Identification of a Prenyl Chalcone as a Competitive Lipoxygenase Inhibitor: Screening, Biochemical Evaluation and Molecular Modeling Studies
Abstract
1. Introduction
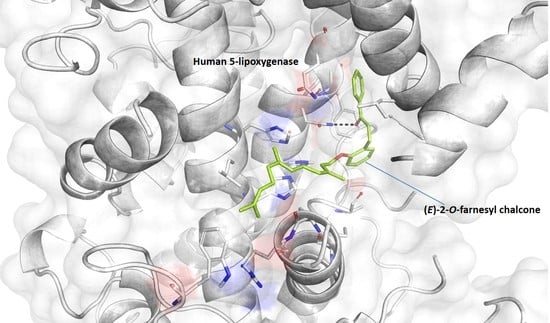
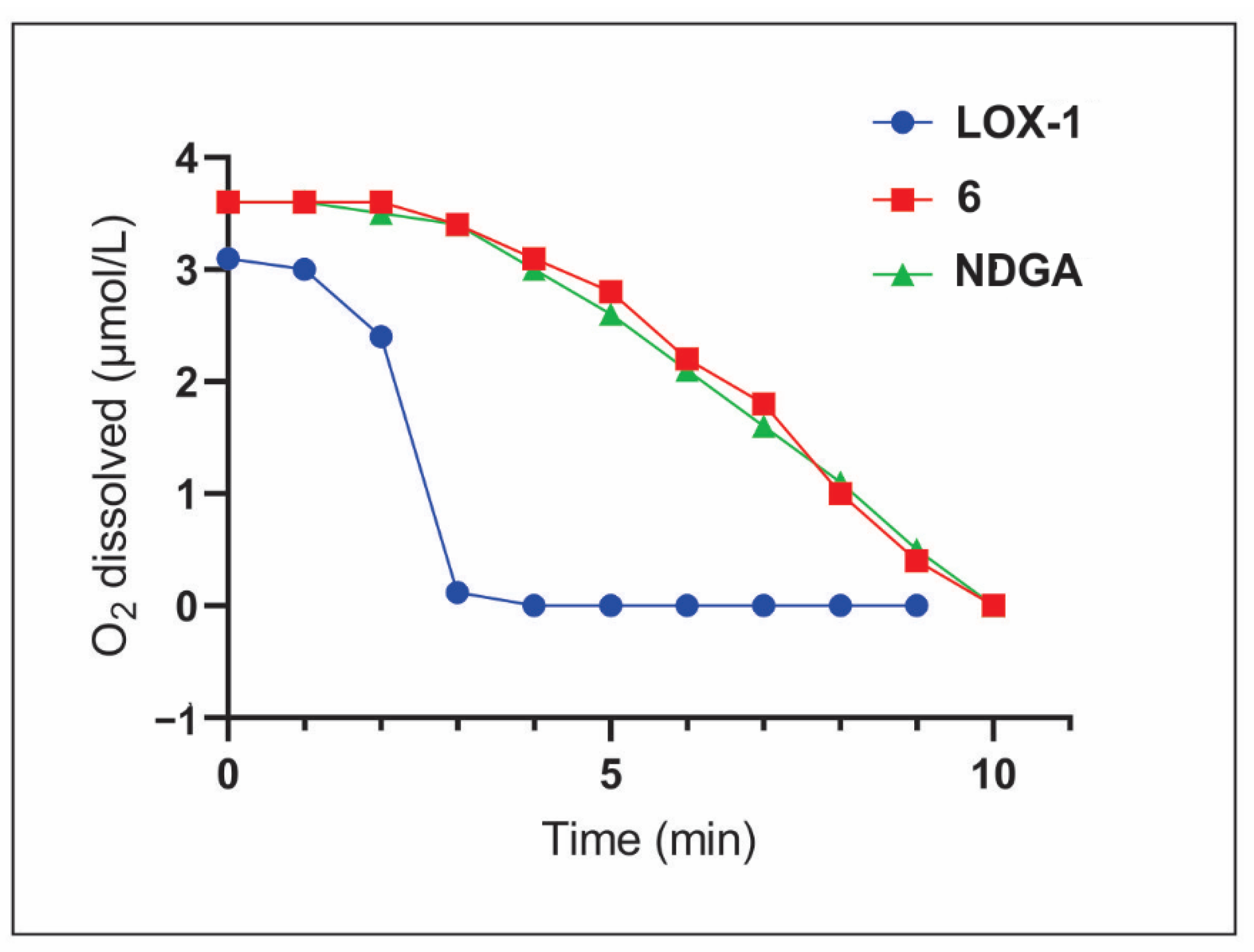
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of Compounds
3.2. LOX Bioassay
3.3. Oxygen Consumption by the Catalytic Activity of LOX
3.4. Docking Simulation
3.5. Theoretical Calculation of log P
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Werz, O. 5-lipoxygenase: Cellular biology and molecular pharmacology. Curr. Drug Targets Inflamm. Allergy 2002, 1, 23–44. [Google Scholar] [CrossRef]
- Steele, V.E.; Holmes, C.A.; Hawk, E.T.; Kopelovich, L.; Lubet, R.A.; Crowell, J.A.; Sigman, C.C.; Kelloff, G.J. Lipoxygenase inhibitors as potential cancer chemopreventives. Cancer Epidemiol. Biomarkers Prev. 1999, 8, 467–483. [Google Scholar]
- Grechkin, A. Recent developments in biochemistry of the plant lipoxygenase pathway. Prog. Lipid Res. 1998, 37, 317–352. [Google Scholar] [CrossRef]
- Brash, A.R. Lipoxygenases: Occurrence, functions, catalysis, and acquisition of substrate. J. Biol. Chem. 1999, 274, 23679–23682. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Masuda, A.; Chen, G.; Bushra, S.; Kamon, M.; Araki, T.; Kinoshita, M.; Ohkawara, B.; Ito, M.; Ohno, K. Inhibition of cyclooxygenase-1 by nonsteroidal anti-inflammatory drugs demethylates MeR2 enhancer and promotes Mbnl1 transcription in myogenic cells. Sci. Rep. 2020, 10, 2558. [Google Scholar] [CrossRef] [PubMed]
- Allison, M.C.; Howatson, A.G.; Torrance, C.J.; Lee, F.D.; Russel, R.I.N. Gastrointestinal damage associated with the use of nonsteroidal anti-inflammatory drugs. N. Engl. J. Med. 1992, 20, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Sibbald, B. Rofecoxib (Vioxx) voluntarity withdrawn from market. CMAJ 2004, 171, 1027–1028. [Google Scholar] [CrossRef]
- Doiron, J.L.; Boudreau, H.; Picot, N.; Villebonet, B.; Surette, M.E.; Touaibia, M. Synthesis and 5-lipoxygenase inhibitory activity of new cinnamoyl and caffeoyl clusters. Bioorg. Med. Chem. Lett. 2009, 19, 1118–1121. [Google Scholar] [CrossRef]
- Kurihara, H.; Kagawa, Y.; Konno, R.; Kim, S.M.; Takahashi, K. Lipoxygenase inhibitors derived from marine macroalgae. Bioorg. Med. Chem. Lett. 2014, 24, 1383–1385. [Google Scholar] [CrossRef]
- Martel-Pelletier, J.; Lajeunesse, D.; Reboul, P.; Pelletier, J.P. Therapeutic role of dual inhibitors of 5-LOX and COX, selective and non-selective non-steroidal anti-inflammatory drugs. Ann. Rheum. Dis. 2003, 62, 501–509. [Google Scholar] [CrossRef]
- Jaismy, J.P.; Manju, S.L.; Ethiraj, K.R.; Elias, G. Safer anti-inflammatory therapy through dual COX-2/5-LOX inhibitors: A structure-based approach. Eur. J. Pharm. Sci. 2018, 121, 356–381. [Google Scholar]
- Carter, G.W.; Young, P.R.; Albert, D.H.; Bouska, J.; Dyer, R.; Bell, R.L.; Summers, J.B.; Brooks, D.W. 5-Lipoxygenase inhibitory activity of zileuton. J. Pharmacol. Exp. Ther. 1991, 256, 929–937. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- De Luca, V.; Salim, V.; Atsumi, S.M.; Yu, F. Mining the biodiversity of plants: A revolution in the making. Science 2012, 206, 1658–1661. [Google Scholar] [CrossRef]
- Hsieh, H.K.; Lee, T.H.; Wang, J.P.; Wang, J.J.; Lin, C.N. Synthesis and anti-inflammatory effect of chalcones and related compounds. Pharm. Res. 1998, 15, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Zeraik, M.L.; Ximenes, V.F.; Regasini, L.O.; Dutra, L.A.; Silva, D.H.S.; Fonseca, L.M.; Coelho, D.; Machado, S.A.; Bolzani, V.S. 4’-Aminochalcones as novel inhibitors of the chlorinating activity of myeloperoxidase. Curr. Med. Chem. 2012, 19, 5405–5413. [Google Scholar] [CrossRef] [PubMed]
- Babu, M.A.; Shakya, N.; Prathipati, P.; Kaskhedikar, S.G.; Saxena, A.K. Development of 3D-QSAR models for 5-lipoxygenase antagonists: Chalcones. Bioorg. Med. Chem. 2002, 10, 4035–4041. [Google Scholar] [CrossRef]
- Irfan, R.; Mousavi, S.; Alazmi, M.; Saleem, R.S.Z. A Comprehensive Review of Aminochalcones. Molecules 2020, 25, 5381. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.P.; Aparoy, P.; Reddy, T.C.; Achari, C.; Sridhar, P.R.; Reddanna, P. Design, synthesis, and biological evaluation of prenylated chalcones as 5-LOX inhibitors. Bioorg. Med. Chem. 2010, 18, 5807–5815. [Google Scholar] [CrossRef]
- Detsi, A.; Majdalani, M.; Kontogiorgis, C.A.; Hadjipavlou-Litina, D.; Kefalas, P. Natural and synthetic 2′-hydroxy-chalcones and aurones: Synthesis, characterization and evaluation of the antioxidant and soybean lipoxygenase inhibitory activity. Bioorg. Med. Chem. 2009, 17, 8073–8085. [Google Scholar] [CrossRef]
- Valli, M.; dos Santos, R.N.; Figueira, L.D.; Nakajima, C.H.; Castro-Gamboa, I.; Andricopulo, A.D.; Bolzani, V.S. Development of a natural products database from the biodiversity of Brazil. J. Nat. Prod. 2013, 76, 439–444. [Google Scholar] [CrossRef]
- Pilon, A.C.; Valli, M.; Dametto, A.C.; Pinto, M.E.F.; Freire, R.T.; Castro-Gamboa, I.; Andricopulo, A.D.; Bolzani, V.S. NuBBEDB: An updated database to uncover chemical and biological information from Brazilian biodiversity. Sci. Rep. 2017, 7, 7215. [Google Scholar] [CrossRef] [PubMed]
- Taraporewala, I.B.; Kauffman, J.M. Synthesis and structure-activity relationships of anti-inflammatory 9,10-dihydro-9-oxo-2-acridine-alkanoic acids and 4-(2-carboxyphenyl)aminobenzenealkanoic acids. J. Pharm. Sci. 1990, 79, 173–178. [Google Scholar] [CrossRef]
- Passalacqua, T.G.; Dutra, L.A.; Almeida, L.; Velásquez, A.M.; Torres, F.A.; Yamasaki, P.R.; Santos, M.B.; Regasini, L.O.; Michels, P.A.; Bolzani, V.S.; et al. Synthesis and evaluation of novel prenylated chalcone derivatives as anti-leishmanial and anti-trypanosomal compounds. Bioorg. Med. Chem. Lett. 2015, 25, 3342–3345. [Google Scholar] [CrossRef] [PubMed]
- Katsori, A.M.; Chatzopoulou, M.; Dimas, K.; Kontogiorgis, C.; Patsilinakos, A.; Trangas, T.; Hadjipavlou-Litina, D. Curcumin analogues as possible anti-proliferative & anti-inflammatory agents. Eur. J. Med. Chem. 2011, 46, 2722–2735. [Google Scholar] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- SwissADME. Available online: http://www.swissadme.ch/ (accessed on 25 November 2020).
- Ha, T.J.; Nihei, K.; Kubo, I. Lipoxygenase inhibitory activity of octyl gallate. J. Agric. Food Chem. 2004, 52, 3177–3181. [Google Scholar] [CrossRef]
- Steczko, J.; Donoho, G.P.; Clemens, J.C.; Dixon, J.E.; Axelrod, B. Conserved histidine residues in soybean lipoxygenase: Functional consequences of their replacement. Biochemistry 1992, 31, 4053–4057. [Google Scholar] [CrossRef] [PubMed]
- Boyington, J.C.; Gaffney, B.J.; Amzel, L.M. The three-dimensional structure of an arachidonic acid 15-lipoxygenase. Science 1993, 260, 1482–1486. [Google Scholar] [CrossRef]
- Amin, S.N.M.; Idris, M.H.M.; Selvaraj, M.; Amin, S.N.M.; Jamari, H.; Kek, T.L.; Salleh, M.Z. Virtual screening, ADME study, and molecular dynamic simulation of chalcone and flavone derivatives as 5-Lipoxygenase (5-LO) inhibitor. Mol. Simul. 2020, 46, 487–496. [Google Scholar] [CrossRef]
- Clapp, C.H.; Grandizio, A.M.; Yang, Y.; Kagey, M.; Turner, D.; Bicker, A.; Muskardin, D. Irreversible inactivation of soybean lipoxygenase-1 by hydrophobic thiols. Biochemistry 2002, 24, 11504–11511. [Google Scholar] [CrossRef] [PubMed]
- Certara. Sybyl; Version 8.0; Certara: Headquarters Princeton, NJ, USA, 2012. [Google Scholar]
- CCDC. GOLD; Version 1.10; CCDC: Hong Kong, China, 2020. [Google Scholar]
- Wallace, A.C.; Laskowski, R.A.; Thornton, J.M. LIGPLOT: A program to generate schematic diagrams of protein-ligand interactions. Prot. Eng. 1995, 8, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.B.; Marques, B.C.; Ayusso, G.M. Chalcones and their B-aryl analogues as myeloperoxidase inhibitors: In silico, in vitro and ex vivo investigations. Bioorg. Chem. 2021, 110, 104773. [Google Scholar] [CrossRef] [PubMed]
- John, G.S.M.; Takeuchi, S.; Venkatraman, G.; Rayala, S.K. Nordihydroguaiaretic Acid in Therapeutics: Beneficial to Toxicity Profiles and the Search for its Analogs. Curr. Cancer Drug Targets 2020, 20, 86–103. [Google Scholar]

| Compound | Structure | IC50 (µM) a | Calculated log P b |
|---|---|---|---|
| 1 | 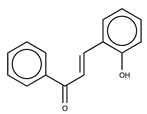 | >50 | 3.09 |
| 2 | 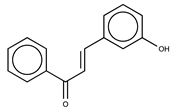 | >50 | 2.87 |
| 3 | 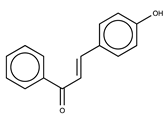 | >50 | 2.87 |
| 4 | 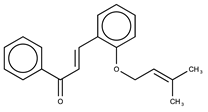 | >50 | 4.52 |
| 5 | 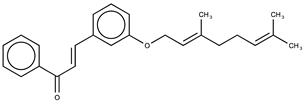 | 11.8 ± 0.9 | 5.94 |
| 6 | 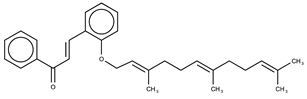 | 5.7 ± 0.8 | 7.46 |
| Curcumin | 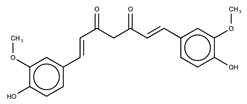 | 10.1 ± 0.5 | |
| Nordihydroguaiaretic acid (NDGA) | 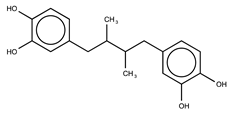 | 2.7 ± 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeraik, M.L.; Pauli, I.; Dutra, L.A.; Cruz, R.S.; Valli, M.; Paracatu, L.C.; de Faria, C.M.Q.G.; Ximenes, V.F.; Regasini, L.O.; Andricopulo, A.D.; et al. Identification of a Prenyl Chalcone as a Competitive Lipoxygenase Inhibitor: Screening, Biochemical Evaluation and Molecular Modeling Studies. Molecules 2021, 26, 2205. https://doi.org/10.3390/molecules26082205
Zeraik ML, Pauli I, Dutra LA, Cruz RS, Valli M, Paracatu LC, de Faria CMQG, Ximenes VF, Regasini LO, Andricopulo AD, et al. Identification of a Prenyl Chalcone as a Competitive Lipoxygenase Inhibitor: Screening, Biochemical Evaluation and Molecular Modeling Studies. Molecules. 2021; 26(8):2205. https://doi.org/10.3390/molecules26082205
Chicago/Turabian StyleZeraik, Maria Luiza, Ivani Pauli, Luiz A. Dutra, Raquel S. Cruz, Marilia Valli, Luana C. Paracatu, Carolina M. Q. G. de Faria, Valdecir F. Ximenes, Luis O. Regasini, Adriano D. Andricopulo, and et al. 2021. "Identification of a Prenyl Chalcone as a Competitive Lipoxygenase Inhibitor: Screening, Biochemical Evaluation and Molecular Modeling Studies" Molecules 26, no. 8: 2205. https://doi.org/10.3390/molecules26082205
APA StyleZeraik, M. L., Pauli, I., Dutra, L. A., Cruz, R. S., Valli, M., Paracatu, L. C., de Faria, C. M. Q. G., Ximenes, V. F., Regasini, L. O., Andricopulo, A. D., & Bolzani, V. S. (2021). Identification of a Prenyl Chalcone as a Competitive Lipoxygenase Inhibitor: Screening, Biochemical Evaluation and Molecular Modeling Studies. Molecules, 26(8), 2205. https://doi.org/10.3390/molecules26082205