Unexpectedly Long Lifetime of the Excited State of Benzothiadiazole Derivative and Its Adducts with Lewis Acids
Abstract
1. Introduction
2. Materials and Methods
2.1. General
2.2. Quantum-Chemical Calculations
2.3. Syntheses
2.4. X-ray Diffraction Analyses
3. Results and Discussion
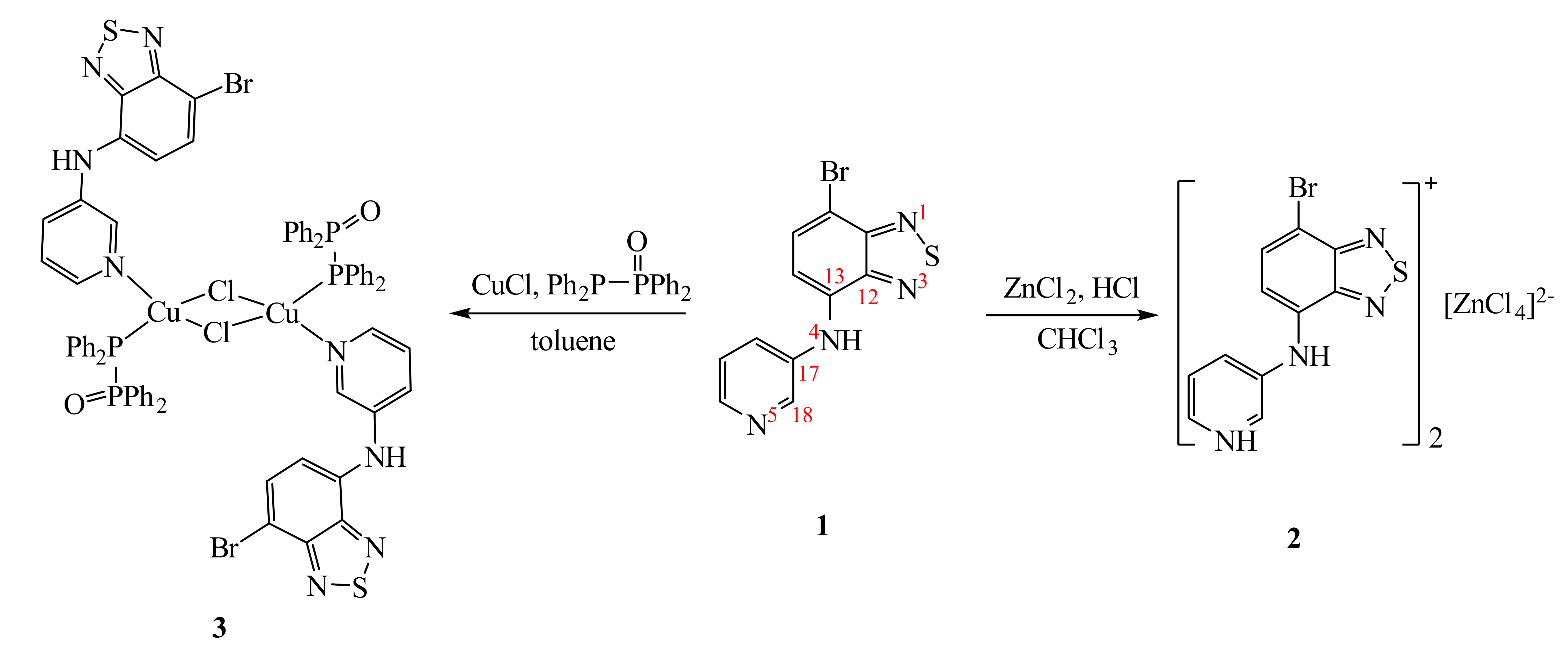
3.1. Synthesis and Structure
3.2. Photophysical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Godfroy, M.; Liotier, J.; Mwalukuku, V.M.; Joly, D.; Huaulmé, Q.; Cabau, L.; Aumaitre, C.; Kervella, Y.; Narbey, S.; Oswald, F.; et al. Benzothiadiazole-based photosensitizers for efficient and stable dye-sensitized solar cells and 8.7% efficiency semi-transparent mini-modules. Sustain. Energy Fuels 2021, 5, 144–153. [Google Scholar] [CrossRef]
- Li, Y.; Qian, Q.; Ling, S.; Fan, T.; Zhang, C.; Zhu, X.; Zhang, Q.; Zhang, Y.; Zhang, J.; Yu, S.; et al. A benzothiadiazole-containing π-conjugated small molecule as promising element for nonvolatile multilevel resistive memory device. J. Solid State Chem. 2021, 294, 121850. [Google Scholar] [CrossRef]
- Neto, B.A.D.; Carvalho, P.H.P.R.; Correa, J.R. Benzothiadiazole Derivatives as Fluorescence Imaging Probes: Beyond Classical Scaffolds. Acc. Chem. Res. 2015, 48, 1560–1569. [Google Scholar] [CrossRef]
- Pritchina, E.A.; Gritsan, N.P.; Rakitin, O.A.; Zibarev, A.V. 2,1,3-Benzochalcogenadiazoles: Regularities and peculiarities over a whole chalcogen pentad O, S, Se, Te and Po. Targets Heterocycl. Syst. 2019, 23, 143–154. [Google Scholar] [CrossRef]
- Rakitin, O.A. Fused 1,2,5-thia- and 1,2,5-selenadiazoles: Synthesis and application in materials chemistry. Tetrahedron Lett. 2020, 61, 152230. [Google Scholar] [CrossRef]
- Martín, R.; Prieto, P.; Carrillo, J.R.; Rodríguez, A.M.; De Cozar, A.; Boj, P.G.; Díaz-García, M.A.; Ramírez, M.G. Design, synthesis and amplified spontaneous emission of 1,2,5-benzothiadiazole derivatives. J. Mater. Chem. C 2019, 7, 9996–10007. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Lu, P.; Wang, Y. Preparation and Photoluminescent Properties of Three 5-Amino Benzothiadiazoles (5-amBTDs). Chem. Asian J. 2020, 15, 3519–3526. [Google Scholar] [CrossRef] [PubMed]
- Korshunov, V.M.; Chmovzh, T.N.; Golovanov, I.S.; Knyazeva, E.A.; Mikhalchenko, L.V.; Saifutyarov, R.S.; Avetisov, I.C.; Woollins, J.D.; Taydakov, I.V.; Rakitin, O.A. Candle light-style OLEDs with benzochalcogenadiazoles cores. Dye. Pigment. 2021, 185, 108917. [Google Scholar] [CrossRef]
- Chulanova, E.A.; Radiush, E.A.; Shundrina, I.K.; Bagryanskaya, I.Y.; Semenov, N.A.; Beckmann, J.; Gritsan, N.P.; Zibarev, A.V. Lewis Ambiphilicity of 1,2,5-Chalcogenadiazoles for Crystal Engineering: Complexes with Crown Ethers. Cryst. Growth Des. 2020, 20, 5868–5879. [Google Scholar] [CrossRef]
- Gautam, P.; Maragani, R.; Mobin, S.M.; Misra, R. Reversible mechanochromism in dipyridylamine-substituted unsymmetrical benzothiadiazoles. RSC Adv. 2014, 4, 52526–52529. [Google Scholar] [CrossRef]
- Shimogawa, H.; Yoshikawa, O.; Aramaki, Y.; Murata, M.; Wakamiya, A.; Murata, Y. 4,7-Bis[3-(dimesitylboryl)thien-2-yl]benzothiadiazole: Solvato-, Thermo-, and Mechanochromism Based on the Reversible Formation of an Intramolecular B−N Bond. Chem. A Eur. J. 2017, 23, 3784–3791. [Google Scholar] [CrossRef] [PubMed]
- Pazini, A.; Maqueira, L.; Santos, F.D.S.; Barreto, A.R.J.; Carvalho, R.D.S.; Valente, F.M.; Back, D.; Aucélio, R.Q.; Cremona, M.; Rodembusch, F.S.; et al. Designing highly luminescent aryloxy-benzothiadiazole derivatives with aggregation-induced enhanced emission. Dye. Pigment. 2020, 178, 108377. [Google Scholar] [CrossRef]
- Sukhikh, T.S.; Ogienko, D.S.; Bashirov, D.A.; Konchenkoa, S.N. Luminescent complexes of 2,1,3-benzothiadiazole derivatives. Russ. Chem. Bull. 2019, 68, 651–661. [Google Scholar] [CrossRef]
- Sukhikh, T.S.; Khisamov, R.M.; Bashirov, D.A.; Komarov, V.Y.; Molokeev, M.S.; Ryadun, A.A.; Benassi, E.; Konchenko, S.N. Tuning of the Coordination and Emission Properties of 4-Amino-2,1,3-Benzothiadiazole by Introduction of Diphenylphosphine Group. Cryst. Growth Des. 2020, 20, 5796–5807. [Google Scholar] [CrossRef]
- Khisamov, R.; Sukhikh, T.; Bashirov, D.; Ryadun, A.; Konchenko, S. Structural and Photophysical Properties of 2,1,3-Benzothiadiazole-Based Phosph(III)azane and Its Complexes. Molecules 2020, 25, 2428. [Google Scholar] [CrossRef]
- Jiang, Q.-J.; Lin, J.-Y.; Hu, Z.-J.; Hsiao, V.K.S.; Chung, M.-Y.; Wu, J.-Y. Luminescent Zinc(II) Coordination Polymers of Bis(pyridin-4-yl)benzothiadiazole and Aromatic Polycarboxylates for Highly Selective Detection of Fe(III) and High-Valent Oxyanions. Cryst. Growth Des. 2021. [Google Scholar] [CrossRef]
- Shen, K.; Ju, Z.; Qin, L.; Wang, T.; Zheng, H. Two stable 3D porous metal-organic frameworks with high selectivity for detection of PA and metal ions. Dyes Pigm. 2017, 136, 515–521. [Google Scholar] [CrossRef]
- Qiu, C.-Q.; Li, L.-Q.; Yao, S.-L.; Liu, S.-J.; Xu, H.; Zheng, T.-F. Two benzothiadiazole-based compounds as multifunctional fluorescent sensors for detection of organic amines and anions. Polyhedron 2021, 199, 115100. [Google Scholar] [CrossRef]
- Sukhikh, T.S.; Khisamov, R.M.; Bashirov, D.A.; Kovtunova, L.M.; Kuratieva, N.V.; Konchenko, S.N. Substituent Effect on the Structure and Photophysical Properties of Phenylamino- and Pyridylamino-2,1,3-Benzothiadiazoles. J. Struct. Chem. 2019, 60, 1670–1680. [Google Scholar] [CrossRef]
- Mocanu, T.; Plyuta, N.; Cauchy, T.; Andruh, M.; Avarvari, N. Dimensionality Control in Crystalline Zinc(II) and Silver(I) Complexes with Ditopic Benzothiadiazole-Dipyridine Ligands. Chemistry 2021, 3, 269–287. [Google Scholar] [CrossRef]
- Kubelka, P. New Contributions to the Optics of Intensely Light-Scattering Materials Part I. J. Opt. Soc. Am. 1948, 38, 448. [Google Scholar] [CrossRef] [PubMed]
- Neese, F. Software update: The ORCA program system, version 4.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2018, 8, 8. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Hansen, A.; Becker, U. Efficient, approximate and parallel Hartree–Fock and hybrid DFT calculations. A ‘chain-of-spheres’ algorithm for the Hartree–Fock exchange. Chem. Phys. 2009, 356, 98–109. [Google Scholar] [CrossRef]
- Plasser, F. TheoDORE: A toolbox for a detailed and automated analysis of electronic excited state computations. J. Chem. Phys. 2020, 152, 084108. [Google Scholar] [CrossRef]
- McKechnie, J.; Payne, D.S.; Sim, W. Tetraphenyldiphosphine monoxide. J. Chem. Soc. 1965, 3500–3501. [Google Scholar] [CrossRef]
- Bruker Apex3 Software Suite: Apex3, SADABS-2016/2 and SAINT, Version 2017.3-0; Bruker AXS Inc.: Madison, WI, USA, 2017.
- Sheldrick, G.M. SHELXT- Integrated space-group and crystal-structure determination. Acta Cryst. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Prescher, C.; Prakapenka, V.B. DIOPTAS: A program for reduction of two-dimensional X-ray diffraction data and data exploration. High Press. Res. 2015, 35, 223–230. [Google Scholar] [CrossRef]
- Coelho, A.A. TOPASandTOPAS-Academic: An optimization program integrating computer algebra and crystallographic objects written in C++. J. Appl. Crystallogr. 2018, 51, 210–218. [Google Scholar] [CrossRef]
- Sukhikh, T.; Komarov, V.; Konchenko, S.; Benassi, E. The hows and whys of peculiar coordination of 4-amino-2,1,3-benzothiadiazole. Polyhedron 2018, 139, 33–43. [Google Scholar] [CrossRef]
- Zehra, S.; Tabassum, S.; Al-Lohedan, H.A.; Arjmand, F. A zwitterionic Zn(II) benzothiazole nanohybrid conjugate as hydrolytic DNA cleavage agent. Inorg. Chem. Commun. 2018, 93, 69–72. [Google Scholar] [CrossRef]
- Pons, R.; Ibáñez, C.; Buades, A.B.; Franconetti, A.; Garcia-Raso, A.; Fiol, J.J.; Terrón, A.; Molins, E.; Frontera, A. Synthesis, X-ray characterization and density functional theory studies of N 6 -benzyl-N 6 -methyladenine–M(II) complexes (M = Zn, Cd): The prominent role of π–π, C–H···π and anion–π interactions. Appl. Organomet. Chem. 2019, 33, e4906. [Google Scholar] [CrossRef]
- Medeiros, G.A.; Correa, J.R.; De Andrade, L.P.; Lopes, T.O.; De Oliveira, H.C.; Diniz, A.B.; Menezes, G.B.; Rodrigues, M.O.; Neto, B.A. A benzothiadiazole-quinoline hybrid sensor for specific bioimaging and surgery procedures in mice. Sens. Actuators B Chem. 2021, 328, 128998. [Google Scholar] [CrossRef]
- Baranov, D.S.; Krivenko, O.L.; Kazantsev, M.S.; Nevostruev, D.A.; Kobeleva, E.S.; Zinoviev, V.A.; Dmitriev, A.A.; Gritsan, N.P.; Kulik, L.V. Synthesis of 2,2′-[2,2′-(arenediyl)bis(anthra[2,3-b]thiophene-5,10-diylidene)]tetrapropanedinitriles and their performance as non-fullerene acceptors in organic photovoltaics. Synth. Met. 2019, 255, 116097. [Google Scholar] [CrossRef]
- Passos, S.T.; Souza, G.C.; Brandão, D.C.; Machado, D.F.; Grisolia, C.K.; Correia, J.R.; da Silva, W.A.; Neto, B.A. Plasma membrane staining with fluorescent hybrid benzothiadiazole and coumarin derivatives: Tuning the cellular selection by molecular design. Dye. Pigment. 2021, 186, 109005. [Google Scholar] [CrossRef]
- Jia, W.; Wang, Q.; Shi, H.; An, Z.; Huang, W. Manipulating the Ultralong Organic Phosphorescence of Small Molecular Crystals. Chem. A Eur. J. 2019, 26, 4437–4448. [Google Scholar] [CrossRef]
- Zhan, G.; Liu, Z.; Bian, Z.; Huang, C. Recent Advances in Organic Light-Emitting Diodes Based on Pure Organic Room Temperature Phosphorescence Materials. Front. Chem. 2019, 7, 305. [Google Scholar] [CrossRef]
- Li, Q.; Tang, Y.; Hu, W.; Li, Z. Fluorescence of Nonaromatic Organic Systems and Room Temperature Phosphorescence of Organic Luminogens: The Intrinsic Principle and Recent Progress. Small 2018, 14, e1801560. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Hamzehpoor, E.; Sakai-Otsuka, Y.; Jadhav, T.; Perepichka, D.F. A Pure-Red Doublet Emission with 90 % Quantum Yield: Stable, Colorless, Iodinated Triphenylmethane Solid. Angew. Chem. 2020, 132, 23230–23234. [Google Scholar] [CrossRef]
- Shi, H.; Zou, L.; Huang, K.; Wang, H.; Sun, C.; Wang, S.; Ma, H.; He, Y.; Wang, J.; Yu, H.-D.; et al. A Highly Efficient Red Metal-free Organic Phosphor for Time-Resolved Luminescence Imaging and Photodynamic Therapy. ACS Appl. Mater. Interfaces 2019, 11, 18103–18110. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, G.D.; Sazama, G.T.; Wu, T.C.; Baldo, M.A.; Swager, T.M. Red Phosphorescence from Benzo[2,1,3]thiadiazoles at Room Temperature. J. Org. Chem. 2016, 81, 4789–4796. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Du, L.; Gong, Y.; Liu, Y.; Yu, C.; Wei, C.; Yuan, W.Z. Crystallization-Induced Red Phosphorescence and Grinding-Induced Blue-Shifted Emission of a Benzobis(1,2,5-thiadiazole)–Thiophene Conjugate. ACS Omega 2019, 4, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Tian, D.; Gao, P.; Wang, K.; Li, Y.; Shu, X.; Zhu, J.; Zhao, Q. Cell-Penetrating Peptides Transport Noncovalently Linked Thermally Activated Delayed Fluorescence Nanoparticles for Time-Resolved Luminescence Imaging. J. Am. Chem. Soc. 2018, 140, 17484–17491. [Google Scholar] [CrossRef]
- Mohanty, M.E.; Madhu, C.; Reddy, V.L.; Paramasivam, M.; Bangal, P.R.; Rao, V.J. Direct observation of the rise of delayed fluorescence in dithienylbenzothiadiazole and its role in the excited state dynamics of a donor–acceptor–donor molecule. Phys. Chem. Chem. Phys. 2017, 19, 9118–9127. [Google Scholar] [CrossRef]
- Livshits, M.Y.; He, W.; Zhang, Z.; Qin, Y.; Rack, J.J. Triplet Excited-State Energetics and Dynamics in Molecular “Roller Wheels”. J. Phys. Chem. C 2019, 123, 16556–16564. [Google Scholar] [CrossRef]
- Goswami, S.; Winkel, R.W.; Schanze, K.S. Photophysics and Nonlinear Absorption of Gold(I) and Platinum(II) Donor–Acceptor–Donor Chromophores. Inorg. Chem. 2015, 54, 10007–10014. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Z.; Wang, X.; He, J.; Wu, J.; Liu, H.; Song, J.; Qu, J.; Chan, W.T.-K.; Wong, W.-Y. Achieving NIR Emission for Donor–Acceptor Type Platinum(II) Complexes by Adjusting Coordination Position with Isomeric Ligands. Inorg. Chem. 2018, 57, 14208–14217. [Google Scholar] [CrossRef]
- Chan, K.T.; Tong, G.S.M.; To, W.-P.; Yang, C.; Du, L.; Phillips, D.L.; Che, C.-M. The interplay between fluorescence and phosphorescence with luminescent gold(i) and gold(iii) complexes bearing heterocyclic arylacetylide ligands. Chem. Sci. 2016, 8, 2352–2364. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.N.; Sil, A.; Patra, S.K. Achieving yellow emission by varying the donor/acceptor units in rod-shaped fluorenyl-alkynyl based?-conjugated oligomers and their binuclear gold(i) alkynyl complexes. Dalton Trans. 2017, 46, 5918–5929. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liu, N.; Ying, L.; Yang, W.; Wu, H.; Xu, W.; Cao, Y. Novel white-light-emitting polyfluorenes with benzothiadiazole and Ir complex on the backbone. Polymer 2009, 50, 1430–1437. [Google Scholar] [CrossRef]
- Sun, L.; Chen, Y.; Kuang, S.; Li, G.; Guan, R.; Liu, J.; Ji, L.; Chao, H. Iridium(III) Anthraquinone Complexes as Two-Photon Phosphorescence Probes for Mitochondria Imaging and Tracking under Hypoxia. Chem. A Eur. J. 2016, 22, 8955–8965. [Google Scholar] [CrossRef] [PubMed]
- Smithen, D.A.; Monro, S.; Pinto, M.; Roque, J.; Diaz-Rodriguez, R.M.; Yin, H.; Cameron, C.G.; Thompson, A.; McFarland, S.A. Bis[pyrrolyl Ru(ii)] triads: A new class of photosensitizers for metal–organic photodynamic therapy. Chem. Sci. 2020, 11, 12047–12069. [Google Scholar] [CrossRef] [PubMed]
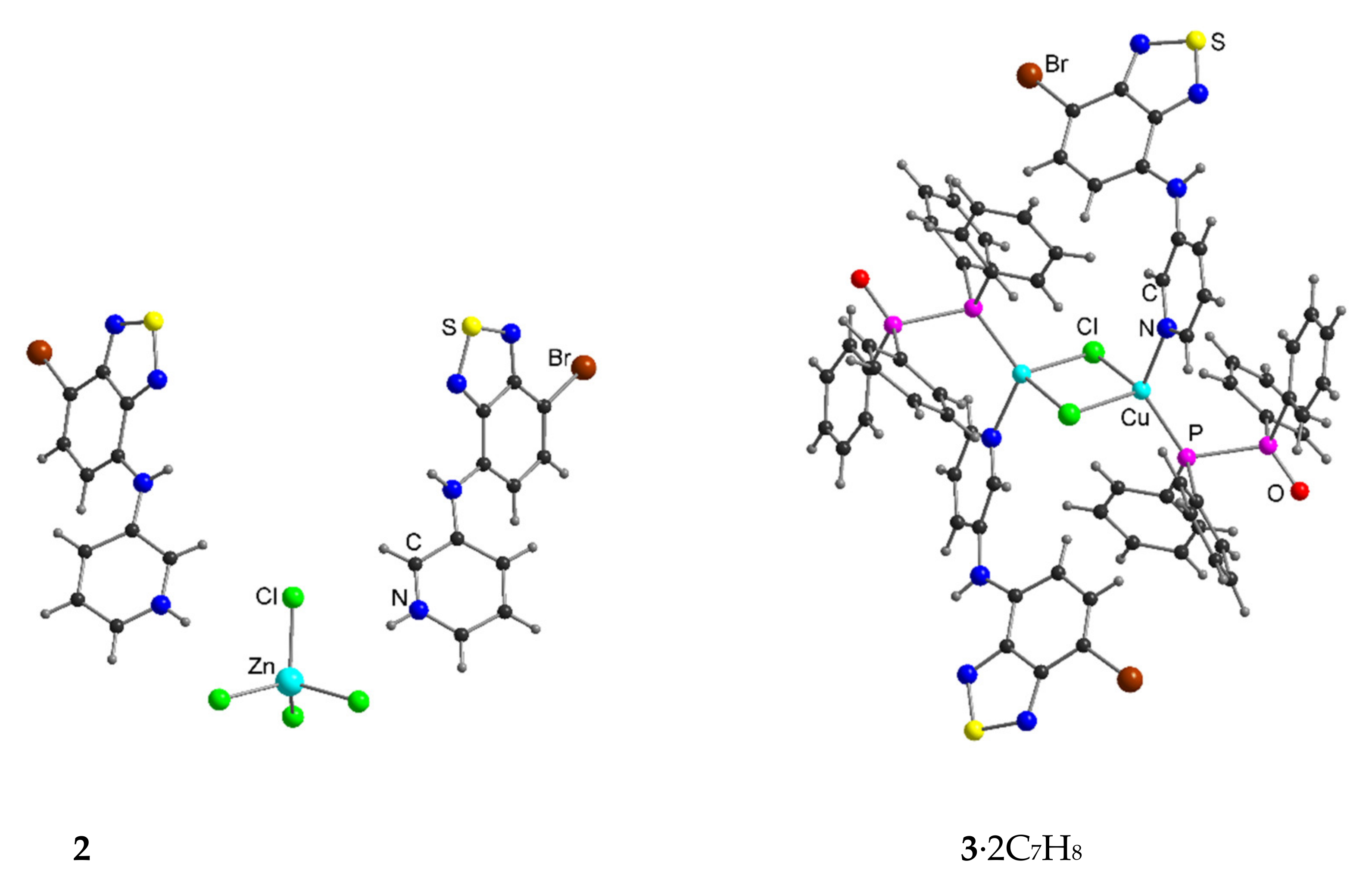
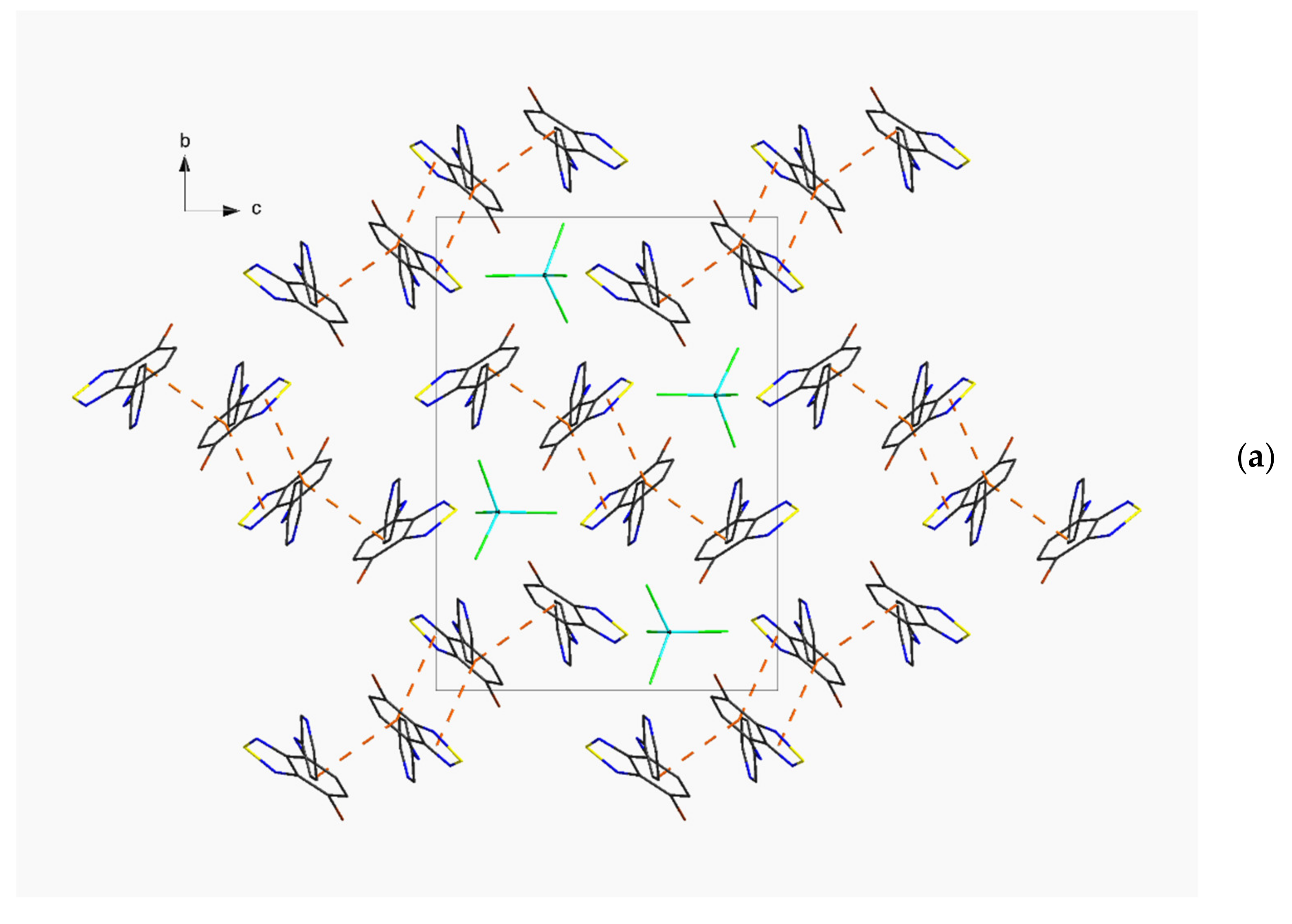
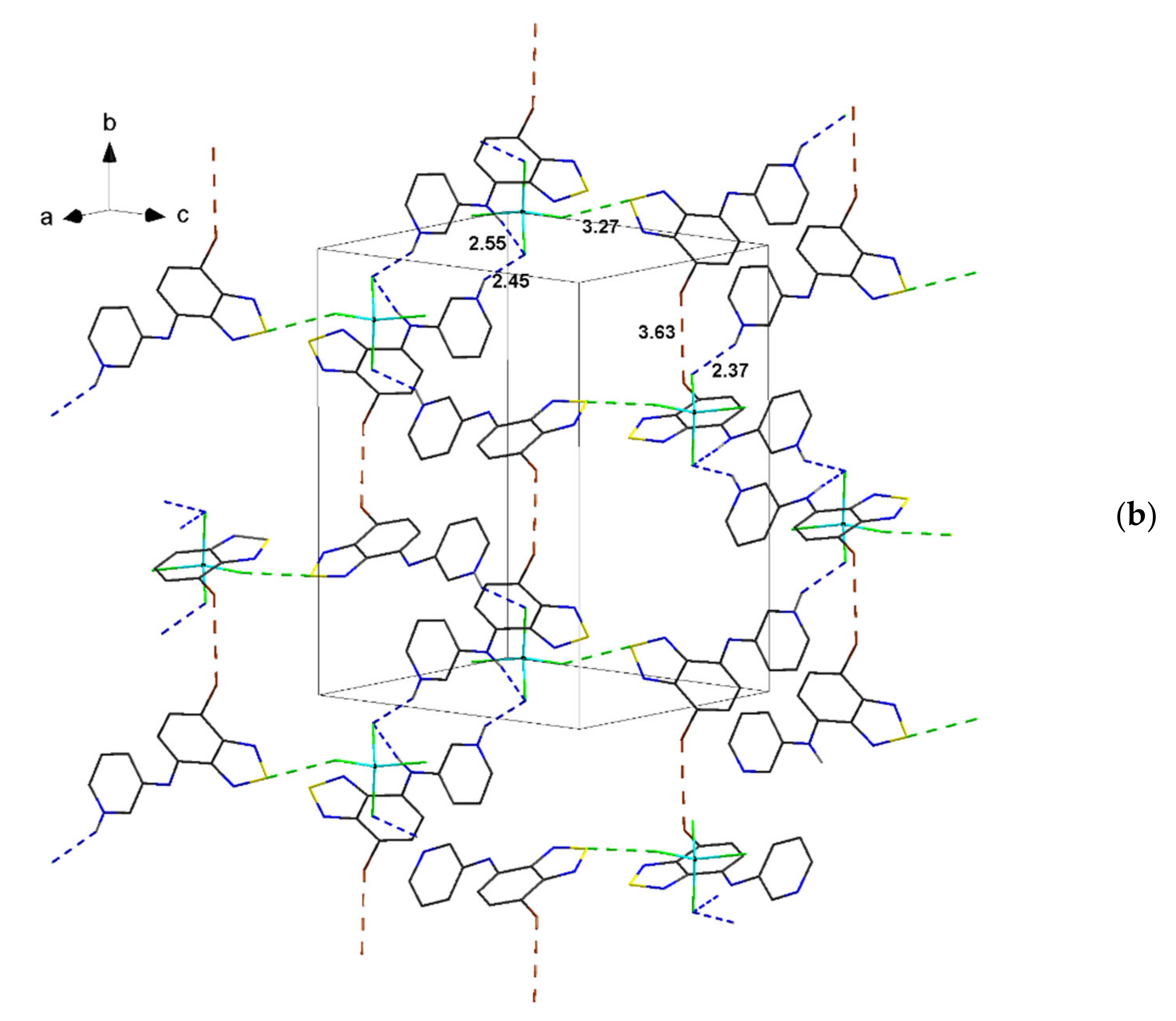
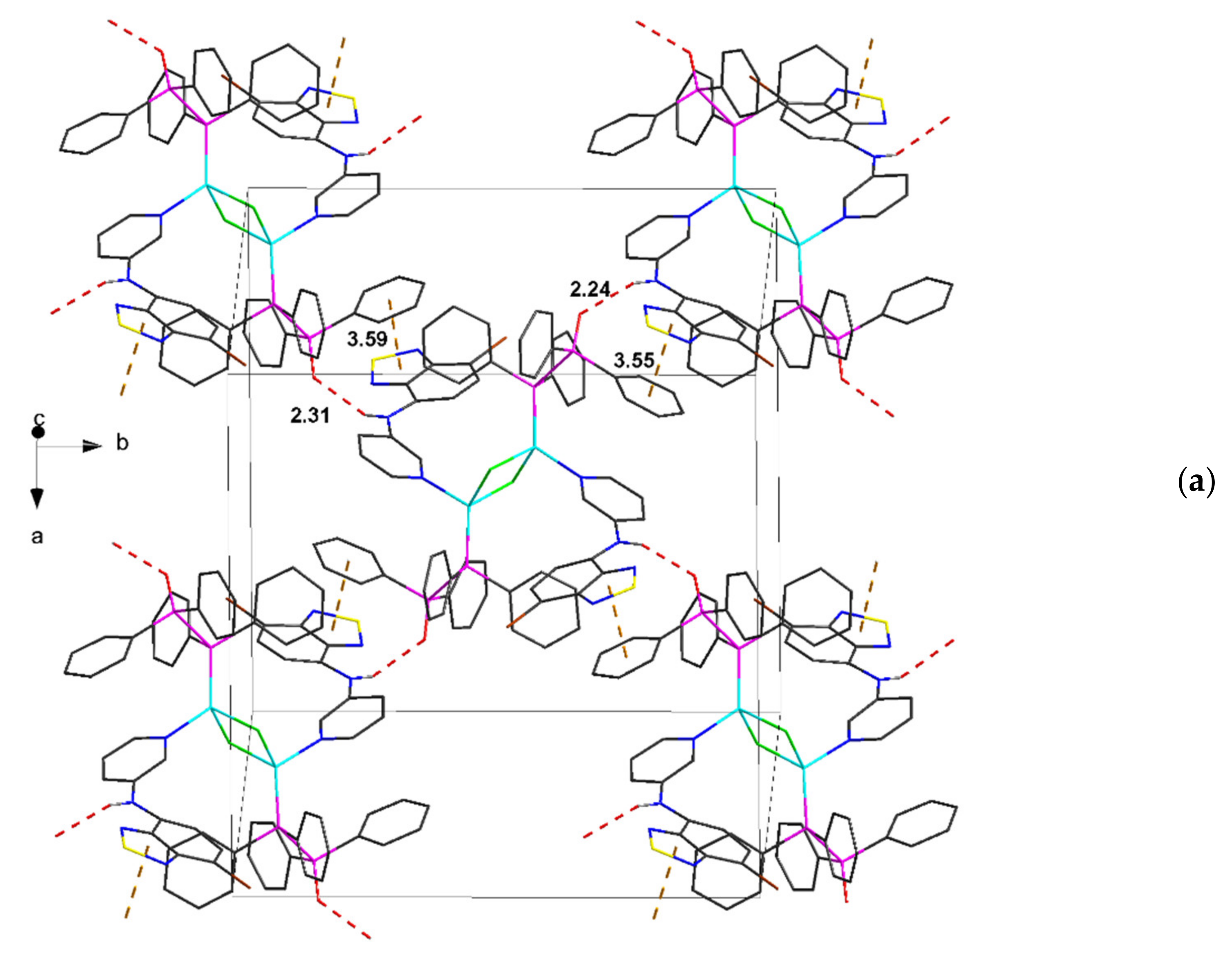
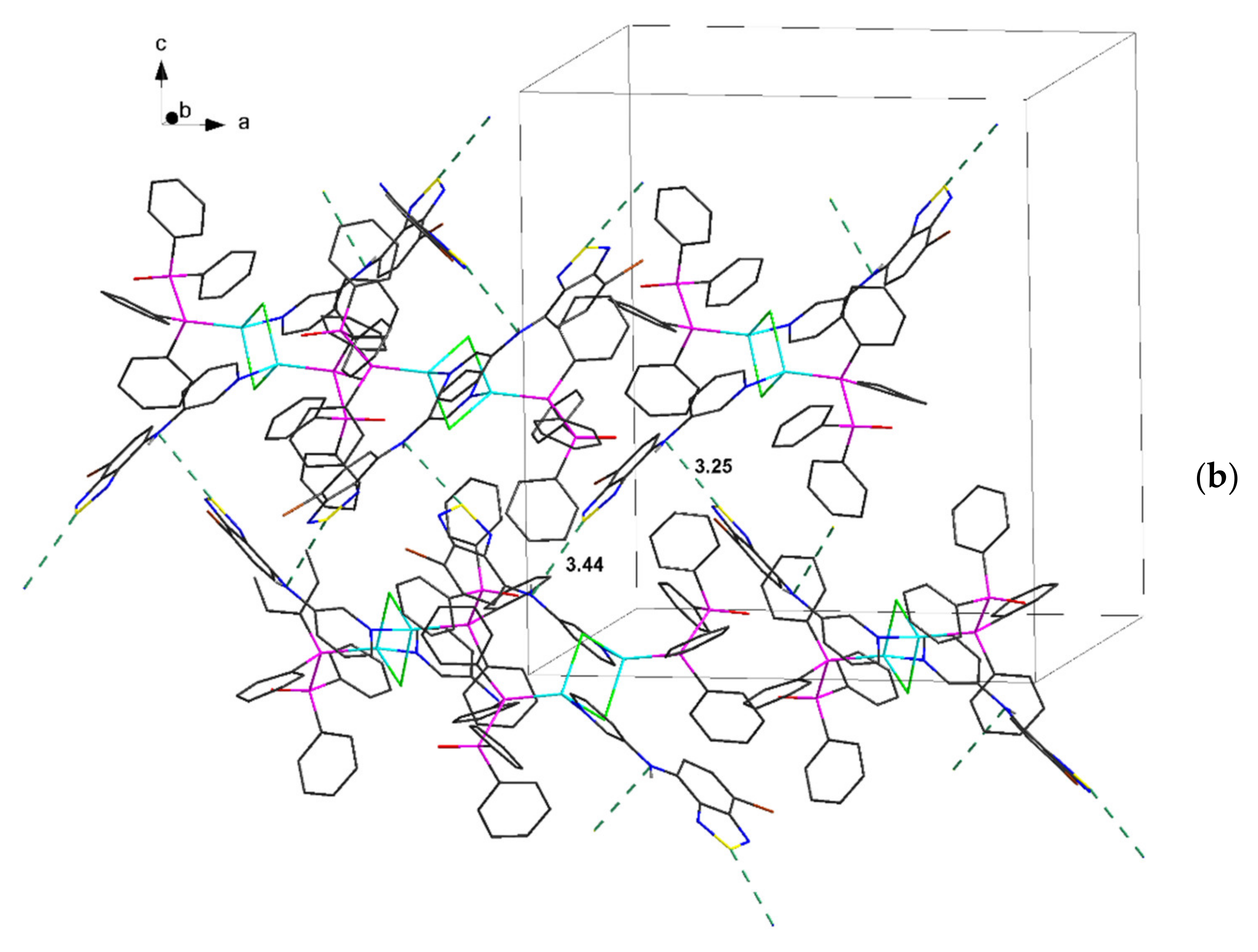
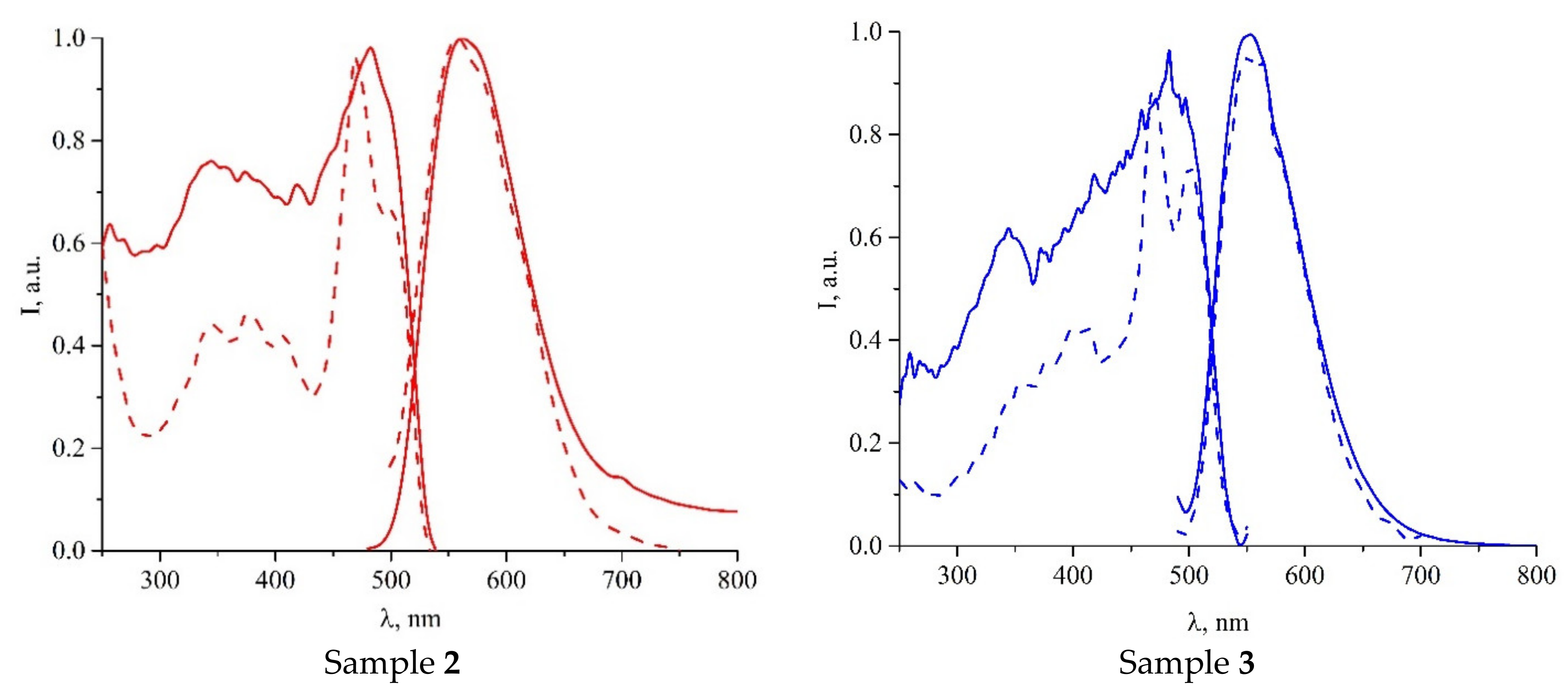
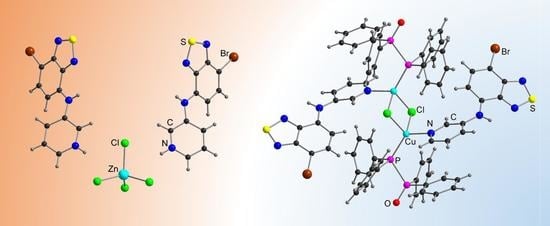
| Compound | C12–C13–N4–C17 | C13–N4–C17–C18 | S···A * | C6 Centroid–C2N2S, Centroid |
|---|---|---|---|---|
| 1 | 180.0 | 149.4 | 2.99 | 3.58 |
| 2 | 158.1, 163.0 | 153.4, 155.3 | 3.27 | 3.57 |
| 3∙2C7H8 | 168.6, 174.0 | 30.9, 36.5 | 3.25, 3.43 | 3.55, 3.59 * |
| Compound | λEm, nm | λEm, nm | τ, μs |
|---|---|---|---|
| 1 | 265–320 (br), 455 | 600 | 9 |
| 2 | 255–315 (br), 445 | 565 | 18 |
| 3∙2C7H8 | 278–320 (br), 470 | 552 | 9 |
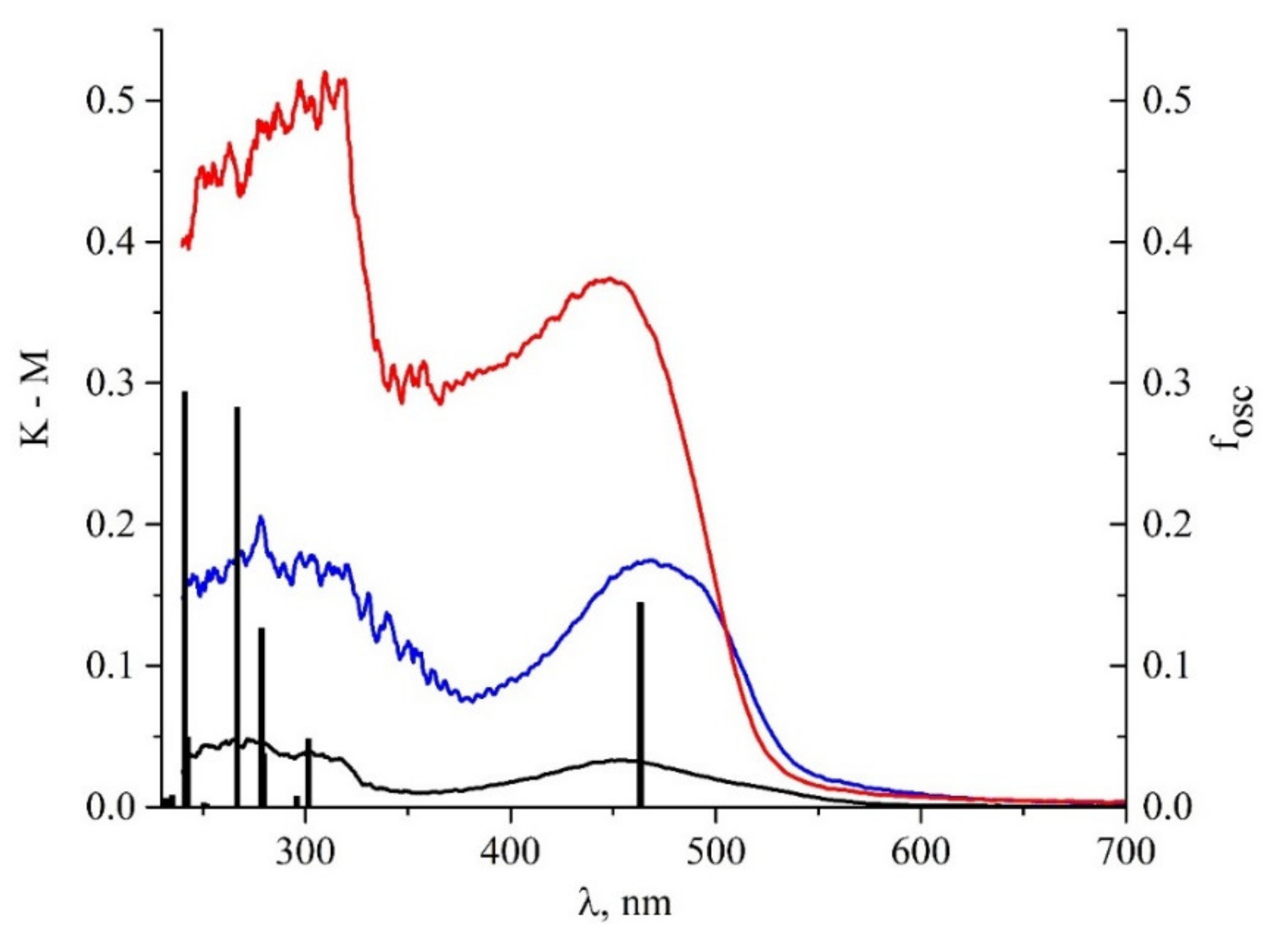
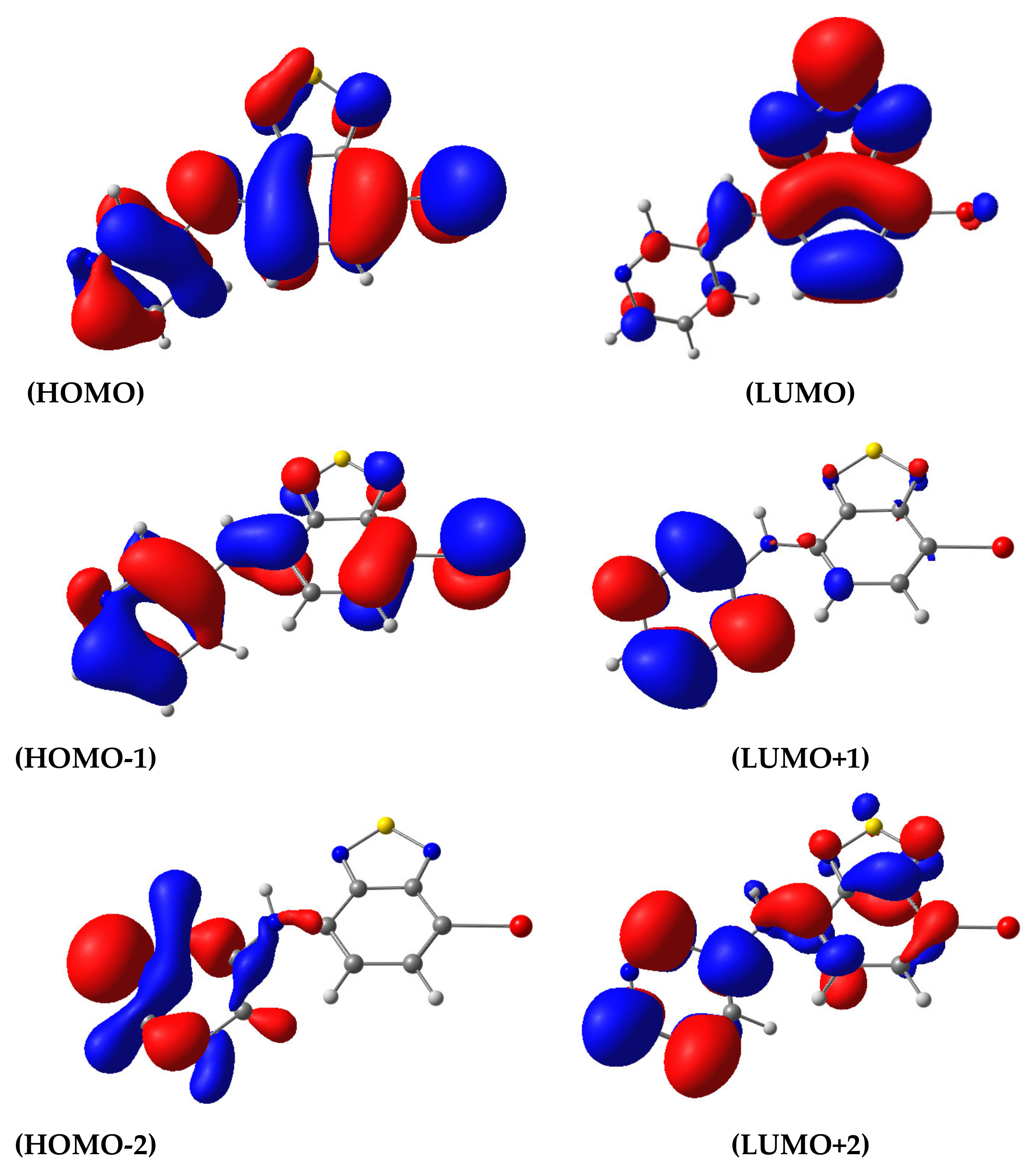
| State | λ, nm | f | Transition | Contribution | CT |
|---|---|---|---|---|---|
| 1 | 463.2 | 0.1446 | H→L | 0.9869 | 0.503 |
| 2 | 301.4 | 0.0481 | H→L+1 | 0.9400 | 0.700 |
| 3 | 295.7 | 0.0075 | H-1→L | 0.9555 | 0.728 |
| 4 | 279.5 | 0.0379 | H-2→L H→L+2 | 0.6953 0.1774 | 0.838 |
| 5 | 278.5 | 0.1268 | H-1→L | 0.95548 | 0.385 |
| 6 | 269.2 | 0.0002 | H-2→L H→L+2 | 0.6953 0.1774 | 0.573 |
| 7 | 266.7 | 0.2827 | H-3→L H→L+2 H→L+3 | 0.178346 0.497742 0.249064 | 0.564 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sukhikh, T.S.; Khisamov, R.M.; Konchenko, S.N. Unexpectedly Long Lifetime of the Excited State of Benzothiadiazole Derivative and Its Adducts with Lewis Acids. Molecules 2021, 26, 2030. https://doi.org/10.3390/molecules26072030
Sukhikh TS, Khisamov RM, Konchenko SN. Unexpectedly Long Lifetime of the Excited State of Benzothiadiazole Derivative and Its Adducts with Lewis Acids. Molecules. 2021; 26(7):2030. https://doi.org/10.3390/molecules26072030
Chicago/Turabian StyleSukhikh, Taisiya S., Radmir M. Khisamov, and Sergey N. Konchenko. 2021. "Unexpectedly Long Lifetime of the Excited State of Benzothiadiazole Derivative and Its Adducts with Lewis Acids" Molecules 26, no. 7: 2030. https://doi.org/10.3390/molecules26072030
APA StyleSukhikh, T. S., Khisamov, R. M., & Konchenko, S. N. (2021). Unexpectedly Long Lifetime of the Excited State of Benzothiadiazole Derivative and Its Adducts with Lewis Acids. Molecules, 26(7), 2030. https://doi.org/10.3390/molecules26072030