Pharmacognostic Evaluation and HPLC–PDA and HS–SPME/GC–MS Metabolomic Profiling of Eleutherococcus senticosus Fruits
Abstract
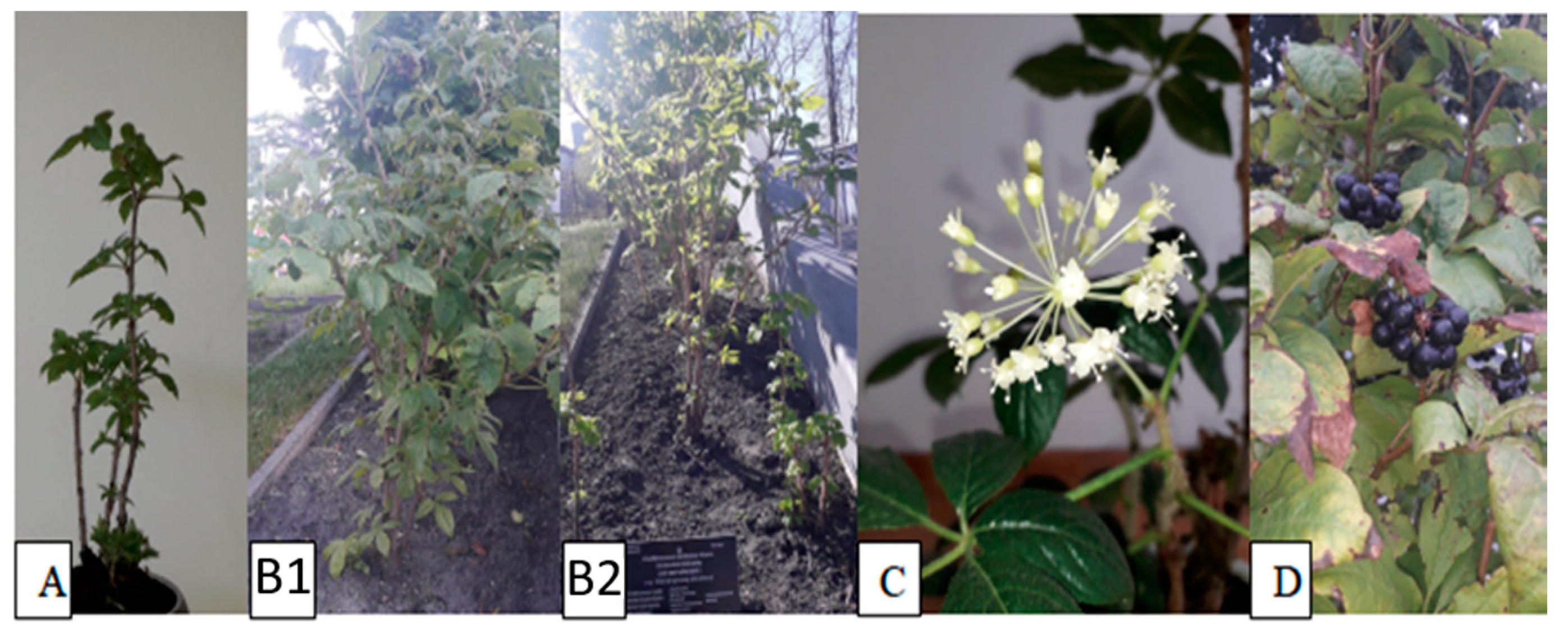
1. Introduction
2. Results and Discussion
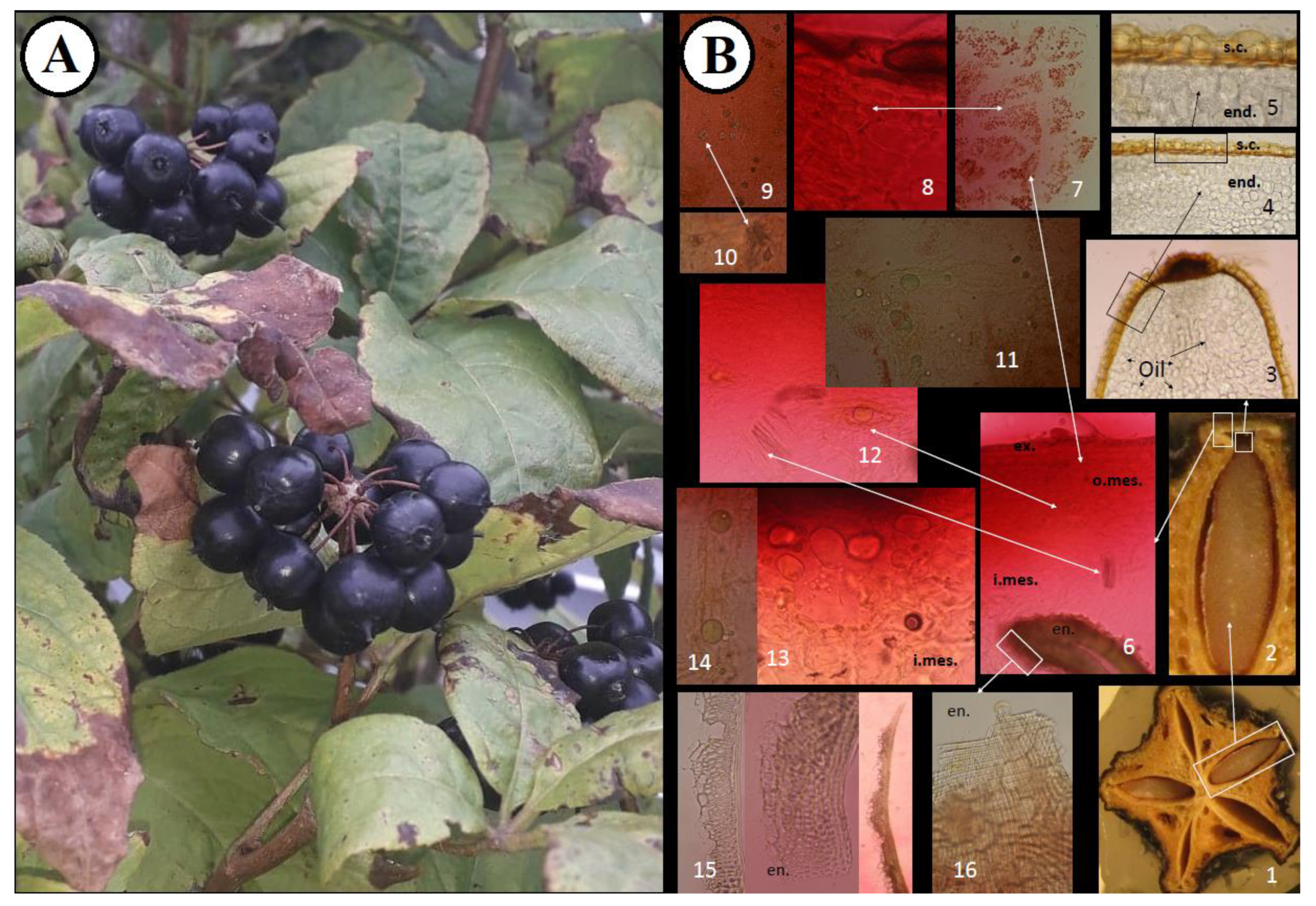
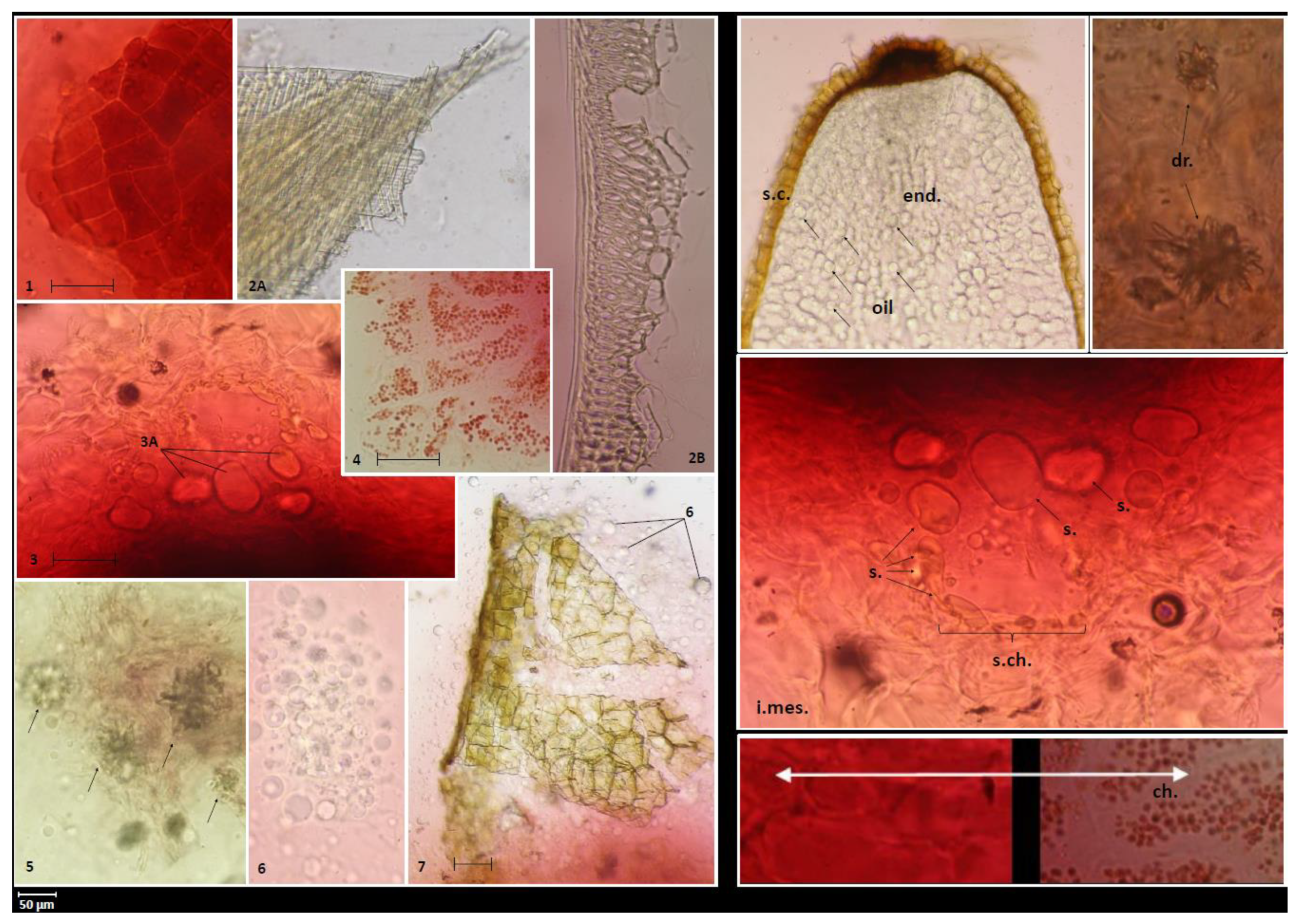
2.1. Microscopic Pharmacognostic Features of Fruits
2.2. HPLC–PDA and GC–MS Metabolite Profiling of the Fruits
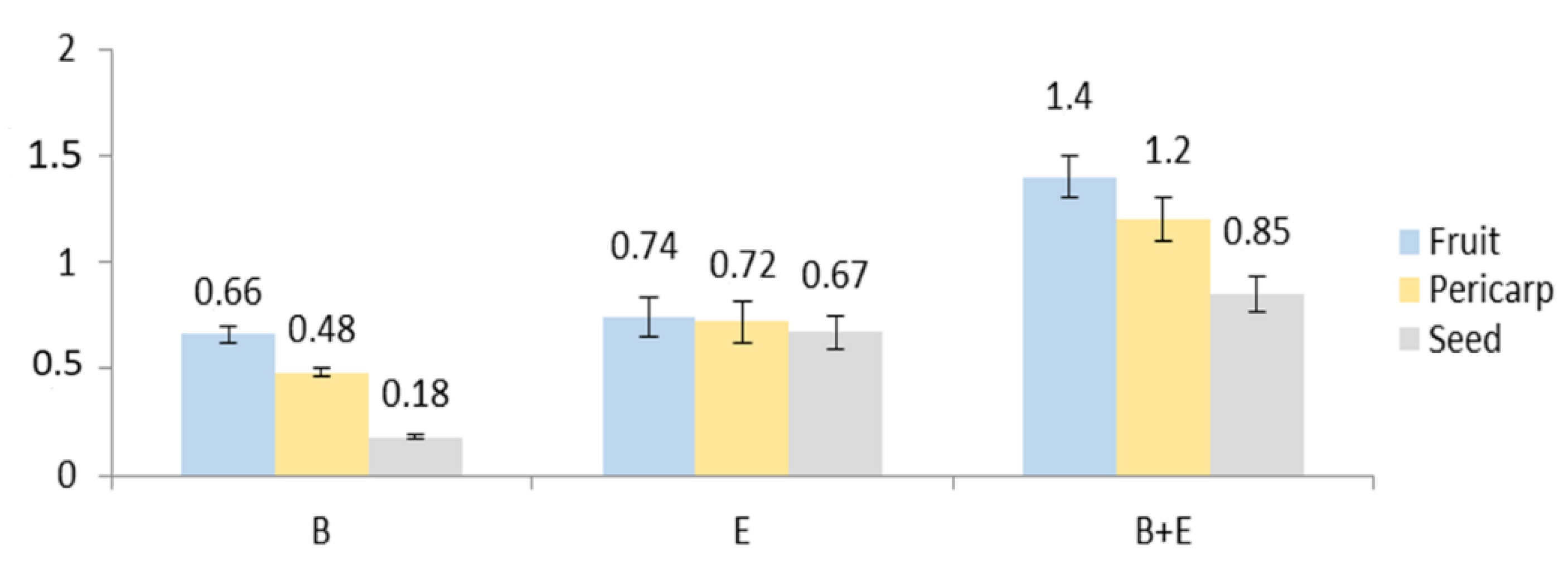
2.2.1. HPLC–PDA Analysis of Eleutherosides B, E, and E1 in the Anatomical Structures of the Fruits
2.2.2. HPLC–PDA Analysis of Phenolic Acids in the Anatomical Structures of the Fruits
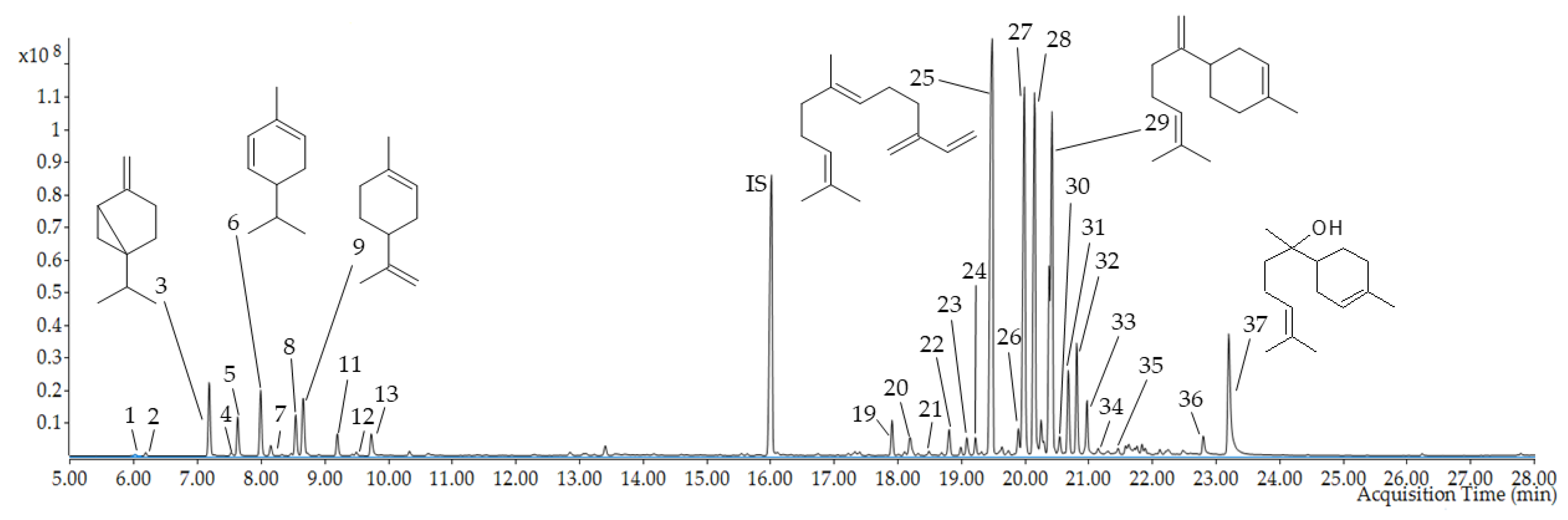
2.3. HS–SPME/GC–MS Analysis of Volatile Metabolites in the Fruits
2.4. GC–MS and HPLC–PDA Profiling of the Fruit Fatty Oil
2.4.1. GC–MS Analysis of Fatty Acids
2.4.2. HPLC–PDA Analysis of Ursolic Acid
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material and Preparation of the Extract
3.3. Microscopic Analysis
3.4. HPLC–PDA Analysis of Eleutherosides B, E, and E1
3.5. HPLC–PDA Analysis of Phenolic Acids
3.6. HS–SPME/GC–MS Analysis of Volatile Compounds
3.7. Isolation of Oil and Preparation of Samples
3.7.1. HPLC–PDA Analysis of Ursolic Acid and Tocopherols in the Oil
3.7.2. GC–MS Analysis of Fatty Acids in the Oil
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Verpoorte, R. Secondary metabolism. In Metabolic Engineering of Plant Secondary Metabolism; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 1–29. [Google Scholar]
- Shikov, A.N.; Pozharitskaya, O.N.; Makarov, V.G.; Wagner, H.; Verpoorte, R.; Heinrich, M. Medicinal plants of the Russian Pharmacopoeia; Their history and applications. J. Ethnopharmacol. 2014, 154, 481–536. [Google Scholar] [CrossRef] [PubMed]
- Li, T.S.C. Comprehensive crop reports: Siberian ginseng. Horttechnology 2001, 11, 79–85. [Google Scholar] [CrossRef]
- Schmidt, M.; Thomsen, M.; Kelber, O.; Kraft, K. Myths and facts in herbal medicines: Eleutherococcus senticosus (Siberian ginseng) and its contraindication in hypertensive patients. Bot. Targets Ther. 2014, 4, 27–32. [Google Scholar] [CrossRef][Green Version]
- Załuski, D.; Olech, M.; Galanty, A.; Verpoorte, R.; Kuźniewski, R.; Nowak, R.; Bogucka-Kocka, A. Phytochemical content and pharma-nutrition study on Eleutherococcus senticosus fruits intractum. Oxidative Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef]
- Lee, J.H.; Lim, J.D.; Choung, M.G. Studies on the anthocyanin profile and biological properties from the fruits of Acanthopanax senticosus (Siberian Ginseng). J. Funct. Foods 2013, 5, 380–388. [Google Scholar] [CrossRef]
- Jang, D.; Lee, J.; Eom, S.H.; Lee, S.M.; Gil, J.; Lim, H.B.; Hyun, T.K. Composition, antioxidant and antimicrobial activities of Eleutherococcus senticosus fruit extracts. J. Appl. Pharm. Sci. 2016, 6, 125–130. [Google Scholar] [CrossRef]
- Graczyk, F.; Orzechowska, B.; Franz, D.; Strzemski, M.; Verpoorte, R.; Załuski, D. The intractum from the Eleutherococcus senticosus fruits affects the innate immunity in human leukocytes: From the ethnomedicinal use to contemporary evidence-based research. J. Ethnopharmacol. 2021, 268. [Google Scholar] [CrossRef]
- Kim, Y.H.; Cho, M.L.; Kim, D.B.; Shin, G.H.; Lee, J.H.; Lee, J.S.; Park, S.O.; Lee, S.J.; Shin, H.M.; Lee, O.H. The antioxidant activity and their major antioxidant compounds from Acanthopanax senticosus and A. koreanum. Molecules 2015, 20, 13281–13295. [Google Scholar] [CrossRef]
- Załuski, D.; Janeczko, Z. Variation in phytochemicals and bioactivity of the fruits of Eleutherococcus species cultivated in Poland. Nat. Prod. Res. 2015, 29, 2207–2211. [Google Scholar] [CrossRef]
- Załuski, D.; Kuźniewski, R.; Janeczko, Z. HPTLC-profiling of eleutherosides, mechanism of antioxidative action of eleutheroside E1, the PAMPA test with LC/MS detection and the structure–activity relationship. Saudi J. Biol. Sci. 2018, 25, 520–528. [Google Scholar] [CrossRef]
- Załuski, D.; Olech, M.; Kuźniewski, R.; Verpoorte, R.; Nowak, R.; Smolarz, H.D. LC-ESI-MS/MS profiling of phenolics from Eleutherococcus spp. inflorescences, structure-activity relationship as antioxidants, inhibitors of hyaluronidase and acetylcholinesterase. Saudi Pharm. J. 2017, 25, 734–743. [Google Scholar] [CrossRef]
- Bączek, K. Accumulation of biologically active compounds in Eleuthero (Eleutherococcus senticosus/Rupr. et Maxim./Maxim.) grown in Poland. Herba Pol. 2009, 55, 7–13. [Google Scholar]
- Gorshkova, T.A.; Salnikov, V.V.; Pogodina, N.M.; Chemikosova, S.B.; Yablokova, E.V.; Ulanov, A.V.; Ageeva, M.V.; Van Dam, J.E.G.; Lozovaya, V.V. Composition and distribution of cell wall phenolic compounds flax (Linum usitatissimum L.) stem tissues. Ann. Bot. 2000, 85, 477–486. [Google Scholar] [CrossRef]
- Hutzler, P.; Fischbach, R.; Heller, W.; Jungblut, T.P.; Reuber, S.; Schmitz, R.; Veit, M.; Weissenböck, G.; Schnitzler, J.P. Tissue localization of phenolic compounds in plants by confocal laser scanning microscopy. J. Exp. Bot. 1998, 49, 953–965. [Google Scholar] [CrossRef]
- Iglesias-Carres, L.; Mas-Capdevila, A.; Bravo, F.I.; Aragonès, G.; Muguerza, B.; Arola-Arnal, A. Optimization of a polyphenol extraction method for sweet orange pulp (Citrus sinensis L.) to identify phenolic compounds consumed from sweet oranges. PLoS ONE 2019, 14. [Google Scholar] [CrossRef]
- Xi, W.; Lu, J.; Qun, J.; Jiao, B. Characterization of phenolic profile and antioxidant capacity of different fruit part from lemon (Citrus limon Burm.) cultivars. J. Food Sci. Technol. 2017, 54, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Um, M.Y.; Lee, H.; Jung, C.H.; Heo, S.H.; Ha, T.Y. Eleutheroside E, an active component of Eleutherococcus senticosus, ameliorates insulin resistance in type 2 diabetic db/db mice. Evid. Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef]
- Bączek, K. Diversity of Eleutherococcus genus in respect of biologically active compounds accumulation. Herba Pol. 2015, 60, 34–43. [Google Scholar] [CrossRef]
- Solomonova, E.; Trusov, N.; Nozdrina, T. Opportunities for using of eleutherococcuses fruits as a new food raw material. In Proceedings of the 1st International Symposium Innovations in Life Sciences (ISILS 2019), Belgorod, Russia, 10–11 October 2019. [Google Scholar]
- Wang, Y.H.; Meng, Y.; Zhai, C.; Wang, M.; Avula, B.; Yuk, J.; Smith, K.M.; Isaac, G.; Khan, I.A. The chemical characterization of Eleutherococcus senticosus and Ci-wu-jia tea using UHPLC-UV-QTOF/MS. Int. J. Mol. Sci. 2019, 20, 475. [Google Scholar] [CrossRef]
- Zhai, C.; Wang, M.; Raman, V.; Rehman, J.U.; Meng, Y.; Zhao, J.; Avula, B.; Wang, Y.H.; Tian, Z.; Khan, I.A.; et al. Eleutherococcus senticosus (Araliaceae) leaf morpho-anatomy, essential oil composition, and its biological activity against Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 2017, 54, 658–669. [Google Scholar] [CrossRef] [PubMed]
- Richter, R.; Hanssen, H.P.; Koenig, W.A.; Koch, A. Essential oil composition of Eleutherococcus senticosus (Rupr. et Maxim.) Maxim roots. J. Essent. Oil Res. 2007, 19, 209–210. [Google Scholar] [CrossRef]
- Strzemski, M.; Płachno, B.J.; Mazurek, B.; Kozłowska, W.; Sowa, I.; Lustofin, K.; Załuski, D.; Rydzik, Ł.; Szczepanek, D.; Sawicki, J.; et al. Morphological, anatomical, and phytochemical studies of Carlina acaulis L. cypsela. Int. J. Mol. Sci. 2020, 21, 9230. [Google Scholar] [CrossRef]
- Blicharska, E.; Tatarczak-Michalewska, M.; Plazińska, A.; Plaziński, W.; Kowalska, A.; Madejska, A.; Szymańska-Chargot, M.; Sroka-Bartnicka, A.; Flieger, J. Solid-phase extraction using octadecyl-bonded silica modified with photosynthetic pigments from Spinacia oleracea L. for the preconcentration of lead(II) ions from aqueous samples. J. Sep. Sci. 2018, 41, 3129–3142. [Google Scholar] [CrossRef]
- Sonestedt, E.; Ericson, U.; Gullberg, B.; Skog, K.; Olsson, H.; Wirfält, E. Do both heterocyclic amines and omega-6 polyunsaturated fatty acids contribute to the incidence of breast cancer in postmenopausal women of the Malmö diet and cancer cohort? Int. J. Cancer 2008, 123, 1637–1643. [Google Scholar] [CrossRef] [PubMed]
- Grilo, E.C.; Costa, P.N.; Gurgel, C.S.S.; de Lima Beserra, A.F.; de Souza Almeida, F.N.; Dimenstein, R. α-tocopherol and gamma-tocopherol concentration in vegetable oils. Food Sci. Technol. 2014, 34, 379–385. [Google Scholar] [CrossRef]
- Ergönül, P.G.; Köseoǧlu, O. Changes in α-, β-, γ- And δ-tocopherol contents of mostly consumed vegetable oils during refining process. CYTA J. Food 2014, 12, 199–202. [Google Scholar] [CrossRef]
- Madrigal, R.V.; Plattner, R.D.; Smith, C.R. Carduus nigrescens seed oil-A rich source of pentacyclic triterpenoids. Lipids 1975, 10, 208–213. [Google Scholar] [CrossRef]
- Zielińska, S.; Dąbrowska, M.; Kozłowska, W.; Kalemba, D.; Abel, R.; Dryś, A.; Szumny, A.; Matkowski, A. Ontogenetic and trans-generational variation of essential oil composition in Agastache rugosa. Ind. Crop. Prod. 2017, 97, 612–619. [Google Scholar] [CrossRef]
- Strzemski, M.; Wójciak-Kosior, M.; Sowa, I.; Rutkowska, E.; Szwerc, W.; Kocjan, R.; Latalski, M. Carlina species as a new source of bioactive pentacyclic triterpenes. Ind. Crop. Prod. 2016, 94. [Google Scholar] [CrossRef]
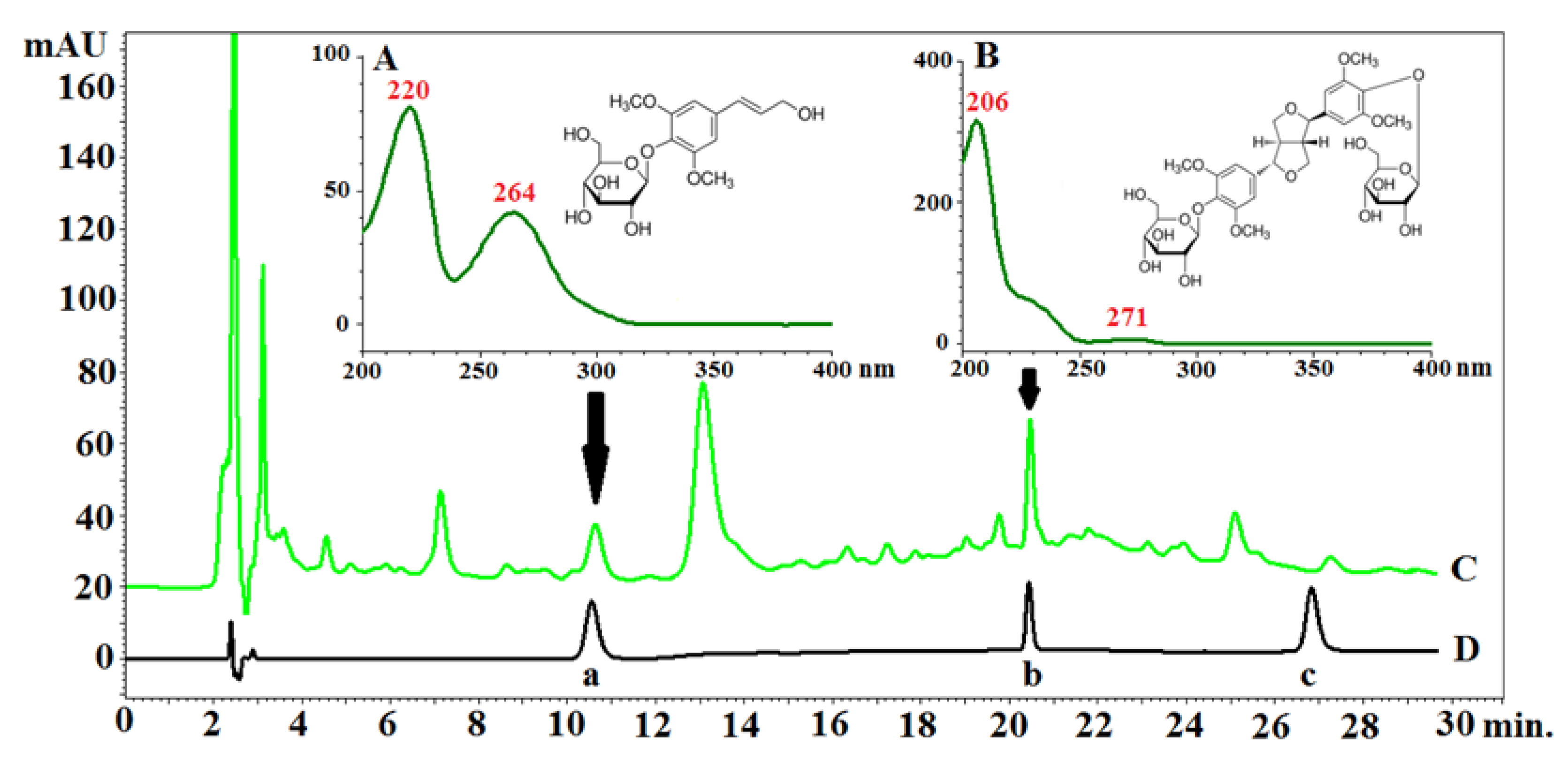



| Sample | 3-CQA | 4-CQA | 5-CQA | PA | Total Content |
|---|---|---|---|---|---|
| Fruit | 1.08 ± 0.96 | 0.07 ± 0.01 | 0.030 ± 0.01 | 0.08 ± 0.07 | 1.26 |
| Pericarp | 0.66 ± 0.2 | 0.03 ± 0.02 | 0.01 ± 0.001 | 0.04 ± 0.03 | 0.74 |
| Seed | 0.076 ± 0.03 | 0.008 ± 0.001 | 0.004 ± 0.002 | 0.008 ± 0.001 | 0.096 |
| No. | RI Lit. | RI Calc. | RT (Min) | Compound Name | Formula | Compound m/z | Match Factor (%) | Content |
|---|---|---|---|---|---|---|---|---|
| 1 | 930 | 926 | 6.01 | α-Thujene | C10H16 | 93; 91; 77 | 94.55 | 0.05 ± 0.02 |
| 2 | 939 | 933 | 6.18 | α-Pinene | C10H16 | 93; 91; 77 | 95.00 | 0.12 ± 0.09 |
| 3 | 975 | 973 | 7.18 | (Z)-Sabinene | C10H16 | 93; 91; 77 | 97.84 | 2.46 ± 1.19 |
| 4 | 985 | 987 | 7.52 | 6-methyl-5-hepten-2-one | C8H14O | 43; 108; 69; 55 | 97.50 | 0.15 ± 0.03 |
| 5 | 990 | 991 | 7.63 | β-Myrcene | C10H16 | 93; 69; 41 | 96.20 | 1.43 ± 0.44 |
| 6 | 1002 | 1005 | 7.99 | α-Phellandrene | C10H16 | 93; 77; 136 | 96.90 | 2.22 ± 0.6 |
| 7 | 1011 | 1010 | 8.15 | 3-Carene | C10H16 | 93; 79; 121 | 97.90 | 0.37 ± 0.012 |
| 8 | 1026 | 1025 | 8.54 | O-Cymene | C10H14 | 119; 134; 91 | 97.90 | 1.40 ± 0.26 |
| 9 | 1029 | 1029 | 8.65 | Limonene | C10H16 | 93; 68; 79 | 98.50 | 2.81 ± 0.54 |
| 10 | 1037 | 1038 | 8.91 | (Z)-β-Ocimene | C10H16 | 93; 91; 79 | 93.24 | 0.05 ± 0.01 |
| 11 | 1050 | 1048 | 9.19 | (E)-β-Ocimene | C10H16 | 93; 91; 79 | 97.10 | 0.86 ± 0.15 |
| 12 | 1059 | 1059 | 9.49 | γ-Terpinene | C10H16 | 93; 136; 77 | 98.60 | 0.11 ± 0.01 |
| 13 | 1070 | 1067 | 9.73 | (Z)-Sabinene hydrate | C10H18O | 71; 93; 43 | 98.50 | 0.90 ± 0.09 |
| 14 | 1088 | 1089 | 10.33 | Terpinolene | C10H16 | 121; 93; 136 | 95.04 | 0.16 ± 0.01 |
| 15 | 1159 | 1159 | 12.30 | Sabina ketone | C9H14O | 81; 96; 67; 55 | 83.10 | 0.08 ± 0.01 |
| 16 | 1177 | 1179 | 12.85 | Terpinen-4-ol | C10H18O | 71; 111; 93 | 96.52 | 0.15 ± 0.02 |
| 17 | 1294 | 1295 | 15.99 | 2-Undecanone (IS) | C11H22O | 58; 43; 71 | 93.20 | 3.79 ± 6.31 |
| 18 | 1294 | 1299 | 16.11 | Methyl myrtenate | C11H16O2 | 105; 137; 91;77 | 94.50 | 0.07 ± 0.02 |
| 19 | 1376 | 1381 | 17.91 | α-Copaene | C15H24 | 161;119; 105; 93 | 98.20 | 1.05 ± 0.16 |
| 20 | 1388 | 1390 | 18.11 | β-Bourbonene | C15H24 | 81; 123; 161 | 94.90 | 0.12 ± 0.02 |
| 21 | 1408 | 1409 | 18.49 | 7-epi-Sesquithujene | C15H24 | 119; 93; 91; 69 | 93.00 | 0.14 ± 0.02 |
| 22 | 1419 | 1426 | 18.81 | β-Caryophyllene | C15H24 | 93; 91; 133; 79 | 97.90 | 0.91 ± 0.14 |
| 23 | 1432 | 1436 | 18.99 | (Z)-β-Copaene | C15H24 | 161; 105; 91; 119 | 99.00 | 0.25 ± 0.04 |
| 24 | 1434 | 1441 | 19.08 | (E)-α-Bergamotene | C15H24 | 119; 93; 91; 69 | 97.60 | 0.54 ± 0.07 |
| 25 | 1456 | 1463 | 19.49 | (E)-β-Farnesene | C15H24 | 69; 93; 133; 161 | 90.00 | 19.46 ± 0.78 |
| 26 | 1466 | 1471 | 19.64 | (Z)-Muurola-4,5-diene | C15H24 | 161; 105; 91; 204 | 94.20 | 0.26 ± 0.04 |
| 27 | 1481 | 1490 | 20.00 | Germacrene d | C15H24 | 161; 105; 91; 119 | 94.60 | 12.88 ± 1.10 |
| 28 | 1497 | 1498 | 20.16 | (E,Z)-α-Farnesene | C15H24 | 93; 119; 69; 107 | 92.10 | 12.84 ± 0.85 |
| 29 | 1511 | 1515 | 20.43 | (E)-β-Bisabolene | C15H24 | 93; 69; 94; 109 | 95.70 | 12.64 ± 0.70 |
| 30 | 1513 | 1522 | 20.55 | γ-Cadinene | C15H24 | 161; 93; 105; 119 | 97.70 | 0.55 ± 0.06 |
| 31 | 1522 | 1530 | 20.68 | β-Sesquiphellandrene | C15H24 | 69; 161; 93; 91 | 95.70 | 2.6 ± 0.22 |
| 32 | 1532 | 1538 | 20.81 | (E)-γ-Bisabolene | C15H24 | 93; 107; 135; 119 | 94.60 | 3.26 ± 0.43 |
| 33 | 1540 | 1548 | 20.97 | (E)-α-Bisabolene | C15H24 | 93; 119; 121; 80 | 95.70 | 1.63 ± 0.20 |
| 34 | 1563 | 1559 | 21.15 | (E)-Nerolidol | C15H26O | 69; 93; 81; 83 | 85.30 | 0.23 ± 0.06 |
| 35 | 1607 | 1619 | 22.12 | β-Oplopenone | C15H24O | 177; 43; 93; 79 | 93.40 | 0.15 ± 0.02 |
| 36 | 1658 | 1665 | 22.80 | α-Bisabolol oxide B | C15H26O2 | 143; 105; 85; 81 | 97.66 | 0.76 ± 0.12 |
| 37 | 1685 | 1692 | 23.20 | α-Bisabolol | C15H26O | 109; 119; 69; 43 | 98.90 | 6.35 ± 0.9 |
| 38 | 2104 | 2107 | 27.78 | 6-Octadecenoic acid, methyl ester, (Z)- | C19H36O2 | 55; 74; 84; 96 | 94.52 | 0.13 ± 0.10 |
| Monoterpene hydrocarbons and oxygenated monoterpenes | 13.17 | |||||||
| Sesquiterpene hydrocarbons and oxygenated sesquiterpenes | 76.62 | |||||||
| Other compounds | 4.07 | |||||||
| Total | 93.86 | |||||||
| Compound Name | FA Abbreviation | RT FAME (Min.) | Mass Data FAME | LOD (% w/w) | LOQ (% w/w) | Content of FA |
|---|---|---|---|---|---|---|
| Palmitic acid | C16:0 | 12.94 ± 0.1 | 270 (M),239,227,213,185,143,129,11197,87,74,69,55 | 0.044 | 0.132 | 2.18 ± 0.04 |
| Stearic acid | C18:0 | 15.08 ± 0.1 | 298 (M),267,255,241,227,213,199,185,171,157,143,129,115,97,87,83,74,69,55 | 0.026 | 0.078 | 0.29 ± 0.00 |
| Oleic acid | C18:1 (n-9) | 15.77 ± 0.1 | 296 (M), 278,264,235,222,180,166,137,123,110,97,83,69,55 | 0.037 | 0.111 | 5.36 ± 0.05 |
| Linoleic acid | C18:2 (n-6) | 16.95 ± 0.2 | 294 (M),263,233,220,205,191,178,164,150,135,123,109,95,81,67,59,55 | 0.013 | 0.039 | 18.24 ± 0.26 |
| α-Linolenic acid | C18:3 (n-3) | 18.56 ± 0.2 | 292 (M),261,236,193,163,149,121,108,95,79,67,55 | 0.015 | 0.045 | 0.40 ± 0.04 |
| Total | 26.47 |
| Compound Name | RT (Min.) | Theoretical Plates | Linear Regression Equation | Concentration Range (µg/mL) | Correlation Coefficient (r) | LOD | LOQ | Content |
|---|---|---|---|---|---|---|---|---|
| Fluorescence Detection | (ng/mL) | (ng/mL) | ||||||
| α-Tocopherol | 8.36 ± 0.1 | 6158 | y = 1851639x + 17320 | 0.48–7.20 | 0.9979 | 12.0 | 38.0 | 0.29 ± 0.00 |
| β- + γ-Tocopherol | 7.25 ± 0.1 | 5779 | y = 4739064x + 115013 | 0.59–8.88 | 0.9997 | 6.0 | 20.0 | 1.36 ± 0.07 |
| δ-Tocopherol | 6.30 ± 0.1 | 5527 | y = 4041480x + 112005 | 0.55–8.34 | 0.9999 | 5.5 | 18.5 | 0.33 ± 0.00 |
| Spectrophotometric Detection | (µg/mL) | (µg/mL) | ||||||
| Ursolic acid | 31.4 ± 0.2 | 6111 | y = 30275.9x + 93277 | 39.20–117.60 | 0.9989 | 0.17 | 0.51 | 35.72 ± 0.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graczyk, F.; Strzemski, M.; Balcerek, M.; Kozłowska, W.; Mazurek, B.; Karakuła, M.; Sowa, I.; Ptaszyńska, A.A.; Załuski, D. Pharmacognostic Evaluation and HPLC–PDA and HS–SPME/GC–MS Metabolomic Profiling of Eleutherococcus senticosus Fruits. Molecules 2021, 26, 1969. https://doi.org/10.3390/molecules26071969
Graczyk F, Strzemski M, Balcerek M, Kozłowska W, Mazurek B, Karakuła M, Sowa I, Ptaszyńska AA, Załuski D. Pharmacognostic Evaluation and HPLC–PDA and HS–SPME/GC–MS Metabolomic Profiling of Eleutherococcus senticosus Fruits. Molecules. 2021; 26(7):1969. https://doi.org/10.3390/molecules26071969
Chicago/Turabian StyleGraczyk, Filip, Maciej Strzemski, Maciej Balcerek, Weronika Kozłowska, Barbara Mazurek, Michał Karakuła, Ireneusz Sowa, Aneta A. Ptaszyńska, and Daniel Załuski. 2021. "Pharmacognostic Evaluation and HPLC–PDA and HS–SPME/GC–MS Metabolomic Profiling of Eleutherococcus senticosus Fruits" Molecules 26, no. 7: 1969. https://doi.org/10.3390/molecules26071969
APA StyleGraczyk, F., Strzemski, M., Balcerek, M., Kozłowska, W., Mazurek, B., Karakuła, M., Sowa, I., Ptaszyńska, A. A., & Załuski, D. (2021). Pharmacognostic Evaluation and HPLC–PDA and HS–SPME/GC–MS Metabolomic Profiling of Eleutherococcus senticosus Fruits. Molecules, 26(7), 1969. https://doi.org/10.3390/molecules26071969








