Synthesis of a Novel Chitosan/Basil Oil Blend and Development of Novel Low Density Poly Ethylene/Chitosan/Basil Oil Active Packaging Films Following a Melt-Extrusion Process for Enhancing Chicken Breast Fillets Shelf-Life
Abstract
1. Introduction
2. Results
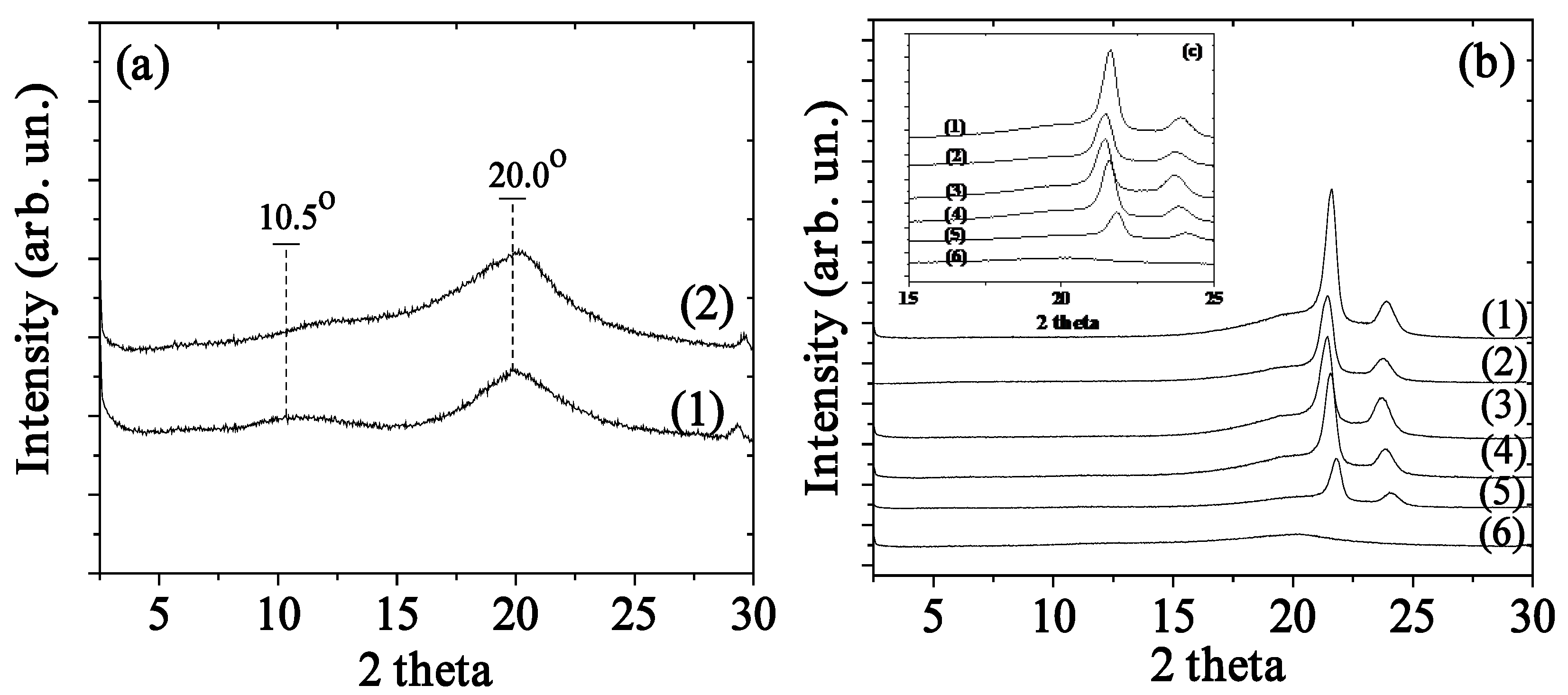
2.1. XRD Analysis
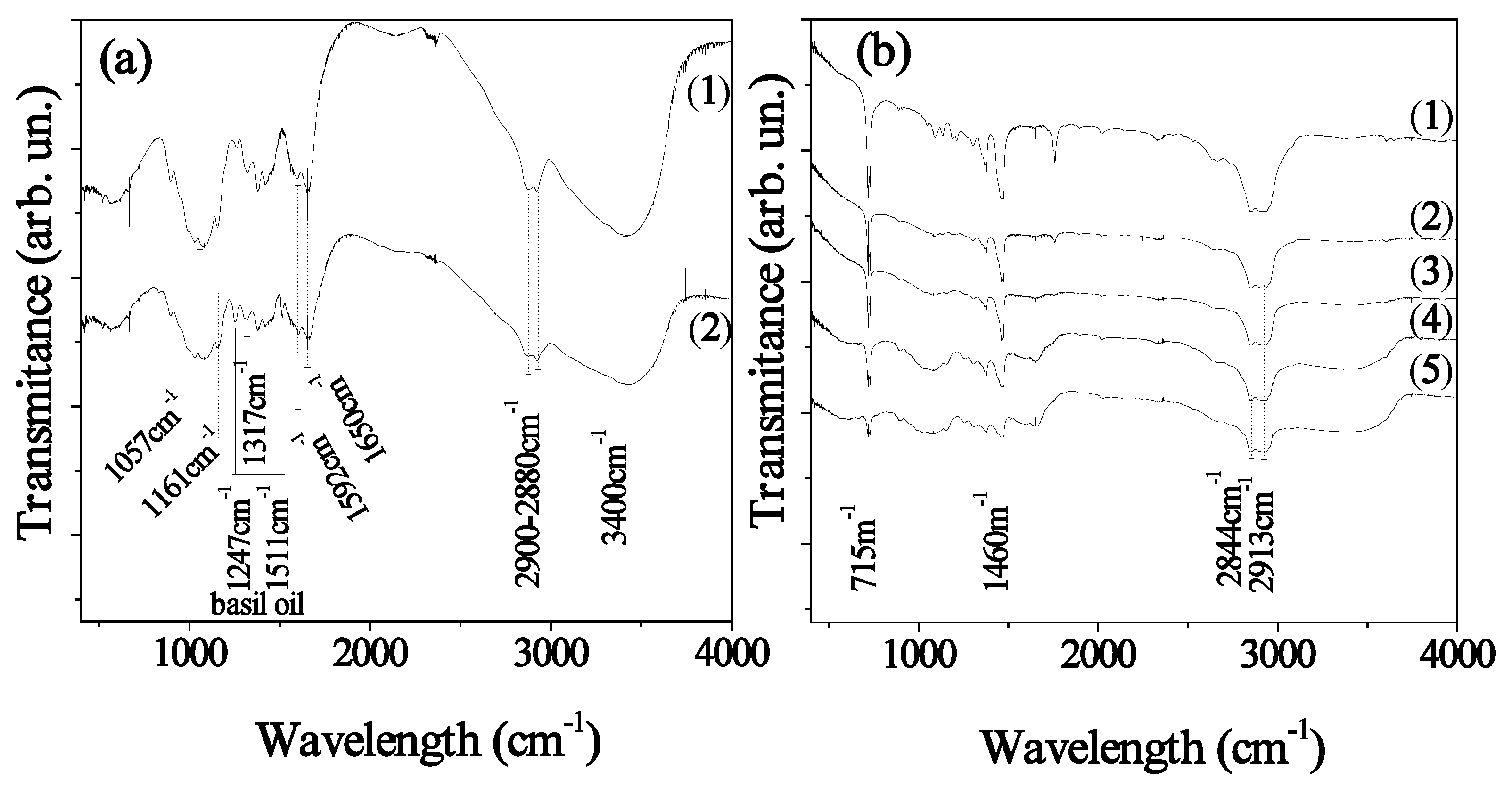
2.2. FTIR Results
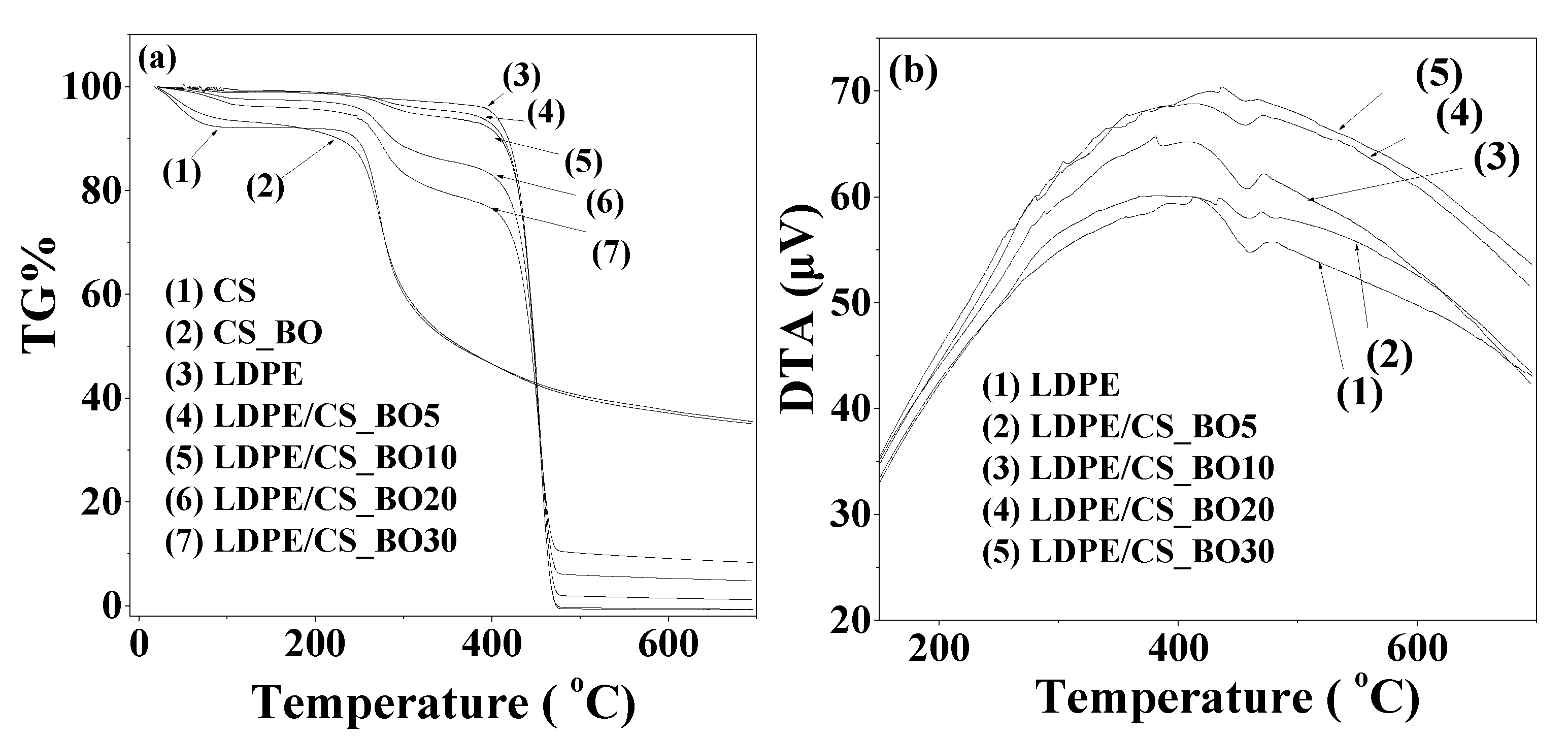
2.3. Thermogravimetric (TG)/Differential Thermal Analysis (DTA) Results
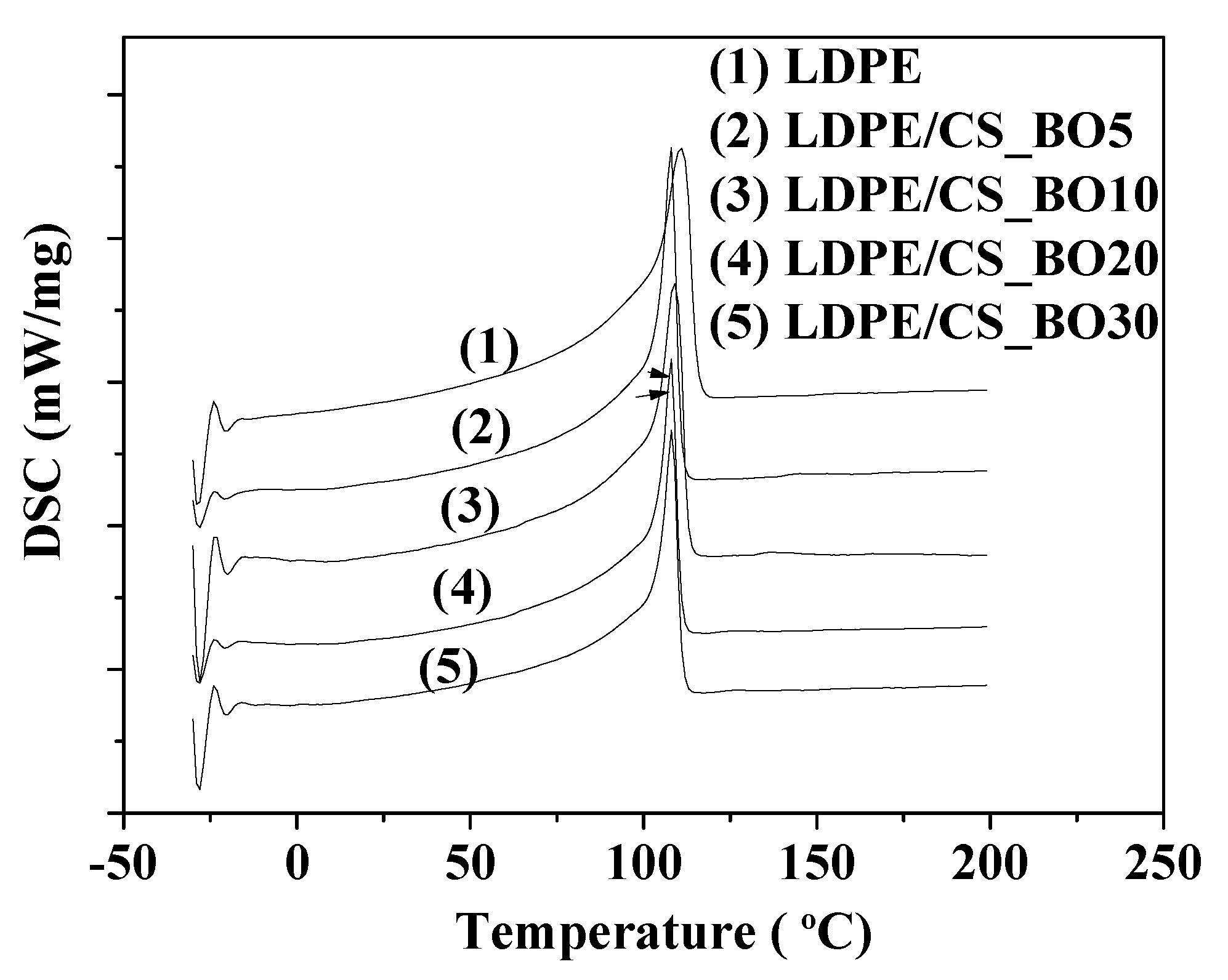
2.4. DSC Results
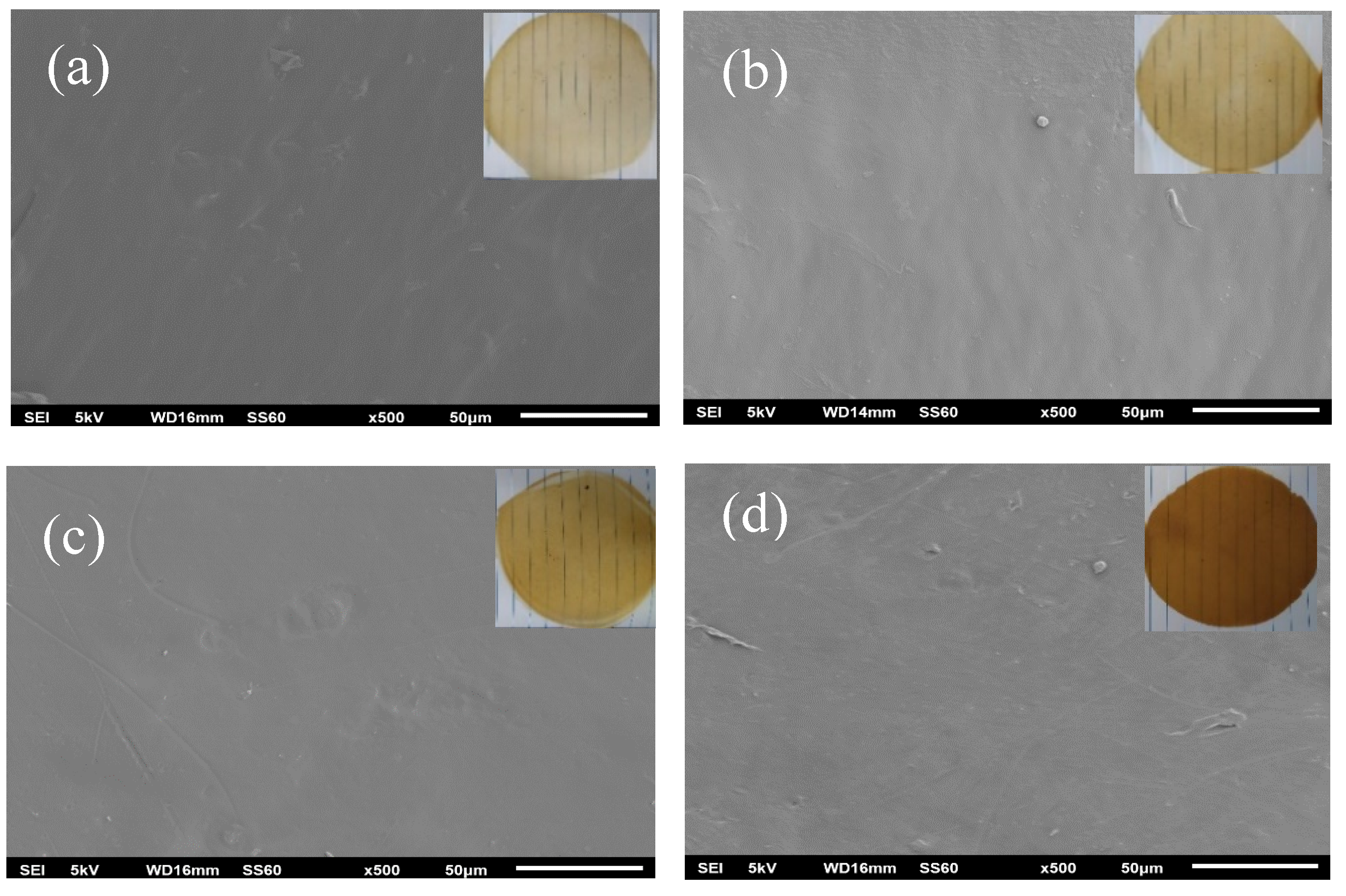
2.5. SEM Morphology
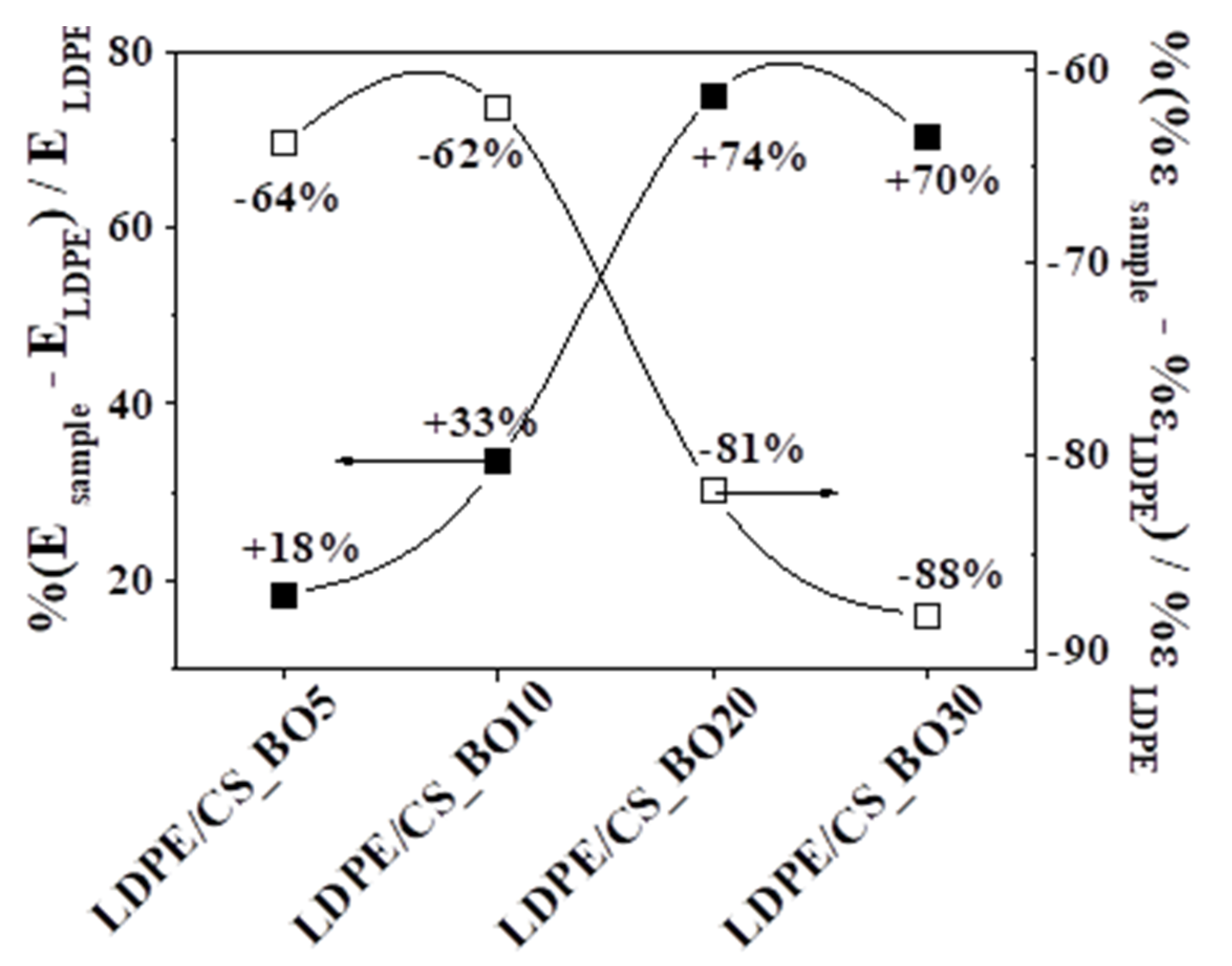
2.6. Tensile Measurements
2.7. Water Sorption
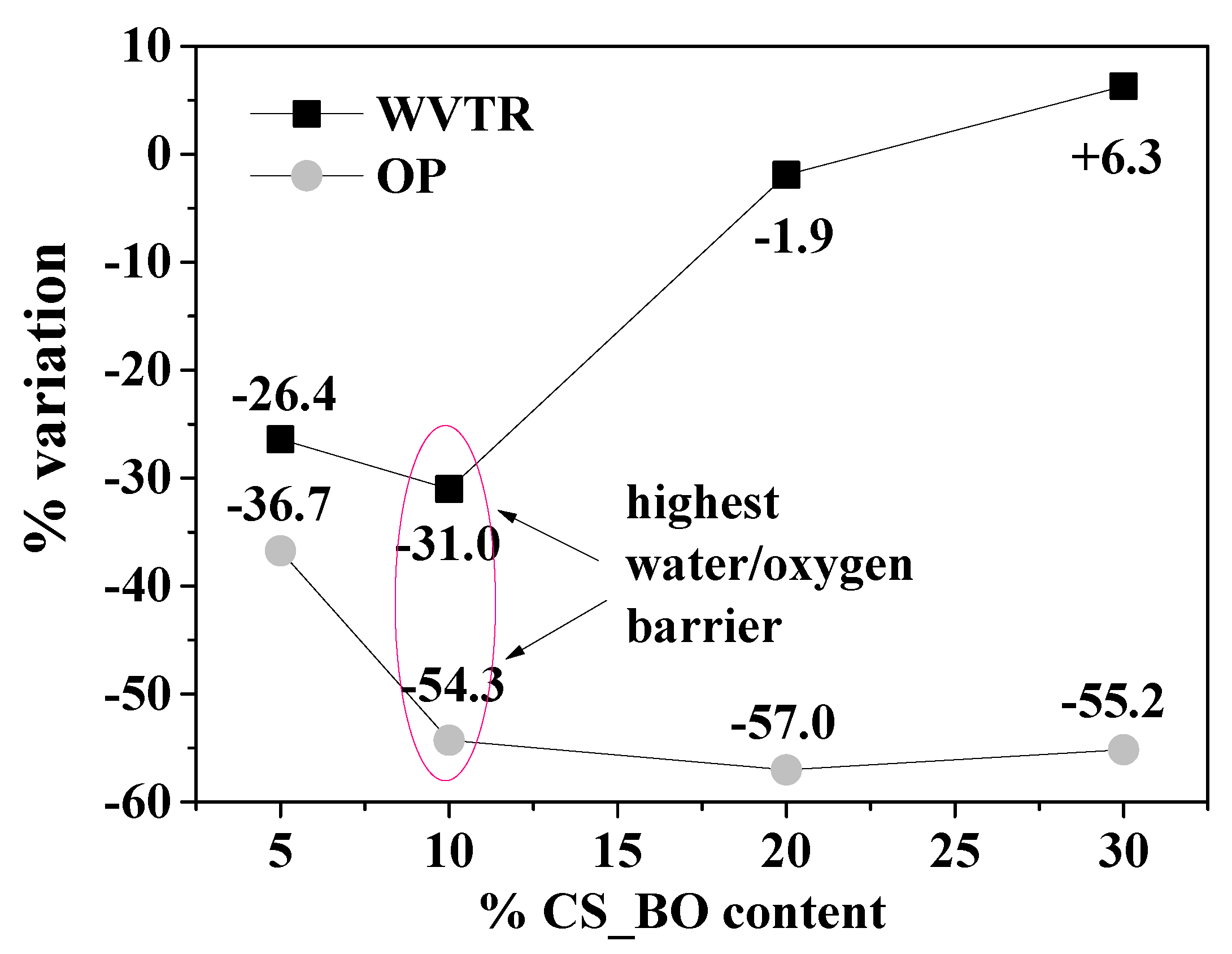
2.8. Water Vapor Transmission Rate (WVTR)
2.9. Oxygen Permeability (OP)
2.10. Overall Migration Rate
2.11. Antioxidant Activity
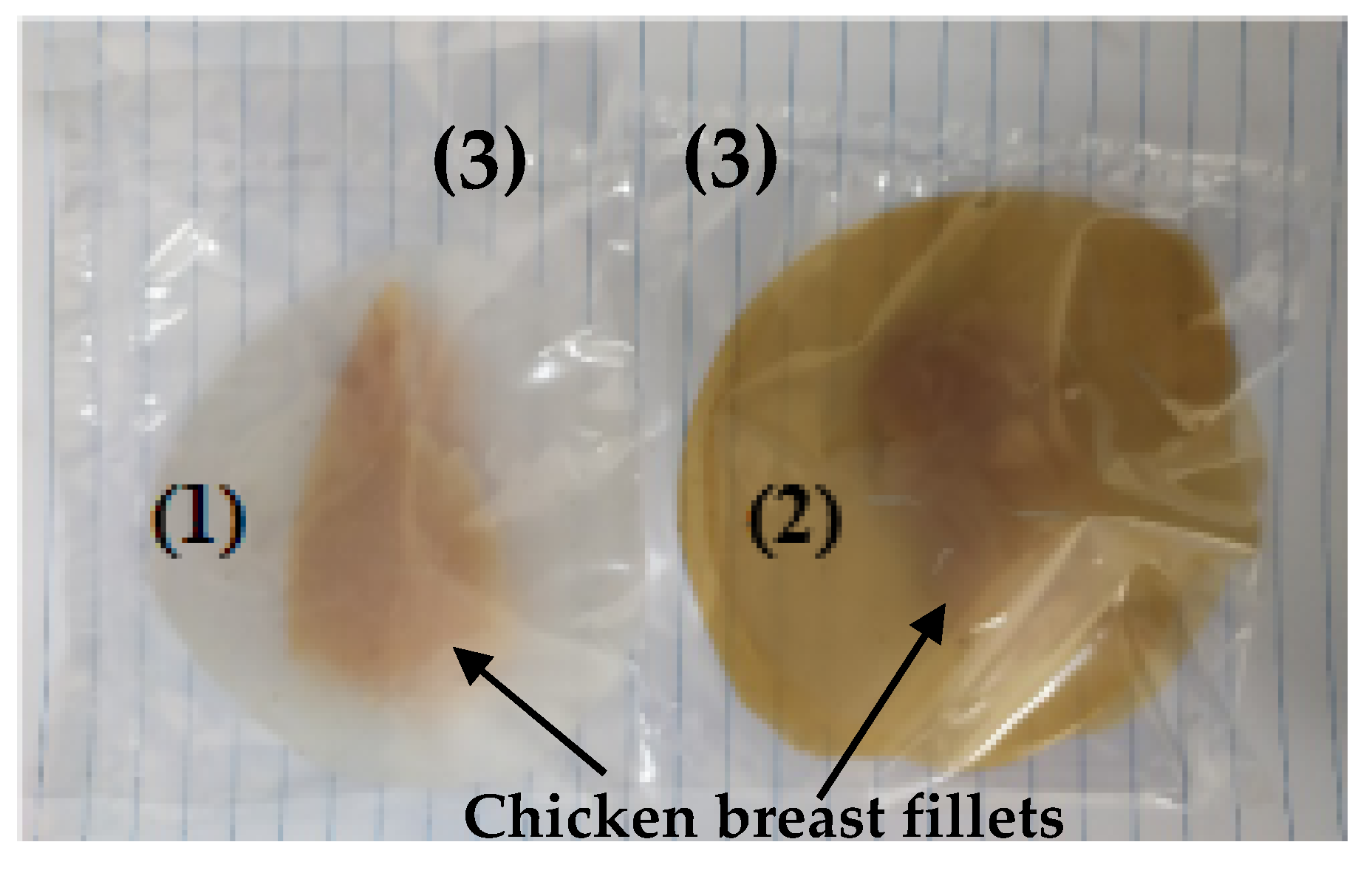
2.12. Lipid Oxidation
2.13. Statistical Analysis of the Experimental Data
3. Materials and Methods
3.1. Materials
3.2. Preparation Methods
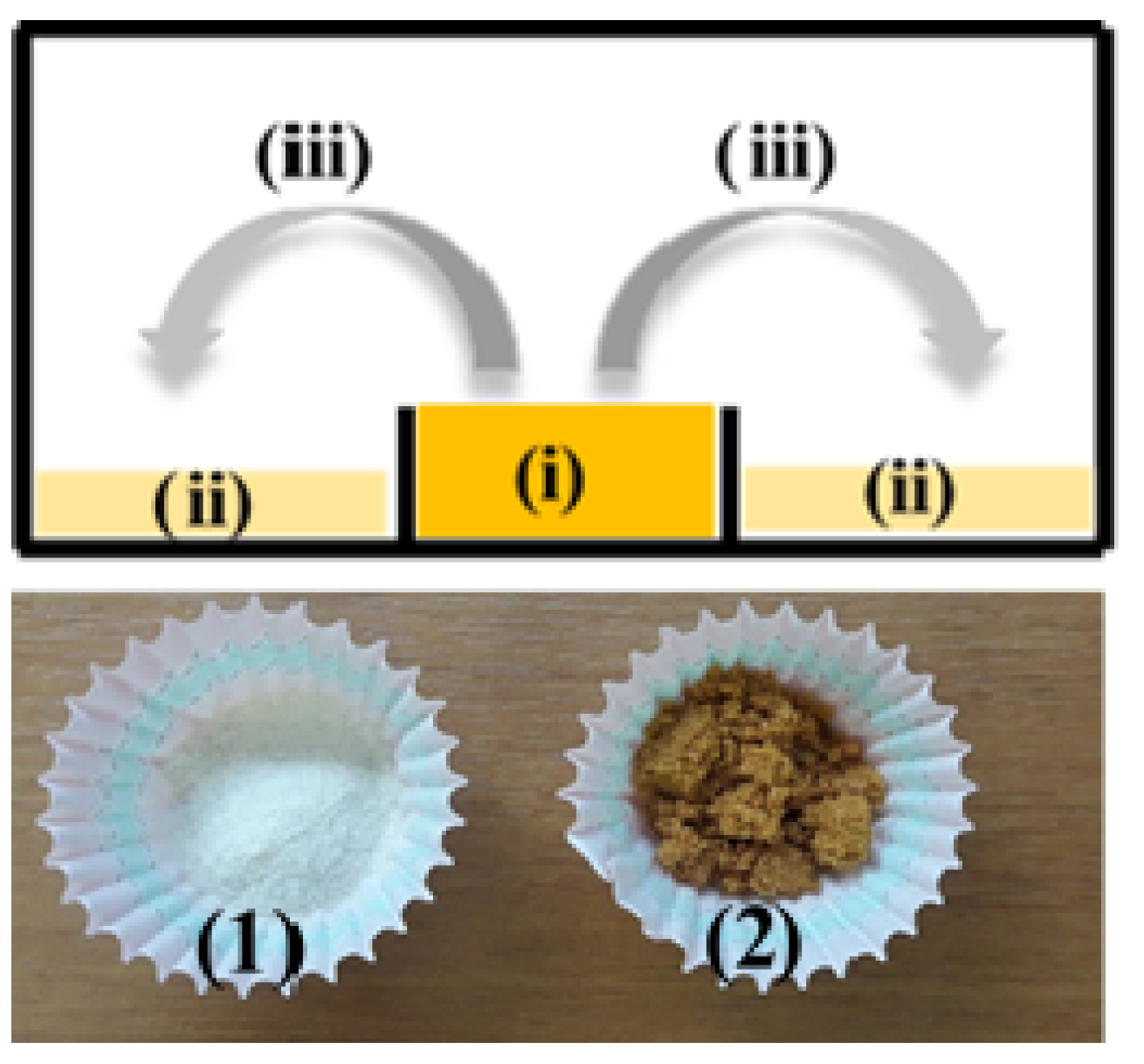
3.2.1. Preparation of CS_BO Hybrid Blend
3.2.2. Preparation of LDPE/CS_BO Active Films
3.3. XRD Analysis
3.4. FTIR Spectrometry
3.5. Thermal Studies TG-DTA/DSC
3.6. Scanning Electron Microscopy (SEM) of LDPE/CS_BO Blends
3.7. Tensile Properties
3.8. Water Sorption
3.9. Water Vapor Transmission Rate (WVTR)
3.10. Oxygen Permeability (OP)
3.11. Overall Migration Test
3.12. Antioxidant Activity
3.13. Lipid Oxidation Test
3.14. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Dasgupta, N.; Ranjan, S.; Mundekkad, D.; Ramalingam, C.; Shanker, R.; Kumar, A. Nanotechnology in Agro-Food: From Field to Plate. Food Res. Int. 2015, 69, 381–400. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Leontiou, A.A. Montmorillonite Composite Materials and Food Packaging. Comp. Mat. Food Pack. 2018, 1–71. [Google Scholar]
- Cazón, P.; Vázquez, M. Applications of Chitosan as Food Packaging Materials. In Sustainable Agriculture Reviews 36: Chitin and Chitosan: Applications in Food, Agriculture, Pharmacy, Medicine and Wastewater Treatment; Crini, G., Lichtfouse, E., Eds.; Sustainable Agriculture Reviews; Springer International Publishing: Cham, Switzerland, 2019; pp. 81–123. ISBN 978-3-030-16581-9. [Google Scholar]
- Almughamisi, M.S.; Khan, Z.A.; Alshitari, W.; Elwakeel, K.Z. Recovery of Chromium(VI) Oxyanions from Aqueous Solution Using Cu(OH)2 and CuO Embedded Chitosan Adsorbents. J. Polym Environ. 2020, 28, 47–60. [Google Scholar] [CrossRef]
- Elwakeel, K.Z.; Elgarahy, A.M.; Mohammad, S.H. Magnetic Schiff’s Base Sorbent Based on Shrimp Peels Wastes for Consummate Sorption of Chromate. Water Sci. Technol. 2017, 76, 35–48. [Google Scholar] [CrossRef]
- Elwakeel, K.Z.; Al-Bogami, A.S.; Elgarahy, A.M. Efficient Retention of Chromate from Industrial Wastewater onto a Green Magnetic Polymer Based on Shrimp Peels. J. Polym Environ. 2018, 26, 2018–2029. [Google Scholar] [CrossRef]
- Elwakeel, K.Z.; Al-Bogami, A.S.; Guibal, E. 2-Mercaptobenzimidazole Derivative of Chitosan for Silver Sorption—Contribution of Magnetite Incorporation and Sonication Effects on Enhanced Metal Recovery. Chem. Eng. J. 2021, 403, 126265. [Google Scholar] [CrossRef]
- Giannakas, A.; Salmas, C.; Leontiou, A.; Tsimogiannis, D.; Oreopoulou, A.; Braouhli, J. Novel LDPE/Chitosan Rosemary and Melissa Extract Nanostructured Active Packaging Films. Nanomaterials 2019, 9, 1105. [Google Scholar] [CrossRef] [PubMed]
- Kusumastuti, Y.; Putri, N.R.E.; Timotius, D.; Syabani, M.W. Rochmadi Effect of Chitosan Addition on the Properties of Low-Density Polyethylene Blend as Potential Bioplastic. Heliyon 2020, 6, e05280. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Marsh, K.S.; Dawson, P. Application of Chitosan-Incorporated LDPE Film to Sliced Fresh Red Meats for Shelf Life Extension. Meat Sci. 2010, 85, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Reesha, K.V.; Satyen Kumar, P.; Bindu, J.; Varghese, T.O. Development and Characterization of an LDPE/Chitosan Composite Antimicrobial Film for Chilled Fish Storage. Int. J. Biol. Macromol. 2015, 79, 934–942. [Google Scholar] [CrossRef]
- Irimia, A.; Vasile, C.; Darie, R.; Stoleru, E.; Pricope, G.; Baklavaridis, A.; Munteanu, B.; Zuburtikudis, I. Effectiveness of Chitosan as Antimicrobial Agent in LDPE/CS Composite Films as Minced Poultry Meat Packaging Materials. Cellul. Chem. Technol. 2014, 48, 325–336. [Google Scholar]
- Huang, Y.; Chen, S.; Bing, X.; Gao, C.; Wang, T.; Yuan, B. Nanosilver migrated into food-simulating solutions from commercially available food fresh containers. Packag. Technol. Sci. 2011, 24, 291–297. [Google Scholar] [CrossRef]
- Arroyo, B.J.; Santos, A.P.; de Melo, E.D.A.; Campos, A.; Lins, L.; Boyano-Orozco, L.C. Chapter 8—Bioactive Compounds and Their Potential Use as Ingredients for Food and Its Application in Food Packaging. In Bioactive Compounds; Campos, M.R.S., Ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 143–156. ISBN 978-0-12-814774-0. [Google Scholar]
- Atarés, L.; Chiralt, A. Essential Oils as Additives in Biodegradable Films and Coatings for Active Food Packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Hemalatha, T.; UmaMaheswari, T.; Senthil, R.; Krithiga, G.; Anbukkarasi, K. Efficacy of Chitosan Films with Basil Essential Oil: Perspectives in Food Packaging. Food Meas. 2017, 11, 2160–2170. [Google Scholar] [CrossRef]
- Barbosa, L.N.; Alves, F.C.B.; Andrade, B.F.M.T.; Albano, M.; Castilho, I.G.; Rall, V.L.M.; Athayde, N.B.; Delbem, N.L.C.; de Oliveira, R.R.; Fernandes, A. Effects of Ocimum Basilicum Linn Essential Oil and Sodium Hexametaphosphate on the Shelf Life of Fresh Chicken Sausage. J. Food Prot. 2014, 77, 981–986. [Google Scholar] [CrossRef]
- Politeo, O.; Jukic, M.; Milos, M. Chemical Composition and Antioxidant Capacity of Free Volatile Aglycones from Basil (Ocimum Basilicum L.) Compared with Its Essential Oil. Food Chem. 2007, 101, 379–385. [Google Scholar] [CrossRef]
- Kathirvel, P.; Ravi, S. Chemical Composition of the Essential Oil from Basil (Ocimum Basilicum Linn.) and Its in Vitro Cytotoxicity against HeLa and HEp-2 Human Cancer Cell Lines and NIH 3T3 Mouse Embryonic Fibroblasts. Nat. Prod. Res. 2012, 26, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Opalchenova, G.; Obreshkova, D. Comparative Studies on the Activity of Basil—an Essential Oil from Ocimum Basilicum L.—Against Multidrug Resistant Clinical Isolates of the Genera Staphylococcus, Enterococcus and Pseudomonas by Using Different Test Methods. J. Microbiol. Meth. 2003, 54, 105–110. [Google Scholar] [CrossRef]
- Suppakul, P.; Miltz, J.; Sonneveld, K.; Bigger, S.W. Characterization of Antimicrobial Films Containing Basil Extracts. Packag. Technol. Sci. 2006, 19, 259–268. [Google Scholar] [CrossRef]
- Wattananawinrat, K.; Threepopnatkul, P.; Kulsetthanchalee, C. Morphological and Thermal Properties of LDPE/EVA Blended Films and Development of Antimicrobial Activity in Food Packaging Film. Energy Procedia 2014, 56, 1–9. [Google Scholar] [CrossRef]
- Giannakas, A.; Grigoriadi, K.; Leontiou, A.; Barkoula, N.-M.; Ladavos, A. Preparation, Characterization, Mechanical and Barrier Properties Investigation of Chitosan–Clay Nanocomposites. Carbohydr. Polym. 2014, 108, 103–111. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Hong, S.-I.; Park, H.-M.; Ng, P.K.W. Preparation and Characterization of Chitosan-Based Nanocomposite Films with Antimicrobial Activity. J. Agric. Food Chem. 2006, 54, 5814–5822. [Google Scholar] [CrossRef]
- Giannakas, A.; Tsagkalias, I.; Achilias, D.S.; Ladavos, A. A Novel Method for the Preparation of Inorganic and Organo-Modified Montmorillonite Essential Oil Hybrids. Appl. Clay Sci. 2017, 146, 362–370. [Google Scholar] [CrossRef]
- Hamdi, M.; Nasri, R.; Li, S.; Nasri, M. Bioactive Composite Films with Chitosan and Carotenoproteins Extract from Blue Crab Shells: Biological Potential and Structural, Thermal, and Mechanical Characterization. Food Hydrocoll. 2019, 89, 802–812. [Google Scholar] [CrossRef]
- Sunilkumar, M.; Francis, T.; Thachil, E.T.; Sujith, A. Low Density Polyethylene-Chitosan Composites: A Study Based on Biodegradation. Chem. Eng. J. 2012, 204–205, 114–124. [Google Scholar] [CrossRef]
- Prasanna, K.; Sailaja, R.R.N. Blends of LDPE/Chitosan Using Epoxy-Functionalized LDPE as Compatibilizer. J. Appl. Polym. Sci. 2012, 124, 3264–3275. [Google Scholar] [CrossRef]
- Vasile, C.; Darie, R.N.; Cheaburu-Yilmaz, C.N.; Pricope, G.-M.; Bračič, M.; Pamfil, D.; Hitruc, G.E.; Duraccio, D. Low Density Polyethylene—Chitosan Composites. Compos. Part. B Eng. 2013, 55, 314–323. [Google Scholar] [CrossRef]
- Plascencia-Jatomea, M.; Rodríguez-Félix, D.E.; del Castillo-Castro, T.; Quiroz-Castillo, J.M.; Rodríguez-Félix, F.; Grijalva-Monteverde, H.; Herrera-Franco, P.J. Preparation of Extruded Polyethylene/Chitosan Blends Compatibilized with Polyethylene-Graft-Maleic Anhydride. Carbohydr. Polym. 2013, 101, 1094–1100. [Google Scholar] [CrossRef]
- Chouliara, E.; Badeka, A.; Savvaidis, I.; Kontominas, M.G. Combined Effect of Irradiation and Modified Atmosphere Packaging on Shelf-Life Extension of Chicken Breast Meat: Microbiological, Chemical and Sensory Changes. Eur. Food Res. Technol. 2008, 226, 877–888. [Google Scholar] [CrossRef]
- Giannakas Na-Montmorillonite Vs. Organically Modified Montmorillonite as Essential Oil Nanocarriers for Melt-Extruded Low-Density Poly-Ethylene Nanocomposite Active Packaging Films with a Controllable and Long-Life Antioxidant Activity. Nanomaterials 2020, 10, 1027. [CrossRef] [PubMed]
- Latou, E.; Mexis, S.F.; Badeka, A.V.; Kontakos, S.; Kontominas, M.G. Combined Effect of Chitosan and Modified Atmosphere Packaging for Shelf Life Extension of Chicken Breast Fillets. LWT Food Sci. Technol. 2014, 55, 263–268. [Google Scholar] [CrossRef]
- Giannakas, A.; Stathopoulou, P.; Tsiamis, G.; Salmas, C. The Effect of Different Preparation Methods on the Development of Chitosan/Thyme Oil/Montmorillonite Nanocomposite Active Packaging Films. J. Food Process. Preserv. 2019, 44, e14327. [Google Scholar] [CrossRef]
- Grigoriadi, K.; Giannakas, A.; Ladavos, A.K.; Barkoula, N.-M. Interplay between Processing and Performance in Chitosan-Based Clay Nanocomposite Films. Polym. Bull. 2015, 72, 1145–1161. [Google Scholar] [CrossRef]
- Vlacha, M.; Giannakas, A.; Katapodis, P.; Stamatis, H.; Ladavos, A.; Barkoula, N.-M. On the Efficiency of Oleic Acid as Plasticizer of Chitosan/Clay Nanocomposites and Its Role on Thermo-Mechanical, Barrier and Antimicrobial Properties—Comparison with Glycerol. Food Hydrocoll. 2016, 57, 10–19. [Google Scholar] [CrossRef]
- Giannakas, A.; Spanos, C.G.; Kourkoumelis, N.; Vaimakis, T.; Ladavos, A. Preparation, Characterization and Water Barrier Properties of PS/Organo-Montmorillonite Nanocomposites. Eur. Polym. J. 2008, 44, 3915–3921. [Google Scholar] [CrossRef]
- Giannakas, A.; Xidas, P.; Triantafyllidis, K.S.; Katsoulidis, A.; Ladavos, A. Preparation and Characterization of Polymer/Organosilicate Nanocomposites Based on Unmodified LDPE. J. Appl. Polym. Sci. 2009, 114, 83–89. [Google Scholar] [CrossRef]
- USFDA. Determination of Overall Migration Residue; USFDA: Hampton, VA, USA, 2014.
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Panea, B.; Ripoll, G.; González, J.; Fernández-Cuello, Á.; Albertí, P. Effect of Nanocomposite Packaging Containing Different Proportions of ZnO and Ag on Chicken Breast Meat Quality. J. Food Eng. 2014, 123, 104–112. [Google Scholar] [CrossRef]


| Code Name | Melting Point T (°C) | Fusion Enthalpy ΔΗf (J/g) | Young’s Modulus E (St. Dev.) (MPa) | Tensile Strength σ (St. Dev.) (MPa) | % Elongation at Break (ε) (St. Dev.) |
|---|---|---|---|---|---|
| LDPE | 110.8 | 113.5 | 203.3 ± 42.4 | 13.7 ± 1.3 | 161.0 ± 72.0 |
| LDPE/CS_BO5 | 108.4 | 97.2 | 240.7 ± 39.6 | 11.6 ± 0.7 | 58.3 ± 10.6 |
| LDPE/CS_BO10 | 109.3 | 103.4 | 271.3 ± 47.1 | 9.7 ± 1.5 | 61.3 ± 25.0 |
| LDPE/CS_BO20 | 107.8 | 81.6 | 355.7 ± 49.1 | 9.5 ± 0.9 | 29.3 ± 9.4 |
| LDPE/CS_BO30 | 108.5 | 79.1 | 346.0 ± 57.4 | 9.3 ± 0.6 | 19.0 ± 11.2 |
| Code Name | WVTR (St. Dev.) (g/m2·day) | % Water Sorption (St. Dev.) | OP (St. Dev.) cm3·mm/m2·day | Total Migration (St. Dev.) (mg/L) | Antioxidant Activity after 24 h (St. Dev.) |
|---|---|---|---|---|---|
| LDPE | 19.49 ± 1.5 | 0.00 ± 0.00 | 182.4 ± 3.4 | 12.4 ± 0.1 | - |
| LDPE/CS_BO5 | 14.35 ± 1.2 | 0.12 ± 0.05 | 115.4 ± 2.8 | 15.5 ± 0.1 | 6.4 ± 0.9 |
| LDPE/CS_BO10 | 13.45 ± 1.8 | 0.17 ± 0.05 | 83.3 ± 3.5 | 17.6 ± 0.1 | 12.8 ± 1.2 |
| LDPE/CS_BO20 | 19.12 ± 1.5 | 0.27 ± 0.05 | 78.4 ± 2.5 | 25.3 ± 0.1 | 22.4 ± 1.4 |
| LDPE/CS_BO30 | 20.71 ± 2.2 | 1.15 ± 0.60 | 81.8 ± 5.4 | 45.4 ± 0.1 | 34.6 ± 1.5 |
| Sig. | IA | |
|---|---|---|
| E | 0.002 | 96 |
| σuts | 0.006 | 88 |
| %ε | 0.002 | 96 |
| WVP | 0.003 | 94 |
| % water sorption | 0.005 | 90 |
| OP | 0.004 | 92 |
| Total migration | 0.002 | 96 |
| TBARS | 0.003 | 94 |
| % Antioxidant activity after 24 h | 0.000 | 100 |
| Code Name | LDPE (g) | CS_BO (g) | Extrusion Temperature (°C) | Extrusion Rotation Speed (rpm) | Extrusion Total Processing Time (min) |
|---|---|---|---|---|---|
| LDPE | 5.00 | - | 140 | 100 | 5 |
| LDPE/CS_BO5 | 4.75 | 0.25 | 140 | 100 | 5 |
| LDPE/CS_BO10 | 4.50 | 0.50 | 140 | 100 | 5 |
| LDPE/CS_BO20 | 3.00 | 1.00 | 140 | 100 | 5 |
| LDPE/CS_BO30 | 3.50 | 1.50 | 140 | 100 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannakas, A.E.; Salmas, C.E.; Leontiou, A.; Baikousi, M.; Moschovas, D.; Asimakopoulos, G.; Zafeiropoulos, N.E.; Avgeropoulos, A. Synthesis of a Novel Chitosan/Basil Oil Blend and Development of Novel Low Density Poly Ethylene/Chitosan/Basil Oil Active Packaging Films Following a Melt-Extrusion Process for Enhancing Chicken Breast Fillets Shelf-Life. Molecules 2021, 26, 1585. https://doi.org/10.3390/molecules26061585
Giannakas AE, Salmas CE, Leontiou A, Baikousi M, Moschovas D, Asimakopoulos G, Zafeiropoulos NE, Avgeropoulos A. Synthesis of a Novel Chitosan/Basil Oil Blend and Development of Novel Low Density Poly Ethylene/Chitosan/Basil Oil Active Packaging Films Following a Melt-Extrusion Process for Enhancing Chicken Breast Fillets Shelf-Life. Molecules. 2021; 26(6):1585. https://doi.org/10.3390/molecules26061585
Chicago/Turabian StyleGiannakas, Aris E., Constantinos E. Salmas, Areti Leontiou, Maria Baikousi, Dimitrios Moschovas, Georgios Asimakopoulos, Nikolaos E. Zafeiropoulos, and Apostolos Avgeropoulos. 2021. "Synthesis of a Novel Chitosan/Basil Oil Blend and Development of Novel Low Density Poly Ethylene/Chitosan/Basil Oil Active Packaging Films Following a Melt-Extrusion Process for Enhancing Chicken Breast Fillets Shelf-Life" Molecules 26, no. 6: 1585. https://doi.org/10.3390/molecules26061585
APA StyleGiannakas, A. E., Salmas, C. E., Leontiou, A., Baikousi, M., Moschovas, D., Asimakopoulos, G., Zafeiropoulos, N. E., & Avgeropoulos, A. (2021). Synthesis of a Novel Chitosan/Basil Oil Blend and Development of Novel Low Density Poly Ethylene/Chitosan/Basil Oil Active Packaging Films Following a Melt-Extrusion Process for Enhancing Chicken Breast Fillets Shelf-Life. Molecules, 26(6), 1585. https://doi.org/10.3390/molecules26061585










