Polyphosphorhydrazone-Based Radical Dendrimers
Abstract
:1. Introduction
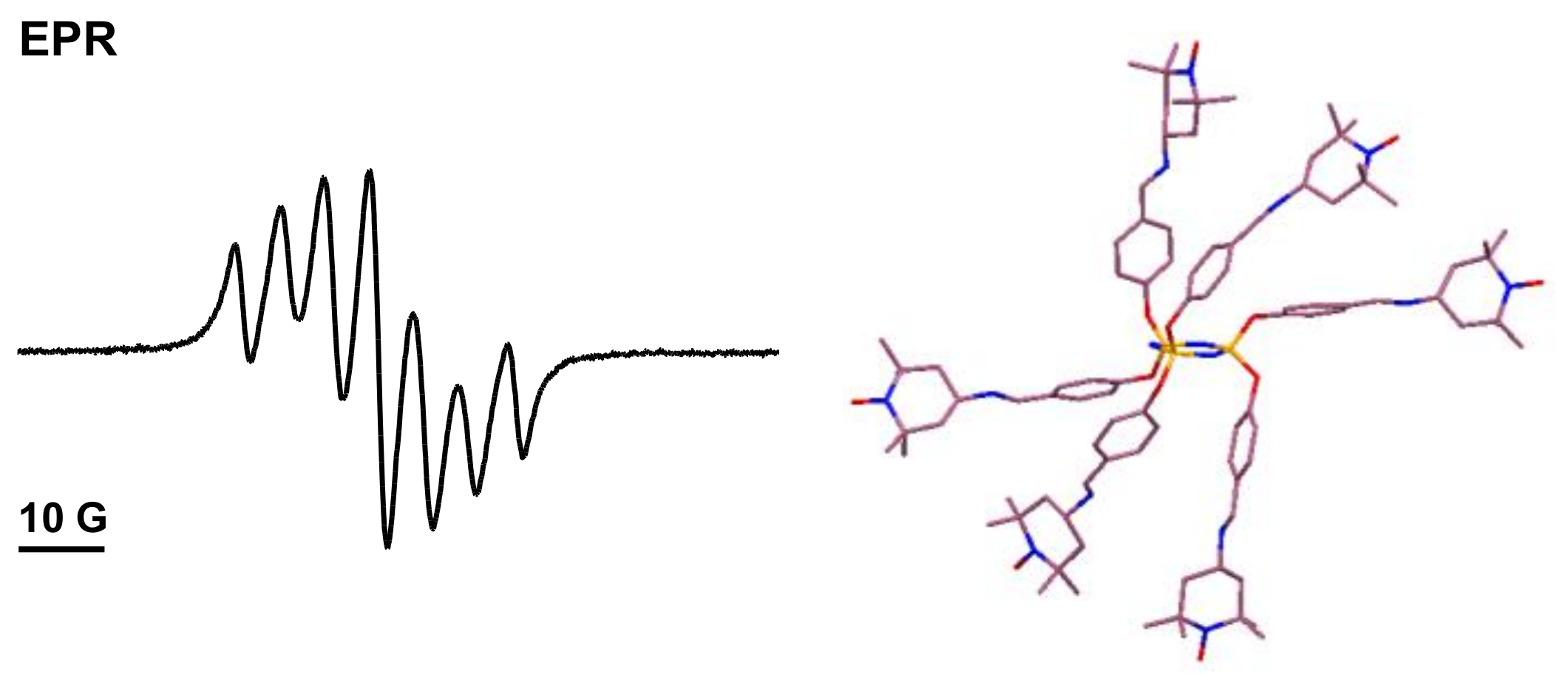
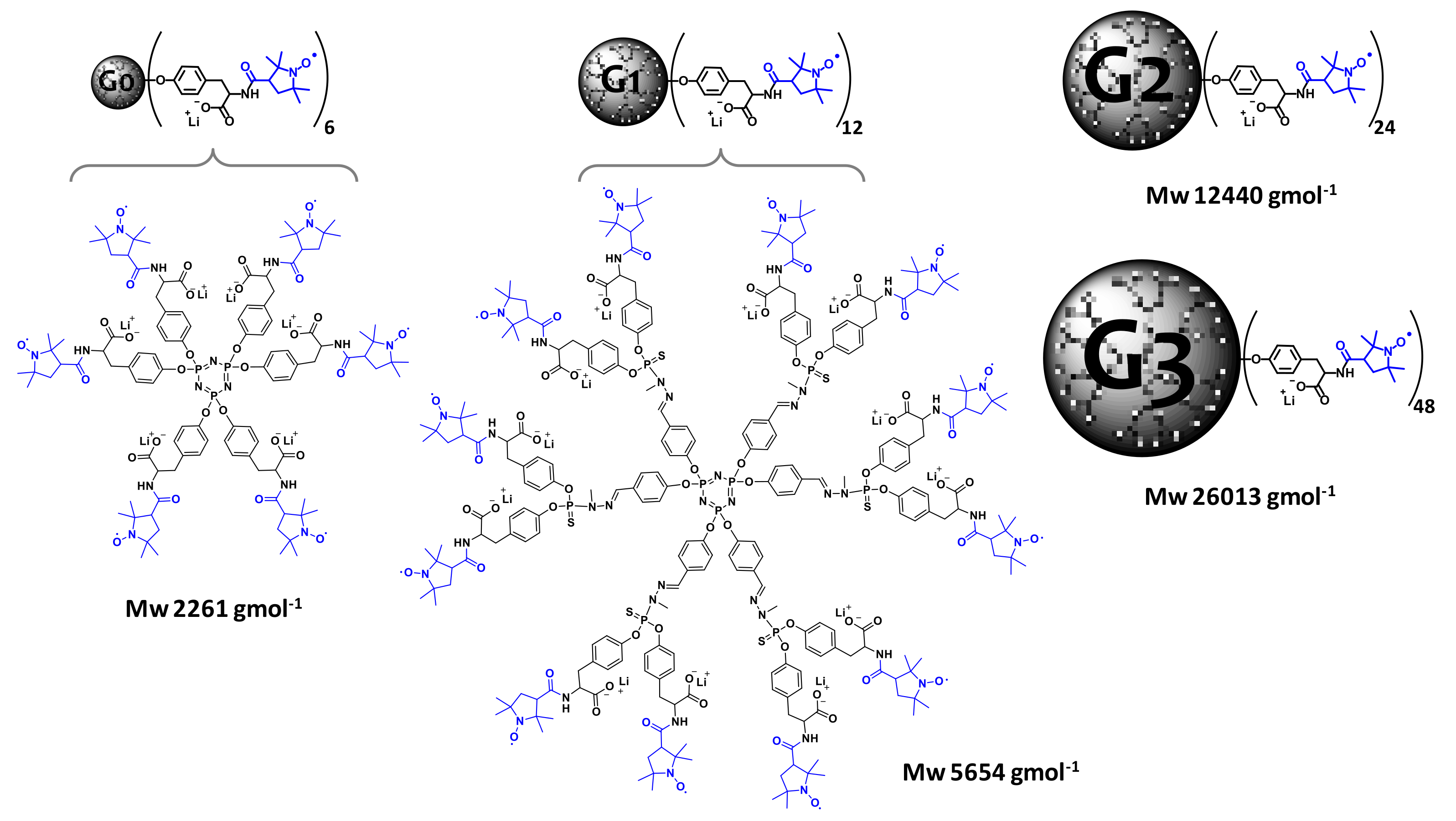
2. Polyphosphorhydrazone-Based Radical Dendrimers
3. Relaxivity Values Obtained So Far with Radical Dendrimers
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Swanson, S.D.; Kukowska-Latallo, J.F.; Patri, A.K.; Chen, C.; Ge, S.; Cao, Z.; Kotlyar, A.; East, A.T.; Baker, J.R. Targeted gadolinium-loaded dendrimer nanoparticles for tumor-specific magnetic resonance contrast enhancement. Int. J. Nanomed. 2008, 3, 201–210. [Google Scholar]
- Caravan, P.; Ellison, J.J.; McMurry, T.J.; Lauffer, R.B. Gadolinium(III) Chelates as MRI Contrast Agents: Structure, Dynamics, and Applications. Chem. Rev. 1999, 99, 2293–2352. [Google Scholar] [CrossRef]
- Wahsner, J.; Gale, E.; Rodríguez-Rodríguez, A.; Caravan, P. Chemistry of MRI contrast agents: Current challenges and new frontiers. Chem. Rev. 2019, 119, 957–1057. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Xu, L.; Kuang, Y.; Xiong, D.; Pei, R. Gadolinium-based nanoscale MRI contrast agents for tumor imaging. J. Mater. Chem. B 2017, 5, 3431–3461. [Google Scholar] [CrossRef] [PubMed]
- McMahon, M.T.; Bulte, J.W.M. Two decades of dendrimers as versatile MRI agents: A tale with and without metals. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 10, e1496. [Google Scholar] [CrossRef] [PubMed]
- Kojima, C.; Turkbey, B.; Ogawa, M.; Bernardo, M.; Regino, C.A.S.; Bryant, L.H., Jr.; Choyke, P.L.; Kono, K.; Kobayashi, H. Dendrimer-based MRI contrast agents: The effects of PEGylation on relaxivity and pharmacokinetics. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 1001–1008. [Google Scholar] [CrossRef] [Green Version]
- Rogosnitzky, M.; Branch, S. Gadolinium-based contrast agent toxicity: A review of known and proposed mechanisms. BioMetals 2016, 29, 365–376. [Google Scholar] [CrossRef] [Green Version]
- Ranga, A.; Agarwal, Y.; Garg, K. Gadolinium based contrast agents in current practice: Risks of accumulation and toxicity in patients with normal renal function. Indian J. Radiol. Imaging 2017, 27, 141–147. [Google Scholar]
- Olchowy, C.; Cebulski, K.; Łasecki, M.; Chaber, R.; Olchowy, A.; Kałwak, K.; Zaleska-Dorobisz, U. The presence of the gadolinium-based contrast agent depositions in the brain and symptoms of gadolinium neurotoxicity—A systematic review. PLoS ONE 2017, 12, e0171704–e0171718. [Google Scholar] [CrossRef] [Green Version]
- Bosman, A.W.; Janssen, R.A.J.; Meijer, E.W. Five Generations of Nitroxyl-Functionalized Dendrimers. Macromolecules 1997, 30, 3606–3611. [Google Scholar] [CrossRef] [Green Version]
- Kashiwagi, Y.; Kurashima, F.; Kikuchi, C.; Anzai, J.-I.; Osa, T. Voltammetric behavior of poly(amidoamine) dendrimers containing nitroxyl radical end groups. Electrochem. Commun. 1999, 1, 305–308. [Google Scholar] [CrossRef]
- Winalski, C.S.; Shortkroff, S.; Mulkern, R.V.; Schneider, E.; Rosen, G.M. Magnetic resonance relaxivity of dendrimer-linked nitroxides. Magn. Reson. Med. 2002, 48, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Maliakal, A.J.; Turro, N.J.; Bosman, A.W.; Cornel, J.; Meijer, E.W. Relaxivity studies on dinitroxide and polynitroxyl functionalized dendrimers: Effect of electron exchange and structure on paramagnetic relaxation enhancement. J. Phys. Chem. A 2003, 107, 8467–8475. [Google Scholar] [CrossRef]
- Yordanov, A.T.; Yamada, K.; Krishna, M.C.; Mitchell, J.B.; Woller, E.; Cloninger, M.; Brechbiel, M.W. Spin-Labeled Dendrimers in EPR Imaging with Low Molecular Weight Nitroxides. Angew. Chem. Int. Ed. 2001, 40, 2690–2692. [Google Scholar] [CrossRef]
- Francese, G.; Dunand, F.A.; Loosli, C.; Merbach, A.E.; Decurtins, S. Functionalization of PAMAM dendrimers with nitronyl nitroxide radicals as models for the outer-sphere relaxation in dentritic potential MRI contrast agents. Magn. Reson. Chem. 2003, 41, 81–83. [Google Scholar] [CrossRef]
- Sebby, K.B.; Walter, E.D.; Usselman, R.J.; Cloninger, M.J.; Singel, D.J. End-Group Distributions of Multiple Generations of Spin-Labeled PAMAM Dendrimers. J. Phys. Chem. B 2011, 115, 4613–4620. [Google Scholar] [CrossRef] [Green Version]
- Rajca, A.; Wang, Y.; Boska, M.; Paletta, J.T.; Olankitwanit, A.; Swanson, M.A.; Mitchell, D.G.; Eaton, S.S.; Eaton, G.R.; Rajca, S. Organic Radical Contrast Agents for Magnetic Resonance Imaging. J. Am. Chem. Soc. 2012, 134, 15724–15727. [Google Scholar] [CrossRef] [Green Version]
- Badetti, E.; Lloveras, V.; Wurst, K.; Sebastián, R.M.; Caminade, A.-M.; Majoral, J.-P.; Veciana, J.; Vidal-Gancedo, J. Synthesis and structural characterization of a dendrimer model compound based on a cyclotriphosphazene core with TEMPO radicals as substituents. Org. Lett. 2013, 15, 3490–3493. [Google Scholar] [CrossRef]
- Badetti, E.; Lloveras, V.; Muñoz-Gómez, J.L.; Sebastián, R.M.; Caminade, A.M.; Majoral, J.P.; Veciana, J.; Vidal-Gancedo, J. Radical dendrimers: A family of five generations of phosphorus dendrimers functionalized with TEMPO radicals. Macromolecules 2014, 47, 7717–7724. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Lloveras, V.; Pulido, D.; Liko, F.; Pinto, L.F.; Albericio, F.; Royo, M.; Vidal-Gancedo, J. Radical Dendrimers Based on Biocompatible Oligoethylene Glycol Dendrimers as Contrast Agents for MRI. Pharmaceutics 2020, 12, 772. [Google Scholar] [CrossRef]
- Pinto, L.F.; Lloveras, V.; Zhang, S.; Liko, F.; Veciana, J.; Muñoz-Gómez, J.L.; Vidal-Gancedo, J. Fully Water-Soluble Polyphosphorhydrazone-Based Radical Dendrimers Functionalized with Tyr-PROXYL Radicals as Metal-Free MRI T 1 Contrast Agents. ACS Appl. Bio Mater. 2020, 3, 369–376. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; Wang, X.; Dai, Y.; Dai, X.; Li, Z.; Luo, Q.; Zheng, X.; Gu, Z.; Zhang, H.; Gong, Q.; et al. Enhancing the Efficacy of Metal-Free MRI Contrast Agents via Conjugating Nitroxides onto PEGylated Cross-Linked Poly(Carboxylate Ester). Adv. Sci. 2020, 7, 2000467. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Sun, J.; Zhu, H.; Wu, H.; Zhang, H.; Gu, Z.; Luo, K. Recent advances in development of dendriticpolymer-based nanomedicines for cancer diagnosis. Wiley Interdiscip. Rev. WIREs Nanomed. Nanobiotechnol. 2020, e1670. [Google Scholar] [CrossRef]
- Chis, A.A.; Dobrea, C.; Morgovan, C.; Arseniu, A.M.; Rus, L.L.; Butuca, A.; Juncan, A.M.; Totan, M.; Vonica-Tincu, A.L.; Cormos, G.; et al. Applications and Limitations of Dendrimers in Biomedicine. Molecules 2020, 25, 3982. [Google Scholar] [CrossRef]
- Ali, B.M.; Boothapandi, M.; Sultan Nasar, A. Nitric oxide, DPPH and hydrogen peroxide radical scavenging activity of TEMPO terminated polyurethane dendrimers: Data supporting antioxidant activity of radical dendrimers. Data Brief 2020, 28, 104972. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.M.; Velavan, B.; Sudhandiran, G.; Sridevi, J.; Sultan Nasar, A. Radical dendrimers: Synthesis, anti-tumor activity and enhanced cytoprotective performance of TEMPO free radical functionalized polyurethane dendrimers. Eur. Polym. J. 2020, 122, 109354. [Google Scholar]
- Launay, N.; Caminade, A.-M.; Majoral, J.P. Synthesis of bowl-shaped dendrimers from generation 1 to generation 8. J. Organomet. Chem. 1997, 529, 51–58. [Google Scholar] [CrossRef]
- Launay, N.; Caminade, A.-M.; Majoral, J.-P. Synthesis and Reactivity of Unusual Phosphorus Dendrimers. A Useful Divergent Growth Approach Up to the Seventh Generation. J. Am. Chem. Soc. 1995, 117, 3282–3283. [Google Scholar] [CrossRef]
- Blais, J.-C.; Turrin, C.-O.; Caminade, A.-M.; Majoral, J.-P. MALDI TOF Mass Spectrometry for the Characterization of Phosphorus-Containing Dendrimers. Scope and Limitations. Anal. Chem. 2000, 72, 5097–5105. [Google Scholar] [CrossRef]
- Majoral, J.P.; Caminade, A.M. Dendrimers containing heteroatoms (si, p, B, ge, or bi). Chem. Rev. 1999, 99, 845–880. [Google Scholar] [CrossRef]
- Caminade, A.-M.; Ouali, A.; Laurent, R.; Turrin, C.-O.; Majoral, J.-P. The dendritic effect illustrated with phosphorus dendrimers. Chem. Soc. Rev. 2015, 44, 3890–3899. [Google Scholar] [CrossRef]
- Caminade, A.-M.; Turrin, C.-O.; Majoral, J.-P. (Eds.) Phosphorus Dendrimers in Biology and Nanomedicine; Pan Stanford: Singapore, 2018; ISBN 9781315110851. [Google Scholar]
- Shimono, S.; Tamura, R.; Ikuma, N.; Takahashi, H.; Sakai, N.; Yamauchi, J. Characterization of the Chiral Paramagnetic Multispin System Built on a Cyclotriphosphazene Scaffold. Chem. Lett. 2004, 33, 932–933. [Google Scholar] [CrossRef]
- Shimono, S.; Takahashi, H.; Sakai, N.; Tamura, R.; Ikuma, N.; Yamauchi, J. Use of Cyclotriphosphazene as a Molecular Scaffold for Building Chiral Multispin Systems. Mol. Cryst. Liq. Cryst. 2005, 440, 37–52. [Google Scholar] [CrossRef]
- Fidan, I.; Önal, E.; Yerli, Y.; Luneau, D.; Ahsen, V.; Hirel, C. Synthetic Access to a Pure Polyradical Architecture: Nucleophilic Insertion of Nitronyl Nitroxide on a Cyclotriphosphazene Scaffold. Chempluschem 2017, 82, 1384–1389. [Google Scholar] [CrossRef] [PubMed]
- Fidan, I.; Önal, E.; Yerli, Y.; Luneau, D.; Ahsen, V.; Hirel, C. Synthesis and Straightforward Quantification Methods of Imino Nitroxide-Based Hexaradical Architecture on a Cyclotriphosphazene Scaffold. Inorg. Chem. 2016, 55, 11447–11453. [Google Scholar] [CrossRef] [PubMed]
- Lloveras, V.; Badetti, E.; Wurst, K.; Vidal-Gancedo, J. Synthesis, X-Ray Structure, Magnetic Properties, and a Study of Intra/Intermolecular Radical-Radical Interactions of a Triradical TEMPO Compound. ChemPhysChem 2015, 16, 3302–3307. [Google Scholar] [CrossRef]
- Dulog, L.; Mohler, H. A λ5-cylotriphosphazene with nitroxyl groups as model for a paramagnetic poly(organo-λ5-phosphazene. Makromol. Chem. 1988, 189, 2611–2615. [Google Scholar] [CrossRef]
- Nguyen, H.V.T.; Chen, Q.; Paletta, J.T.; Harvey, P.; Jiang, Y.; Zhang, H.; Boska, M.D.; Ottaviani, M.F.; Jasanoff, A.; Rajca, A.; et al. Nitroxide-Based Macromolecular Contrast Agents with Unprecedented Transverse Relaxivity and Stability for Magnetic Resonance Imaging of Tumors. ACS Cent. Sci. 2017, 3, 800–811. [Google Scholar] [CrossRef]
- Niidome, T.; Gokuden, R.; Watanabe, K.; Mori, T.; Naganuma, T.; Utsumi, H.; Ichikawa, K.; Katayama, Y. Nitroxyl radicals-modified dendritic poly(l-lysine) as a contrast agent for Overhauser-enhanced MRI. J. Biomater. Sci. Polym. Ed. 2014, 25, 1425–1439. [Google Scholar] [CrossRef]
- Lloveras, V.; Liko, F.; Pinto, L.F.; Muñoz-Gómez, J.L.; Veciana, J.; Vidal-Gancedo, J. Tuning Spin-Spin Interactions in Radical Dendrimers. ChemPhysChem 2018, 19, 1895–1902. [Google Scholar] [CrossRef]
- Lloveras, V.; Liko, F.; Muñoz-Gómez, J.L.; Veciana, J.; Vidal-Gancedo, J. Redox-Active PTM Radical Dendrimers as Promising Multifunctional Molecular Switches. Chem. Mater. 2019, 31, 9400–9412. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lloveras, V.; Vidal-Gancedo, J. Polyphosphorhydrazone-Based Radical Dendrimers. Molecules 2021, 26, 1230. https://doi.org/10.3390/molecules26051230
Lloveras V, Vidal-Gancedo J. Polyphosphorhydrazone-Based Radical Dendrimers. Molecules. 2021; 26(5):1230. https://doi.org/10.3390/molecules26051230
Chicago/Turabian StyleLloveras, Vega, and José Vidal-Gancedo. 2021. "Polyphosphorhydrazone-Based Radical Dendrimers" Molecules 26, no. 5: 1230. https://doi.org/10.3390/molecules26051230
APA StyleLloveras, V., & Vidal-Gancedo, J. (2021). Polyphosphorhydrazone-Based Radical Dendrimers. Molecules, 26(5), 1230. https://doi.org/10.3390/molecules26051230






