Increased Stress Resistance and Lifespan in Chaenorhabditis elegans Wildtype and Knockout Mutants—Implications for Depression Treatment by Medicinal Herbs
Abstract
1. Introduction
2. Materials and Methods
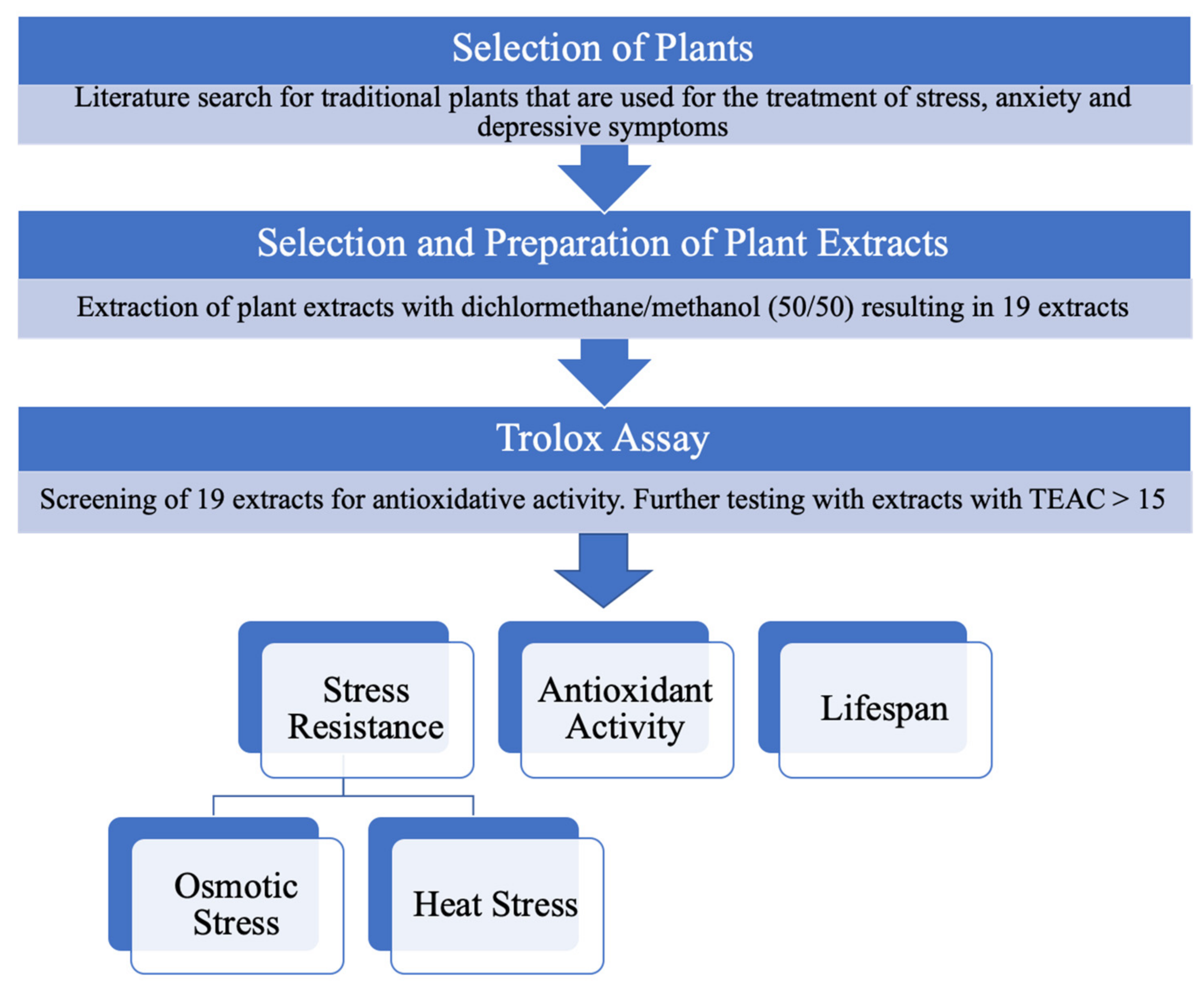
2.1. Selection of Plants
2.2. Substances and Reagents
2.3. Plant Material and Extraction Preparation and Reference Compounds
2.4. C. elegans Strains and Culture Conditions
2.5. Trolox Antioxidant Activity
2.6. H2DCFDA Antioxidant Activity
2.7. Osmotic Stress Resistance Assay
2.8. Heat Stress Assay
2.9. Lifespan Assay
2.10. Extract Analyses by HPLC-HRMS
2.11. Statistical Analysis
3. Results
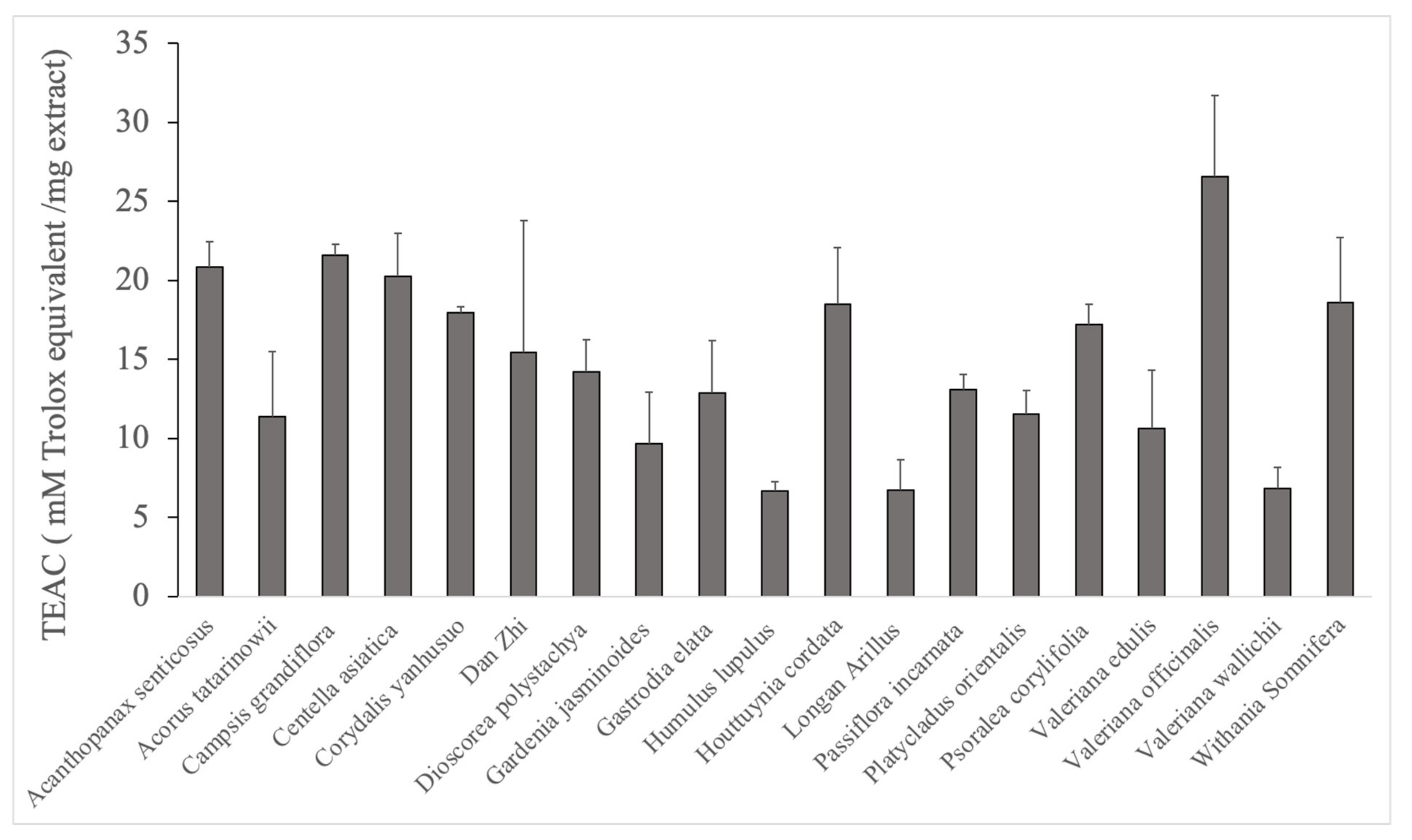
3.1. Trolox Antioxidant Activity
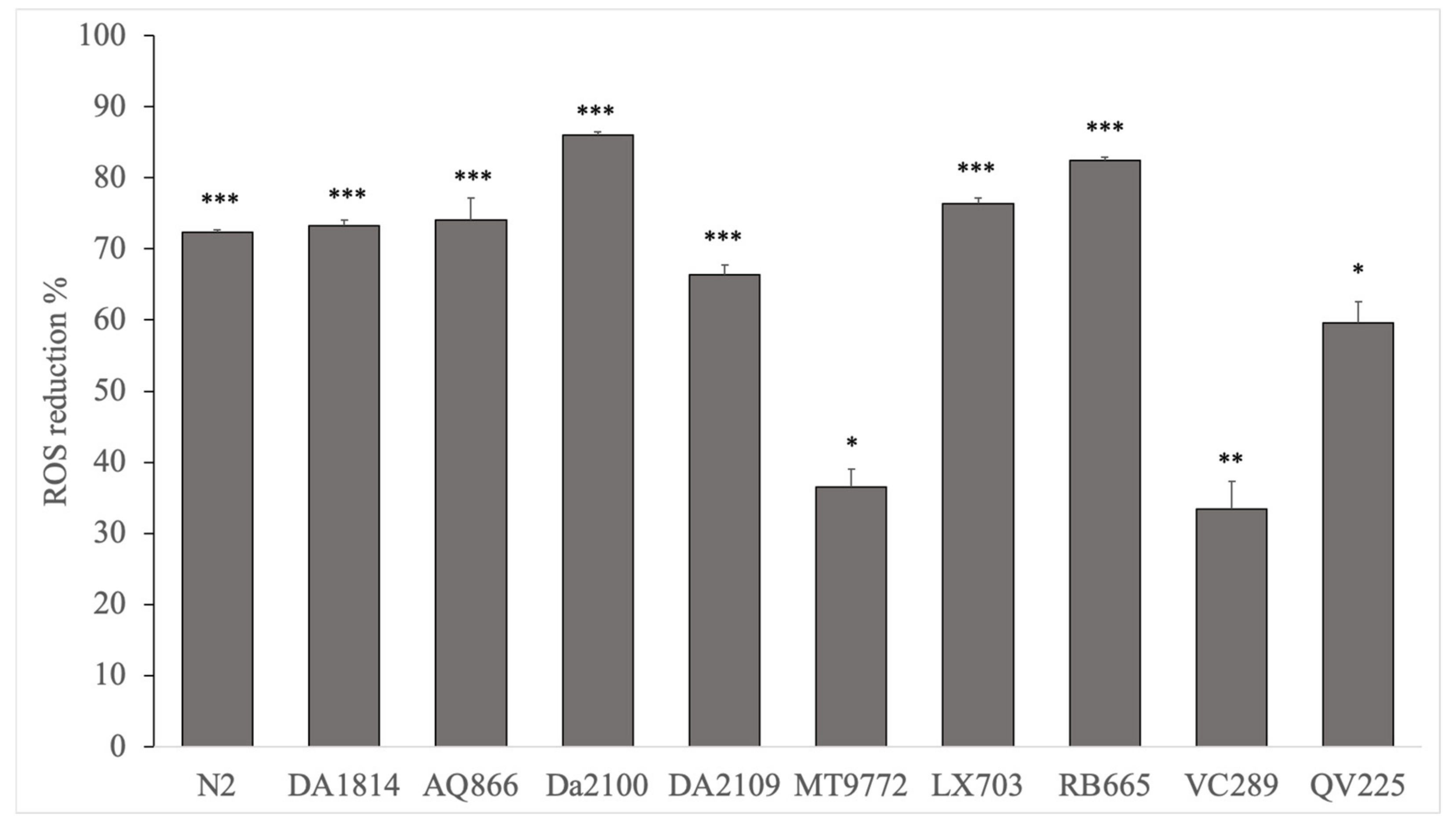
3.2. H2DCFDA Antioxidant Activity
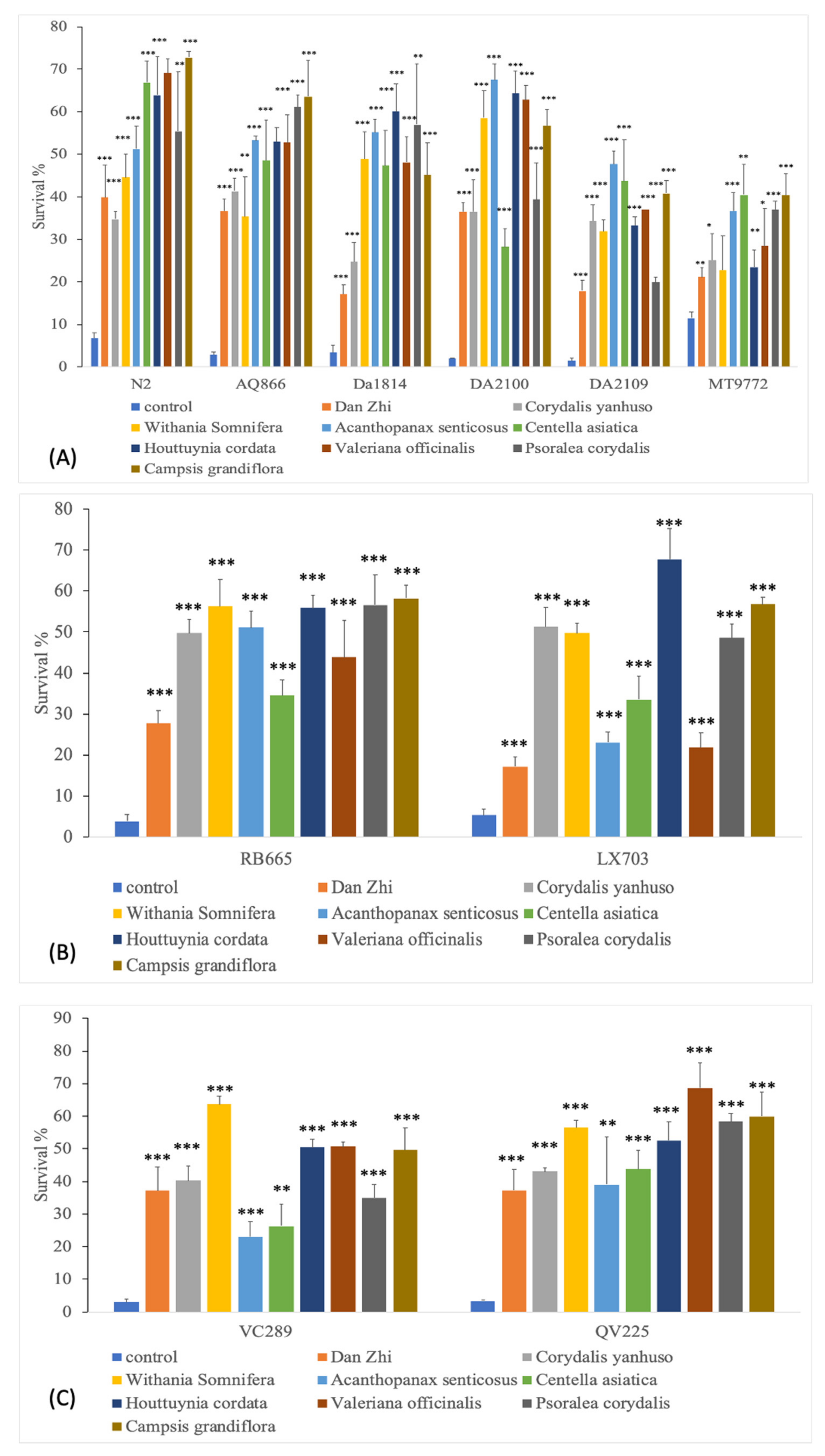
3.3. Acute Osmotic Stress Resistance Assay
3.4. Heat Stress Assay
3.5. Lifespan Assay
3.6. Extract Analyses by HPLC-HRMS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Vindegaard, N.; Benros, M.E. COVID-19 pandemic and mental health consequences: Systematic review of the current evidence. Brain Behav. Immun. 2020, 89, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Torales, J.; O’Higgins, M.; Castaldelli-Maia, J.M.; Ventriglio, A. The outbreak of COVID-19 coronavirus and its impact on global mental health. Int. J. Soc. Psychiatry 2020, 66, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Semo, B.-W.; Frissa, S.M. The mental health impact of the COVID-19 pandemic: Implications for sub-saharan Africa. Psychol. Res. Behav. Manag. 2020, 13, 713–720. [Google Scholar] [CrossRef]
- Wang, P.S.; Aguilar-Gaxiola, S.; Alonso, J.; Angermeyer, M.C.; Borges, G.; Bromet, E.J.; Bruffaerts, R.; de Girolamo, G.; de Graaf, R.; Gureje, O.; et al. Use of mental health services for anxiety, mood, and substance disorders in 17 countries in the WHO world mental health surveys. Lancet 2007, 370, 841–850. [Google Scholar] [CrossRef]
- Bong, C.-L.; Brasher, C.; Chikumba, E.; McDougall, R.; Mellin-Olsen, J.; Enright, A. The COVID-19 Pandemic: Effects on low- and middle-income countries. Anesth. Analg. 2020, 131, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Zar, H.J.; Dawa, J.; Fischer, G.B.; Castro-Rodriguez, J.A. Challenges of COVID-19 in children in low- and middle-income countries. Paediatr. Respir. Rev. 2020, 35, 70–74. [Google Scholar] [CrossRef]
- Ataguba, O.A.; Ataguba, J.E. Social determinants of health: The role of effective communication in the COVID-19 pandemic in developing countries. Glob. Health Action 2020, 13, 1788263. [Google Scholar] [CrossRef]
- Mendenhall, E.; Kohrt, B.A.; Norris, S.A.; Ndetei, D.; Prabhakaran, D. Non-communicable disease syndemics: Poverty, depression, and diabetes among low-income populations. Lancet 2017, 389, 951–963. [Google Scholar] [CrossRef]
- Yatham, S.; Sivathasan, S.; Yoon, R.; da Silva, T.L.; Ravindran, A.V. Depression, anxiety, and post-traumatic stress disorder among youth in low and middle income countries: A review of prevalence and treatment interventions. Asian J. Psychiatr. 2018, 38, 78–91. [Google Scholar] [CrossRef]
- Lotfaliany, M.; Hoare, E.; Jacka, F.N.; Kowal, P.; Berk, M.; Mohebbi, M. Variation in the prevalence of depression and patterns of association, sociodemographic and lifestyle factors in community-dwelling older adults in six low- and middle-income countries. J. Affect. Disord. 2019, 251, 218–226. [Google Scholar] [CrossRef]
- Kim, A.W.; Nyengerai, T.; Mendenhall, E. Evaluating the mental health impacts of the COVID-19 pandemic: Perceived risk of COVID-19 infection and childhood trauma predict adult depressive symptoms in urban South Africa. Psychol. Med. 2020. [Google Scholar] [CrossRef]
- World Health Organization. Policy Brief: COVID-19 and the Need for Action on Mental Health 2020. Available online: https://reliefweb.int/report/world/policy-brief-covid-19-and-need-action-mental-health-13-may-2020 (accessed on 10 March 2021).
- Hacimusalar, Y.; Eşel, E. Suggested biomarkers for major depressive disorder. Noro Psikiyatr. Ars. 2018, 55, 280–290. [Google Scholar] [CrossRef]
- Crider, A.; Feng, T.; Pandya, C.D.; Davis, T.; Nair, A.; Ahmed, A.O.; Baban, B.; Turecki, G.; Pillai, A. Complement component 3a receptor deficiency attenuates chronic stress-induced monocyte infiltration and depressive-like behavior. Brain Behav. Immun. 2018, 70, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Okamoto, Y.; Takagaki, K.; Okada, G.; Toki, S.; Inoue, T.; Tanabe, H.; Kobayakawa, M.; Yamawaki, S. Direct and indirect influences of childhood abuse on depression symptoms in patients with major depressive disorder. BMC Psychiatry 2015, 15, 244. [Google Scholar] [CrossRef] [PubMed]
- Sousa, C.; Mason, W.A.; Herrenkohl, T.I.; Prince, D.; Herrenkohl, R.C.; Russo, M.J. Direct and indirect effects of child abuse and environmental stress: A lifecourse perspective on adversity and depressive symptoms. Am. J. Orthopsychiatry 2018, 88, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Dell’Osso, L.; Carmassi, C.; Mucci, F.; Marazziti, D. Depression, serotonin and tryptophan. Curr. Pharm. Des. 2016, 22, 949–954. [Google Scholar] [CrossRef]
- Grace, A.A. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat. Rev. Neurosci. 2016, 17, 524–532. [Google Scholar] [CrossRef]
- Chockalingam, R.; Gott, B.M.; Conway, C.R. Tricyclic antidepressants and monoamine oxidase inhibitors: Are they too old for a new look? Handb. Exp. Pharmacol. 2019, 250, 37–48. [Google Scholar] [CrossRef]
- Schmidt, M.V.; Sterlemann, V.; Müller, M.B. Chronic stress and individual vulnerability. Ann. N. Y. Acad. Sci. 2008, 1148, 174–183. [Google Scholar] [CrossRef]
- Liu, T.; Zhong, S.; Liao, X.; Chen, J.; He, T.; Lai, S.; Jia, Y. A meta-analysis of oxidative stress markers in depression. PLoS ONE 2015, 10, e0138904. [Google Scholar] [CrossRef]
- Maes, M.; Ruckoanich, P.; Chang, Y.S.; Mahanonda, N.; Berk, M. Multiple aberrations in shared inflammatory and oxidative & nitrosative stress (IO & NS) pathways explain the co-association of depression and cardiovascular disorder (CVD), and the increased risk for CVD and due mortality in depressed patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 769–783. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, T.P. Depressive disorders: Treatment failures and poor prognosis over the last 50 years. Pharmacol. Res. Perspect 2019, 7, e00472. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.M. SSRI Antidepressant medications: Adverse effects and tolerability. Prim. Care Companion J. Clin. Psychiatry 2001, 3, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Fathinezhad, Z.; Sewell, R.D.E.; Lorigooini, Z.; Rafieian-Kopaei, M. Depression and treatment with effective herbs. Curr. Pharm. Des. 2019, 25, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Ismail, H.; Amanat, M.A.; Iqbal, A.; Mirza, B. Medicinal plants: A complementary and alternative antidepressant therapy. Curr. Pharm. Des. 2018, 24, 2609–2624. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, R.; Huang, X. Meta-analysis of the clinical effectiveness of traditional Chinese medicine formula Chaihu-Shugan-San in depression. J. Ethnopharmacol. 2012, 141, 571–577. [Google Scholar] [CrossRef]
- World Health Organization. WHO Traditional Medicine Strategy: 2014–2023. Available online: https://www.who.int/medicines/publications/traditional/trm_strategy14_23/en/ (accessed on 10 March 2021).
- Sarris, J.; Panossian, A.; Schweitzer, I.; Stough, C.; Scholey, A. Herbal medicine for depression, anxiety and insomnia: A review of psychopharmacology and clinical evidence. Eur. Neuropsychopharmacol. 2011, 21, 841–860. [Google Scholar] [CrossRef] [PubMed]
- Farahani, M.S.; Bahramsoltani, R.; Farzaei, M.H.; Abdollahi, M.; Rahimi, R. Plant-derived natural medicines for the management of depression: An overview of mechanisms of action. Rev. Neurosci. 2015, 26, 305–321. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Hood, S.D.; Drummond, P.D. Multiple antidepressant potential modes of action of curcumin: A review of its anti-inflammatory, monoaminergic, antioxidant, immune-modulating and neuroprotective effects. J. Psychopharmacol. 2012, 26, 1512–1524. [Google Scholar] [CrossRef]
- Gerzson, M.F.B.; Victoria, F.N.; Radatz, C.S.; de Gomes, M.G.; Boeira, S.P.; Jacob, R.G.; Alves, D.; Jesse, C.R.; Savegnago, L. In vitro antioxidant activity and in vivo antidepressant-like effect of α-(phenylselanyl) acetophenone in mice. Pharmacol. Biochem. Behav. 2012, 102, 21–29. [Google Scholar] [CrossRef]
- Ng, Q.X.; Venkatanarayanan, N.; Ho, C.Y.X. Clinical use of Hypericum perforatum (St John’s wort) in depression: A meta-analysis. J. Affect. Disord. 2017, 210, 211–221. [Google Scholar] [CrossRef]
- Kober, M.; Pohl, K.; Efferth, T. Molecular mechanisms underlying St. John’s wort drug interactions. Curr. Drug Metab. 2008, 9, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Rangaraju, S.; Levey, D.F.; Nho, K.; Jain, N.; Andrews, K.D.; Le-Niculescu, H.; Salomon, D.R.; Saykin, A.J.; Petrascheck, M.; Niculescu, A.B. Mood, stress and longevity: Convergence on ANK3. Mol. Psychiatry 2016, 21, 1037–1049. [Google Scholar] [CrossRef]
- Dwyer, D.S. Crossing the worm-brain barrier by using Caenorhabditis elegans to explore fundamentals of human psychiatric illness. Mol. Neuropsychiatry 2018, 3, 170–179. [Google Scholar] [CrossRef]
- Yuan, P.; Pan, L.Y.; Xiong, L.G.; Tong, J.W.; Li, J.; Huang, J.A.; Gong, Y.S.; Liu, Z.H. Black tea increases hypertonic stress resistance in C. elegans. Food Funct. 2018, 9, 3798–3806. [Google Scholar] [CrossRef] [PubMed]
- Alammar, N.; Wang, L.; Saberi, B.; Nanavati, J.; Holtmann, G.; Shinohara, R.T.; Mullin, G.E. The impact of peppermint oil on the irritable bowel syndrome: A meta-analysis of the pooled clinical data. BMC Complement. Altern. Med. 2019, 19, 21. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, J.; Li, T.; Liu, R.H. Blueberry extract promotes longevity and stress tolerance via DAF-16 in Caenorhabditis elegans. Food Funct. 2018, 9, 5273–5282. [Google Scholar] [CrossRef]
- Naß, J.; Abdelfatah, S.; Efferth, T. Induction of stress resistance and extension of lifespan in Chaenorhabditis elegans serotonin-receptor knockout strains by withanolide A. Phytomedicine 2021, 84, 153482. [Google Scholar] [CrossRef] [PubMed]
- Naß, J.; Abdelfatah, S.; Efferth, T. Ursolic acid enhances stress resistance, reduces ROS accumulation and prolongs life span in C. elegans serotonin-deficient mutants. Food Funct. 2021. [Google Scholar] [CrossRef]
- Naß, J.; Efferth, T. Ursolic acid ameliorates stress and reactive oxygen species in C. elegans knockout mutants by the dopamine Dop1 and Dop3 receptors. Phytomedicine 2021, 81, 153439. [Google Scholar] [CrossRef] [PubMed]
- Stiernagle, T. Maintenance of C. elegans. WormBook 2006, 1–11. [Google Scholar] [CrossRef]
- Yoon, D.S.; Lee, M.-H.; Cha, D.S. Measurement of intracellular ROS in Caenorhabditis elegans using 2′,7′-dichlorodihydrofluorescein diacetate. Bio-Protocol 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Solomon, A.; Bandhakavi, S.; Jabbar, S.; Shah, R.; Beitel, G.J.; Morimoto, R.I. Caenorhabditis elegans OSR-1 regulates behavioral and physiological responses to hyperosmotic environments. Genetics 2004, 167, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Zevian, S.C.; Yanowitz, J.L. Methodological considerations for heat shock of the nematode Caenorhabditis elegans. Methods 2014, 68, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Sutphin, G.L.; Kaeberlein, M. Measuring Caenorhabditis elegans life span on solid media. J. Vis. Exp. 2009, 27, 1152. [Google Scholar] [CrossRef]
- Lu, X.; Saeed, M.E.M.; Hegazy, M.-E.F.; Kampf, C.J.; Efferth, T. Chemopreventive property of Sencha tea extracts towards sensitive and multidrug-resistant leukemia and multiple myeloma cells. Biomolecules 2020, 10, 1000. [Google Scholar] [CrossRef]
- Chen, N.; Chen, J.; Yao, B.; Li, Z. QSAR Study on antioxidant tripeptides and the antioxidant activity of the designed tripeptides in free radical systems. Molecules 2018, 23, 1407. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.M.; Nabavi, S.F.; Sureda, A.; Caprioli, G.; Iannarelli, R.; Sokeng, A.J.T.; Braidy, N.; Khanjani, S.; Moghaddam, A.H.; Atanasov, A.G.; et al. The water extract of tutsan (Hypericum androsaemum L.) red berries exerts antidepressive-like effects and in vivo antioxidant activity in a mouse model of post-stroke depression. Biomed. Pharmacother. 2018, 99, 290–298. [Google Scholar] [CrossRef]
- Di Lorenzo, A.; Nabavi, S.F.; Sureda, A.; Moghaddam, A.H.; Khanjani, S.; Arcidiaco, P.; Nabavi, S.M.; Daglia, M. Antidepressive-like effects and antioxidant activity of green tea and GABA green tea in a mouse model of post-stroke depression. Mol. Nutr. Food Res. 2016, 60, 566–579. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.-G.; Teng, J.-F.; Wong, V.K.-W.; Zhou, X.-G.; Qiu, W.-Q.; Tang, Y.; Wu, J.-M.; Xiong, R.; Pan, R.; Wang, Y.-L.; et al. Novel steroidal saponin isolated from Trillium tschonoskii Maxim. exhibits anti-oxidative effect via autophagy induction in cellular and Caenorhabditis elegans models. Phytomedicine 2019, 65, 153088. [Google Scholar] [CrossRef] [PubMed]
- Chandler-Brown, D.; Choi, H.; Park, S.; Ocampo, B.R.; Chen, S.; Le, A.; Sutphin, G.L.; Shamieh, L.S.; Smith, E.D.; Kaeberlein, M. Sorbitol treatment extends lifespan and induces the osmotic stress response in Caenorhabditis elegans. Front. Genet. 2015, 6, 316. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Y.; Shen, H.; Li, H.; Wang, Z.; Chen, G. Nox2 and Nox4 participate in ROS-induced neuronal apoptosis and brain injury during ischemia-reperfusion in rats. Acta Neurochir. Suppl. 2020, 127, 47–54. [Google Scholar] [CrossRef]
- Fang, E.F.; Waltz, T.B.; Kassahun, H.; Lu, Q.; Kerr, J.S.; Morevati, M.; Fivenson, E.M.; Wollman, B.N.; Marosi, K.; Wilson, M.A.; et al. Tomatidine enhances lifespan and healthspan in C. elegans through mitophagy induction via the SKN-1/Nrf2 pathway. Sci. Rep. 2017, 7, 46208. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-C.; Wu, J.; Zhang, H.-X.; Zhang, H.-S.; Qiao, T.-T.; Zhang, J.-X.; Zhang, G.-L.; Sui, J.; Li, L.-W.; Zhang, L.-R.; et al. Antidepressant-like and anti-oxidative efficacy of Campsis grandiflora flower. J. Pharm. Pharmacol. 2015, 67, 1705–1715. [Google Scholar] [CrossRef]
- Kim, D.-H.; Han, K.-M.; Chung, I.-S.; Kim, D.-K.; Kim, S.-H.; Kwon, B.-M.; Jeong, T.-S.; Park, M.-H.; Ahn, E.-M.; Baek, N.-I. Triterpenoids from the flower of Campsis grandiflora K. Schum. as human acyl-CoA: Cholesterol acyltransferase inhibitors. Arch. Pharm. Res. 2005, 28, 550–556. [Google Scholar] [CrossRef]
- Jiang, H.; Zheng, G.; Lv, J.; Chen, H.; Lin, J.; Li, Y.; Fan, G.; Ding, X. Identification of Centella asiatica’s effective ingredients for inducing the neuronal differentiation. Evid. Based Complement. Altern. Med. 2016, 2016, 9634750. [Google Scholar] [CrossRef] [PubMed]
- Bauer, R.; Pröbstle, A.; Lotter, H.; Wagner-Redecker, W.; Matthiesen, U. Cyclooxygenase inhibitory constituents from Houttuynia cordata. Phytomedicine 1996, 2, 305–308. [Google Scholar] [CrossRef]
- Koul, B.; Taak, P.; Kumar, A.; Kumar, A.; Sanyal, I. Genus Psoralea: A review of the traditional and modern uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2019, 232, 201–226. [Google Scholar] [CrossRef]
- Ruan, B.; Kong, L.-Y.; Takaya, Y.; Niwa, M. Studies on the chemical constituents of Psoralea corylifolia L. J. Asian Nat. Prod. Res. 2007, 9, 41–44. [Google Scholar] [CrossRef]
- Rosa, A.; Maccioni, D.; Maxia, A. Fatty acid and triacylglycerol composition of seed and pericarp oils of the medicinal crop Withania somnifera (L.) Dunal cultivated in Sardinia (Italy). Nat. Prod. Res. 2020. [Google Scholar] [CrossRef]
- Triantaphyllou, K.; Blekas, G.; Boskou, D. Antioxidative properties of water extracts obtained from herbs of the species Lamiaceae. Int. J. Food Sci. Nutr. 2001, 52, 313–317. [Google Scholar] [CrossRef]
- González-Burgos, E.; Liaudanskas, M.; Viškelis, J.; Žvikas, V.; Janulis, V.; Gómez-Serranillos, M.P. Antioxidant activity, neuroprotective properties and bioactive constituents analysis of varying polarity extracts from Eucalyptus globulus leaves. J. Food Drug Anal. 2018, 26, 1293–1302. [Google Scholar] [CrossRef]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Załuski, D.; Smolarz, H.D. TLC profiling, nutritional and pharmacological properties of Siberian ginseng (Eleutherococcus senticosus) cultivated in Poland. Pak. J. Pharm. Sci. 2016, 29, 1497–1502. [Google Scholar]
- Tafet, G.E.; Nemeroff, C.B. The links between stress and depression: Psychoneuroendocrinological, genetic, and environmental interactions. J. Neuropsychiatry Clin. Neurosci. 2016, 28, 77–88. [Google Scholar] [CrossRef]
- Dailly, E.; Chenu, F.; Renard, C.E.; Bourin, M. Dopamine, depression and antidepressants. Fundam. Clin. Pharmacol. 2004, 18, 601–607. [Google Scholar] [CrossRef]
- Hashimoto, K. Essential Role of Keap1-Nrf2 signaling in mood disorders: Overview and future perspective. Front. Pharmacol. 2018, 9, 1182. [Google Scholar] [CrossRef]
- Hu, Q.; D’Amora, D.R.; MacNeil, L.T.; Walhout, A.J.M.; Kubiseski, T.J. The oxidative stress response in Caenorhabditis elegans requires the GATA transcription factor ELT-3 and SKN-1/Nrf2. Genetics 2017, 206, 1909–1922. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.; Snoek, L.B.; de Bono, M.; Kammenga, J.E. Worms under stress: C. elegans stress response and its relevance to complex human disease and aging. Trends Genet. 2013, 29, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Durg, S.; Dhadde, S.B.; Vandal, R.; Shivakumar, B.S.; Charan, C.S. Withania somnifera (Ashwagandha) in neurobehavioural disorders induced by brain oxidative stress in rodents: A systematic review and meta-analysis. J. Pharm. Pharmacol. 2015, 67, 879–899. [Google Scholar] [CrossRef] [PubMed]
- Mager, W.H.; de Boer, A.H.; Siderius, M.H.; Voss, H.P. Cellular responses to oxidative and osmotic stress. Cell Stress Chaperones 2000, 5, 73–75. [Google Scholar] [CrossRef]
- Slimen, I.B.; Najar, T.; Ghram, A.; Dabbebi, H.; Ben-Mrad, M.; Abdrabbah, M. Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage. A review. Int. J. Hyperth. 2014, 30, 513–523. [Google Scholar] [CrossRef]
- Denzel, M.S.; Lapierre, L.R.; Mack, H.I.D. Emerging topics in C. elegans aging research: Transcriptional regulation, stress response and epigenetics. Mech. Ageing Dev. 2019, 177, 4–21. [Google Scholar] [CrossRef]
- Anjaneyulu, J.; Vidyashankar, R.; Godbole, A. Differential effect of Ayurvedic nootropics on C. elegans models of Parkinson’s disease. J. Ayurveda Integr. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Y.; Wang, C.; Zhang, X.; Wang, Z.; Liang, X.; Alachkar, A.; Civelli, O. A natural product with high affinity to sigma and 5-HT7 receptors as novel therapeutic drug for negative and cognitive symptoms of schizophrenia. Neurochem. Res. 2019, 44, 2536–2545. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yuan, Z.-Z.; Ma, X.; Wang, C.; Suo, Y.-R.; Wang, H.-L.; Wang, X.-Y.; Bai, B. Triterpenoids with antioxidant activities from Myricaria squamosa. J. Asian Nat. Prod. Res. 2018, 20, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Antioxidant and anti-inflammatory mechanisms of neuroprotection by ursolic acid: Addressing brain injury, cerebral ischemia, cognition deficit, anxiety, and depression. Oxid. Med. Cell Longev. 2019, 2019, 8512048. [Google Scholar] [CrossRef]
- Ramos-Hryb, A.B.; Cunha, M.P.; Pazini, F.L.; Lieberknecht, V.; Prediger, R.D.S.; Kaster, M.P.; Rodrigues, A.L.S. Ursolic acid affords antidepressant-like effects in mice through the activation of PKA, PKC, CAMK-II and MEK1/2. Pharmacol. Rep. 2017, 69, 1240–1246. [Google Scholar] [CrossRef]
- Gannon, J.M.; Brar, J.; Rai, A.; Chengappa, K.N.R. Effects of a standardized extract of Withania somnifera (Ashwagandha) on depression and anxiety symptoms in persons with schizophrenia participating in a randomized, placebo-controlled clinical trial. Ann. Clin. Psychiatry 2019, 31, 123–129. [Google Scholar]
- Choudhary, D.; Bhattacharyya, S.; Bose, S. Efficacy and safety of Ashwagandha (Withania somnifera (L.) Dunal) root extract in improving memory and cognitive functions. J. Diet. Suppl. 2017, 14, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Akhoon, B.A.; Pandey, S.; Tiwari, S.; Pandey, R. Withanolide A offers neuroprotection, ameliorates stress resistance and prolongs the life expectancy of Caenorhabditis elegans. Exp. Gerontol. 2016, 78, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Montilla, M.P.; Agil, A.; Navarro, M.C.; Jiménez, M.I.; García-Granados, A.; Parra, A.; Cabo, M.M. Antioxidant activity of maslinic acid, a triterpene derivative obtained from Olea europaea. Planta Med. 2003, 69, 472–474. [Google Scholar] [CrossRef] [PubMed]
- Ou, H.-C.; Pandey, S.; Hung, M.-Y.; Huang, S.-H.; Hsu, P.-T.; Day, C.-H.; Pai, P.; Viswanadha, V.P.; Kuo, W.-W.; Huang, C.-Y. Luteolin: A natural flavonoid enhances the survival of HUVECs against oxidative stress by modulating AMPK/PKC pathway. Am. J. Chin. Med. 2019, 47, 541–557. [Google Scholar] [CrossRef] [PubMed]
- Kempuraj, D.; Thangavel, R.; Kempuraj, D.D.; Ahmed, M.E.; Selvakumar, G.P.; Raikwar, S.P.; Zaheer, S.A.; Iyer, S.S.; Govindarajan, R.; Chandrasekaran, P.N.; et al. Neuroprotective effects of flavone luteolin in neuroinflammation and neurotrauma. Biofactors 2020. [Google Scholar] [CrossRef]
- Pan, H.; Hu, X.-Z.; Jacobowitz, D.M.; Chen, C.; McDonough, J.; van Shura, K.; Lyman, M.; Marini, A.M. Alpha-linolenic acid is a potent neuroprotective agent against soman-induced neuropathology. Neurotoxicology 2012, 33, 1219–1229. [Google Scholar] [CrossRef]
- Rodeghiero, F.; Castaman, G.C.; Tosetto, A.; Lattuada, A.; Mannucci, P.M. Platelet von Willebrand factor assay: Results using two methods for platelet lysis. Thromb. Res. 1990, 59, 259–267. [Google Scholar] [CrossRef]
- Efferth, T.; Koch, E. Complex interactions between phytochemicals. The multi-target therapeutic concept of phytotherapy. Curr. Drug Targets 2011, 12, 122–132. [Google Scholar] [CrossRef]
| Species | Family | Traditional/Common Name | Drug | Traditional Use | Origin |
|---|---|---|---|---|---|
| Acorus tatarinowii Schott. | Acoraceae | Shi Chang Pu | Rhizome |
| Traditional Chinese Medicine |
| Campsis grandiflora (Thunb.) K. Schum. | Bignoniaceae | Chinese trumpet vine | Flower |
| Traditional Chinese Medicine |
| Corydalis yanhusuo W.T.Wang | Papaveraceae | Yan hu suo | Rhizome |
| Traditional Chinese Medicine |
| Dioscorea polystachya Turcz. | Dioscoreaceae | Shan yao; Chinese yam | Rhizome |
| Traditional Chinese Medicine |
| Acanthopanax senticosus (Rupr. & Maxim.) Harms | Araliaceae | Eleutherococcus senticosus; Siberian Ginseng | Herb |
| Traditional Chinese Medicine |
| Gardenia jasminoides J. Ellis | Rubiaceae | Danh-danh; Cape jasmine | Fruit |
| Traditional Chinese Medicine |
| Gastrodia elata Bl. | Orchidaceae | Chì jiàn | Rhizome |
| Traditional Chinese Medicine |
| Houttuynia cordata Thunb. | Saururaceae | Yu Xing Cao; Fish mint | Herb |
| Traditional Indian Medicine/Traditional Chinese Medicine |
| Centella asiatica (L.) Urban | Apiaceae | Gotu Kola; Centella | Herb |
| Traditional Indian Medicine/Traditional Chinese Medicine |
| Longan Arillus Lour. | Sapindaceae | Long Yan Rou | Fruit |
| Traditional Chinese Medicine |
| Humulus lupulus L. | Cannabaceae | Hops | Flower |
| Traditional European Medicine |
| Passiflora incarnata L. | Passifloraceae | Maypop; Passion Flower | Herb |
| Traditional European Medicine |
| Platycladus orientalis (L.) Franco | Cupressaceae | Bai Zi Ren; Oriental thuja | Seed |
| Traditional Chinese Medicine |
| Psoralea corylifolia L. | Fabaceae | Bu Gu Zhi; Babchi | Fruit |
| Traditional Indian Medicine/Traditional Chinese Medicine |
| Valeriana edulis Nutt. ex Torr. & A.Gray | Caprifoliaceae | Tobacco root | Rhizome herb |
| Traditional European Medicine |
| Valeriana officinalis L. | Caprifoliaceae | Valerian | Rhizome herb |
| Traditional European Medicine |
| Valeriana wallichii DC. | Valerianaceae | Indian Valerian | Rhizome herb |
| Traditional Indian Medicine |
| Withania somnifera (L.) Dunal | Solanaceae | Ashwagandha; Winter-cherry | Herb |
| Traditional Chinese Medicine |
| Dan-zhi-xiao-yao-san | - | Atractylodis macrocephale rhizoma, Bupleuri radix, Angelicae sinensis, poria, Glycyrrihizae radix, tree peony bark, Gardenia jasminoides, Paeonia lactiflora Pall, mint and roasted ginger |
| Traditional Chinese Medicine |
| Mutant | Protein | Human Homologue | Role in Depression |
|---|---|---|---|
| AQ866 | Ser-4 | 5-hydroxytryptamine receptor 1A (HTR1A) | Mutations are connected to depression and anxiety |
| DA1814 | Ser-1 | 5-hydroxytryptamine receptor 2B (HTR2B) | agonism induces an SSRI-like response |
| DA2100 | Ser-7 | 5-hydroxytryptamine receptor 7 (HTR7) | 5-HT7 antagonism antidepressant-like effects |
| DA2109 | Ser-1/Ser-7 | HTR2B/HTR7 | See Da1814/DA2100 |
| MT9772 | Mod-5 | Serotonin transporter (SERT) | gene-environment interaction of SERT and stress |
| RB665 | Dop-1 | Dopamine receptor D1 (D1) | Stimulation improves depression symptoms |
| LX703 | Dop-3 | Dopamine receptor D2 (D2) | Enhanced dopamine signalling improves symptoms |
| VC289 | Prdx-2 | Peroxiredoxin 2 (Prdx2) | protective role in cells; oxidative stress protection |
| QV225 | Skn-1 | Nuclear factor, erythroid 2 like 2 (Nrf2) | Lower expressions of Nrf2 correlated to depressive disorder |
| Stress Reduction (%) | N2 | AQ866 | DA1814 | Da2100 | DA2109 | MT9772 | RB665 | LX703 | VC289 | QV225 | Stress Reduction Mean (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Acanthopanax senticosus | 72.4 ± 0.3 *** | 74.1 ± 3.1 *** | 73.3 ± 0.7 *** | 86.1 ± 0.4 *** | 66.4 ± 1.4 *** | 36.5 ± 0.8 * | 82.4 ± 0.5 *** | 76.4 ± 2.6 *** | 33.4 ± 3.9 ** | 59.6 ± 3.0 * | 66.8 ± 18.0 |
| Campsis grandiflora | 72.3 ± 3.2 *** | 15.6 ± 18.8 n.s. | 48.8 ± 8.0 *** | 59.6 ± 6.8 ** | 58.7 ± 8.3 ** | 34.1 ± 4.9 *** | 43.4 ± 0.9 ** | 20.2 ± 1.1 ** | 47.9 ± 3.9 *** | 71.1 ± 0.8 *** | 47.2 ± 19.4 |
| Centella asiatica | 79.0 ± 0.4 ** | 63.9 ± 2.2 *** | 63.0 ± 3.5 *** | 2.9 ± 7.7 n.s. | 51.0 ± 4.9 *** | 67.7 ± 1.0 *** | 48.3 ± 3.5 ** | 51.7 ± 4.2 *** | 28.3 ± 2.4 ** | 78.0 ± 1.3 ** | 53.4 ± 23.3 |
| Corydalis yanhuso | 59.8 ± 4.3 *** | 81.5 ± 0.4 *** | 62.2 ± 3.6 *** | 82.8 ± 0.6 *** | 66.3 ± 4.9 *** | 26.7 ± 6.3 * | 76.4 ± 5.2 ** | 75.8 ± 1.2 *** | 65.2 ± 17.0 ** | 69.9 ± 1.5 *** | 66.7 ± 16.1 |
| Dan Zhi | 80.2 ± 2.5 *** | 67.6 ± 3.3 ** | 22.2 ± 11.1 * | 79.8 ± 1.4 ** | 24.2 ± 9.2 * | 22.2 ± 6.2 ** | 35.9 ± 6.2 ** | 17.0 ± 14.2 n.s. | 34.1 ± 12.7 *** | 46.6 ± 5.5 ** | 43.0 ± 24.5 |
| Houttuynia cordata | 73.5 ± 4.2 *** | 69.4 ± 2.0 *** | 70.6 ± 3.0 *** | 86.1 ± 4.7 *** | 61.2 ± 2.4 *** | 23.4 ± 13.2 n.s. | 74.3 ± 4.4 *** | 85.5 ± 2.0 *** | 50.0 ± 7.8 *** | 66.9 ± 3.1 *** | 66.1 ± 18.4 |
| Psoralea corydalis | 45.4 ± 2.0 *** | 72.8 ± 4.9 *** | 71.9 ± 1.2 *** | 58.9 ± 2.4 *** | 29.4 ± 45.8 n.s. | 40.1 ± 14.1 * | 45.7 ± 19.9 * | 56.5 ± 3.5 *** | 39.0 ± 3.3 *** | 78.5 ± 0.9 ** | 53.8 ± 16.6 |
| Valeriana officinalis | 81.4 ± 2.5 *** | 48.3 ± 1.8 ** | 43.2 ± 2.5 *** | 64.4 ± 8.2 ** | 37.1 ± 7.8 ** | 39. 2± 7.2 ** | 36.3 ± 2.5 ** | 10.9 ± 6.2 n.s. | 49.9 ± 1.1 *** | 70.1 ± 0.6 ** | 48.1 ± 20.0 |
| Withania somnifera | 71.4 ± 7.1 *** | 77.6 ± 0.8 *** | 72.2 ± 3.9 *** | 85.9 ± 1.1 *** | 65.6 ± 5.0 *** | 27.7 ± 4.6 * | 77.1 ± 3.7 *** | 77.6 ± 1.3 *** | 87.8 ± 9.7 ** | 70.7 ± 8.9 ** | 71.4 ± 16.8 |
| Control | Acanthopanax senticosus | Campsis grandiflora | Centella asiatica | Corydalis yanhusuo | Dan Zhi | Houttuynia cordata | Psoralea corylifolia | Valeriana officinalis | Withania somnifera | |
|---|---|---|---|---|---|---|---|---|---|---|
| N2 | 100 ± 11,5 | 153.8 ± 7.5 * | 284.6 ± 8.1 *** | 238.5 ± 6.5 *** | 173.1 ± 8.9 ** | 250.0 ± 6.2 *** | 215.4 ± 7.1 *** | 196.2 ± 7.8 *** | 296.2 ± 5.2 *** | 196.2 ± 9.8 ** |
| AQ866 | 100 ± 9.1 | 136.4 ± 10.0 * | 277.3 ± 8.2 *** | 295.5 ± 7.7 *** | 159.1 ± 8.6 ** | 190.9 ± 9.5 ** | 195.5 ± 11.6 ** | 250.0 ± 7.3 *** | 190.9 ± 9.5 *** | 209.1 ± 8.7 *** |
| DA1814 | 100 ± 0 | 228.6 ± 10.4 *** | 195.2 ± 9.8 *** | 176.2 ± 8.1 *** | 123.8 ± 7.7 * | 214.3 ± 11.1 *** | 257.1 ± 11.1 *** | 209.5 ± 9.1*** | 328.6 ± 5.8 *** | 338.1 ± 16.9 *** |
| DA2100 | 100 ± 0 | 109.1 ± 4.2 n.s. | 222.7 ± 8.2 *** | 268.2 ± 6.8 *** | 100.0 ± 9.1 n.s. | 363.6 ± 3.8 *** | 331.8 ± 5.5 *** | 191.3 ± 7.3 *** | 209.1 ± 6.5 *** | 127.3 ± 25.0 n.s. |
| DA2109 | 100 ± 8.7 | 156.5 ± 5.6 *** | 169.6 ± 7.7 *** | 195.7 ± 8.9 *** | 152.2 ± 22.9 n.s. | 169.6 ± 10.3 ** | 173.9 ± 7.5 *** | 166.7 ± 11.4 *** | 243.5 ± 10.7 *** | 191.3 ± 18.2 * |
| MT9772 | 100 ± 8.3 | 91.7 ± 9.1 n.s. | 220.8 ± 9.4 *** | 162.5 ± 5.1 ** | 133.3 ± 15.6 n.s. | 212.5 ± 7.8*** | 175.0 ± 11.9 ** | 235.0 ± 12.5 * | 191.7 ± 10.9 ** | 290.0 ± 8.7 ** |
| RB665 | 100 ± 0 | 395.0 ± 11.4 *** | 210.0 ± 9.5 *** | 310.0 ± 8.1 *** | 220.0 ± 9.1 *** | 200.0 ± 10.0 *** | 320.0 ± 6.3 *** | 228.6 ± 8.5 *** | 250.0 ± 8.0 *** | 252.4 ± 24.1 ** |
| LX703 | 100 ± 4.8 | 223.8 ± 10.6 *** | 228.6 ± 8.3 *** | 238.1 ± 8.0 *** | 200.0 ± 11.9 *** | 271.4 ± 7.0 *** | 195.2 ± 7.3 *** | 147.8 ± 6.3 *** | 233.3 ± 4.1 *** | 139.1 ± 37.7 * |
| VC289 | 100 ± 8.7 | 113.0 ± 7.7 n.s. | 173.9 ± 10.0 * | 156.5 ± 8.3 * | 169.6 ± 7.7 ** | 152.2 ± 8.6 * | 152.2 ± 11.4 * | 190.0 ± 8.8 * | 204.3 ± 10.6 ** | 170.0 ± 15.6 n.s. |
| QV225 | 100 ± 0 | 195.0 ± 17.9 *** | 235.0 ± 8.5 *** | 190.0 ± 10.5 *** | 110.0 ± 13.6 n.s. | 210.0 ± 11.9 *** | 220.0 ± 9.1 *** | 206.5 ± 15.8 *** | 180.0 ± 13.9*** | 210.5 ± 8.8 *** |
| Overall lifespan extension (%) | 180.3 ± 88.9 | 221.8 ± 38.2 | 223.1 ± 55.2 | 154.1 ± 38.4 | 223.4 ± 60.2 | 223.6 ± 61.2 | 206.5 ± 34.5 | 232.8 ± 48.5 | 210.5 ± 65.8 |
| Control | Acanthopanax senticosus | Campsis grandiflora | Centella asiatica | Corydalis yanhusuo | Dan Zhi | Houttuynia cordata | Psoralea corylifolia | Valeriana officinalis | Withania somnifera | |
|---|---|---|---|---|---|---|---|---|---|---|
| N2 | 100.0 ± 1.02 | 113.7 ± 0.89 *** | 117.7 ± 3.02 *** | 108.1 ± 2.35 *** | 114.7 ± 1.77 *** | 117.8± 2.59 *** | 116.2 ± 1.31 *** | 108.6 ± 0.47 *** | 106.1 ± 2.87 ** | 103.0 ± 0.49 ** |
| AQ866 | 100.0 ± 1.33 | 153.0 ± 0.87 *** | 125.3 ± 3.19 *** | 155.3 ± 2.58 *** | 150.6 ± 0.44 *** | 98.8 ± 5.70 *** | 151.3 ± 0.88 *** | 144.0 ± 0.93 *** | 141.3 ± 3.77 *** | 152.0 ± 0.44 *** |
| DA1814 | 100.0 ± 1.18 | 137.6 ± 1.28 *** | 129.4 ± 1.82 *** | 126.5 ± 2.79 *** | 111.8 ± 2.12 ** | 131.8 ± 2.05 *** | 128.8 ± 0.91 *** | 135.9 ± 0.43 * | 138.8 ± 1.69 *** | 137.0 ± 1.29 *** |
| DA2100 | 100.0 ± 1.23 | 130.2 ± 0.95 ** | 125.5 ± 1.46 ** | 130.2 ± 1.42 *** | 129.6 ± 1.90 *** | 137.7 ± 1.35 *** | 126.5 ± 2.44 *** | 128.4 ± 0.96 * | 132.0 ± 1.87 *** | 129.6 ± 1.43 *** |
| DA2109 | 100.0 ± 1.84 | 127.0 ± 1.45 ** | 128.2 ± 1.91 *** | 120.2 ± 2.55 *** | 131.3 ± 0.93 * | 126.4 ± 1.94 ** | 128.2 ± 1.44 * | 122.0 ± 3.02 * | 133.7 ± 1.38 ** | 130.7 ± 1.41 * |
| MT9772 | 100.0 ± 2.04 | 150.3 ± 0.45 *** | 149.0 ± 1.37 *** | 180.0 ± 1.22 * | 148.3 ± 0.92 * | 166.0 ± 1.64 *** | 149.0 ± 0.46 ** | 121.8 ± 1.12 *** | 152.8 ± 2.01 * | 146.9 ± 0.93 * |
| RB665 | 100.0 ± 1.92 | 139.1 ± 0.92 *** | 157.7 ± 2.03 *** | 151.9 ± 3.38 *** | 141.6 ± 1.36 *** | 148.1 ± 4.33 *** | 139.1 ± 0.92 *** | 132.1 ± 1.46 *** | 135.9 ± 12.73 *** | 143.0 ± 0.90 *** |
| LX703 | 100.0 ± 0.62 | 134.2 ± 0.93 ** | 118.0 ± 3.52 *** | 114.9 ± 3.24 *** | 139.1 ± 0.45 * | 128.8 ± 2.49 *** | 138.5 ± 0.90 *** | 129.2 ± 0.96 ** | 116.1 ± 3.74 *** | 135.4 ± 0.92 *** |
| VC289 | 100.0 ± 2.21 | 114.9 ± 1.44 ** | 112.2 ± 1.48 * | 112.7 ± 1.47 * | 107.2 ± 1.55 n.s. | 107.7 ± 1.03 n.s. | 106.1 ± 1.56 * | 110.5 ± 1.50 ** | 108.3 ± 1.53 * | 115.5 ± 0.96 * |
| QV225 | 100.0 ± 3.37 | 150.6 ± 1.87 *** | 146.6 ± 1.92 *** | 125.3 ± 2.24 *** | 124.2 ± 4.52 *** | 122.5 ± 3.21 *** | 118.5 ± 4.74 ** | 106.2 ± 2.65 *** | 132.6 ± 3.81 *** | 117.4 ± 5.26 ** |
| Overall lifespan extension (%) | 135.1 ± 14.0 | 131 ± 15.1 | 130 ± 22.8 | 129.8 ± 15.3 | 128.6 ± 19.3 | 130.2 ± 14.5 | 123.9 ± 12.5 | 129.8 ± 15.0 | 131.1 ± 15.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naß, J.; Kampf, C.J.; Efferth, T. Increased Stress Resistance and Lifespan in Chaenorhabditis elegans Wildtype and Knockout Mutants—Implications for Depression Treatment by Medicinal Herbs. Molecules 2021, 26, 1827. https://doi.org/10.3390/molecules26071827
Naß J, Kampf CJ, Efferth T. Increased Stress Resistance and Lifespan in Chaenorhabditis elegans Wildtype and Knockout Mutants—Implications for Depression Treatment by Medicinal Herbs. Molecules. 2021; 26(7):1827. https://doi.org/10.3390/molecules26071827
Chicago/Turabian StyleNaß, Janine, Christopher J. Kampf, and Thomas Efferth. 2021. "Increased Stress Resistance and Lifespan in Chaenorhabditis elegans Wildtype and Knockout Mutants—Implications for Depression Treatment by Medicinal Herbs" Molecules 26, no. 7: 1827. https://doi.org/10.3390/molecules26071827
APA StyleNaß, J., Kampf, C. J., & Efferth, T. (2021). Increased Stress Resistance and Lifespan in Chaenorhabditis elegans Wildtype and Knockout Mutants—Implications for Depression Treatment by Medicinal Herbs. Molecules, 26(7), 1827. https://doi.org/10.3390/molecules26071827







