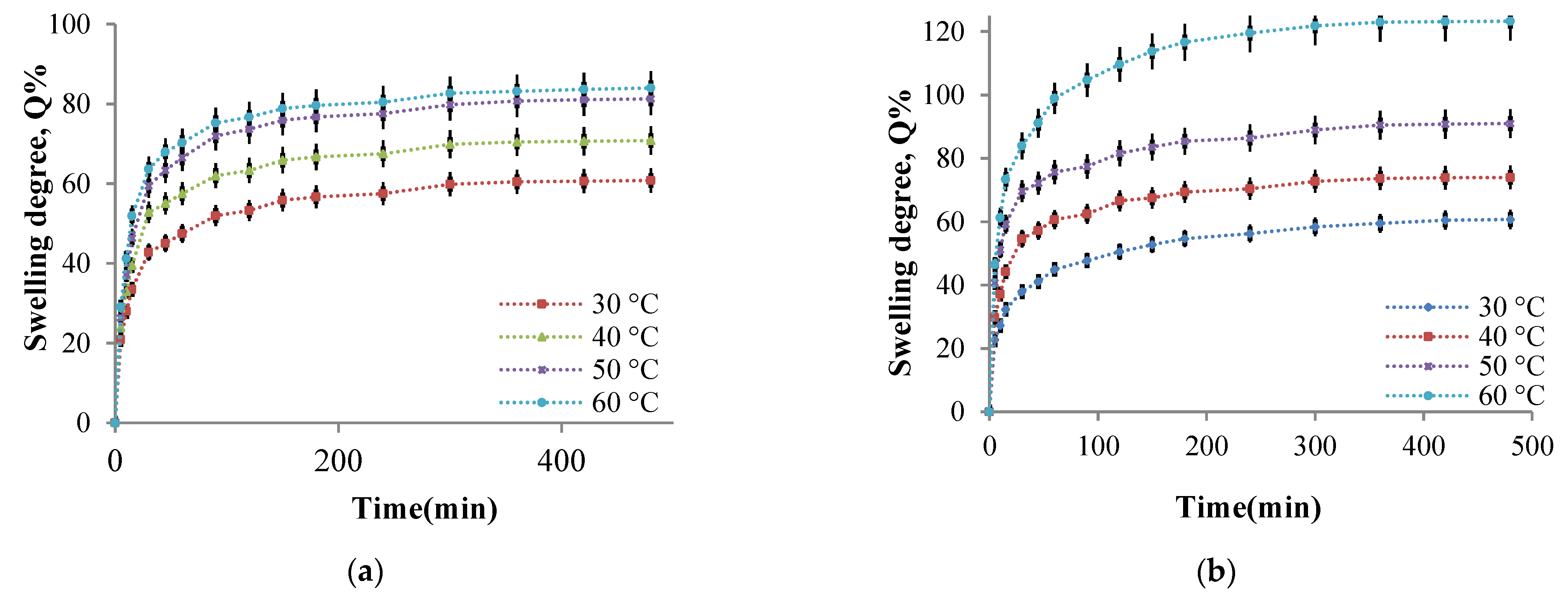
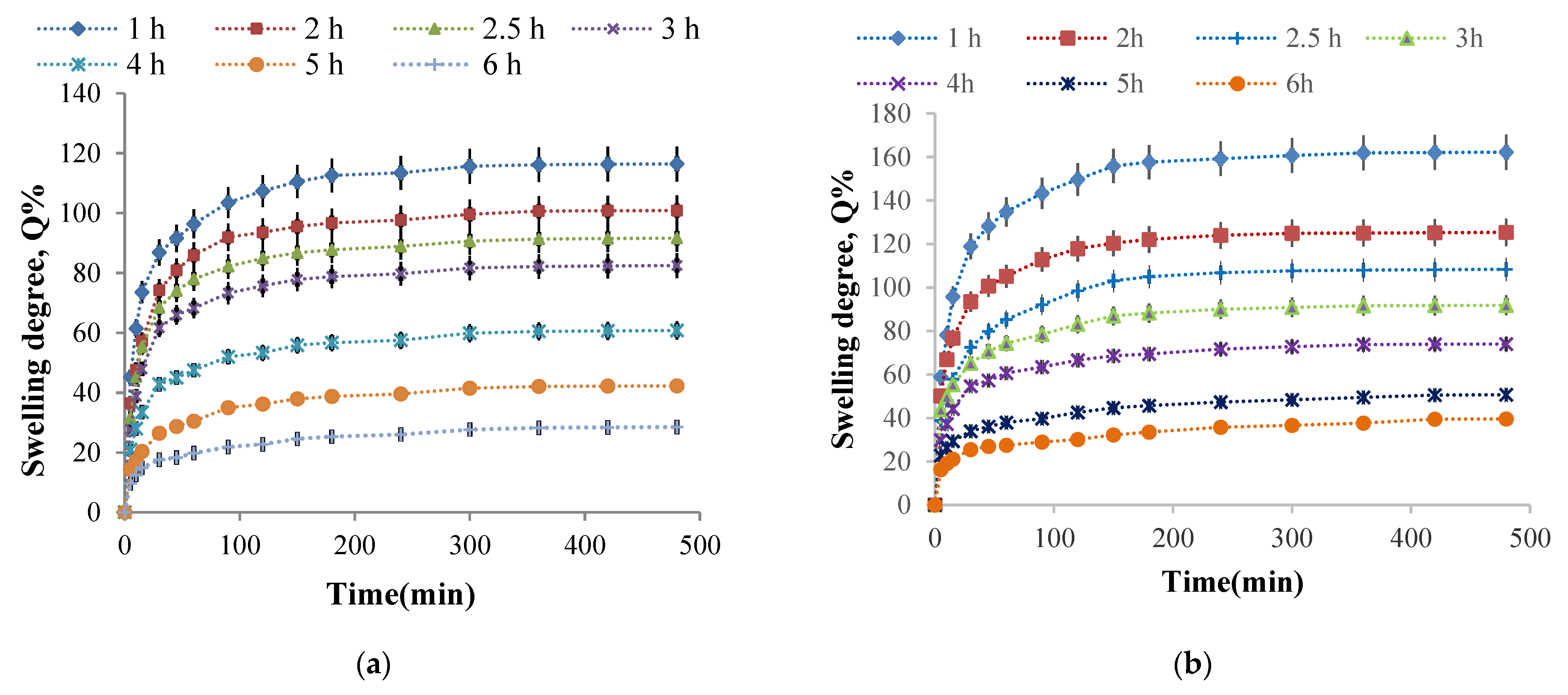
2.2.4. Hydrogel Films Ability to Absorb Aqueous Solutions
- The molar ratio CHO/NH2 influence on the swelling degree value
- The influence of the cross-linking temperature on the hydrogel films swelling degree
- The influence of the cross-linking time on the hydrogel films swelling degree
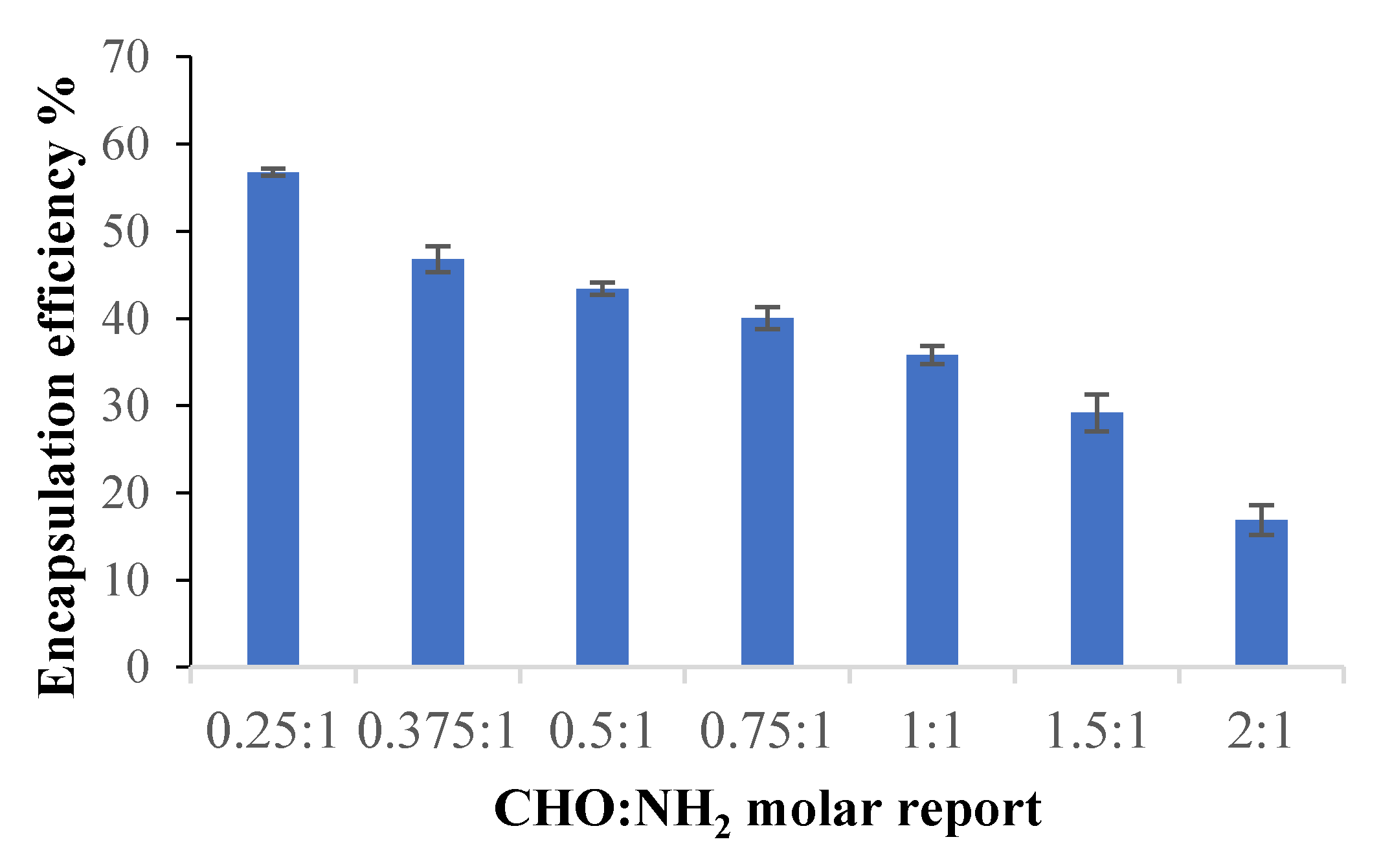
2.2.5. Encapsulation Efficiency
2.2.6. In Vitro Release Kinetics of Curcumin from Hydrogel Films
2.2.7. Antioxidant Activity
3. Materials and Methods
3.1. Materials
3.2. Preparation of Oxidized Carboxymethylcellulose
3.3. Obtaining Hydrogel Films Based on CS and CMCOx
3.4. Characterisation Methods
3.4.1. FTIR Spectroscopy
3.4.2. 1H NMR and 13C NMR Spectra of CMC and CMCOx
3.4.3. Oxidation Reaction Kinetics and Quantitative Determination of Aldehyde Groups Obtained in CMCOx
- Aldehyde groups determination
- Oxidation kinetics studies
3.4.4. Molecular WEIGHT determination of CMC and CMCOx by the Viscometric Method
3.4.5. Scanning Electron Microscopy
3.4.6. The Amino Groups’ CI% Determination into Shiff Bases in Hydrogel Films
3.4.7. Hydrogel Films Ability to Absorb Aqueous Solutions
3.4.8. Encapsulation Efficiency
3.4.9. In Vitro Release Kinetics of Curcumin from Hydrogel Films
3.4.10. Antioxidant Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zagórska-Dziok, M.; Sobczak, M. Hydrogel-Based Active Substance Release Systems for Cosmetology and Dermatology Application: A Review. Pharmaceutics 2020, 12, 396. [Google Scholar] [CrossRef]
- Svensson, A.; Ofenloch, R.; Bruze, M.; Naldi, L.; Cazzaniga, S.; Elsner, P.; Goncalo, M.; Schuttelaar, M.-L.; Diepgen, T. Prevalence of skin disease in a population-based sample of adults from five European countries. Br. J. Dermatol. 2018, 178, 1111–1118. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Singh, T.R.R.; Morrow, D.I.J.; Woolfson, A.D. Microneedle-Mediated Intradermal Delivery. In Microneedle-Mediated Transdermal and Intradermal Drug Delivery; Donnelly, R.F., Singh, T.R.R., Morrow, D.I., Woolfson, A.D., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; pp. 113–151. [Google Scholar]
- Larrañeta, E.; Stewart, S.; Ervine, M.; Al-Kasasbeh, R.; Donnelly, R.F. Hydrogels for Hydrophobic Drug Delivery. Classification, Synthesis and Applications. J. Funct. Biomater. 2018, 9, 13. [Google Scholar] [CrossRef]
- Benson, H.A.; Grice, J.E.; Mohammed, Y.; Namjoshi, S.; Roberts, M.S. Topical and Transdermal Drug Delivery: From Simple Potions to Smart Technologies. Curr. Drug Deliv. 2019, 16, 444–460. [Google Scholar] [CrossRef] [PubMed]
- Ruela, A.L.M.; Perissinato, A.G.; Lino, M.E.D.S.; Mudrik, P.S.; Pereira, G.R. Evaluation of skin absorption of drugs from topical and transdermal formulations. Braz. J. Pharm. Sci. 2016, 52, 527–544. [Google Scholar] [CrossRef]
- Miao, T.; Wang, J.; Zeng, Y.; Liu, G.; Chen, X. Polysaccharide-Based Controlled Release Systems for Therapeutics Delivery and Tissue Engineering: From Bench to Bedside. Adv. Sci. 2018, 5, 1700513. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Park, J.H.; Robinson, J.R. Bioadhesive-based dosage forms: The next generation. J. Pharm. Sci. 2000, 89, 850–866. [Google Scholar] [CrossRef]
- Sogias, I.A.; Williams, A.C.; Khutoryanskiy, V.V. Why is Chitosan Mucoadhesive? Biomacromolecules 2008, 9, 1837–1842. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Bhuiyan, M.A.R.; Islam, M.N. Chitin and Chitosan: Structure, Properties and Applications in Biomedical Engineering. J. Polym. Environ. 2017, 25, 854–866. [Google Scholar] [CrossRef]
- Aguilar, A.; Zein, N.; Harmouch, E.; Hafdi, B.; Bornert, F.; Offner, D.; Clauss, F.; Fioretti, F.; Huck, O.; Benkirane-Jessel, N.; et al. Application of Chitosan in Bone and Dental Engineering. Molecules 2019, 24, 3009. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Yu, S.; Sun, B.; Gao, S.; Guo, S.; Zhao, K. Biomedical Applications of Chitosan and Its Derivative Nanoparticles. Polymers 2018, 10, 462. [Google Scholar] [CrossRef] [PubMed]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.S.; Liu, S.; Chen, Y.Y.; Meerasa, A.; Gu, F.X. Size-tunable nanoparticles composed of dextran-b-poly(D,L-lactide) for drug delivery applications. Nano Res. 2011, 5, 49–61. [Google Scholar] [CrossRef]
- Guo, P.; Martin, C.R.; Zhao, Y.; Ge, J.; Zare, R.N. General Method for Producing Organic Nanoparticles Using Nanoporous Membranes. Nano Lett. 2010, 10, 2202–2206. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xie, B.; Xia, K.; Zhao, C.; Li, Y.; Li, D.; Han, J. Facile synthesis and characterization of cross-linked chitosan quaternary ammonium salt membrane for antibacterial coating of piezoelectric sensors. Int. J. Biol. Macromol. 2018, 120, 745–752. [Google Scholar] [CrossRef]
- Sengiz, C.; Congur, G.; Eksin, E.; Erdem, A. Multiwalled Carbon Nanotubes-Chitosan Modified Single-Use Biosensors for Electrochemical Monitoring of Drug-DNA Interactions. Electroanalysis 2015, 27, 1855–1863. [Google Scholar] [CrossRef]
- Wei, P.-R.; Cheng, S.-H.; Liao, W.-N.; Kao, K.-C.; Weng, C.-F.; Lee, C.-H. Synthesis of chitosan-coated near-infrared layered double hydroxide nanoparticles for in vivo optical imaging. J. Mater. Chem. 2012, 22, 5503–5513. [Google Scholar] [CrossRef]
- Martínez-Martínez, M.; Rodríguez-Berna, G.; Bermejo, M.; Gonzalez-Alvarez, I.; Gonzalez-Alvarez, M.; Merino, V. Covalently crosslinked organophosphorous derivatives-chitosan hydrogel as a drug delivery system for oral administration of camptothecin. Eur. J. Pharm. Biopharm. 2019, 136, 174–183. [Google Scholar] [CrossRef]
- Kim, B.S.; Yeo, T.Y.; Yun, Y.H.; Lee, B.K.; Cho, Y.W.; Han, S.S. Facile preparation of biodegradable glycol chitosan hydrogels using divinyladipate as a crosslinker. Macromol. Res. 2009, 17, 734–738. [Google Scholar] [CrossRef]
- Gonçalves, V.L.; Laranjeira, M.C.M.; Fávere, V.T.; Pedrosa, R.C. Effect of crosslinking agents on chitosan microspheres in controlled release of diclofenac sodium. Polímeros 2005, 15, 6–12. [Google Scholar] [CrossRef]
- Iurciuc-Tincu, C.-E.; Atanase, L.I.; Ochiuz, L.; Jérôme, C.; Sol, V.; Martin, P.; Popa, M. Curcumin-loaded polysaccharides-based complex particles obtained by polyelectrolyte complexation and ionic gelation. I-Particles obtaining and characterization. Int. J. Biol. Macromol. 2020, 147, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, B.; Seitz, D.; Mencke, A.; Kokott, A.; Ziegler, G. Glutaraldehyde and oxidised dextran as crosslinker reagents for chitosan-based scaffolds for cartilage tissue engineering. J. Mater. Sci. Mater. Med. 2009, 20, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Huang-Lee, L.L.H.; Cheung, D.T.; Nimni, M.E. Biochemical changes and cytotoxicity associated with the degradation of polymeric glutaraldehyde derived crosslinks. J. Biomed. Mater. Res. 1990, 24, 1185–1201. [Google Scholar] [CrossRef]
- Aramwit, P.; Ekasit, S.; Yamdech, R. The development of non-toxic ionic-crosslinked chitosan-based microspheres as carriers for the controlled release of silk sericin. Biomed. Microdevices 2015, 17. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.K.; Halder, M.; Srivastava, U.; Singh, Y. Antibacterial PEG-Chitosan Hydrogels for Controlled Antibiotic/Protein Delivery. ACS Appl. Bio Mater. 2019, 2, 5313–5322. [Google Scholar] [CrossRef]
- Akakuru, O.U.; Isiuku, B.O. Chitosan Hydrogels and their Glutaraldehyde-Crosslinked Counterparts as Potential Drug Release and Tissue Engineering Systems-Synthesis, Characterization, Swelling Kinetics and Mechanism. J. Phys. Chem. Biophys. 2017, 7, 100256. Available online: https://www.longdom.org/open-access/chitosan-hydrogels-and-their-glutaraldehydecrosslinked-counterpartsas-potential-drug-release-and-tissue-engineering-systems--synth-2161-0398-1000256.pdf (accessed on 1 November 2020).
- Baron, R.I.; Culica, M.E.; Biliuta, G.; Bercea, M.; Gherman, S.; Zavastin, D.; Ochiuz, L.; Avadanei, M.; Coseri, S. Physical Hydrogels of Oxidized Polysaccharides and Poly(Vinyl Alcohol) for Wound Dressing Applications. Materials 2019, 12, 1569. [Google Scholar] [CrossRef]
- George, D.; Maheswari, P.U.; Begum, K.M.S. Synergic formulation of onion peel quercetin loaded chitosan-cellulose hydrogel with green zinc oxide nanoparticles towards controlled release, biocompatibility, antimicrobial and anticancer activity. Int. J. Biol. Macromol. 2019, 132, 784–794. [Google Scholar] [CrossRef]
- Patrulea, V.; Ostafe, V.; Borchard, G.; Jordan, O. Chitosan as a starting material for wound healing applications. Eur. J. Pharm. Biopharm. 2015, 97, 417–426. [Google Scholar] [CrossRef]
- Larasati, Y.A.; Yoneda-Kato, N.; Nakamae, I.; Yokoyama, T.; Meiyanto, E.; Kato, J.-Y. Curcumin targets multiple enzymes involved in the ROS metabolic pathway to suppress tumor cell growth. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Iurciuc-Tincu, C.-E.; Cretan, M.S.; Purcar, V.; Popa, M.; Daraba, O.M.; Atanase, L.I.; Ochiuz, L. Drug Delivery System Based on pH-Sensitive Biocompatible Poly(2-vinyl pyridine)-b-poly(ethylene oxide) Nanomicelles Loaded with Curcumin and 5-Fluorouracil. Polymers 2020, 12, 1450. [Google Scholar] [CrossRef] [PubMed]
- (Tincu), C.-E.I.; Atanase, L.; Jérôme, C.; Sol, V.; Martin, P.; Popa, M.; Ochiuz, L. Polysaccharides-Based Complex Particles’ Protective Role on the Stability and Bioactivity of Immobilized Curcumin. Int. J. Mol. Sci. 2021, 22, 3075. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; McClements, D.J. Formulation of More Efficacious Curcumin Delivery Systems Using Colloid Science: Enhanced Solubility, Stability, and Bioavailability. Molecules 2020, 25, 2791. [Google Scholar] [CrossRef]
- Rakotoarisoa, M.; Angelov, B.; Garamus, V.M.; Angelova, A. Curcumin- and Fish Oil-Loaded Spongosome and Cubosome Nanoparticles with Neuroprotective Potential against H2O2-Induced Oxidative Stress in Differentiated Human SH-SY5Y Cells. ACS Omega 2019, 4, 3061–3073. [Google Scholar] [CrossRef]
- Zamarioli, C.M.; Martins, R.M.; Carvalho, E.C.; Freitas, L.A. Nanoparticles containing curcuminoids (Curcuma longa): Development of topical delivery formulation. Rev. Bras. Farm. 2015, 25, 53–60. [Google Scholar] [CrossRef]
- Anjali, T. Modification of carboxymethyl cellulose through oxidation. Carbohydr. Polym. 2012, 87, 457–460. [Google Scholar] [CrossRef]
- Hosoya, T.; Bacher, M.; Potthast, A.; Elder, T.; Rosenau, T. Insights into degradation pathways of oxidized anhydroglucose units in cellulose by β-alkoxy-elimination: A combined theoretical and experimental approach. Cellulose 2018, 25, 3797–3814. [Google Scholar] [CrossRef]
- Li, H.; Wu, B.; Mu, C.; Lin, W. Concomitant degradation in periodate oxidation of carboxymethyl cellulose. Carbohydr. Polym. 2011, 84, 881–886. [Google Scholar] [CrossRef]
- Mansouri, L.; Benghanem, S.; Elkolli, M.; Mahmoud, B. Chemical and biological behaviors of hydrogels based on oxidized carboxymethylcellulose coupled to chitosan. Polym. Bull. 2019, 77, 85–105. [Google Scholar] [CrossRef]
- Lan, W.; He, L.; Liu, Y. Preparation and Properties of Sodium Carboxymethyl Cellulose/Sodium Alginate/Chitosan Composite Film. Coatings 2018, 8, 291. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, R.; Cao, J. Characterization of surface chemistry and crystallization behavior of polypropylene composites reinforced with wood flour, cellulose, and lignin during accelerated weathering. Appl. Surf. Sci. 2015, 332, 253–259. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to Read and Interpret FTIR Spectroscope of Organic Material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Venkateshwarlu, G.; Subrahmanyam, B. Conformations of α,β-unsaturated ketones: An IR spectroscopic study. Proc. Indian Acad. Sci. (Chem. Sci.) 1990, 102, 45–50. [Google Scholar] [CrossRef]
- Afinjuomo, F.; Fouladian, P.; Barclay, T.G.; Song, Y.; Petrovsky, N.; Garg, S. Influence of Oxidation Degree on the Physicochemical Properties of Oxidized Inulin. Polymers 2020, 12, 1025. [Google Scholar] [CrossRef] [PubMed]
- Lisman, A.; Butruk, B.; Wasiak, I.; Ciach, T. Dextran/Albumin hydrogel sealant for Dacron(R) vascular prosthesis. J. Biomater. Appl. 2014, 28, 1386–1396. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Zhang, L.; Zhang, B.; He, M.; Shi, W.; Yang, S.; Cui, Y.; Yin, G. Preparation and Characterization of Edible Dialdehyde Carboxymethyl Cellulose Crosslinked Feather Keratin Films for Food Packaging. Polymers 2020, 12, 158. [Google Scholar] [CrossRef] [PubMed]
- Dacrory, S.; Abou-Yousef, H.; Kamel, S.; Abou-Zeid, R.E.; Abdel-Aziz, M.S.; ElBadry, M. Functionalization and cross-linking of carboxymethyl cellulose in aqueous media. Cellul. Chem. Technol. 2019, 53, 23–33. [Google Scholar] [CrossRef]
- Dyatlov, V.; Gumnikova, V.; Grebeneva, T.; Kruppa, I.; Rustamov, I.; Kireev, V.; Maleev, V. Study of the Chemical Structure of Dialdehyde Carboxymethyl Cellulose Produced by Periodate Oxidation under Different Conditions. Int. Polym. Sci. Technol. 2015, 42, 19–26. [Google Scholar] [CrossRef]
- Gu, H.; He, J.; Huang, Y.; Guo, Z. Fabrication of oxidized sodium carboxymethylcellulose from viscose fibers and their viscosity behaviors. Fibers Polym. 2013, 14, 1266–1270. [Google Scholar] [CrossRef]
- Kono, H.; Oshima, K.; Hashimoto, H.; Shimizu, Y.; Tajima, K. NMR characterization of sodium carboxymethyl cellulose: Substituent distribution and mole fraction of monomers in the polymer chains. Carbohydr. Polym. 2016, 146, 1–9. [Google Scholar] [CrossRef]
- Hou, Q.X.; Liu, W.; Liu, Z.H.; Bai, L.L. Characteristics of Wood Cellulose Fibers Treated with Periodate and Bisulfite. Ind. Eng. Chem. Res. 2007, 46, 7830–7837. [Google Scholar] [CrossRef]
- Eremeeva, T.; Bykova, T. SEC of mono-carboxymethyl cellulose (CMC) in a wide range of pH.; Mark–Houwink constants. Carbohydr. Polym. 1998, 36, 319–326. [Google Scholar] [CrossRef]
- Marin, L.; Harabagiu, V.; Van Der Lee, A.; Arvinte, A.; Barboiu, M. Structure-directed functional properties of symmetrical and unsymmetrical Br-substituted Schiff-bases. J. Mol. Struct. 2013, 1049, 377–385. [Google Scholar] [CrossRef]
- Cheaburu-Yilmaz, C.N.; Dumitriu, R.P.; Nistor, M.-T.; Lupusoru, C.; Popa, M.I.; Profire, L.; Vasile, C. Biocompatible and Biodegradable Chitosan/Clay Nanocomposites as New Carriers for Theophylline Controlled Release. Br. J. Pharm. Res. 2015, 6, 228–254. [Google Scholar] [CrossRef]
- Dovbeshko, G.I.; Gridina, N.Y.; Kruglova, E.B.; Pashchuk, O.P. FTIR spectroscopy studies of nucleic acid damage. Talanta 2000, 53, 233–246. [Google Scholar] [CrossRef]
- Fukuda, H. Polyelectrolyte complexes of chitosan with sodium carboxymethylcellulose. Bull. Chem. Soc. Jpn. 1980, 53, 837–840. [Google Scholar] [CrossRef]
- Benghanem, S.; Chetouani, A.; Elkolli, M.; Bounekhel, M.; Benachour, D. Grafting of oxidized carboxymethyl cellulose with hydrogen peroxide in presence of Cu(II) to chitosan and biological elucidation. Biocybern. Biomed. Eng. 2017, 37, 94–102. [Google Scholar] [CrossRef]
- Lee, S.H. Preparation and Evaluation of Crosslinked Polyelectrolyte Complex Membranes. Polym. J. 2000, 32, 716–721. [Google Scholar] [CrossRef]
- Fukuda, H.; Kikuchi, Y. Polyelectrolyte Complexes of Sodium Carboxymethylcellulose with Chitosan. Makromol. Chem. 1979, 180, 1631–1633. Available online: https://onlinelibrary.wiley.com/doi/pdf/10.1002/macp.1979.021800629 (accessed on 1 March 2021). [CrossRef]
- Strätz, J.; Liedmann, A.; Trutschel, M.-L.; Mäder, K.; Groth, T.; Fischer, S. Development of hydrogels based on oxidized cellulose sulfates and carboxymethyl chitosan. Cellulose 2019, 26, 7371–7382. [Google Scholar] [CrossRef]
- Lü, S.; Liu, M.; Ni, B. An injectable oxidized carboxymethylcellulose/N-succinyl-chitosan hydrogel system for protein delivery. Chem. Eng. J. 2010, 160, 779–787. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Wang, Q.Z.; Chen, X.G.; Liu, N.; Wang, S.X.; Liu, C.S.; Meng, X.H. Protonation constants of chitosan with different molecular weight and degree of deacetylation. Carbohydr. Polym. 2006, 65, 194–201. [Google Scholar] [CrossRef]
- Szymańska, E.; Winnicka, K. Stability of Chitosan—A Challenge for Pharmaceutical and Biomedical Applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Sandhu, S.K.; Sharma, I.; Kaur, I.P. Development and Evaluation of Curcumin-loaded Elastic Vesicles as an Effective Topical Anti-inflammatory Formulation. AAPS PharmSciTech 2014, 16, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Ternullo, S.; Werning, L.V.S.; Holsæter, A.M.; Škalko-Basnet, N. Curcumin-In-Deformable Liposomes-In-Chitosan-Hydrogel as a Novel Wound Dressing. Pharmaceutics 2019, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Kulikowska, A.; Wasiak, I.; Ciach, T. Carboxymethyl Cellulose Oxidation to Form Aldehyde Groups. Biomed. Eng. 2013, 4, 11–18. Available online: http://yadda.icm.edu.pl/baztech/element/bwmeta1.element.baztech-4e71d122-c444-458c-9083-57e262ced33f (accessed on 1 May 2018).
- Caner, C.; Vergano, P.J.; Wiles, J.L. Chitosan Film Mechanical and Permeation Properties as Affected by Acid, Plasticizer, and Storage. J. Food Sci. 2006, 63, 1049–1053. [Google Scholar] [CrossRef]
- McSweeny, J.D.; Rowell, R.M.; Chen, G.C.; Eberhardt, T.L.; Min, S.-H. Periodate and hypobromite modification of southern pine wood to improve sorption of copper ion. Bioresources 2008, 3, 204–216. Available online: https://bioresources.cnr.ncsu.edu//BioRes_03/BioRes_03_1_0204_McSweeny_RCEM_Periodate_Pine_Copper.pdf (accessed on 1 May 2018).
- Dean, J.A. Lange’s Handbook of Chemistry, 15th ed.; McGraw-Hill Book Company, Inc.: New York, NY, USA; Toronto, ON, Canada; London, UK, 1999; p. 1292. Available online: http://depa.fquim.unam.mx/amyd/archivero/ManualdeLange_9164.pdf (accessed on 1 May 2018).
- Choi, C.W.; Kim, S.C.; Hwang, S.S.; Choi, B.K.; Ahn, H.J.; Lee, M.Y.; Park, S.H.; Kim, S.K. Antioxidant activity and free radical scavenging capacity between Korean medicinal plants and flavonoids by assay-guided comparison. Plant Sci. 2002, 163, 1161–1168. [Google Scholar] [CrossRef]



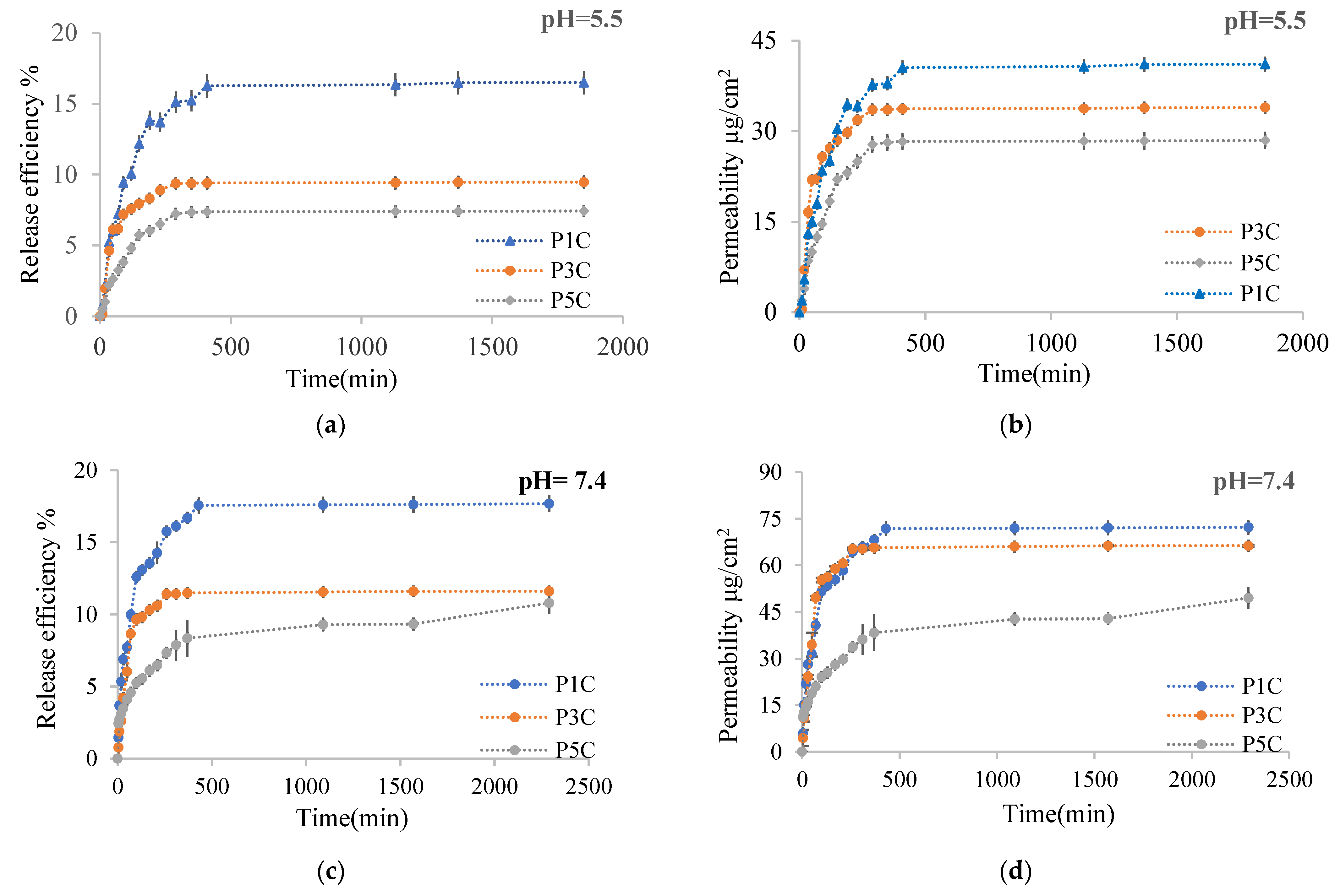
| Sample | Ratio | Efabs% on Skin Membrane, % | Ef% in the Receptor, % | Total Ef, % | P (µg/cm2) in the Receptor (after 48 h) | P (µg/cm2) in Skin Membrane, 48 h | Total P (µg/cm2), 48 h | n | R2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH 5.5 | pH 7.4 | pH 5.5 | pH 7.4 | pH 5.5 | pH 7.4 | pH 5.5 | pH 7.4 | pH 5.5 | pH 7.4 | pH 5.5 | pH 7.4 | pH 5.5 | pH 7.4 | pH 5.5 | pH 7.4 | ||
| P1C | 0.25:1 | 47 | 8.6 | 16.5 | 18 | 64 | 26 | 41.1 | 71 | 117 | 35.1 | 158 | 106 | 0.63 | 0.43 | 0.92 | 0.97 |
| P3C | 0.5:1 | 31.6 | 3.6 | 9.5 | 12 | 41 | 15 | 33.9 | 66.3 | 113 | 20.4 | 147 | 87 | 0.48 | 0.53 | 0.81 | 0.91 |
| P5C | 1:01 | 23.3 | 3.2 | 7.4 | 11 | 31 | 14 | 28.5 | 49.5 | 90 | 14.9 | 118 | 64 | 0.53 | 0.31 | 0.97 | 0.99 |
| Sample | IC50, µmoles/mL |
|---|---|
| Ascorbic acid | 0.031 ± 0.00053 |
| Curcumin | 0.051 ± 0.00033 |
| P1C | 0.054 ± 0.00039 |
| P3C | 0.046 ± 0.00039 |
| P5C | 0.039 ± 0.00012 |
| M1 | 0.082 ± 0.0032 |
| M3 | 0.081 ± 0.00041 |
| M5 | 0.092 ± 0.00846 |
| P5 + M5 | 0.035 ± 0.00012 |
| CS | 0.4 ± 0.0103 |
| P5 (without curcumin) | 10.02 ± 0.28 |
| Samples Code * | The Molar Ratio (-CH=O/-NH2) | Moles of Aldehyde Groups from CMCOx (×103) |
|---|---|---|
| P1 | 0.25:1 | 0.4375 |
| P2 | 0.375:1 | 0.656 |
| P3 | 0.5:1 | 0.875 |
| P4 | 0.75:1 | 1.3125 |
| P5 | 1:1 | 1.75 |
| P6 | 1.5:1 | 2.625 |
| P7 | 2:1 | 3.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dellali, M.; Iurciuc, C.E.; Savin, C.L.; Spahis, N.; Djennad, M.; Popa, M. Hydrogel Films Based on Chitosan and Oxidized Carboxymethylcellulose Optimized for the Controlled Release of Curcumin with Applications in Treating Dermatological Conditions. Molecules 2021, 26, 2185. https://doi.org/10.3390/molecules26082185
Dellali M, Iurciuc CE, Savin CL, Spahis N, Djennad M, Popa M. Hydrogel Films Based on Chitosan and Oxidized Carboxymethylcellulose Optimized for the Controlled Release of Curcumin with Applications in Treating Dermatological Conditions. Molecules. 2021; 26(8):2185. https://doi.org/10.3390/molecules26082185
Chicago/Turabian StyleDellali, Mohamed, Camelia Elena Iurciuc (Tincu), Corina Lenuța Savin, Nawel Spahis, M’hamed Djennad, and Marcel Popa. 2021. "Hydrogel Films Based on Chitosan and Oxidized Carboxymethylcellulose Optimized for the Controlled Release of Curcumin with Applications in Treating Dermatological Conditions" Molecules 26, no. 8: 2185. https://doi.org/10.3390/molecules26082185
APA StyleDellali, M., Iurciuc, C. E., Savin, C. L., Spahis, N., Djennad, M., & Popa, M. (2021). Hydrogel Films Based on Chitosan and Oxidized Carboxymethylcellulose Optimized for the Controlled Release of Curcumin with Applications in Treating Dermatological Conditions. Molecules, 26(8), 2185. https://doi.org/10.3390/molecules26082185




.png)



