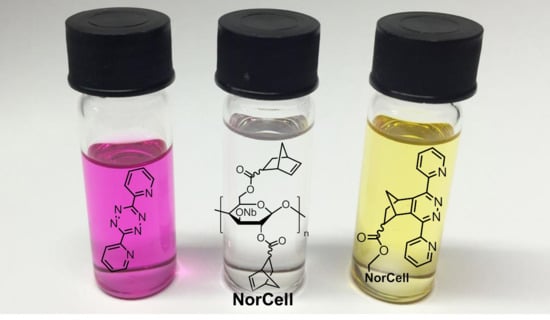
Highly Norbornylated Cellulose and Its “Click” Modification by an Inverse-Electron Demand Diels–Alder (iEDDA) Reaction
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Synthesis
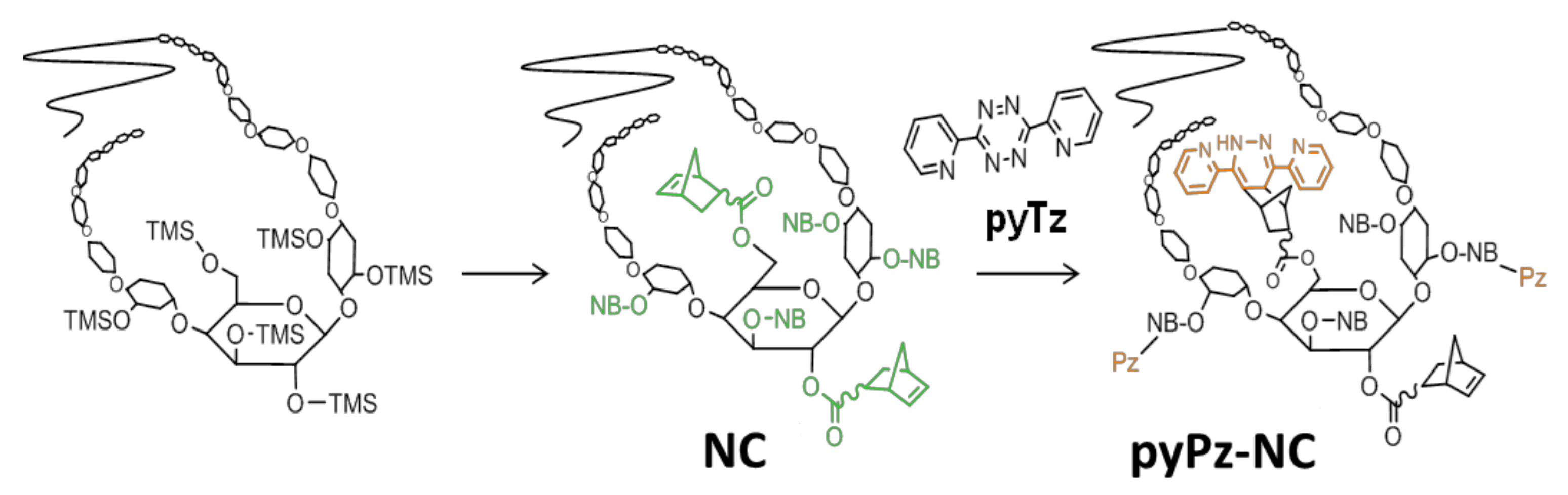
3.2.1. Synthesis of 2,3,6-norbornoyl Cellulose (NC)
3.2.2. Synthesis of pyPz-NC via iEDDA
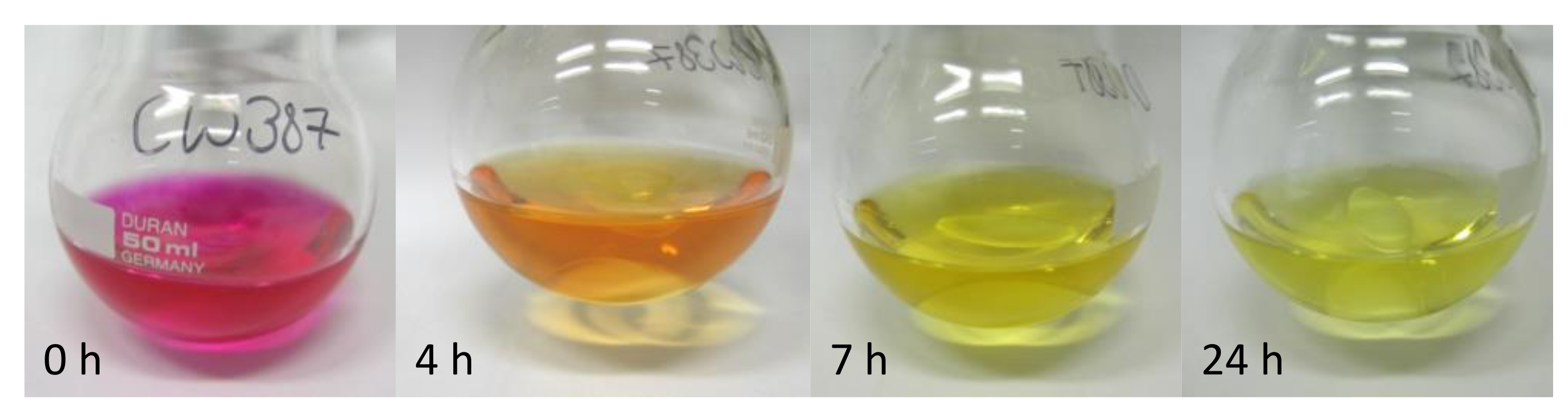
3.3. Determination of the Rate Constant of the iEDDA Reaction
3.4. Measurements
3.4.1. NMR Spectroscopy
3.4.2. Infrared Spectroscopy (ATR-FTIR)
3.4.3. Elemental Analysis
3.4.4. UV/Vis Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Appendix A
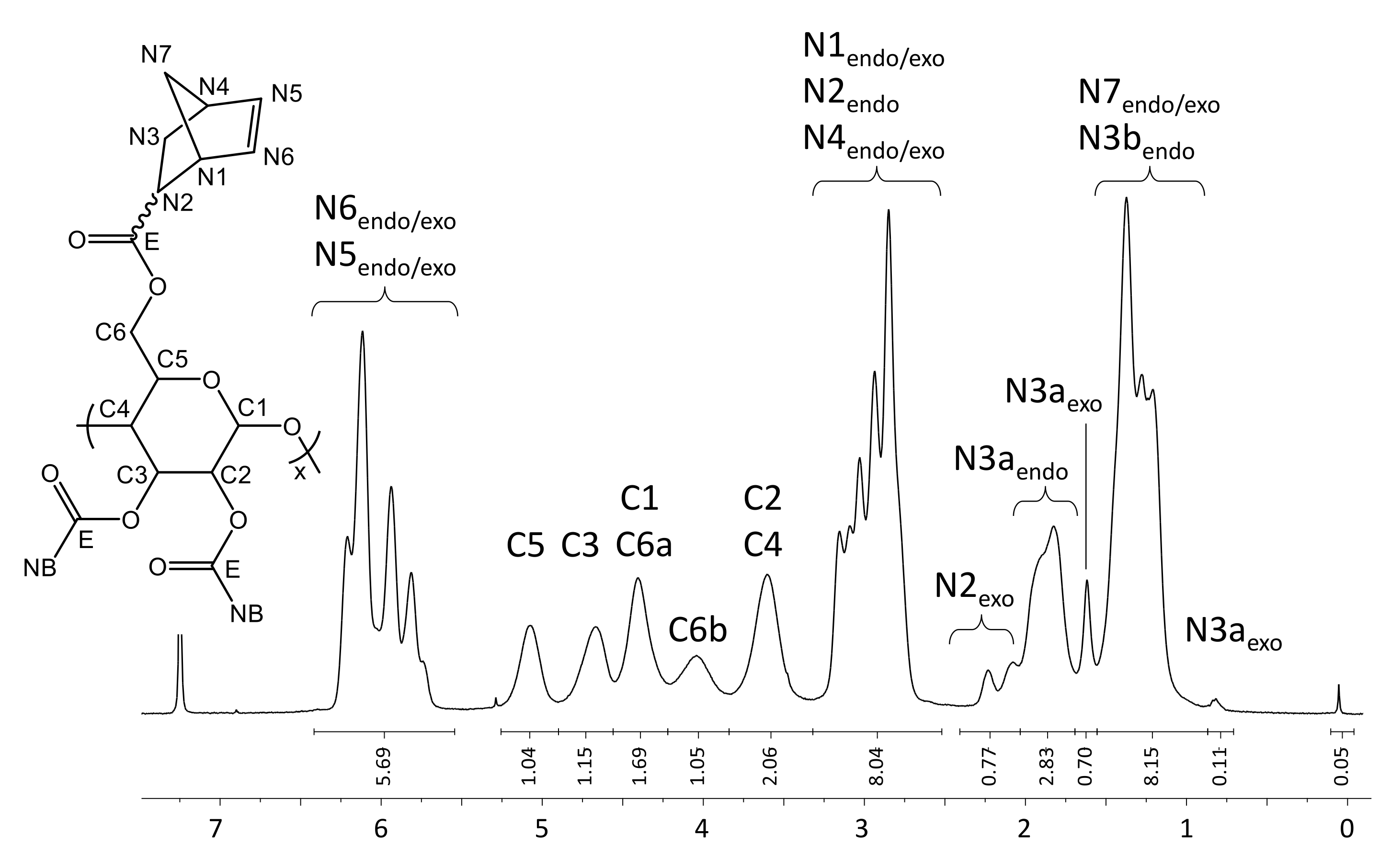
Appendix A.1. Characterization Data for NC
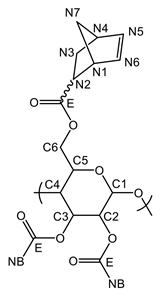
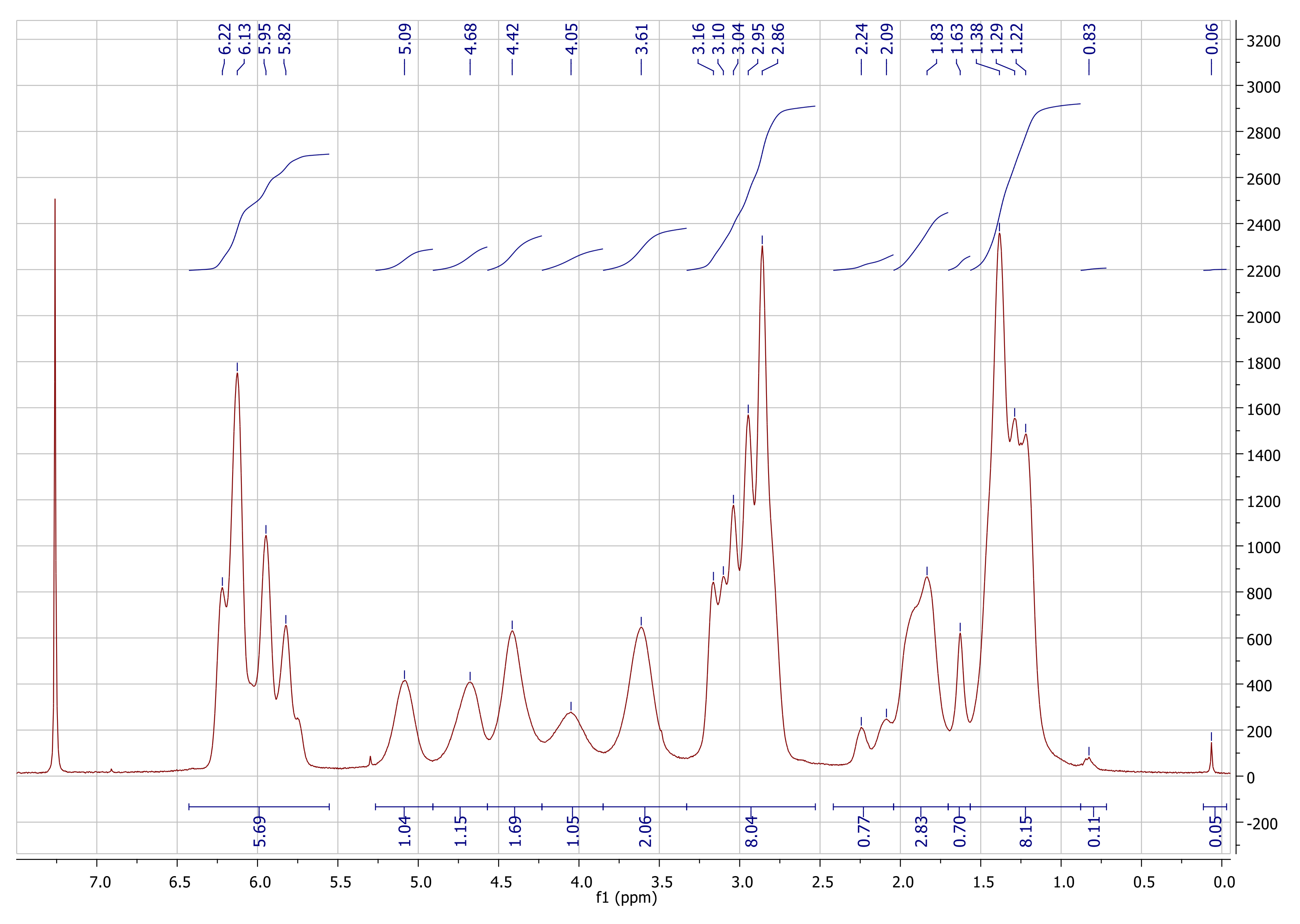
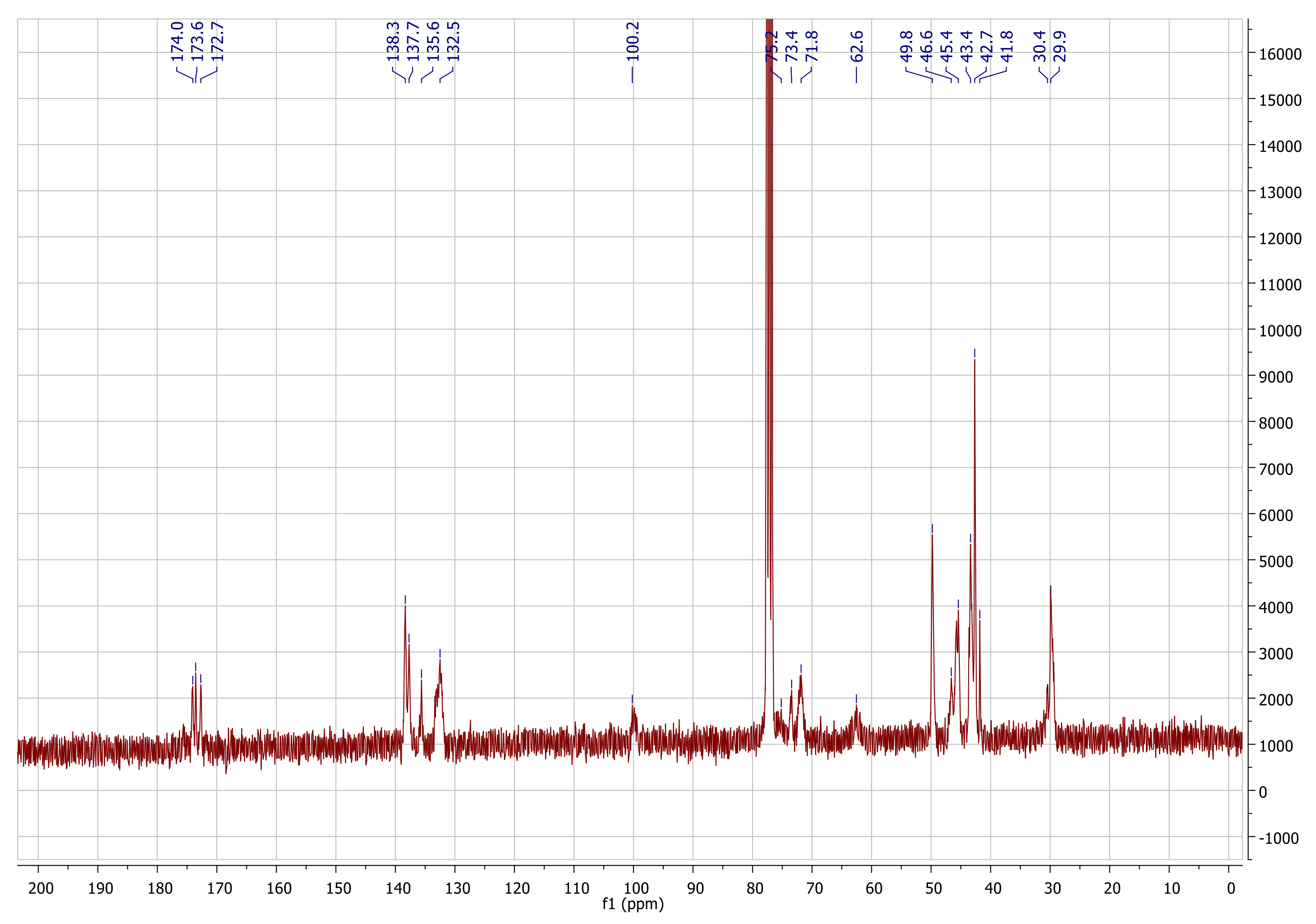
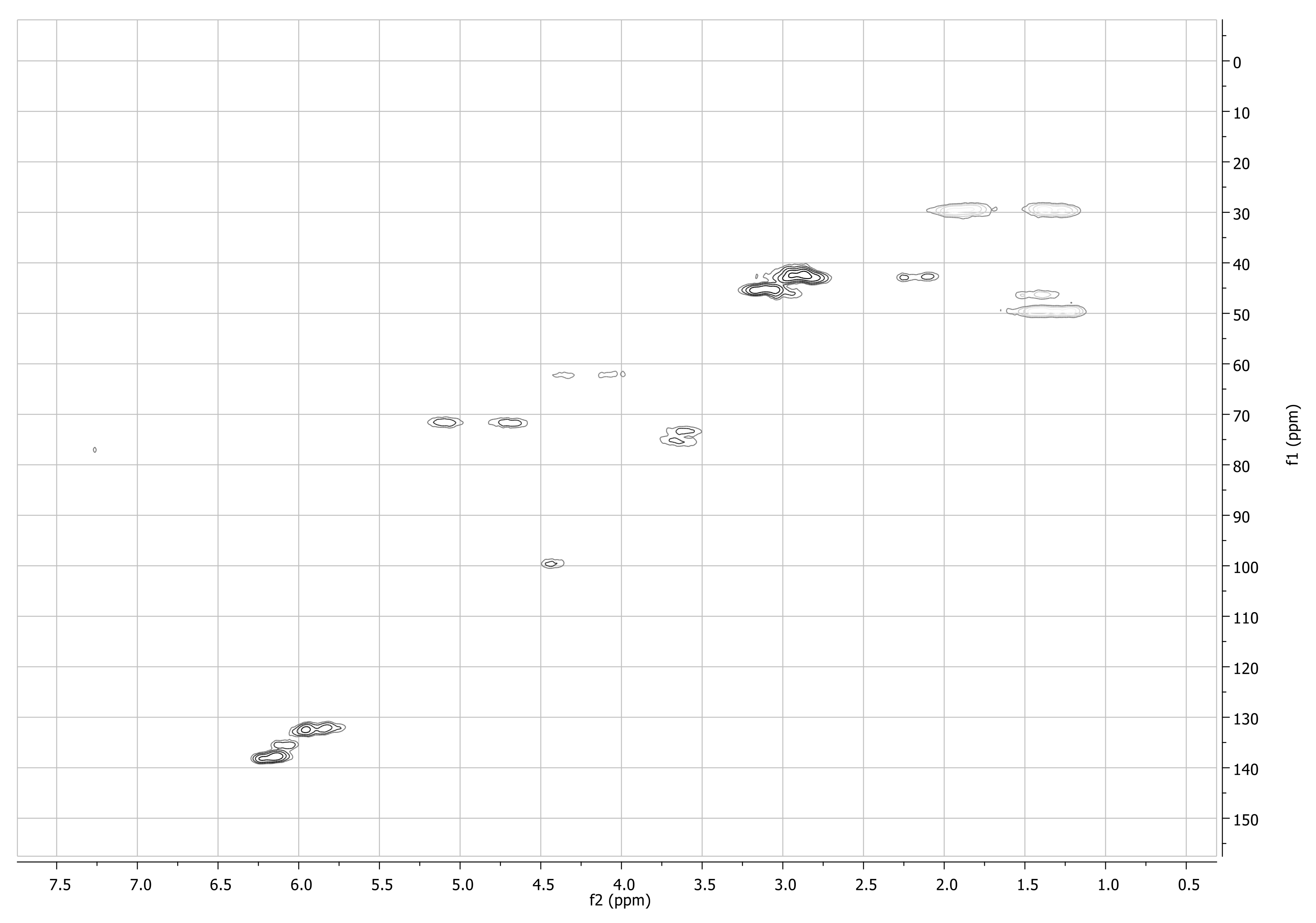
Appendix A.2. Characterization of pyPz-NC
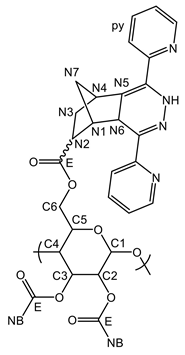
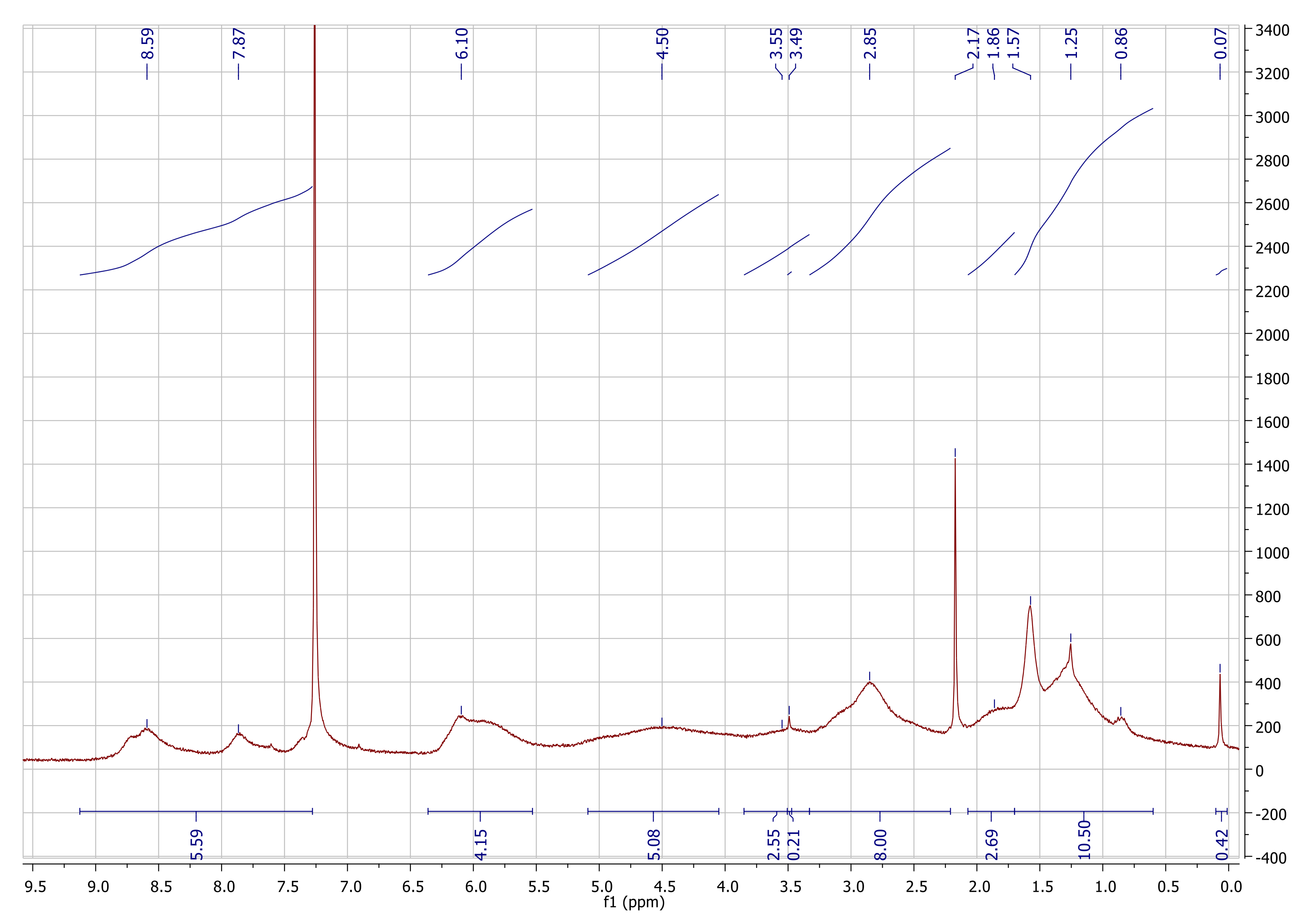
References
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Moses, J.E.; Moorhouse, A.D. The growing applications of click chemistry. Chem. Soc. Rev. 2007, 36, 1249–1262. [Google Scholar] [CrossRef]
- Liang, L.; Astruc, D. The copper(I)-catalyzed alkyne-azide cycloaddition (CuAAC) “click” reaction and its applications. An overview. Coord. Chem. Rev. 2011, 255, 2933–2945. [Google Scholar] [CrossRef]
- Navarro, J.R.G.; Conzatti, G.; Yu, Y.; Fall, A.B.; Mathew, R.; Edén, M.; Bergström, L. Multicolor Fluorescent Labeling of Cellulose Nanofibrils by Click Chemistry. Biomacromolecules 2015, 16, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Orelma, H.; Johansson, L.-S.; Filpponen, I.; Rojas, O.J.; Laine, J. Generic Method for Attaching Biomolecules via Avidin–Biotin Complexes Immobilized on Films of Regenerated and Nanofibrillar Cellulose. Biomacromolecules 2012, 13, 2802–2810. [Google Scholar] [CrossRef] [PubMed]
- Filpponen, I.; Kontturi, E.; Nummelin, S.; Rosilo, H.; Kolehmainen, E.; Ikkala, O.; Laine, J. Generic Method for Modular Surface Modification of Cellulosic Materials in Aqueous Medium by Sequential “Click” Reaction and Adsorption. Biomacromolecules 2012, 13, 736–742. [Google Scholar] [CrossRef]
- Koschella, A.; Hartlieb, M.; Heinze, T. A “click-chemistry” approach to cellulose-based hydrogels. Carbohydr. Polym. 2011, 86, 154–161. [Google Scholar] [CrossRef]
- Koschella, A.; Richter, M.; Heinze, T. Novel cellulose-based polyelectrolytes synthesized via the click reaction. Carbohydr. Res. 2010, 345, 1028–1033. [Google Scholar] [CrossRef]
- Chen, J.; Lin, N.; Huang, J.; Dufresne, A. Highly alkynyl-functionalization of cellulose nanocrystals and advanced nanocomposites thereof via click chemistry. Polym. Chem. 2015, 6, 4385–4395. [Google Scholar] [CrossRef]
- Montañez, M.I.; Hed, Y.; Utsel, S.; Ropponen, J.; Malmström, E.; Wågberg, L.; Hult, A.; Malkoch, M. Bifunctional Dendronized Cellulose Surfaces as Biosensors. Biomacromolecules 2011, 12, 2114–2125. [Google Scholar] [CrossRef] [PubMed]
- Copper: Health Information Summary, Environmental Fact Sheet, ARD-EHP-9 New Hampshire Department of Environmental Services. 2005. Available online: http://des.nh.gov/organization/commissioner/pip/factsheets/ard/documents/ard-ehp-9.pdf (accessed on 3 March 2021).
- Eskici, G.; Axelsen, P.H. Copper and Oxidative Stress in the Pathogenesis of Alzheimer’s Disease. Biochem. 2012, 51, 6289–6311. [Google Scholar] [CrossRef] [PubMed]
- Codelli, J.A.; Baskin, J.M.; Agard, N.J.; Bertozzi, C.R. Second-Generation Difluorinated Cyclooctynes for Copper-Free Click Chemistry. J. Am. Chem. Soc. 2008, 130, 11486–11493. [Google Scholar] [CrossRef] [PubMed]
- Sletten, E.M.; Bertozzi, C.R. A Hydrophilic Azacyclooctyne for Cu-Free Click Chemistry. Org. Lett. 2008, 10, 3097–3099. [Google Scholar] [CrossRef]
- Baskin, J.M.; Prescher, J.A.; Laughlin, S.T.; Agard, N.J.; Chang, P.V.; Miller, I.A.; Lo, A.; Codelli, J.A.; Bertozzi, C.R. Copper-free click chemistry for dynamic in vivo imaging. Proc. Natl. Acad. Sci. 2007, 104, 16793–16797. [Google Scholar] [CrossRef]
- Agard, N.J.; Prescher, J.A.; Bertozzi, C.R. A Strain-Promoted [3 + 2] Azide−Alkyne Cycloaddition for Covalent Modification of Biomolecules in Living Systems. J. Am. Chem. Soc. 2004, 126, 15046–15047. [Google Scholar] [CrossRef]
- Ning, X.; Guo, J.; Wolfert, M.A.; Boons, G.-J. Visualizing Metabolically Labeled Glycoconjugates of Living Cells by Copper-Free and Fast Huisgen Cycloadditions. Angew. Chem. Int. Ed. 2008, 47, 2253–2255. [Google Scholar] [CrossRef]
- Blackman, M.L.; Royzen, M.; Fox, J.M. Tetrazine Ligation: Fast Bioconjugation Based on Inverse-Electron-Demand Diels−Alder Reactivity. J. Am. Chem. Soc. 2008, 130, 13518–13519. [Google Scholar] [CrossRef] [PubMed]
- Knall, A.-C.; Hollauf, M.; Saf, R.; Slugovc, C. A trifunctional linker suitable for conducting three orthogonal click chemistries in one pot. Org. Biomol. Chem. 2016, 14, 10576–10580. [Google Scholar] [CrossRef]
- Wu, H.; Devaraj, N.K. Advances in Tetrazine Bioorthogonal Chemistry Driven by the Synthesis of Novel Tetrazines and Dienophiles. Accounts Chem. Res. 2018, 51, 1249–1259. [Google Scholar] [CrossRef] [PubMed]
- Knall, A.-C.; Kovačič, S.; Hollauf, M.; Reishofer, D.; Saf, R.; Slugovc, C. Inverse electron demand Diels–Alder (iEDDA) functionalisation of macroporous poly(dicyclopentadiene) foams. Chem. Commun. 2013, 49, 7325–7327. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, N.K.; Weissleder, R. Biomedical Applications of Tetrazine Cycloadditions. Accounts Chem. Res. 2011, 44, 816–827. [Google Scholar] [CrossRef]
- Knall, A.-C.; Slugovc, C. Inverse electron demand Diels-Alder (iEDDA)-initiated conjugation: A (high) potential click chemistry scheme. Chem. Soc. Rev. 2013, 42, 5131–5142. [Google Scholar] [CrossRef]
- Meng, X.; Edgar, K.J. “Click” reactions in polysaccharide modification. Prog. Polym. Sci. 2016, 53, 52–85. [Google Scholar] [CrossRef]
- Fox, S.C.; Li, B.; Xu, D.; Edgar, K.J. Regioselective Esterification and Etherification of Cellulose: A Review. Biomacromolecules 2011, 12, 1956–1972. [Google Scholar] [CrossRef]
- Stein, A.; Klemm, D. Syntheses of cellulose derivatives via O-triorganosilyl celluloses, 1. Effective synthesis of organic cellulose esters by acylation of trimethylsilyl celluloses. Die Makromol. Chem. Rapid Commun. 1988, 9, 569–573. [Google Scholar] [CrossRef]
- Kono, H.; Hashimoto, H.; Shimizu, Y. NMR characterization of cellulose acetate: Chemical shift assignments, substituent effects, and chemical shift additivity. Carbohydr. Polym. 2015, 118, 91–100. [Google Scholar] [CrossRef]
- Knall, A.-C.; Hollauf, M.; Slugovc, C. Kinetic studies of inverse electron demand Diels–Alder reactions (iEDDA) of norbornenes and 3,6-dipyridin-2-yl-1,2,4,5-tetrazine. Tetrahedron Lett. 2014, 55, 4763–4766. [Google Scholar] [CrossRef]
- Bakkali, H.; Marie, C.; Ly, A.; Thobie-Gautier, C.; Graton, J.; Pipelier, M.; Sengmany, S.; Léonel, E.; Nédélec, J.-Y.; Evain, M.; et al. Functionalized 2,5-Dipyridinylpyrroles by Electrochemical Reduction of 3,6-Dipyridinylpyridazine Precursors. Eur. J. Org. Chem. 2008, 2008, 2156–2166. [Google Scholar] [CrossRef]
- Bruson, H.A. The Chemistry of Acrylonitrile. I. Cyanoethylation of Active Methylene Groups. J. Am. Chem. Soc. 1942, 64, 2457–2461. [Google Scholar] [CrossRef]
- Xia, Y.; LaRock, R.C. Castor oil-based thermosets with varied crosslink densities prepared by ring-opening metathesis polymerization (ROMP). Polym. 2010, 51, 2508–2514. [Google Scholar] [CrossRef]
- Gottlieb, H.E.; Kotlyar, V.; Nudelman, A. NMR Chemical Shifts of Common Laboratory Solvents as Trace Impurities. J. Org. Chem. 1997, 62, 7512–7515. [Google Scholar] [CrossRef] [PubMed]
- Bendoraitiene, J.; Kavaliauskaite, R.; Klimaviciute, R.; Zemaitaitis, A. Peculiarities of Starch Cationization with Glycidyltrimethylammonium Chloride. Starch Stärke 2006, 58, 623–631. [Google Scholar] [CrossRef]
- Leitgeb, A.; Wappel, J.; Slugovc, C. The ROMP toolbox upgraded. Polym. 2010, 51, 2927–2946. [Google Scholar] [CrossRef]
- Meng, X.; Matson, J.B.; Edgar, K.J. Olefin cross-metathesis, a mild, modular approach to functionalized cellulose esters. Polym. Chem. 2014, 5, 7021–7033. [Google Scholar] [CrossRef]
- Meng, X.; Edgar, K.J. Synthesis of amide-functionalized cellulose esters by olefin cross-metathesis. Carbohydr. Polym. 2015, 132, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, L.; Malmström, E.; Carlmark, A. Surface-initiated ring-opening metathesis polymerisation from cellulose fibres. Polym. Chem. 2012, 3, 727–733. [Google Scholar] [CrossRef]



| c(Alkene) (M) | Pseudo First Order Rate Constant kapp (s−1) | |
|---|---|---|
| NC | NM | |
| 0.005 | 6.55 × 10−5 ± 3.37 × 10−6 | 1.02 × 10−4 ± 2.90 × 10−6 |
| 0.010 | 1.26 × 10−4 ± 3.96 × 10−6 | 1.86 × 10−4 ± 4.83 × 10−6 |
| 0.015 | 1.79 × 10−4 ± 4.10 × 10−6 | 2.77 × 10−4 ± 2.79 × 10−6 |
| Alkene:Tz | NC/ NM (µL) | THF (µL) | pyTz (µL) |
|---|---|---|---|
| 5:1 | 250 | 750 | 1000 |
| 10:1 | 500 | 500 | 1000 |
| 15:1 | 750 | 250 | 1000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wappl, C.; Schallert, V.; Slugovc, C.; Knall, A.-C.; Spirk, S. Highly Norbornylated Cellulose and Its “Click” Modification by an Inverse-Electron Demand Diels–Alder (iEDDA) Reaction. Molecules 2021, 26, 1358. https://doi.org/10.3390/molecules26051358
Wappl C, Schallert V, Slugovc C, Knall A-C, Spirk S. Highly Norbornylated Cellulose and Its “Click” Modification by an Inverse-Electron Demand Diels–Alder (iEDDA) Reaction. Molecules. 2021; 26(5):1358. https://doi.org/10.3390/molecules26051358
Chicago/Turabian StyleWappl, Christina, Viktor Schallert, Christian Slugovc, Astrid-Caroline Knall, and Stefan Spirk. 2021. "Highly Norbornylated Cellulose and Its “Click” Modification by an Inverse-Electron Demand Diels–Alder (iEDDA) Reaction" Molecules 26, no. 5: 1358. https://doi.org/10.3390/molecules26051358
APA StyleWappl, C., Schallert, V., Slugovc, C., Knall, A.-C., & Spirk, S. (2021). Highly Norbornylated Cellulose and Its “Click” Modification by an Inverse-Electron Demand Diels–Alder (iEDDA) Reaction. Molecules, 26(5), 1358. https://doi.org/10.3390/molecules26051358