Induction of Cytokines by Nucleic Acid Nanoparticles (NANPs) Depends on the Type of Delivery Carrier
Abstract
1. Introduction
2. Results
2.1. Physicochemical Characterization of Dendrimers
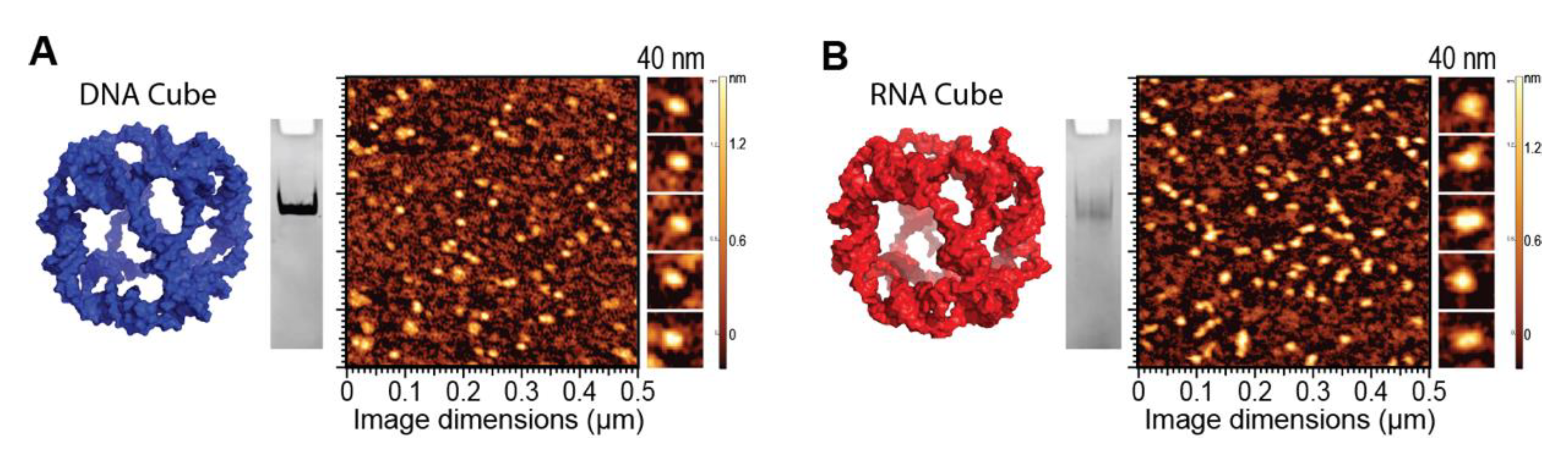
2.2. NANP Synthesis and Characterization
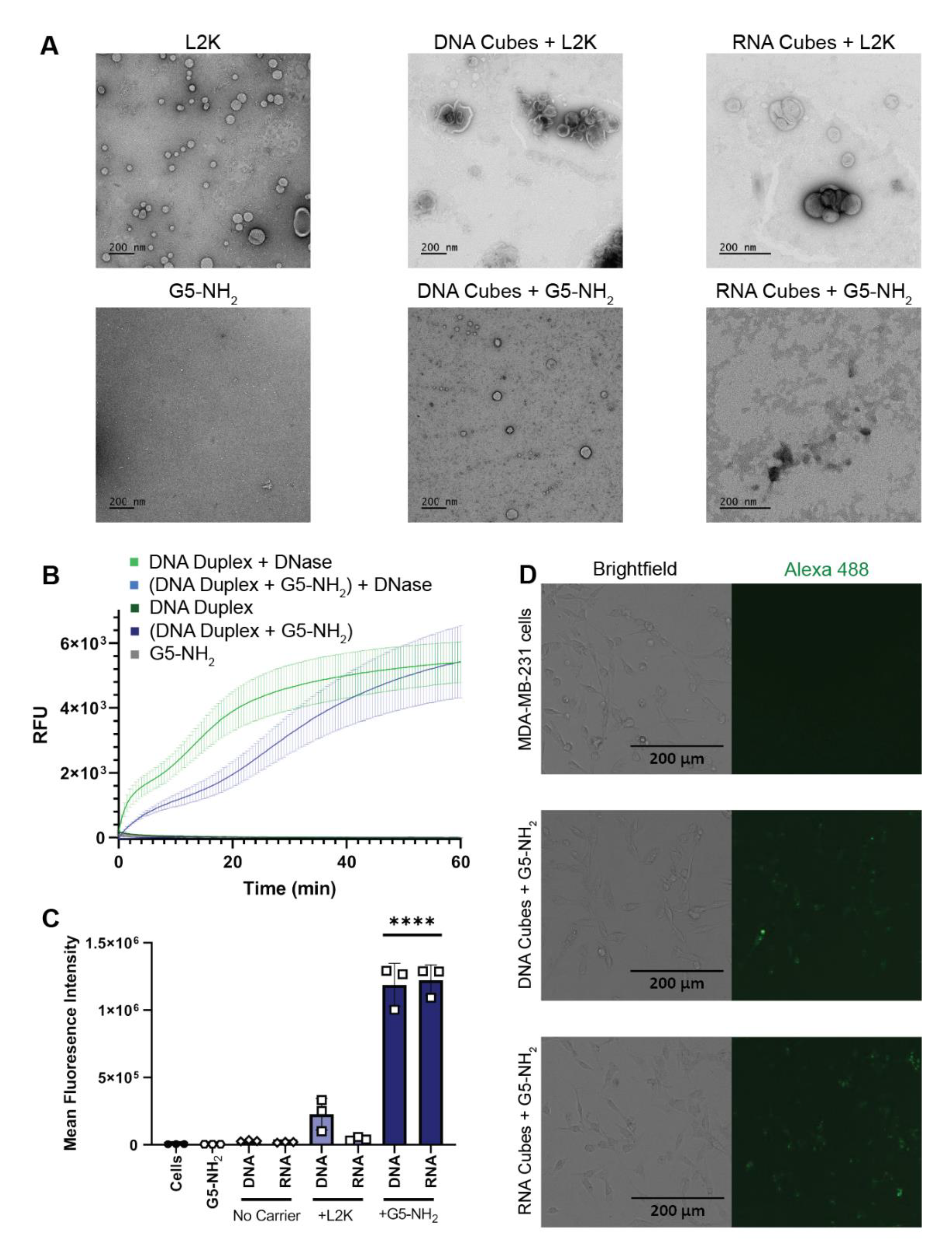
2.3. NANP Complexation with G5-NH2 Dendrimers
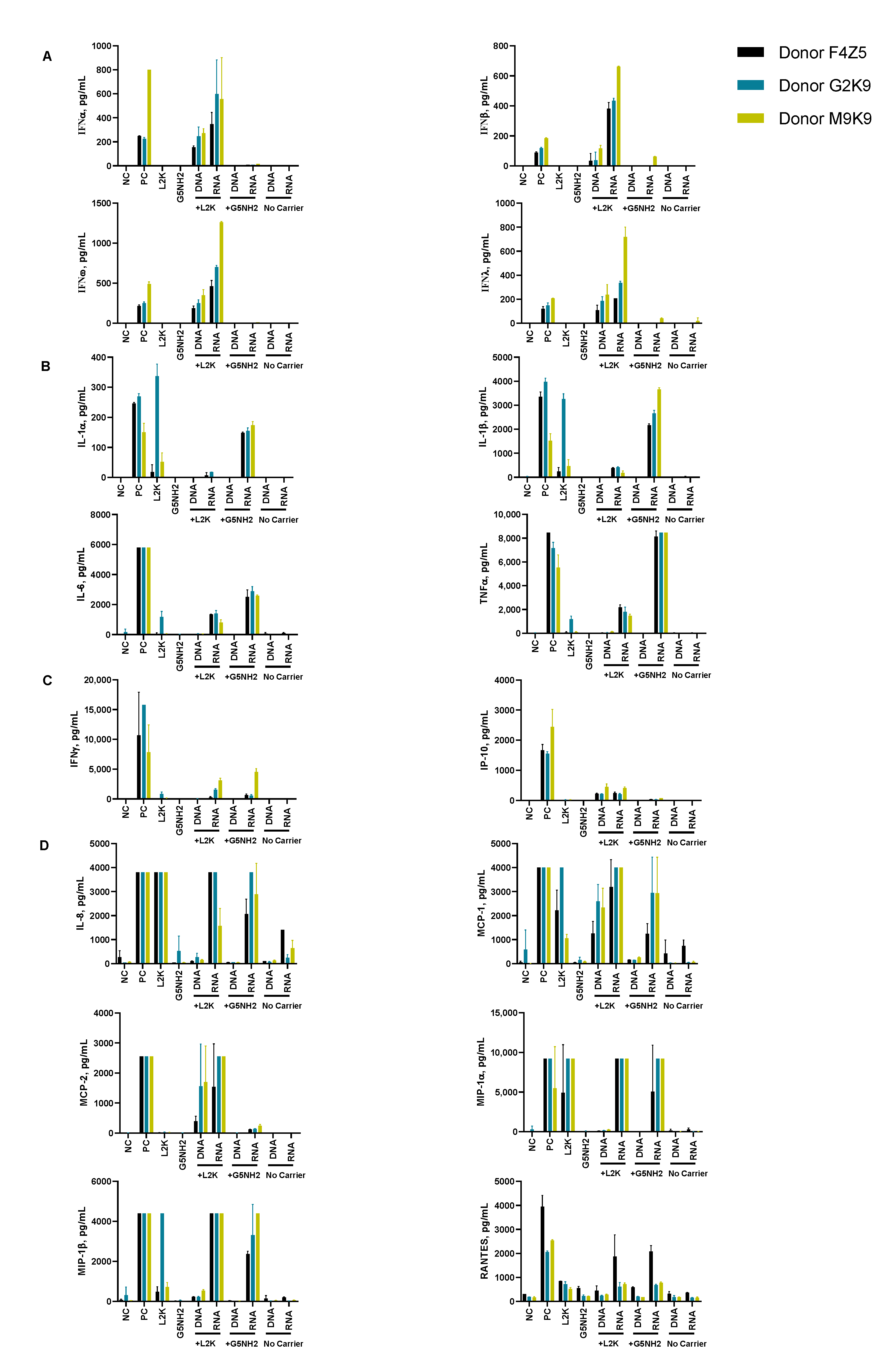
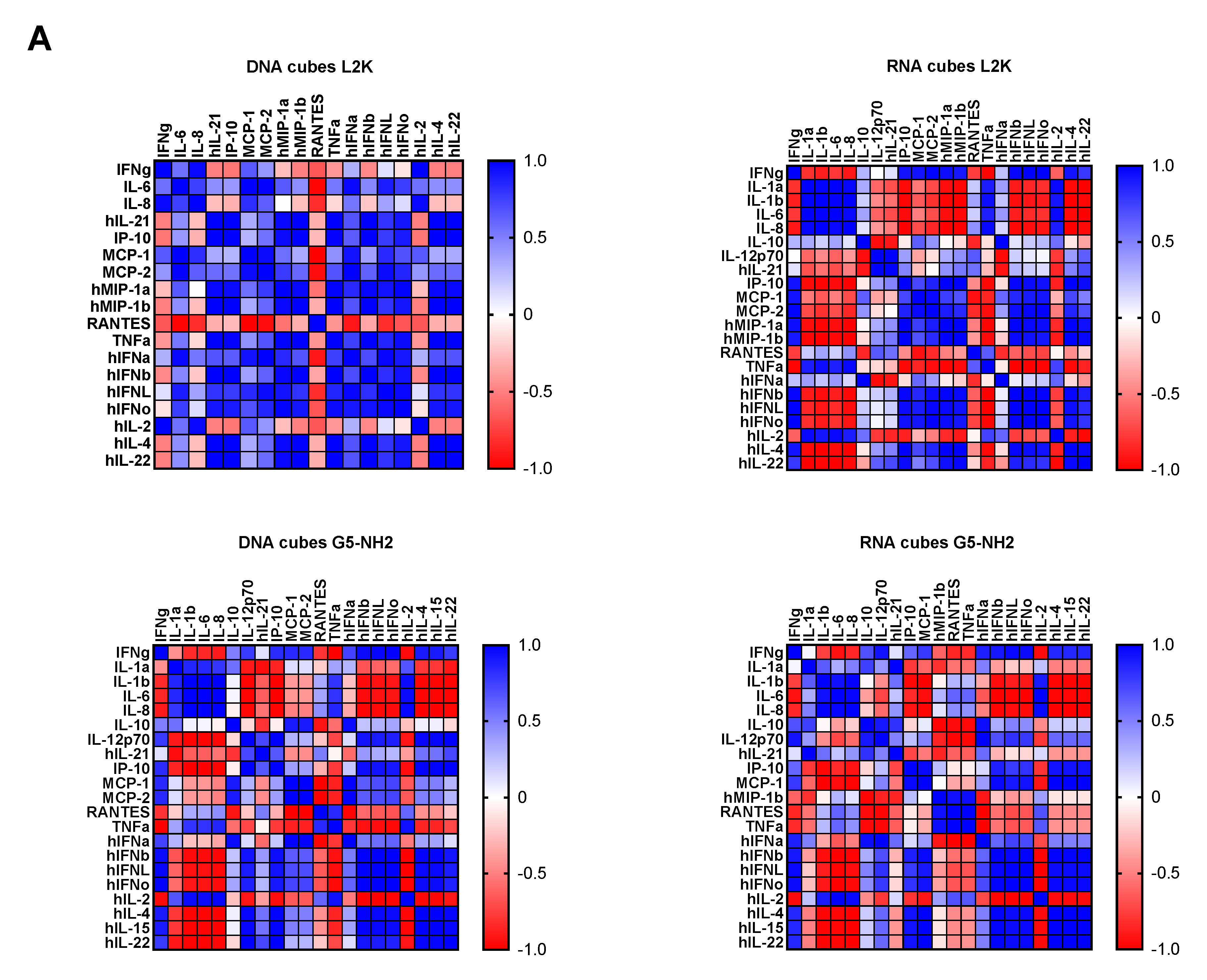
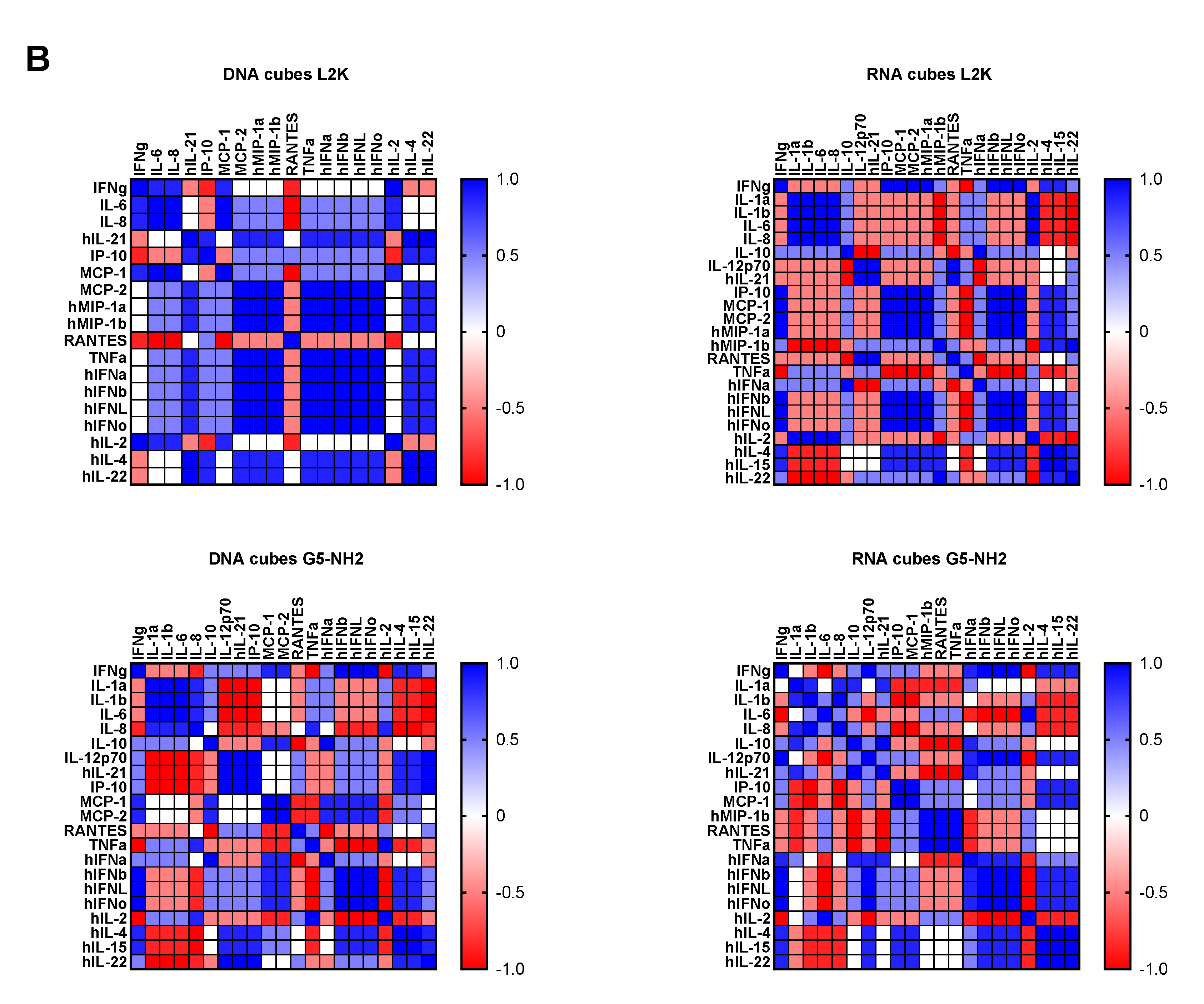
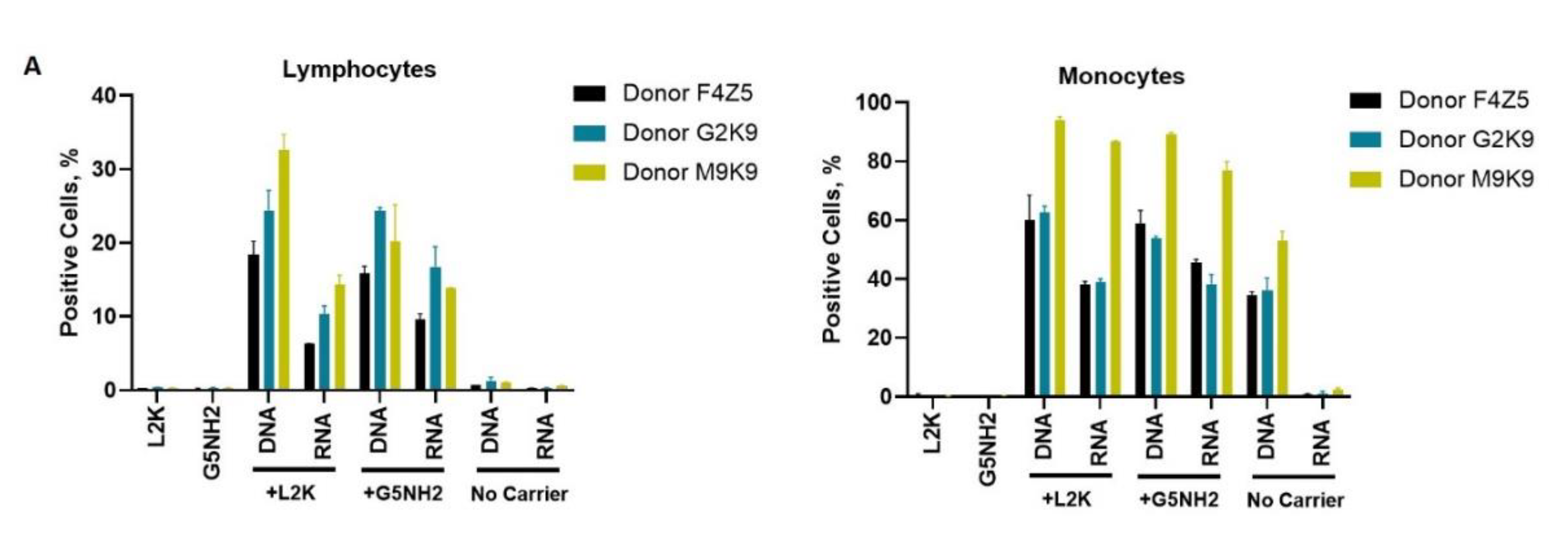
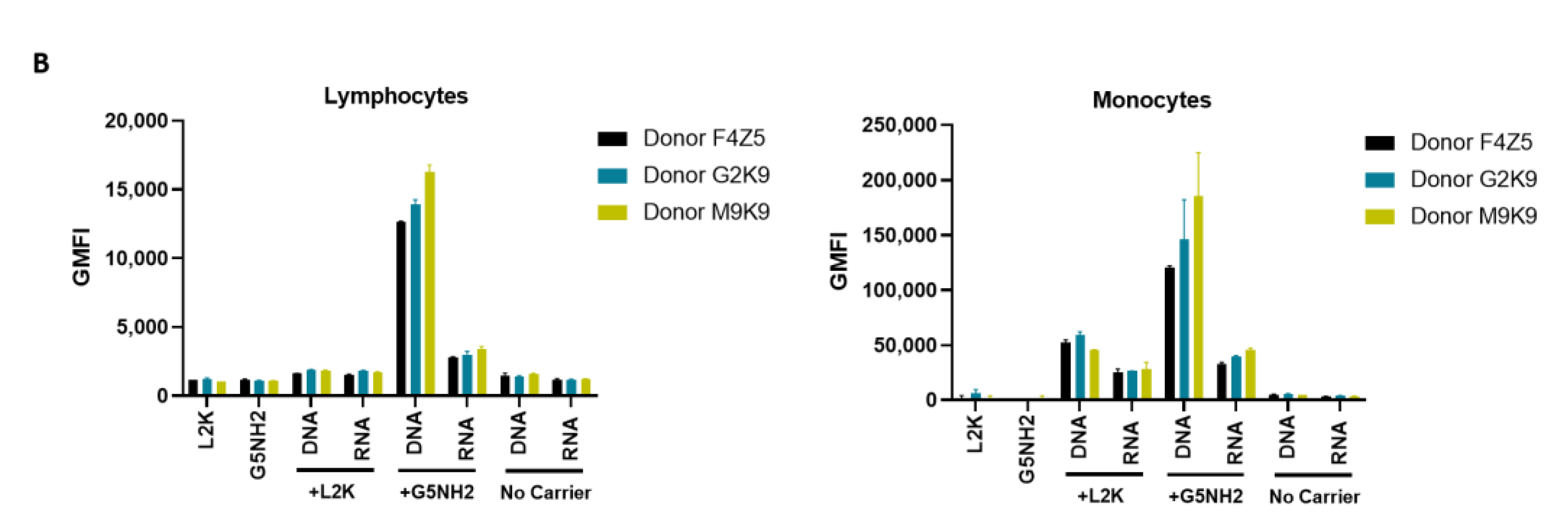
2.4. Cytokine Response in PBMCs Depends on the Type of Carrier and Correlates with NANP Uptake by the Cells
3. Discussion
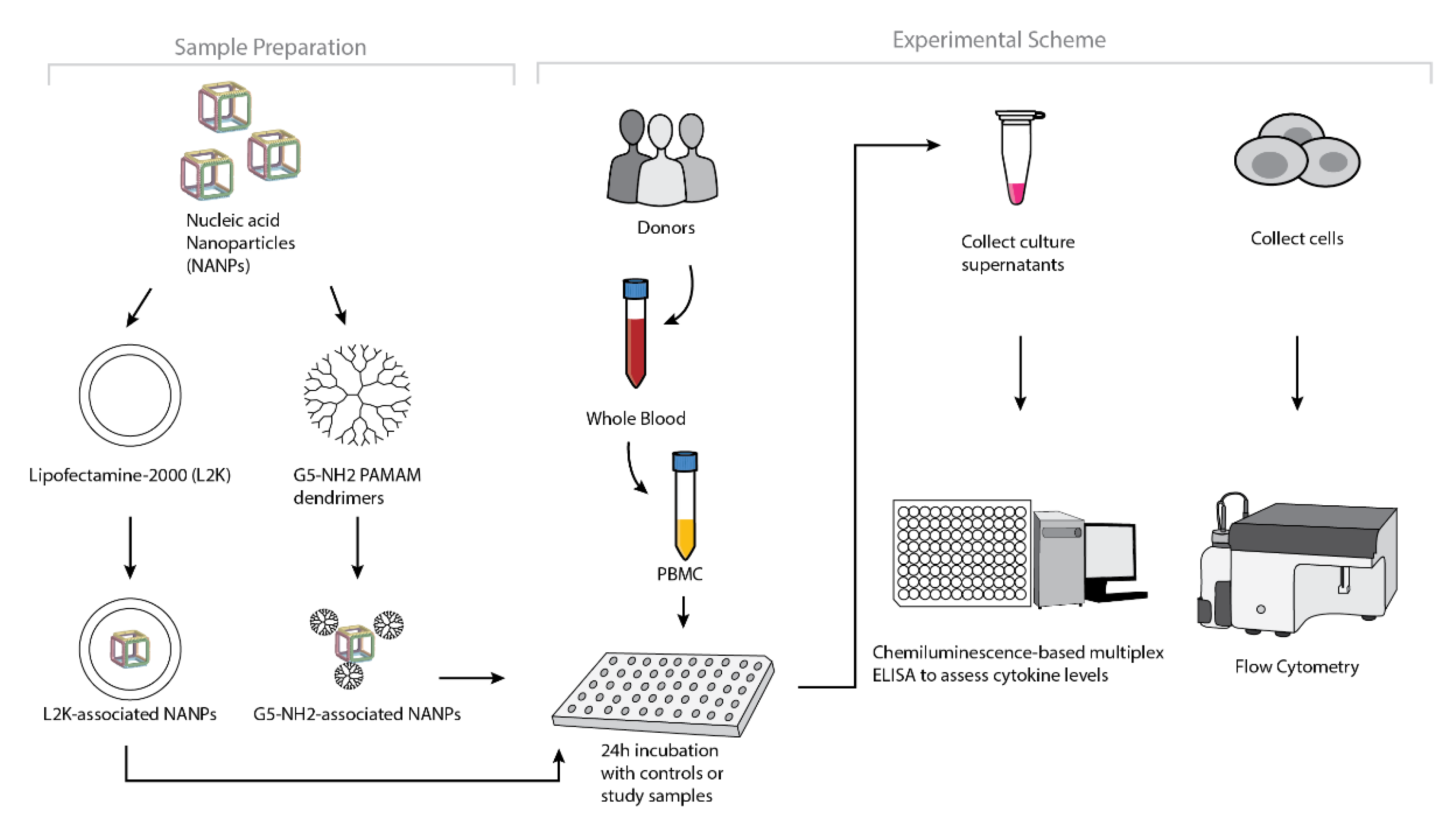
4. Materials and Methods
4.1. Materials
4.2. Physicocheimcal Characterization of Dendrimers
4.3. Preparation of NANPs
4.4. Physicochemical Characterization of NANPs
4.5. Complexing NANPs and Dendrimers
4.6. Transmission Electron Microscopy
4.7. Uptake by Cancer Cell Line MDA-MB-231
4.8. Research Donor Blood
4.9. In Vitro Cytokine Release
4.10. Uptake by Flow Cytometry
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chandler, M.; Afonin, K.A. Smart-Responsive Nucleic Acid Nanoparticles (NANPs) with the Potential to Modulate Immune Behavior. Nanomaterials 2019, 9, 611. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.B.; Halman, J.R.; Satterwhite, E.; Zakharov, A.V.; Bui, M.N.; Benkato, K.; Goldsworthy, V.; Kim, T.; Hong, E.; Dobrovolskaia, M.A.; et al. Programmable Nucleic Acid Based Polygons with Controlled Neuroimmunomodulatory Properties for Predictive QSAR Modeling. Small 2017, 13. [Google Scholar] [CrossRef] [PubMed]
- Ke, W.; Hong, E.; Saito, R.F.; Rangel, M.C.; Wang, J.; Viard, M.; Richardson, M.; Khisamutdinov, E.F.; Panigaj, M.; Dokholyan, N.V.; et al. RNA–DNA fibers and polygons with controlled immunorecognition activate RNAi, FRET and transcriptional regulation of NF-κB in human cells. Nucleic Acids Res. 2019, 47, 1350–1361. [Google Scholar] [CrossRef]
- Panigaj, M.; Johnson, M.B.; Ke, W.; McMillan, J.; Goncharova, E.A.; Chandler, M.; Afonin, K.A. Aptamers as Modular Components of Therapeutic Nucleic Acid Nanotechnology. ACS Nano 2019, 13, 12301–12321. [Google Scholar] [CrossRef]
- Sajja, S.; Chandler, M.; Fedorov, D.; Kasprzak, W.K.; Lushnikov, A.Y.; Viard, M.; Shah, A.; Dang, D.; Dahl, J.; Worku, B.; et al. Dynamic Behavior of RNA Nanoparticles Analyzed by AFM on a Mica/Air Interface. Langmuir 2018, 34, 15099–15108. [Google Scholar] [CrossRef]
- Halman, J.R.; Kim, K.-T.; Gwak, S.-J.; Pace, R.; Johnson, M.B.; Chandler, M.R.; Rackley, L.; Viard, M.; Marriott, I.; Lee, J.S.; et al. A cationic amphiphilic co-polymer as a carrier of nucleic acid nanoparticles (Nanps) for controlled gene silencing, immunostimulation, and biodistribution. Nanomed. Nanotechnol. Biol. Med. 2020, 23, 102094. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.B.; Halman, J.R.; Miller, D.K.; Cooper, J.S.; Khisamutdinov, E.F.; Marriott, I.; Afonin, K.A. The immunorecognition, subcellular compartmentalization, and physicochemical properties of nucleic acid nanoparticles can be controlled by composition modification. Nucleic Acids Res. 2020, 48, 11785–11798. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, S.; Greschner, A.A.; Gauthier, M.A. Progress Toward Absorption, Distribution, Metabolism, Elimination, and Toxicity of DNA Nanostructures. Adv. Ther. 2019, 2, 1900144. [Google Scholar] [CrossRef]
- Zeng, Y.; Nixon, R.L.; Liu, W.; Wang, R. The Applications of Functionalized DNA Nanostructures in Bioimaging and Cancer Therapy. Biomaterials 2020, 268, 120560. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Franco, E. RNA nanotechnology in synthetic biology. Curr. Opin. Biotechnol. 2020, 63, 135–141. [Google Scholar] [CrossRef]
- Green, L.N.; Subramanian, H.K.K.; Mardanlou, V.; Kim, J.; Hariadi, R.F.; Franco, E. Autonomous dynamic control of DNA nanostructure self-assembly. Nat. Chem. 2019, 11, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Afonin, K.A.; Viard, M.; Koyfman, A.Y.; Martins, A.N.; Kasprzak, W.K.; Panigaj, M.; Desai, R.; Santhanam, A.; Grabow, W.W.; Jaeger, L.; et al. Multifunctional RNA Nanoparticles. Nano Lett. 2014, 14, 5662–5671. [Google Scholar] [CrossRef] [PubMed]
- Khisamutdinov, E.F.; Li, H.; Jasinski, D.L.; Chen, J.; Fu, J.; Guo, P. Enhancing immunomodulation on innate immunity by shape transition among RNA triangle, square and pentagon nanovehicles. Nucleic Acids Res. 2014, 42, 9996–10004. [Google Scholar] [CrossRef] [PubMed]
- Rackley, L.; Stewart, J.M.; Salotti, J.; Krokhotin, A.; Shah, A.; Halman, J.R.; Juneja, R.; Smollett, J.; Lee, L.; Roark, K.; et al. RNA Fibers as Optimized Nanoscaffolds for siRNA Coordination and Reduced Immunological Recognition. Adv. Funct. Mater. 2018, 28, 1805959. [Google Scholar] [CrossRef] [PubMed]
- Jasinski, D.; Haque, F.; Binzel, D.W.; Guo, P. Advancement of the Emerging Field of RNA Nanotechnology. ACS Nano 2017, 11, 1142–1164. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wen, Y.; Wang, L.; Zhou, C.; Li, Q.; Xu, L.; Li, L.; Shi, J.; Li, Z.; Ren, S.; et al. PCR-Free Colorimetric DNA Hybridization Detection Using a 3D DNA Nanostructured Reporter Probe. ACS Appl. Mater. Interfaces 2017, 9, 38281–38287. [Google Scholar] [CrossRef]
- Zeng, D.; Zhang, H.; Zhu, D.; Lihua, W.; San, L.; Wang, Z.; Wang, C.; Wang, Y.; Wang, L.; Zuo, X.; et al. A novel ultrasensitive electrochemical DNA sensor based on double tetrahedral nanostructures. Biosens. Bioelectron. 2015, 71, 434–438. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, Q.; Yang, B.; Ju, H.; Lei, J. Pixel Counting of Fluorescence Spots Triggered by DNA Walkers for Ultrasensitive Quantification of Nucleic Acid. Anal. Chem. 2018, 90, 6357–6361. [Google Scholar] [CrossRef]
- Douglas, S.M.; Bachelet, I.; Church, G.M. A Logic-Gated Nanorobot for Targeted Transport of Molecular Payloads. Science 2012, 335, 831–834. [Google Scholar] [CrossRef]
- Jiang, Q.; Song, C.; Nangreave, J.; Liu, X.; Lin, L.; Qiu, D.; Wang, Z.-G.; Zou, G.; Liang, X.; Yan, H.; et al. DNA Origami as a Carrier for Circumvention of Drug Resistance. J. Am. Chem. Soc. 2012, 134, 13396–13403. [Google Scholar] [CrossRef]
- Du, Y.; Jiang, Q.; Beziere, N.; Song, L.; Zhang, Q.; Peng, D.; Chi, C.; Yang, X.; Guo, H.; Diot, G.; et al. DNA-Nanostructure-Gold-Nanorod Hybrids for Enhanced In Vivo Optoacoustic Imaging and Photothermal Therapy. Adv. Mater. 2016, 28, 10000–10007. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.M.; Keller, A. DNA Nanostructures in the Fight Against Infectious Diseases. Adv. NanoBiomed Res. 2020. [Google Scholar] [CrossRef]
- Afonin, K.A.; Dobrovolskaia, M.A.; Church, G.; Bathe, M. Opportunities, Barriers, and a Strategy for Overcoming Translational Challenges to Therapeutic Nucleic Acid Nanotechnology. ACS Nano 2020, 14, 9221–9227. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A. Nucleic Acid Nanoparticles at a Crossroads of Vaccines and Immunotherapies. Molecules 2019, 24, 4620. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A.; Bathe, M. Opportunities and challenges for the clinical translation of structured DNA assemblies as gene therapeutic delivery and vaccine vectors. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1657. [Google Scholar] [CrossRef] [PubMed]
- Chandler, M.; Johnson, M.B.; Panigaj, M.; Afonin, K.A. Innate immune responses triggered by nucleic acids inspire the design of immunomodulatory nucleic acid nanoparticles (NANPs). Curr. Opin. Biotechnol. 2020, 63, 8–15. [Google Scholar] [CrossRef]
- Veneziano, R.; Ratanalert, S.; Zhang, K.; Zhang, F.; Yan, H.; Chiu, W.; Bathe, M. Designer nanoscale DNA assemblies programmed from the top down. Science 2016, 352, 1534. [Google Scholar] [CrossRef]
- Li, S.; Jiang, Q.; Liu, S.; Zhang, Y.; Tian, Y.; Chen, L.; Wang, J.; Zou, Y.; Anderson, G.J.; Han, J.-Y.; et al. A DNA nanorobot functions as a cancer therapeutic in response to a molecular trigger in vivo. Nat. Biotechnol. 2018, 36, 258–264. [Google Scholar] [CrossRef]
- Hong, E.; Halman, J.R.; Shah, A.B.; Khisamutdinov, E.F.; Dobrovolskaia, M.A.; Afonin, K.A. Structure and Composition Define Immunorecognition of Nucleic Acid Nanoparticles. Nano Lett. 2018, 18, 4309–4321. [Google Scholar] [CrossRef]
- Halman, J.R.; Satterwhite, E.; Roark, B.; Chandler, M.; Viard, M.; Ivanina, A.; Bindewald, E.; Kasprzak, W.K.; Panigaj, M.; Bui, M.N.; et al. Functionally-interdependent shape-switching nanoparticles with controllable properties. Nucleic Acids Res. 2017, 45, 2210–2220. [Google Scholar] [CrossRef]
- Hong, E.; Halman, J.R.; Shah, A.; Cedrone, E.; Truong, N.; Afonin, K.A.; Dobrovolskaia, M.A. Toll-Like Receptor-Mediated Recognition of Nucleic Acid Nanoparticles (NANPs) in Human Primary Blood Cells. Molecules 2019, 24, 1094. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Hou, W.; Cao, X.; Wen, S.; Shen, M.; Shi, X. Dendrimer-entrapped gold nanoparticles modified with folic acid for targeted gene delivery applications. Biomater. Sci. 2013, 1, 1172–1180. [Google Scholar] [CrossRef] [PubMed]
- Krämer, M.; Stumbé, J.F.; Grimm, G.; Kaufmann, B.; Krüger, U.; Weber, M.; Haag, R. Dendritic polyamines: Simple ac-cess to new materials with defined treelike structures for application in nonviral gene delivery. ChemBioChem 2004, 5, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Zarebkohan, A.; Najafi, F.; Moghimi, H.R.; Hemmati, M.; Deevband, M.R.; Kazemi, B. Synthesis and characterization of a PAMAM dendrimer nanocarrier functionalized by SRL peptide for targeted gene delivery to the brain. Eur. J. Pharm. Sci. 2015, 78, 19–30. [Google Scholar] [CrossRef]
- Abedi-Gaballu, F.; Dehghan, G.; Ghaffari, M.; Yekta, R.; Abbaspour-Ravasjani, S.; Baradaran, B.; Dolatabadi, J.E.N.; Hamblin, M.R. PAMAM dendrimers as efficient drug and gene delivery nanosystems for cancer therapy. Appl. Mater. Today 2018, 12, 177–190. [Google Scholar] [CrossRef]
- Wang, H.; Shi, H.-B.; Yin, S.-K. Polyamidoamine dendrimers as gene delivery carriers in the inner ear: How to improve transfection efficiency. Exp. Ther. Med. 2011, 2, 777–781. [Google Scholar] [CrossRef]
- Guillot-Nieckowski, M.; Joester, D.; Stöhr, M.; Losson, M.; Adrian, M.; Wagner, B.; Kansy, M.; Heinzelmann, H.; Pugin, R.; Diederich, F.; et al. Self-assembly, DNA Complexation, and pH Response of Amphiphilic Dendrimers for Gene Transfection. Langmuir 2007, 23, 737–746. [Google Scholar] [CrossRef][Green Version]
- Janiszewska, J.; Posadas, I.; Játiva, P.; Bugaj-Zarebska, M.; Urbanczyk-Lipkowska, Z.; Ceña, V. Second generation am-phiphilic poly-lysine dendrons inhibit glioblastoma cell proliferation without toxicity for neurons or astrocytes. PLoS ONE 2016, 11, e0165704. [Google Scholar] [CrossRef]
- Hou, W.; Wei, P.; Kong, L.; Guo, R.; Wang, S.; Shi, X. Partially PEGylated dendrimer-entrapped gold nanoparticles: A promising nanoplatform for highly efficient DNA and siRNA delivery. J. Mater. Chem. B 2016, 4, 2933–2943. [Google Scholar] [CrossRef]
- Perez, A.P.; Cosaka, M.L.; Romero, E.L.; Morilla, M.J. Uptake and intracellular traffic of siRNA dendriplexes in glioblas-toma cells and macrophages. Int. J. Nanomed. 2011, 6, 2715. [Google Scholar]
- Ofek, P.; Fischer, W.; Calderón, M.; Haag, R.; Satchi-Fainaro, R. In vivo delivery of small interfering RNA to tumors and their vasculature by novel dendritic nanocarriers. FASEB J. 2010, 24, 3122–3134. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Quan, X.; Li, L.; Zhou, J. Computer Simulation of DNA Condensation by PAMAM Dendrimer. Macromol. Theory Simul. 2018, 27, 1700070. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; Patri, A.K.; Potter, T.M.; Rodriguez, J.; Hall, J.B.; McNeil, S.E. Dendrimer-induced leukocyte procoagulant activity depends on particle size and surface charge. Nanomedicine 2012, 7, 245–256. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; Patri, A.K.; Simak, J.; Hall, J.B.; Semberova, J.; Lacerda, S.H.D.P.; McNeil, S.E. Nanoparticle Size and Surface Charge Determine Effects of PAMAM Dendrimers on Human Platelets in Vitro. Mol. Pharm. 2011, 9, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Ilinskaya, A.N.; Man, S.; Patri, A.K.; Clogston, J.D.; Crist, R.M.; Cachau, R.E.; McNeil, S.E.; Dobrovolskaia, M.A. Inhibition of phosphoinositol 3 kinase contributes to nanoparticle-mediated exaggeration of endotoxin-induced leukocyte procoagulant activity. Nanomedicine 2014, 9, 1311–1326. [Google Scholar] [CrossRef]
- Hong, S.; Leroueil, P.R.; Janus, E.K.; Peters, J.L.; Kober, M.-M.; Islam, M.T.; Orr, B.G.; Baker, J.J.R.; Holl, M.M.B. Interaction of Polycationic Polymers with Supported Lipid Bilayers and Cells: Nanoscale Hole Formation and Enhanced Membrane Permeability. Bioconjugate Chem. 2006, 17, 728–734. [Google Scholar] [CrossRef]
- Leroueil, P.R.; Berry, S.A.; Duthie, K.; Han, G.; Rotello, V.; McNerny, D.Q.; Baker, J.J.R.; Orr, B.G.; Holl, M.M.B. Wide Varieties of Cationic Nanoparticles Induce Defects in Supported Lipid Bilayers. Nano Lett. 2008, 8, 420–424. [Google Scholar] [CrossRef]
- Qin, L.; Cao, D.; Huang, H.; Ji, G.; Feng, M.; Chen, J.; Pan, S. Improvement of Cellular Uptake and Transfection Ability of pDNA Using α-Cyclodextrin-Polyamidoamine Conjugates as Gene Delivery System. J. Biomed. Nanotechnol. 2016, 12, 261–273. [Google Scholar] [CrossRef]
- Thomas, T.P.; Majoros, I.; Kotlyar, A.; Mullen, D.; Holl, M.M.B.; Baker, J.R., Jr. Cationic Poly(amidoamine) Dendrimer Induces Lysosomal Apoptotic Pathway at Therapeutically Relevant Concentrations. Biomacromolecules 2009, 10, 3207–3214. [Google Scholar] [CrossRef]
- Afonin, K.A.; Viard, M.; Kagiampakis, I.; Case, C.L.; Dobrovolskaia, M.A.; Hofmann, J.; Vrzak, A.; Kireeva, M.; Kasprzak, W.K.; KewalRamani, V.N.; et al. Triggering of RNA Interference with RNA–RNA, RNA–DNA, and DNA–RNA Nanoparticles. ACS Nano 2015, 9, 251–259. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; Shurin, M.R.; Shvedova, A.A. Current understanding of interactions between nanoparticles and the immune system. Toxicol. Appl. Pharmacol. 2016, 299, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A.; McNeil, S.E. Understanding the correlation between in vitro and in vivo immunotoxicity tests for nanomedicines. J. Control. Release 2013, 172, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Ribeiro, A.M.; Pérez-Betancourt, Y. Cationic Nanostructures for Vaccines Design. Biomimetics 2020, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Zhang, Z.; Mo, Y.; Tong, R.; Zhong, Z.; Chen, Z.; He, D.; Wan, R.; Gao, M.; Mo, Y.; et al. Activation of NLRP3 inflammasome in hepatocytes after exposure to cobalt nanoparticles: The role of oxidative stress. Toxicol. Vitr. 2020, 69, 104967. [Google Scholar] [CrossRef]
- Liu, X.; Lu, B.; Fu, J.; Zhu, X.; Song, E.; Song, Y. Amorphous Silica Nanoparticles Induce Inflammation via Activation of NLRP3 Inflammasome and HMGB1/TLR4/MyD88/NF-kB Signaling Pathway in HUVEC cells. J. Hazard. Mater. 2021, 404, 124050. [Google Scholar] [CrossRef]
- Ilinskaya, A.N.; Clogston, J.D.; McNeil, S.E.; Dobrovolskaia, M.A. Induction of oxidative stress by Taxol® vehicle Cremophor-EL triggers production of interleukin-8 by peripheral blood mononuclear cells through the mechanism not requiring de novo synthesis of mRNA. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1925–1938. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, C.; Finco, D.; Fort, M.M.; Gliddon, D.; Harper, K.; Helms, W.S.; Mitchell, J.A.; O’Lone, R.; Parish, S.T.; Piche, M.-S.; et al. Cytokine release: A workshop proceedings on the state-of-the-science, current challenges and future directions. Cytokine 2016, 85, 101–108. [Google Scholar] [CrossRef]
- Hofmann, J.N.; Yu, K.; Bagni, R.K.; Lan, Q.; Rothman, N.; Purdue, M.P. Intra-individual variability over time in serum cytokine levels among participants in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Cytokine 2011, 56, 145–148. [Google Scholar] [CrossRef]
- Ma, M.; Percopo, C.M.; Sturdevant, D.E.; Sek, A.C.; Komarow, H.D.; Rosenberg, H.F. Cytokine Diversity in Human Peripheral Blood Eosinophils: Profound Variability of IL-16. J. Immunol. 2019, 203, 520–531. [Google Scholar] [CrossRef]
- Mueller, S.C.; Marz, R.W.; Schmolz, M.; Drewelow, B. Intraindividual long term stability and response corridors of cytokines in healthy volunteers detected by a standardized whole-blood culture system for bed-side application. BMC Med Res. Methodol. 2012, 12, 112. [Google Scholar] [CrossRef]
- Sahdo, B.; Fransén, K.; Idosa, B.A.; Eriksson, P.; Söderquist, B.; Kelly, A.; Särndahl, E. Cytokine Profile in a Cohort of Healthy Blood Donors Carrying Polymorphisms in Genes Encoding the NLRP3 Inflammasome. PLoS ONE 2013, 8, e75457. [Google Scholar] [CrossRef]
- A Nicola, N. Cytokine pleiotropy and redundancy: A view from the receptor. Stem Cells 1994, 12, 3. [Google Scholar] [PubMed]
- Ozaki, K.; Leonard, W.J. Cytokine and Cytokine Receptor Pleiotropy and Redundancy. J. Biol. Chem. 2002, 277, 29355–29358. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.L.; Han, Y.; Wei, T.; Aras, S.; Chaturvedi, P.; Tyler, S.; Rani, M.R.S.; Ransohoff, R.M. Regulation of monocyte chemoattractant protein (MCP)-1 transcription by interferon-gamma (IFN-γ) in human astrocytoma cells: Postinduction refractory state of the gene, governed by its upstream elements. FASEB J. 2001, 15, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Padgett, L.E.; Araujo, D.J.; Hedrick, C.C.; Olingy, C.E. Functional crosstalk between T cells and monocytes in cancer and atherosclerosis. J. Leukoc. Biol. 2020, 108, 297–308. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; Afonin, K.A. Use of human peripheral blood mononuclear cells to define immunological properties of nucleic acid nanoparticles. Nat. Protoc. 2020, 15, 3678–3698. [Google Scholar] [CrossRef]
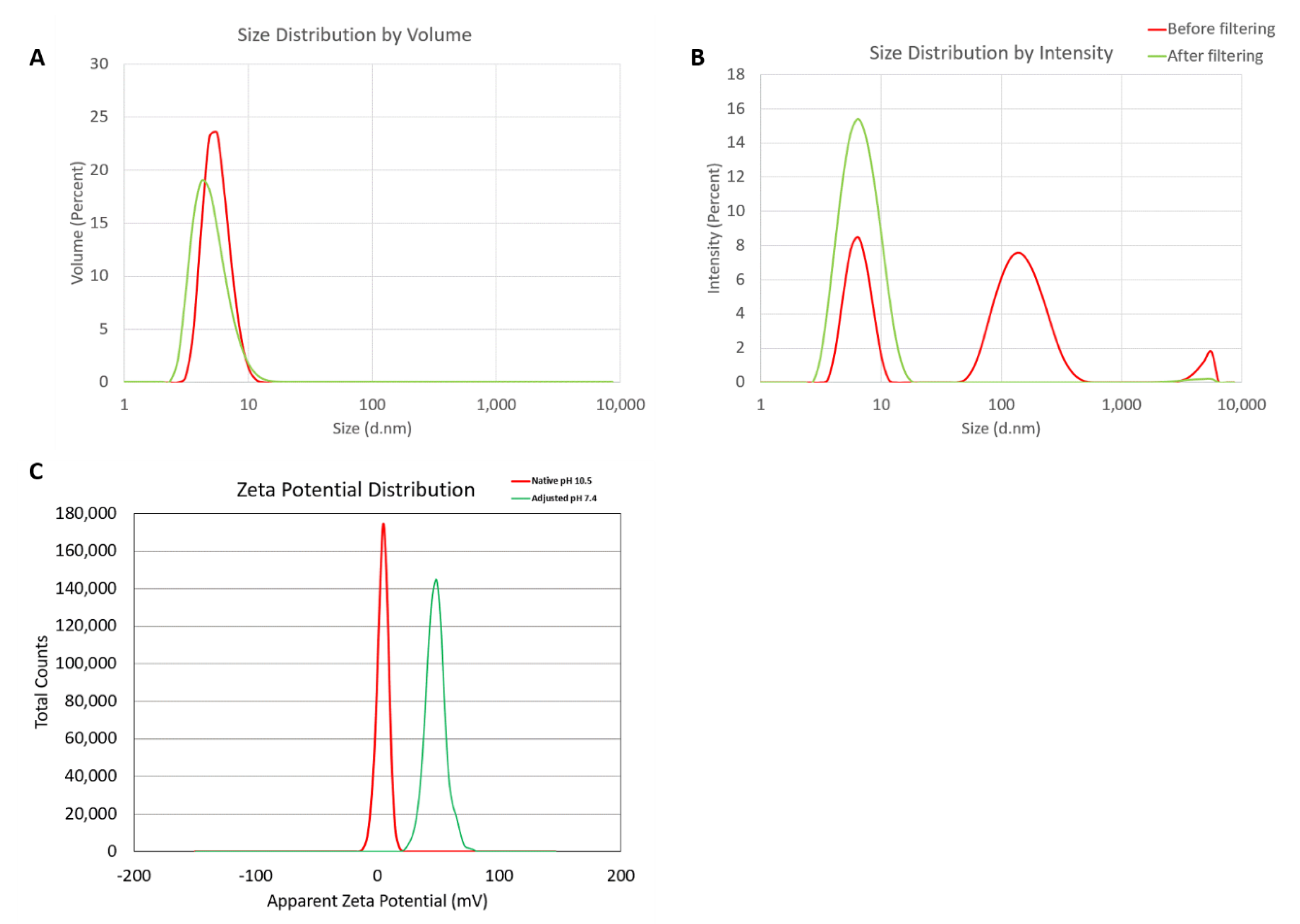
| Sample | Z-Avg, nm | PdI | Int-Peak, nm | %Int | Vol-Peak, nm | %Vol |
|---|---|---|---|---|---|---|
| Before filtering | 30.4 ± 11.0 | 0.759 ± 0.188 | 156.5 ± 11.4 | 59.7 ± 2.0 | 5.7 ± 0.1 | 100 ± 0 |
| After filtering | 6.3 ± 0.1 | 0.143 ± 0.022 | 7.0 ± 0.1 | 99.1 ± 1.1 | 5.1 ± 0.1 | 100 ± 0 |
| Sample | pH | ZP, mV |
|---|---|---|
| G5-NH2 | 10.5 (native) | +4.6 ± 0.9 |
| G5-NH2 | 7.4 | +48.2 ± 3.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avila, Y.I.; Chandler, M.; Cedrone, E.; Newton, H.S.; Richardson, M.; Xu, J.; Clogston, J.D.; Liptrott, N.J.; Afonin, K.A.; Dobrovolskaia, M.A. Induction of Cytokines by Nucleic Acid Nanoparticles (NANPs) Depends on the Type of Delivery Carrier. Molecules 2021, 26, 652. https://doi.org/10.3390/molecules26030652
Avila YI, Chandler M, Cedrone E, Newton HS, Richardson M, Xu J, Clogston JD, Liptrott NJ, Afonin KA, Dobrovolskaia MA. Induction of Cytokines by Nucleic Acid Nanoparticles (NANPs) Depends on the Type of Delivery Carrier. Molecules. 2021; 26(3):652. https://doi.org/10.3390/molecules26030652
Chicago/Turabian StyleAvila, Yelixza I., Morgan Chandler, Edward Cedrone, Hannah S. Newton, Melina Richardson, Jie Xu, Jeffrey D. Clogston, Neill J. Liptrott, Kirill A. Afonin, and Marina A. Dobrovolskaia. 2021. "Induction of Cytokines by Nucleic Acid Nanoparticles (NANPs) Depends on the Type of Delivery Carrier" Molecules 26, no. 3: 652. https://doi.org/10.3390/molecules26030652
APA StyleAvila, Y. I., Chandler, M., Cedrone, E., Newton, H. S., Richardson, M., Xu, J., Clogston, J. D., Liptrott, N. J., Afonin, K. A., & Dobrovolskaia, M. A. (2021). Induction of Cytokines by Nucleic Acid Nanoparticles (NANPs) Depends on the Type of Delivery Carrier. Molecules, 26(3), 652. https://doi.org/10.3390/molecules26030652









