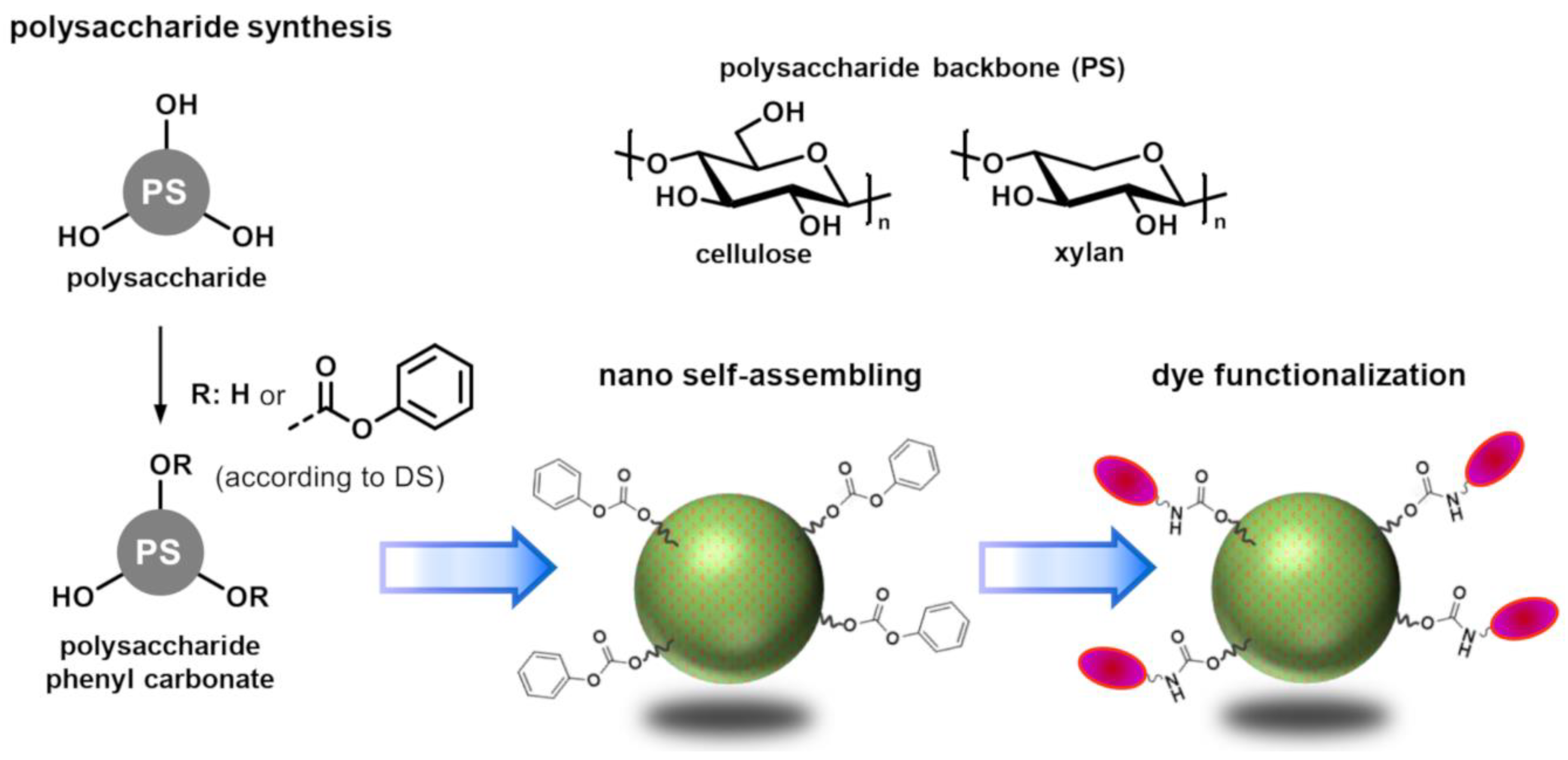
Reactive Nanoparticles Derived from Polysaccharide Phenyl Carbonates
Abstract
:1. Introduction
2. Results and Discussion
2.1. Evaluation of the Self-Assembling Behaviour
2.2. Functionalization of Reactive Nanoparticles
2.3. Bio-Compatibility Studies
3. Materials and Methods
3.1. Materials
3.2. Measurements
3.3. Preparation and Functionalization of Nanoparticles
3.4. Bio-Compatibility Studies
3.4.1. Cell Culture
3.4.2. PrestoBlue Cytotoxicity Assay
3.4.3. SYTOX Staining
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Roduner, E. Size matters: Why nanomaterials are different. Chem. Soc. Rev. 2006, 35, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Tomalia, D.A. In quest of a systematic framework for unifying and defining nanoscience. J. Nanoparticle Res. 2009, 11, 1251–1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boles, M.A.; Engel, M.; Talapin, D.V. Self-Assembly of Colloidal Nanocrystals: From Intricate Structures to Functional Materials. Chem. Rev. 2016, 116, 11220–11289. [Google Scholar] [CrossRef]
- Letchford, K.; Burt, H. A review of the formation and classification of amphiphilic block copolymer nanoparticulate structures: Micelles, nanospheres, nanocapsules and polymersomes. Eur. J. Pharm. Biopharm. 2007, 65, 259–269. [Google Scholar] [CrossRef]
- Calzoni, E.; Cesaretti, A.; Polchi, A.; Di Michele, A.; Tancini, B.; Emiliani, C. Biocompatible Polymer Nanoparticles for Drug Delivery Applications in Cancer and Neurodegenerative Disorder Therapies. J. Funct. Biomater. 2019, 10, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sur, S.; Rathore, A.; Dave, V.; Reddy, K.R.; Chouhan, R.S.; Sadhu, V. Recent developments in functionalized polymer nanoparticles for efficient drug delivery system. Nano-Struct. Nano-Objects 2019, 20, 100397. [Google Scholar] [CrossRef]
- Gao, D.; Hu, D.; Liu, X.; Zhang, X.; Yuan, Z.; Sheng, Z.; Zheng, H. Recent Advances in Conjugated Polymer Nanoparticles for NIR-II Imaging and Therapy. ACS Appl. Polym. Mater. 2020, 2, 4241–4257. [Google Scholar] [CrossRef]
- Suárez-García, S.; Solórzano, R.; Novio, F.; Alibés, R.; Busqué, F.; Ruiz-Molina, D. Coordination polymers nanoparticles for bioimaging. Coord. Chem. Rev. 2021, 432, 213716. [Google Scholar] [CrossRef]
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef]
- Bugnicourt, L.; Ladavière, C. Interests of chitosan nanoparticles ionically cross-linked with tripolyphosphate for biomedical applications. Prog. Polym. Sci. 2016, 60, 1–17. [Google Scholar] [CrossRef]
- Gericke, M.; Schulze, P.; Heinze, T. Nanoparticles Based on Hydrophobic Polysaccharide Derivatives—Formation Principles, Characterization Techniques, and Biomedical Applications. Macromol. Biosci. 2020, 20, e1900415. [Google Scholar] [CrossRef]
- Daus, S.; Heinze, T. Xylan-Based Nanoparticles: Prodrugs for Ibuprofen Release. Macromol. Biosci. 2009, 10, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Kühne, M.; Lindemann, H.; Grune, C.; Schröder, D.; Cseresnyés, Z.; Godmann, M.; Koschella, A.; Figge, M.T.; Eggeling, C.; Fischer, D.; et al. Biocompatible sulfated valproic acid-coupled polysaccharide-based nanocarriers with HDAC inhibitory activity. J. Control. Release 2021, 329, 717–730. [Google Scholar] [CrossRef]
- Schulze, P.; Gericke, M.; Heinze, T. Reactive nanoparticles with activated ester moieties from cellulose acetate phthalate derivatives. Cellulose 2019, 26, 475–490. [Google Scholar] [CrossRef]
- Elschner, T.; Heinze, T. Cellulose Carbonates: A Platform for Promising Biopolymer Derivatives with Multifunctional Capabilities. Macromol. Biosci. 2015, 15, 735–746. [Google Scholar] [CrossRef]
- Gabriel, L.; Gericke, M.; Heinze, T. Modular synthesis of non-charged and ionic xylan carbamate derivatives from xylan carbonates. Carbohydr. Polym. 2019, 207, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Wondraczek, H.; Elschner, T.; Heinze, T. Synthesis of highly functionalized dextran alkyl carbonates showing nanosphere formation. Carbohydr. Polym. 2011, 83, 1112–1118. [Google Scholar] [CrossRef]
- Gericke, M.; Gabriel, L.; Geitel, K.; Benndorf, S.; Trivedi, P.; Fardim, P.; Heinze, T. Synthesis of xylan carbonates—An approach towards reactive polysaccharide derivatives showing self-assembling into nanoparticles. Carbohydr. Polym. 2018, 193, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Elschner, T.; Ganske, K.; Heinze, T. Synthesis and aminolysis of polysaccharide carbonates. Cellulose 2013, 20, 339–353. [Google Scholar] [CrossRef]
- Elschner, T.; Kötteritzsch, M.; Heinze, T. Synthesis of Cellulose Tricarbonates in 1-Butyl-3-methylimidazolium Chloride/Pyridine. Macromol. Biosci. 2013, 14, 161–165. [Google Scholar] [CrossRef]
- Schulze, P.; Gericke, M.; Scholz, F.; Wondraczek, H.; Miethe, P.; Heinze, T. Incorporation of Hydrophobic Dyes within Cellulose Acetate and Acetate Phthalate Based Nanoparticles. Macromol. Chem. Phys. 2016, 217, 1823–1833. [Google Scholar] [CrossRef]
- Hornig, S.; Heinze, T. Efficient Approach to Design Stable Water-Dispersible Nanoparticles of Hydrophobic Cellulose Esters. Biomacromolecules 2008, 9, 1487–1492. [Google Scholar] [CrossRef] [PubMed]
- Wondraczek, H.; Petzold-Welcke, K.; Fardim, P.; Heinze, T. Nanoparticles from conventional cellulose esters: Evaluation of preparation methods. Cellulose 2013, 20, 751–760. [Google Scholar] [CrossRef]
- Geissler, A.; Biesalski, M.; Heinze, T.; Zhang, K. Formation of nanostructured cellulose stearoyl esters via nanoprecipitation. J. Mater. Chem. A 2014, 2, 1107–1116. [Google Scholar] [CrossRef]
- Galindo-Rodriguez, S.; Allémann, E.; Fessi, H.; Doelker, E. Physicochemical Parameters Associated with Nanoparticle Formation in the Salting-Out, Emulsification-Diffusion, and Nanoprecipitation Methods. Pharm. Res. 2004, 21, 1428–1439. [Google Scholar] [CrossRef]
- Nikolajski, M.; Wotschadlo, J.; Clement, J.H.; Heinze, T. Amino-Functionalized Cellulose Nanoparticles: Preparation, Characterization, and Interactions with Living Cells. Macromol. Biosci. 2012, 12, 920–925. [Google Scholar] [CrossRef]
- Heinze, T.; Michealis, N.; Hornig, S. Reactive polymeric nanoparticles based on unconventional dextran derivatives. Eur. Polym. J. 2007, 43, 697–703. [Google Scholar] [CrossRef]
- Aschenbrenner, E.; Bley, K.; Koynov, K.; Makowski, M.; Kappl, M.; Landfester, K.; Weiss, C.K. Using the Polymeric Ouzo Effect for the Preparation of Polysaccharide-Based Nanoparticles. Langmuir 2013, 29, 8845–8855. [Google Scholar] [CrossRef] [PubMed]
- Berruyer, P.; Gericke, M.; Moutzouri, P.; Jakobi, D.; Bardet, M.; Karlson, L.; Schantz, S.; Heinze, T.; Emsley, L. Advanced characterization of regioselectively substituted methylcellulose model compounds by DNP enhanced solid-state NMR spectroscopy. Carbohydr. Polym. 2021, 262, 117944. [Google Scholar] [CrossRef] [PubMed]
- Santander-Ortega, M.J.; Stauner, T.; Loretz, B.; Ortega-Vinuesa, J.L.; González, D.B.; Wenz, G.; Schaefer, U.F.; Lehr, C.M. Nanoparticles made from novel starch derivatives for transdermal drug delivery. J. Control. Release 2010, 141, 85–92. [Google Scholar] [CrossRef]
- Mazumder, S.; Dewangan, A.K.; Pavurala, N. Enhanced dissolution of poorly soluble antiviral drugs from nanoparticles of cellulose acetate based solid dispersion matrices. Asian J. Pharm. Sci. 2017, 12, 532–541. [Google Scholar] [CrossRef]
- Sauraj; Kumar, S.U.; Kumar, V.; Priyadarshi, R.; Gopinath, P.; Negi, Y.S. pH-responsive prodrug nanoparticles based on xylan-curcumin conjugate for the efficient delivery of curcumin in cancer therapy. Carbohydr. Polym. 2018, 188, 252–259. [Google Scholar] [CrossRef]
- Cui, L.; Cohen, J.A.; Broaders, K.E.; Beaudette, T.T.; Fréchet, J.M.J. Mannosylated dextran nanoparticles: A pH-sensitive system engineered for immunomodulation through mannose targeting. Bioconjugate Chem. 2011, 22, 949–957. [Google Scholar] [CrossRef]
- Rosenfeldt, S.; Mickoleit, F.; Jörke, C.; Clement, J.H.; Markert, S.; Jérôme, V.; Schwarzinger, S.; Freitag, R.; Schüler, D.; Uebe, R.; et al. Towards standardized purification of bacterial magnetic nanoparticles for future in vivo applications. Acta Biomater. 2021, 120, 293–303. [Google Scholar] [CrossRef] [PubMed]
- DIN Deutsches Institut für Normen e.V. Biologische Beurteilung von Medizinprodukten—Teil 5: Prüfungen auf In-vitro-Zytotoxizität (ISO 10993-5:2009); Deutsche Fassung EN ISO 10993-5:2009. 2009. Available online: https://www.din.de/de/mitwirken/normenausschuesse/nafuo/veroeffentlichungen/wdc-beuth:din21:113571989 (accessed on 1 March 2020).
- Schlenk, F.; Werner, S.; Rabel, M.; Jacobs, F.; Bergemann, C.; Clement, J.H.; Fischer, D. Comprehensive analysis of the in vitro and ex ovo hemocompatibility of surface engineered iron oxide nanoparticles for biomedical applications. Arch. Toxicol. 2017, 91, 3271–3286. [Google Scholar] [CrossRef]
- Stetefeld, J.; McKenna, S.A.; Patel, T.R. Dynamic light scattering: A practical guide and applications in biomedical sciences. Biophys. Rev. 2016, 8, 409–427. [Google Scholar] [CrossRef] [PubMed]
- Rangan, S.R.S. A new human cell line (FaDu) from a hypopharyngeal carcinoma. Cancer 1972, 29, 117–121. [Google Scholar] [CrossRef]

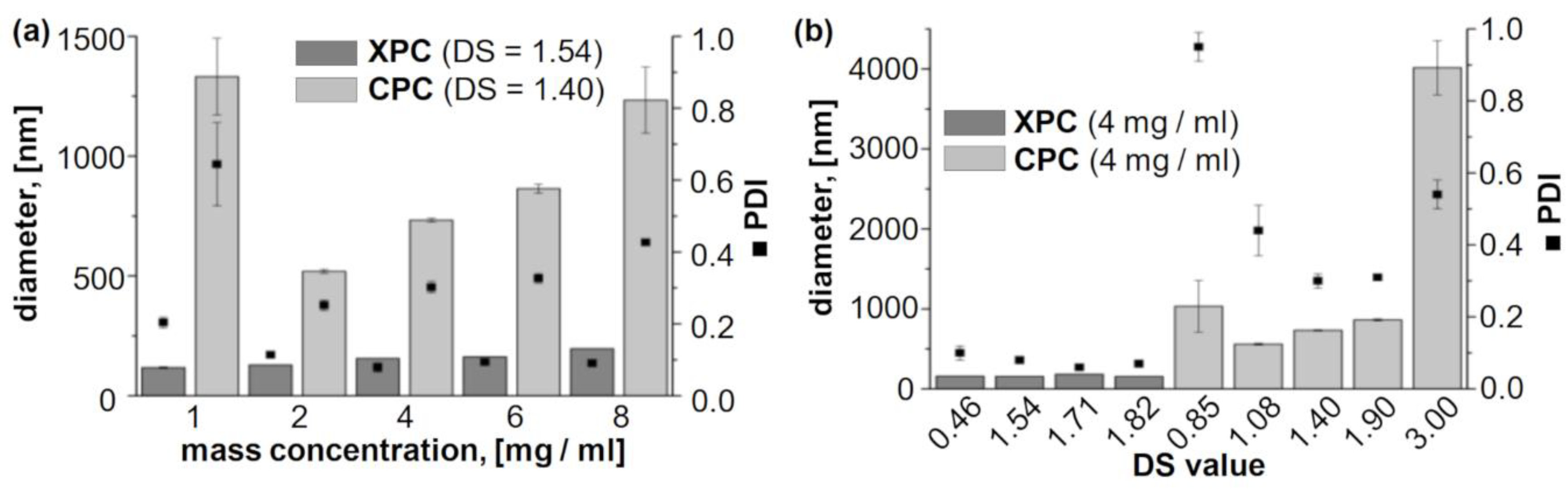


| ID | PS Backbone | DScarbonate | Mass Concentration, [mg/mL] | Diameter, [nm] 1 | PDI 2 |
|---|---|---|---|---|---|
| CPC-NP 1 | cellulose | 3.00 | 1 | 1005 ± 116 | 0.73 ± 0.04 |
| CPC-NP 2 | cellulose | 3.00 | 2 | 2796 ± 571 | 0.31 ± 0.10 |
| CPC-NP 3 | cellulose | 3.00 | 4 | 4015 ± 179 | 0.54 ± 0.04 |
| CPC-NP 4 | cellulose | 3.00 | 6 | 3192 ± 337 | 0.59 ± 0.16 |
| CPC-NP 5 | cellulose | 3.00 | 8 | 4255 ± 684 | 0.39 ± 0.09 |
| CPC-NP 6 | cellulose | 1.90 | 1 | 403 ± 2.4 | 0.17 ± 0.00 |
| CPC-NP 7 | cellulose | 1.90 | 2 | 457 ± 1 | 0.31 ± 0.00 |
| CPC-NP 8 | cellulose | 1.90 | 4 | 862 ± 14 | 0.31 ± 0.00 |
| CPC-NP 9 | cellulose | 1.90 | 6 | 1080 ± 42 | 0.33 ± 0.00 |
| CPC-NP 10 | cellulose | 1.90 | 8 | 1805 ± 136 | 0.41 ± 0.00 |
| CPC-NP 11 | cellulose | 1.40 | 1 | 1332 ± 159 | 0.64 ± 0.12 |
| CPC-NP 12 | cellulose | 1.40 | 2 | 519 ± 8 | 0.25 ± 0.02 |
| CPC-NP 13 | cellulose | 1.40 | 4 | 733 ± 7 | 0.30 ± 0.02 |
| CPC-NP 14 | cellulose | 1.40 | 6 | 864 ± 19 | 0.33 ± 0.01 |
| CPC-NP 15 | cellulose | 1.40 | 8 | 1234 ± 137 | 0.43 ± 0.01 |
| CPC-NP 16 | cellulose | 1.08 | 1 | 226 ± 5 | 0.13 ± 0.01 |
| CPC-NP 17 | cellulose | 1.08 | 2 | 251 ± 2 | 0.14 ± 0.01 |
| CPC-NP 18 | cellulose | 1.08 | 4 | 560 ± 11 | 0.44 ± 0.07 |
| CPC-NP 19 | cellulose | 1.08 | 6 | 345 ± 1 | 0.21 ± 0.02 |
| CPC-NP 20 | cellulose | 1.08 | 8 | 309 ± 2 | 0.18 ± 0.01 |
| CPC-NP 21 | cellulose | 0.85 | 1 | 1443 ± 189 | 0.47 ± 0.03 |
| CPC-NP 22 | cellulose | 0.85 | 2 | 1596 ± 118 | 0.81 ± 0.04 |
| CPC-NP 23 | cellulose | 0.85 | 4 | 1033 ± 321 | 0.95 ± 0.04 |
| CPC-NP 24 | cellulose | 0.85 | 6 | 614 ± 43 | 0.66 ± 0.08 |
| CPC-NP 25 | cellulose | 0.85 | 8 | 977 ± 289 | 0.82 ±0.09 |
| XPC-NP 1 | xylan | 1.82 | 1 | 102 ± 1 | 0.09 ± 0.03 |
| XPC-NP 2 | xylan | 1.82 | 2 | 123 ± 2 | 0.10 ± 0.00 |
| XPC-NP 3 | xylan | 1.82 | 4 | 155 ± 0 | 0.07 ± 0.01 |
| XPC-NP 4 | xylan | 1.82 | 6 | 177 ± 1 | 0.07 ± 0.00 |
| XPC-NP 5 | xylan | 1.82 | 8 | 205 ± 1 | 0.09 ± 0.01 |
| XPC-NP 6 | xylan | 1.71 | 1 | 130 ± 0 | 0.08 ± 0.00 |
| XPC-NP 7 | xylan | 1.71 | 2 | 150 ± 1 | 0.09 ± 0.01 |
| XPC-NP 8 | xylan | 1.71 | 4 | 183 ± 0 | 0.06 ± 0.01 |
| XPC-NP 9 | xylan | 1.71 | 6 | 204 ± 1 | 0.06 ± 0.02 |
| XPC-NP 10 | xylan | 1.71 | 8 | 214 ± 1 | 0.10 ± 0.02 |
| XPC-NP 11 | xylan | 1.54 | 1 | 118 ± 3 | 0.20 ± 0.02 |
| XPC-NP 12 | xylan | 1.54 | 2 | 130 ± 0 | 0.11 ± 0.00 |
| XPC-NP 13 | xylan | 1.54 | 4 | 156 ± 0 | 0.08 ± 0.01 |
| XPC-NP 14 | xylan | 1.54 | 6 | 163 ± 0 | 0.09 ± 0.00 |
| XPC-NP 15 | xylan | 1.54 | 8 | 197 ± 1 | 0.09 ± 0.01 |
| XPC-NP 16 | xylan | 0.46 | 1 | 115 ± 5 | 0.37 ± 0.03 |
| XPC-NP 17 | xylan | 0.46 | 2 | 122 ± 1 | 0.20 ± 0.00 |
| XPC-NP 18 | xylan | 0.46 | 4 | 159 ± 0 | 0.10 ± 0.02 |
| XPC-NP 19 | xylan | 0.46 | 6 | 214 ± 1 | 0.22 ± 0.02 |
| XPC-NP 20 | xylan | 0.46 | 8 | 223 ± 2 | 0.13 ± 0.02 |
| Added Component | Percentage of Dead Cells | |
|---|---|---|
| after 3 h | after 24 h | |
| None (positive control) | 11.2 | n.d. |
| Triton X-100 (negative control) | 99.6 | n.d. |
| XPC-NP (25 µg/cm²) | 5.9 | 8.8 |
| XPC-NP (100 µg/cm²) | 6.5 | 9.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gericke, M.; Geitel, K.; Jörke, C.; Clement, J.H.; Heinze, T. Reactive Nanoparticles Derived from Polysaccharide Phenyl Carbonates. Molecules 2021, 26, 4026. https://doi.org/10.3390/molecules26134026
Gericke M, Geitel K, Jörke C, Clement JH, Heinze T. Reactive Nanoparticles Derived from Polysaccharide Phenyl Carbonates. Molecules. 2021; 26(13):4026. https://doi.org/10.3390/molecules26134026
Chicago/Turabian StyleGericke, Martin, Katja Geitel, Cornelia Jörke, Joachim H. Clement, and Thomas Heinze. 2021. "Reactive Nanoparticles Derived from Polysaccharide Phenyl Carbonates" Molecules 26, no. 13: 4026. https://doi.org/10.3390/molecules26134026
APA StyleGericke, M., Geitel, K., Jörke, C., Clement, J. H., & Heinze, T. (2021). Reactive Nanoparticles Derived from Polysaccharide Phenyl Carbonates. Molecules, 26(13), 4026. https://doi.org/10.3390/molecules26134026







