Divergent and Overlapping Roles for Selected Phytochemicals in the Regulation of Pathological Cardiac Hypertrophy
Abstract
1. Introduction
2. Results
2.1. Six Phytochemicals Attenuated Pathological Cardiac Hypertrophy
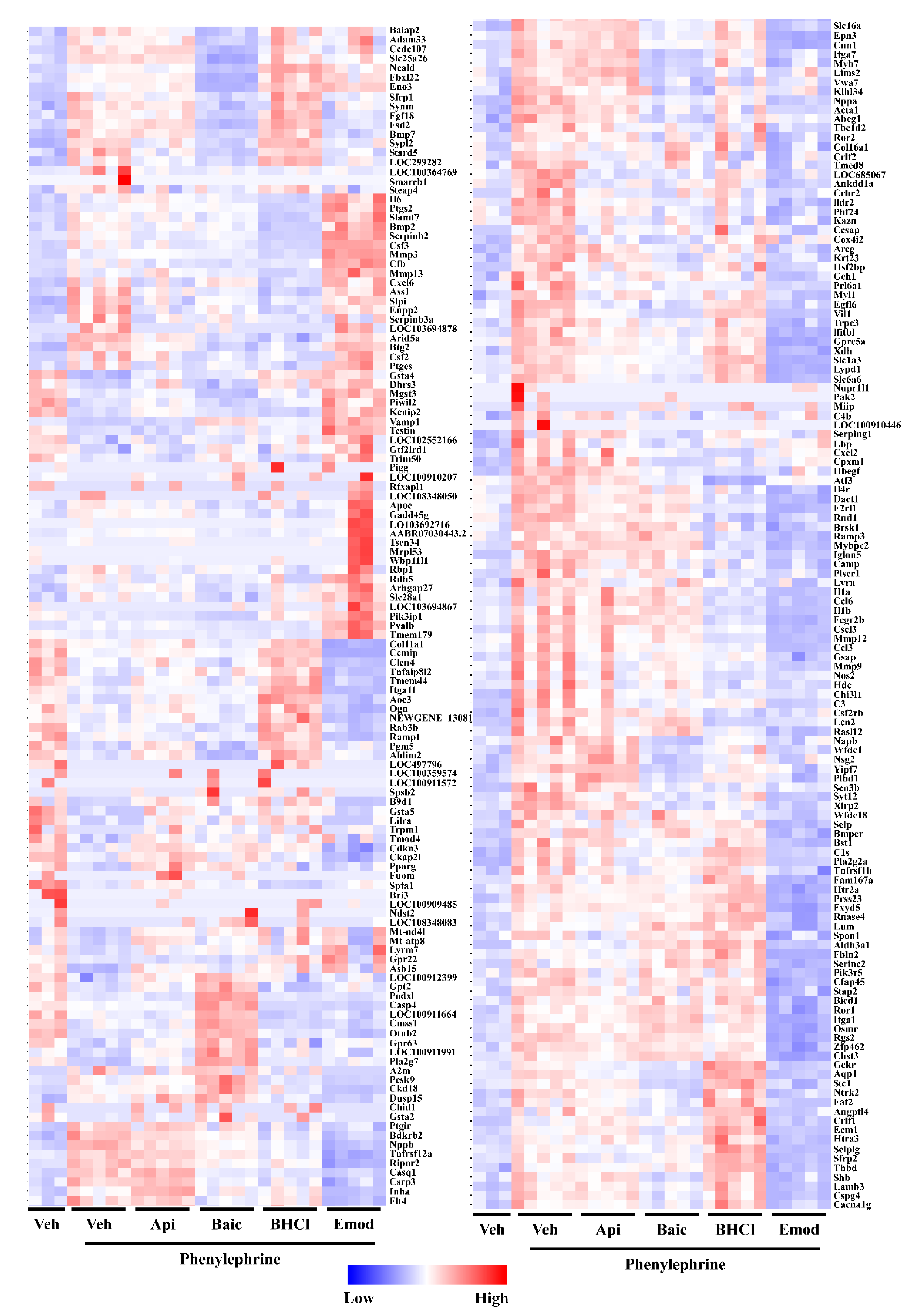
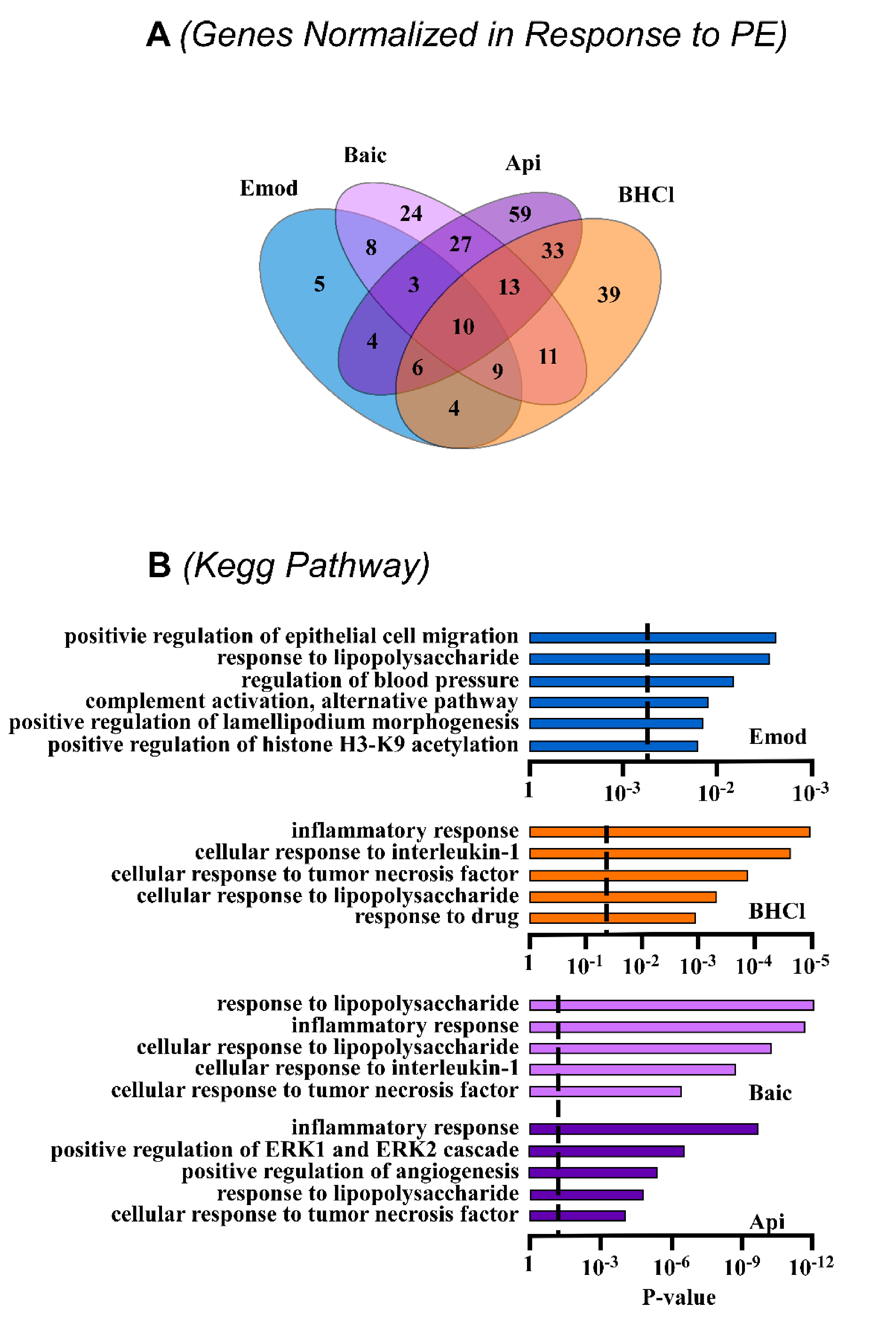
2.2. Phytochemicals Regulate Overlapping and Divergent Changes in Gene Expression
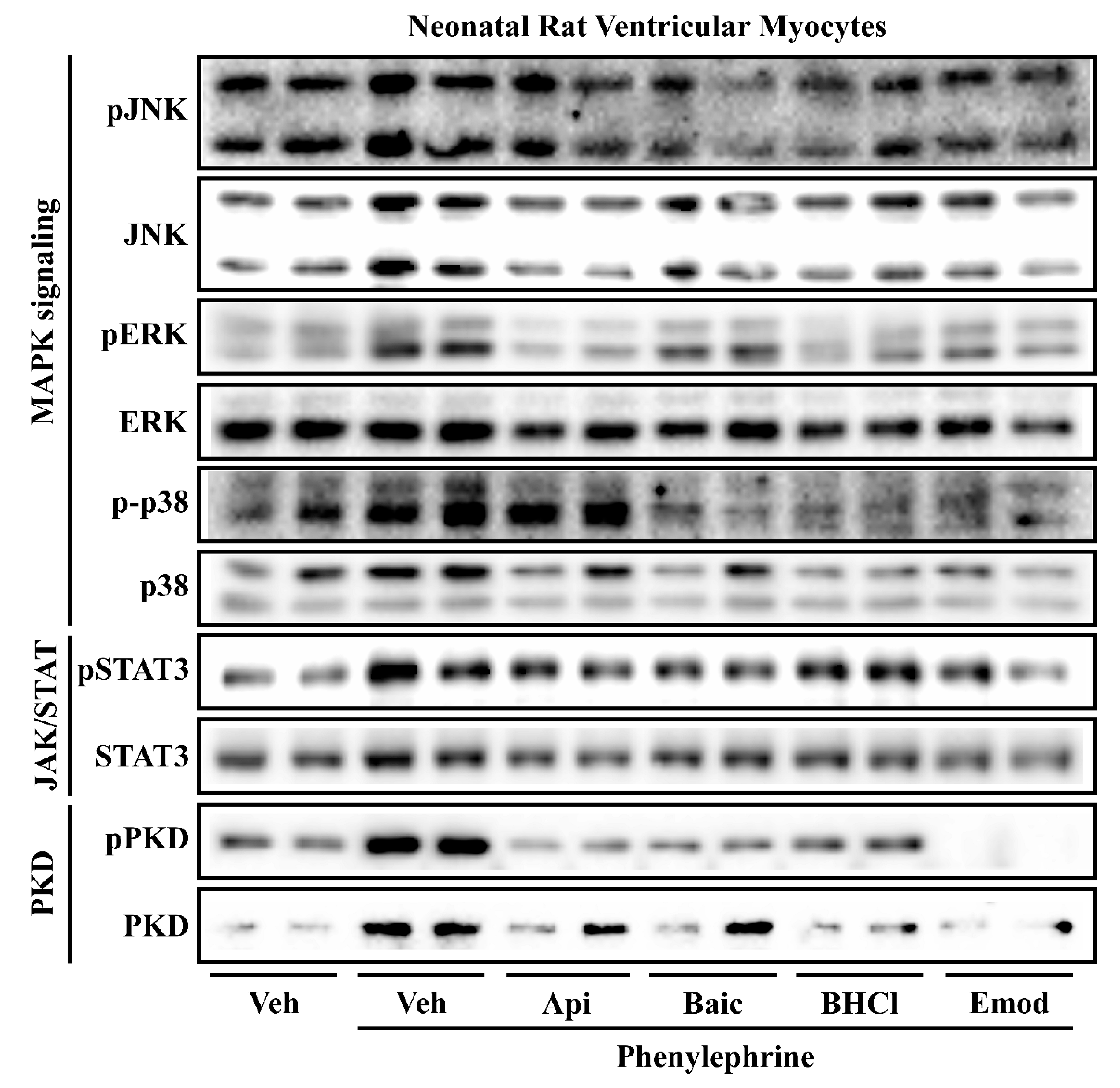
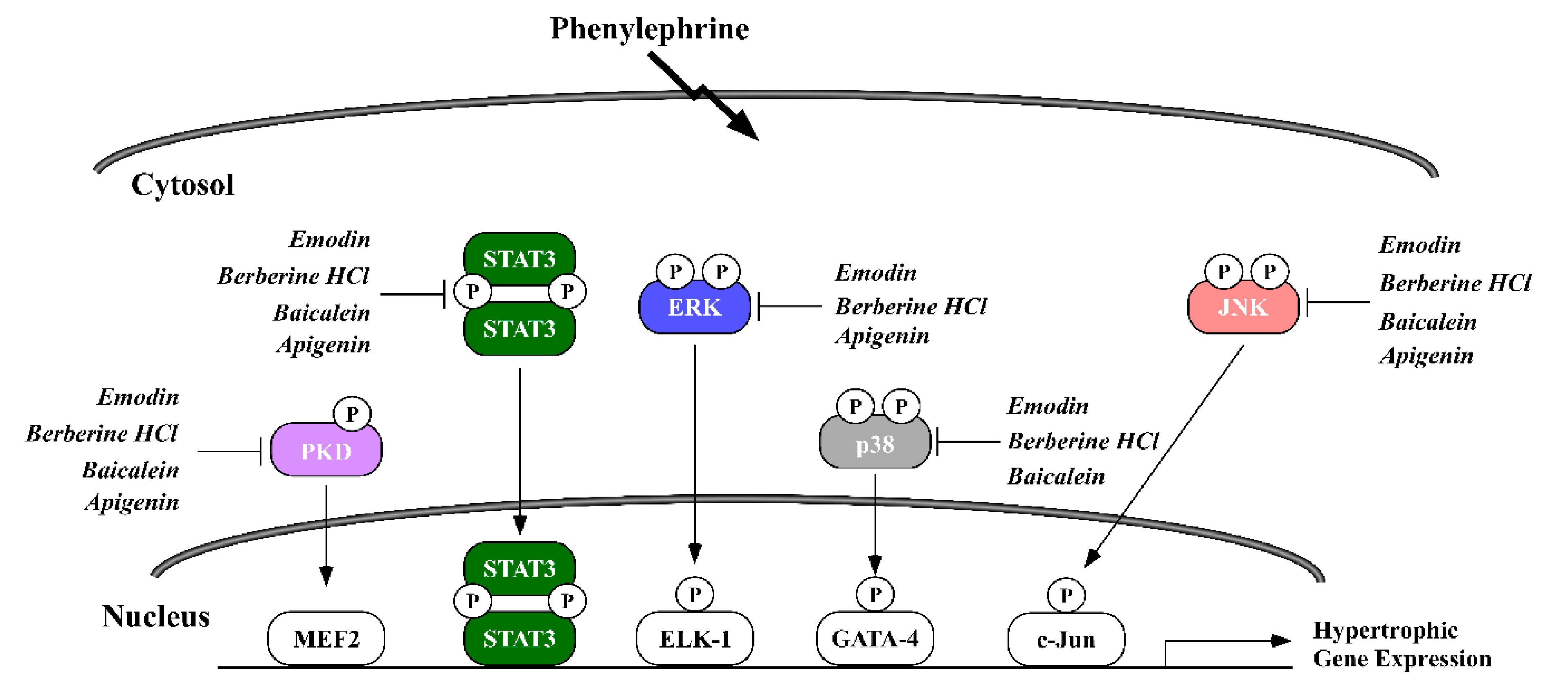
2.3. Phytochemicals Regulate Overlapping and Divergent Changes in Signal Transduction
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Neonatal Rat Ventricular Myocyte (NRVM) Culture
4.3. Immunohistochemistry
4.4. Cell Viability
4.5. Immunoblotting
4.6. RNA Sequencing
4.7. Real-Time qPCR
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, D.; Wu, J.; Jing, Z.; Duan, X.; Cui, Y.; Liu, S. Efficacy and safety of ginkgo injections in the treatment of angina pectoris caused by coronary heart disease in China: A network Meta-analysis and systematic review. J. Tradit. Chin. Med. 2019, 39, 285–296. [Google Scholar]
- García-Conesa, M.T.; Chambers, K.; Combet, E.; Pinto, P.; Garcia-Aloy, M.; Andrés-Lacueva, C.; Pascual-Teresa, S.D.; Mena, P.; Ristic, A.K.; Hollands, W.J.; et al. Meta-analysis of the effects of foods and derived products containing ellagitannins and anthocyanins on cardiometabolic biomarkers: Analysis of factors influencing variability of the individual responses. Int. J. Mol. Sci. 2018, 19, 694. [Google Scholar] [CrossRef]
- Viguiliouk, E.; Glenn, A.J.; Nishi, S.K.; Chiavaroli, L.; Seider, M.; Khan, T.; Bonaccio, M.; Iacoviello, L.; Mejia, S.B.; Jenkins, D.J.A.; et al. Associations between Dietary Pulses Alone or with Other Legumes and Cardiometabolic Disease Outcomes: An Umbrella Review and Updated Systematic Review and Meta-analysis of Prospective Cohort Studies. Adv. Nutr. 2019, 10, S308–S319. [Google Scholar] [CrossRef] [PubMed]
- Kwok, C.S.; Gulati, M.; Michos, E.D.; Potts, J.; Wu, P.; Watson, L.; Loke, Y.K.; Mallen, C.; Mamas, M.A. Dietary components and risk of cardiovascular disease and all-cause mortality: A review of evidence from meta-analyses. Eur. J. Prev. Cardiol. 2019, 26, 1415–1429. [Google Scholar] [CrossRef]
- Al-Shafei, A.I.M.; El-Gendy, O.A.A. Regular consumption of green tea improves pulse pressure and induces regression of left ventricular hypertrophy in hypertensive patients. Physiol. Rep. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Pop, R.M.; Popolo, A.; Trifa, A.P.; Stanciu, L.A. Phytochemicals in cardiovascular and respiratory diseases: Evidence in oxidative stress and inflammation. Oxid. Med. Cell. Longev. 2018, 1603872. [Google Scholar] [CrossRef]
- Evans, L.; Ferguson, B. Food Bioactive HDAC Inhibitors in the Epigenetic Regulation of Heart Failure. Nutrients 2018, 10, 1120. [Google Scholar] [CrossRef]
- Nienaber, J.J.; Tachibana, H.; Naga Prasad, S.V.; Esposito, G.; Wu, D.; Mao, L.; Rockman, H.A. Inhibition of receptor-localized PI3K preserves cardiac β-adrenergic receptor function and ameliorates pressure overload heart failure. J. Clin. Invest. 2003. [Google Scholar] [CrossRef]
- Devereux, R.B.; Wachtell, K.; Gerdts, E.; Boman, K.; Nieminen, M.S.; Papademetriou, V.; Rokkedal, J.; Harris, K.; Aurup, P.; Dahlöf, B. Prognostic significance of left ventricular mass change during treatment of hypertension. J. Am. Med. Assoc. 2004. [Google Scholar] [CrossRef] [PubMed]
- Godoy, L.D.; Lucas, J.E.; Bender, A.J.; Romanick, S.S.; Ferguson, B.S. Targeting the epigenome: Screening bioactive compounds that regulate histone deacetylase activity. Mol. Nutr. Food Res. 2016, 1600744. [Google Scholar] [CrossRef] [PubMed]
- Antos, C.L.; McKinsey, T.A.; Dreitz, M.; Hollingsworth, L.M.; Zhang, C.L.; Schreiber, K.; Rindt, H.; Gorczynski, R.J.; Olson, E.N. Dose-dependent blockade to cardiomyocyte hypertrophy by histone deacetylase inhibitors. J. Biol. Chem. 2003, 278, 28930–28937. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.A.; Ludwig, S.; Flory, E.; Gambaryan, S.; Singh, K.; Finn, P.; Pfeffer, M.A.; Kelly, R.A.; Pfeffer, J.M. Activation of cardiac c-Jun NH2-terminal kinases and p38-mitogen-activated protein kinases with abrupt changes in hemodynamic load. Hypertension 2001, 37, 1222–1228. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Cheng, G.; Jin, R.; Afzal, M.R.; Samanta, A.; Xuan, Y.T.; Girgis, M.; Elias, H.K.; Zhu, Y.; Davani, A.; et al. Deletion of Interleukin-6 Attenuates Pressure Overload-Induced Left Ventricular Hypertrophy and Dysfunction. Circ. Res. 2016. [Google Scholar] [CrossRef]
- Monovich, L.; Vega, R.B.; Meredith, E.; Miranda, K.; Rao, C.; Capparelli, M.; Lemon, D.D.; Phan, D.; Koch, K.A.; Chapo, J.A.; et al. A novel kinase inhibitor establishes a predominant role for protein kinase D as a cardiac class IIa histone deacetylase kinase. FEBS Lett. 2010. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.W.; Bender, A.; Burnett, L.; Godoy, L.; Shen, Y.; Staten, D.; Zhou, T.; Angermann, J.E.; Ferguson, B.S. Emodin and emodin-rich rhubarb inhibits histone deacetylase (HDAC) activity and cardiac myocyte hypertrophy. J. Nutr. Biochem. 2020, 108339. [Google Scholar] [CrossRef]
- Chen, H.M.; Hsu, J.H.; Liou, S.F.; Chen, T.J.; Chen, L.Y.; Chiu, C.C.; Yeh, J.L. Baicalein, an active component of Scutellaria baicalensis Georgi, prevents lysophosphatidylcholine-induced cardiac injury by reducing reactive oxygen species production, calcium overload and apoptosis via MAPK pathways. BMC Complement. Altern. Med. 2014, 14, 233. [Google Scholar] [CrossRef]
- Ye, B.; Chen, X.; Dai, S.; Han, J.; Liang, X.; Lin, S.; Cai, X.; Huang, Z.; Huang, W. Emodin alleviates myocardial ischemia/reperfusion injury by inhibiting gasdermin D-mediated pyroptosis in cardiomyocytes. Drug Des. Devel. Ther. 2019, Volume 13, 975–990. [Google Scholar] [CrossRef]
- Zhang, Y.; Liao, P.; Zhu, M.; Li, W.; Hu, D.; Guan, S.; Chen, L. Baicalin Attenuates Cardiac Dysfunction and Myocardial Remodeling in a Chronic Pressure-Overload Mice Model. Cell. Physiol. Biochem. 2017, 41, 849–864. [Google Scholar] [CrossRef]
- Wu, J.; Nakashima, S.; Nakamura, S.; Matsuda, H. Effects of Sanoshashinto on left ventricular hypertrophy and gut microbiota in spontaneously hypertensive rats. J. Nat. Med. 2020, 74, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Zong, J.; Zhang, D.P.; Zhou, H.; Bian, Z.Y.; Deng, W.; Dai, J.; Yuan, Y.; Gan, H.W.; Guo, H.P.; Tang, Q.Z. Baicalein protects against cardiac hypertrophy through blocking MEK-ERK1/2 signaling. J. Cell. Biochem. 2013, 114, 1058–1065. [Google Scholar] [CrossRef]
- Zhao, F.; Fu, L.; Yang, W.; Dong, Y.; Yang, J.; Sun, S.; Hou, Y. Cardioprotective effects of baicalein on heart failure via modulation of Ca2 + handling proteins in vivo and in vitro. Life Sci. 2016, 145, 213–223. [Google Scholar] [CrossRef]
- Wang, A.W.; Song, L.; Miao, J.; Wang, H.X.; Tian, C.; Jiang, X.; Han, Q.Y.; Yu, L.; Liu, Y.; Du, J.; et al. Baicalein attenuates angiotensin II-induced cardiac remodeling via inhibition of AKT/mTOR, ERK1/2, NF-κB, and calcineurin signaling pathways in mice. Am. J. Hypertens. 2015, 28, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.M.; Liou, S.F.; Hsu, J.H.; Chen, T.J.; Cheng, T.L.; Chiu, C.C.; Yeh, J.L. Baicalein inhibits HMGB1 release and MMP-2/-9 expression in lipopolysaccharide-induced cardiac hypertrophy. Am. J. Chin. Med. 2014, 42, 785–797. [Google Scholar] [CrossRef]
- Xiao, J. Dietary flavonoid aglycones and their glycosides: Which show better biological significance? Crit. Rev. Food Sci. Nutr. 2017, 57, 1874–1905. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhang, J.D.; Wang, B.; Lv, Y.J.; Jiang, H.; Liu, G.L.; Qiao, Y.; Ren, M.; Guo, X.F. Quercetin Inhibits Left Ventricular Hypertrophy in Spontaneously Hypertensive Rats and Inhibits Angiotensin II-Induced H9C2 Cells Hypertrophy by Enhancing PPAR-γ Expression and Suppressing AP-1 Activity. PLoS ONE 2013, 8, e72548. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Cao, J.; Zhang, G.; Wang, Y. Kaempferol Attenuates Cardiac Hypertrophy via Regulation of ASK1/MAPK Signaling Pathway and Oxidative Stress. Planta Med. 2017, 83, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, J.; Samojedny, A.; Paul, M.; Pietsz, G.; Wilczok, T. Effect of apigenin, kaempferol and resveratrol on the expression of interleukin-1β and tumor necrosis factor-α genes in J774.2 macrophages. Pharmacol. Rep. 2005, 57, 390–394. [Google Scholar] [PubMed]
- Palacz-Wrobel, M.; Borkowska, P.; Paul-Samojedny, M.; Kowalczyk, M.; Fila-Danilow, A.; Suchanek-Raif, R.; Kowalski, J. Effect of apigenin, kaempferol and resveratrol on the gene expression and protein secretion of tumor necrosis factor alpha (TNF-α) and interleukin-10 (IL-10) in RAW-264.7 macrophages. Biomed. Pharmacother. 2017. [Google Scholar] [CrossRef] [PubMed]
- Caltagirone, S.; Rossi, C.; Poggi, A.; Ranelletti, F.O.; Natali, P.G.; Brunetti, M.; Aiello, F.B.; Piantelli, M. Flavonoids apigenin and quercetin inhibit melanoma growth and metastatic potential. Int. J. Cancer 2000. [Google Scholar] [CrossRef]
- Janssen, K.; Mensink, R.P.; Cox, F.J.J.; Harryvan, J.L.; Hovenier, R.; Hollman, P.C.; Katan, M.B. Effects of the flavonoids quercetin and apigenin on hemostasis in healthy volunteers: Results from an in vitro and a dietary supplement study. Am. J. Clin. Nutr. 1998. [Google Scholar] [CrossRef]
- Cos, P.; Ying, L.; Calomme, M.; Hu, J.P.; Cimanga, K.; Van Poel, B.; Pieters, L.; Vlietinck, A.J.; Vanden Berghe, D. Structure-activity relationship and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers. J. Nat. Prod. 1998. [Google Scholar] [CrossRef] [PubMed]
- Mahfoudi, R.; Djeridane, A.; Benarous, K.; Gaydou, E.M.; Yousfi, M. Structure-activity relationships and molecular docking of thirteen synthesized flavonoids as horseradish peroxidase inhibitors. Bioorg. Chem. 2017, 74, 201–211. [Google Scholar] [CrossRef]
- Wright, B.; Watson, K.A.; McGuffin, L.J.; Lovegrove, J.A.; Gibbins, J.M. GRID and docking analyses reveal a molecular basis for flavonoid inhibition of Src family kinase activity. J. Nutr. Biochem. 2015. [Google Scholar] [CrossRef]
- Mistry, B.; Patel, R.V.; Keum, Y.S.; Kim, D.H. Evaluation of the biological potencies of newly synthesized berberine derivatives bearing benzothiazole moieties with substituted functionalities. J. Saudi Chem. Soc. 2017, 21, 210–219. [Google Scholar] [CrossRef]
- Shimmyo, Y.; Kihara, T.; Akaike, A.; Niidome, T.; Sugimoto, H. Flavonols and flavones as BACE-1 inhibitors: Structure-activity relationship in cell-free, cell-based and in silico studies reveal novel pharmacophore features. Biochim. Biophys. Acta Gen. Subj. 2008. [Google Scholar] [CrossRef]
- Scafuri, B.; Bontempo, P.; Altucci, L.; De Masi, L.; Facchiano, A. Molecular docking simulations on histone deacetylases (Hdac)-1 and-2 to investigate the flavone binding. Biomedicines 2020, 8, 568. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, K.; Wang, Y.; Guo, R.; Liu, H.; Jia, C.; Sun, X.; Wu, C.; Wang, W.; Du, J.; et al. A machine learning-driven study indicates emodin improves cardiac hypertrophy by modulation of mitochondrial SIRT3 signaling. Pharmacol. Res. 2020, 155, 104739. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Wang, J.; Pang, Y.; Dang, X.; Ren, H.; Liu, Y.; Chen, M.; Shang, D. Emodin suppresses silica-induced lung fibrosis by promoting Sirt1 signaling via direct contact. Mol. Med. Rep. 2016, 14, 4643–4649. [Google Scholar] [CrossRef]
- Ruppert, C.; Deiss, K.; Herrmann, S.; Vidal, M.; Oezkur, M.; Gorski, A.; Weidemann, F.; Lohse, M.J.; Lorenz, K. Interference with ERKThr188 phosphorylation impairs pathological but not physiological cardiac hypertrophy. Proc. Natl. Acad. Sci. USA 2013, 110, 7440–7445. [Google Scholar] [CrossRef] [PubMed]
- Slone, S.; Anthony, S.R.; Wu, X.; Benoit, J.B.; Aube, J.; Xu, L.; Tranter, M. Activation of HuR downstream of p38 MAPK promotes cardiomyocyte hypertrophy. Cell. Signal. 2016. [Google Scholar] [CrossRef] [PubMed]
- Zechner, D.; Thuerauf, D.J.; Hanford, D.S.; McDonough, P.M.; Glembotski, C.C. A role for the p38 mitogen-activated protein kinase pathway in myocardial cell growth, sarcomeric organization, and cardiac-specific gene expression. J. Cell Biol. 1997. [Google Scholar] [CrossRef]
- Wang, Y.; Su, B.; Sah, V.P.; Brown, J.H.; Han, J.; Chien, K.R. Cardiac hypertrophy induced by Mitogen-activated Protein Kinase Kinase 7, a specific activator for c-Jun NH2-terminal kinase in ventricular muscle cells. J. Biol. Chem. 1998. [Google Scholar] [CrossRef]
- Liu, W.; Zi, M.; Jin, J.; Prehar, S.; Oceandy, D.; Kimura, T.E.; Lei, M.; Neyses, L.; Weston, A.H.; Cartwright, E.J.; et al. Cardiac-specific deletion of Mkk4 reveals its role in pathological hypertrophic remodeling but not in physiological cardiac growth. Circ. Res. 2009. [Google Scholar] [CrossRef]
- Dorn, G.W.; Force, T. Protein kinase cascades in the regulation of cardiac hypertrophy. J. Clin. Invest. 2005, 115, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Harrison, B.C.; Kim, M.-S.; van Rooij, E.; Plato, C.F.; Papst, P.J.; Vega, R.B.; McAnally, J.A.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N.; et al. Regulation of Cardiac Stress Signaling by Protein Kinase D1. Mol. Cell. Biol. 2006. [Google Scholar] [CrossRef]
- Golia, E.; Limongelli, G.; Natale, F.; Fimiani, F.; Maddaloni, V.; Pariggiano, I.; Bianchi, R.; Crisci, M.; D’Acierno, L.; Giordano, R.; et al. Inflammation and cardiovascular disease: From pathogenesis to therapeutic target. Curr. Atheroscler. Rep. 2014, 16, 435. [Google Scholar] [CrossRef] [PubMed]
- Ross, R. Atherosclerosis—An Inflammatory Disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef]
- Purcell, N.H.; Tang, G.; Yu, C.; Mercurio, F.; DiDonato, J.A.; Lin, A. Activation of NF-κB is required for hypertrophic growth of primary rat neonatal ventricular cardiomyocytes. Proc. Natl. Acad. Sci. USA 2001. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Wang, G.; Zheng, N.; Cheng, W.; Ouyang, K.; Lin, H.; Liao, Y.; Liu, J. HIMF (Hypoxia-Induced Mitogenic Factor)-IL (Interleukin)-6 signaling mediates cardiomyocyte-fibroblast crosstalk to promote cardiac hypertrophy and fibrosis. Hypertension 2019, 73, 1058–1070. [Google Scholar] [CrossRef]
- Lai, N.C.; Gao, M.H.; Tang, E.; Tang, R.; Guo, T.; Dalton, N.D.; Deng, A.; Tang, T. Pressure overload-induced cardiac remodeling and dysfunction in the absence of interleukin 6 in mice. Lab. Investig. 2012. [Google Scholar] [CrossRef] [PubMed]
- Shimada, B.K.; Yang, Y.; Zhu, J.; Wang, S.; Suen, A.; Kronstadt, S.M.; Jeyaram, A.; Jay, S.M.; Zou, L.; Chao, W. Extracellular miR-146a-5p Induces Cardiac Innate Immune Response and Cardiomyocyte Dysfunction. ImmunoHorizons 2020, 4, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Y.L.; Lin, Q.Y.; Liu, Y.; Guan, X.M.; Ma, X.L.; Cao, H.J.; Liu, Y.; Bai, J.; Xia, Y.L.; et al. CXCL1-CXCR2 axis mediates angiotensin II-induced cardiac hypertrophy and remodelling through regulation of monocyte infiltration. Eur. Heart J. 2018. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Yue, Q.; Du, W.; Li, Y.; Liu, F.; Yang, L.; Zhao, R.; Hu, J. C-C chemokine receptor 5 signaling contributes to cardiac remodeling and dysfunction under pressure overload. Mol. Med. Rep. 2021, 23. [Google Scholar] [CrossRef]
- Wang, M.; Firrman, J.; Liu, L.S.; Yam, K. A review on flavonoid apigenin: Dietary intake, ADME, antimicrobial effects, and interactions with human gut microbiota. Biomed Res. Int. 2019, 7010467. [Google Scholar] [CrossRef]
- Pang, H.; Xue, W.; Shi, A.; Li, M.; Li, Y.; Cao, G.; Yan, B.; Dong, F.; Xiao, W.; He, G.; et al. Multiple-Ascending-Dose Pharmacokinetics and Safety Evaluation of Baicalein Chewable Tablets in Healthy Chinese Volunteers. Clin. Drug Investig. 2016. [Google Scholar] [CrossRef]
- Li, G.; Zhao, M.; Qiu, F.; Sun, Y.; Zhao, L. Pharmacokinetic interactions and tolerability of berberine chloride with simvastatin and fenofibrate: An open-label, randomized, parallel study in healthy chinese subjects. Drug Des. Devel. Ther. 2019. [Google Scholar] [CrossRef]
- Lin, S.P.; Chu, P.M.; Tsai, S.Y.; Wu, M.H.; Hou, Y.C. Pharmacokinetics and tissue distribution of resveratrol, emodin and their metabolites after intake of Polygonum cuspidatum in rats. J. Ethnopharmacol. 2012, 144, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Hayasaka, N.; Shimizu, N.; Komoda, T.; Mohri, S.; Tsushida, T.; Eitsuka, T.; Miyazawa, T.; Nakagawa, K. Absorption and Metabolism of Luteolin in Rats and Humans in Relation to in Vitro Anti-inflammatory Effects. J. Agric. Food Chem. 2018, 66, 11320–11329. [Google Scholar] [CrossRef] [PubMed]
- Mullen, W.; Edwards, C.A.; Crozier, A. Absorption, excretion and metabolite profiling of methyl-, glucuronyl-, glucosyl- and sulpho-conjugates of quercetin in human plasma and urine after ingestion of onions. Br. J. Nutr. 2006, 96, 107. [Google Scholar] [CrossRef] [PubMed]
- Lampe, J.W.; Chang, J.L. Interindividual differences in phytochemical metabolism and disposition. Semin. Cancer Biol. 2007, 17, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Gradolatto, A.; Basly, J.P.; Berges, R.; Teyssier, C.; Chagnon, M.C.; Siess, M.H.; Canivenc-Lavier, M.C. Pharmacokinetics and metabolism of apigenin in female and male rats after a single oral administration. Drug Metab. Dispos. 2005. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Li, J.; Ren, Y.; Wang, H.; Cong, Z.; Wu, G.; Du, L.; Li, H.; Zhang, X. Pharmacokinetics and tissue distribution of emodin loaded nanoemulsion in rats. J. Drug Deliv. Sci. Technol. 2015. [Google Scholar] [CrossRef]
- Reid, B.G.; Stratton, M.S.; Bowers, S.; Cavasin, M.A.; Demos-Davies, K.M.; Susano, I.; McKinsey, T.A. Discovery of novel small molecule inhibitors of cardiac hypertrophy using high throughput, high content imaging. J. Mol. Cell. Cardiol. 2016, 97, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, B.S.; Nam, H.; Hopkins, R.G.; Morrison, R.F. Impact of reference gene selection for target gene normalization on experimental outcome using real- time qRT-PCR in adipocytes. PLoS ONE 2010. [Google Scholar] [CrossRef] [PubMed]
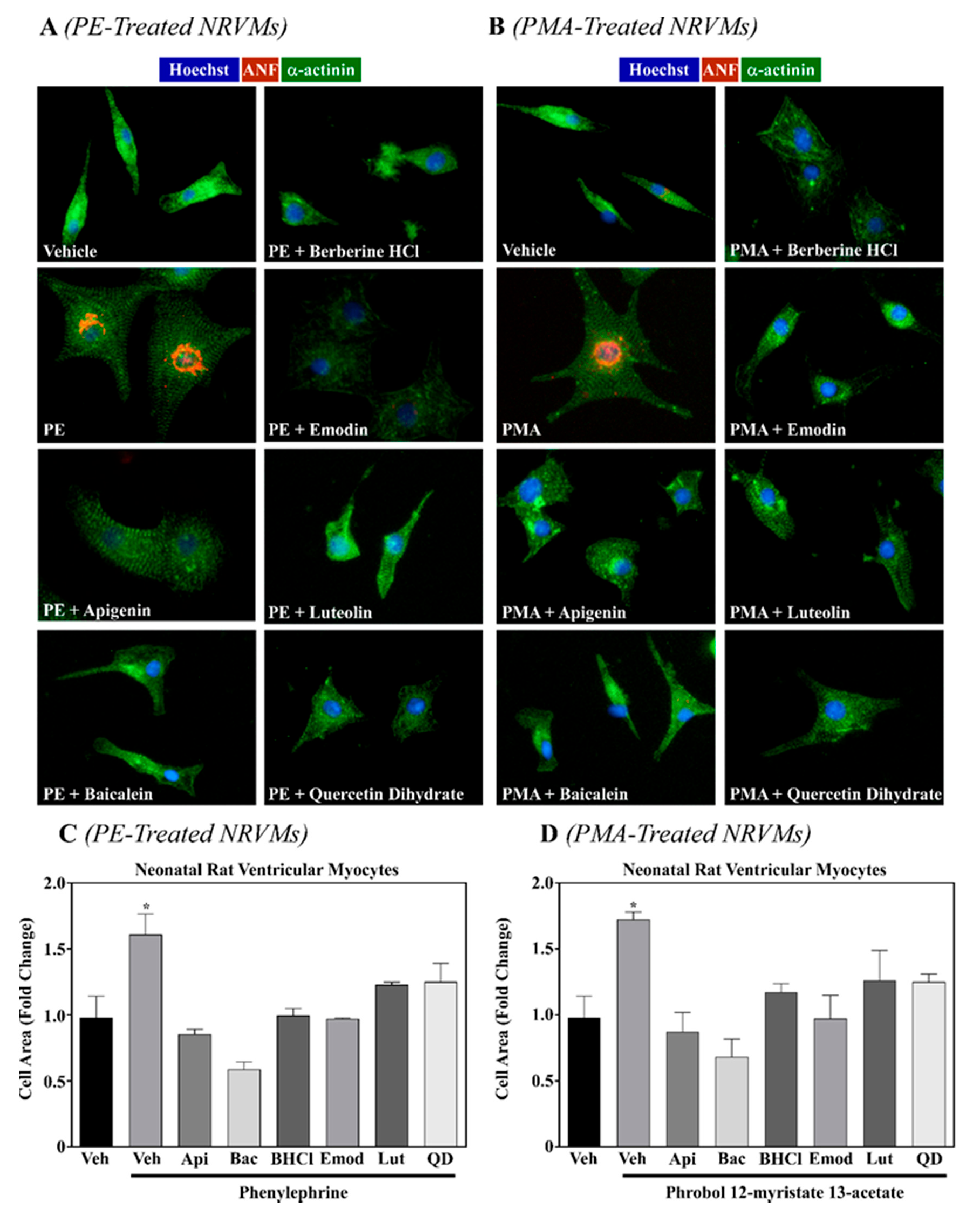
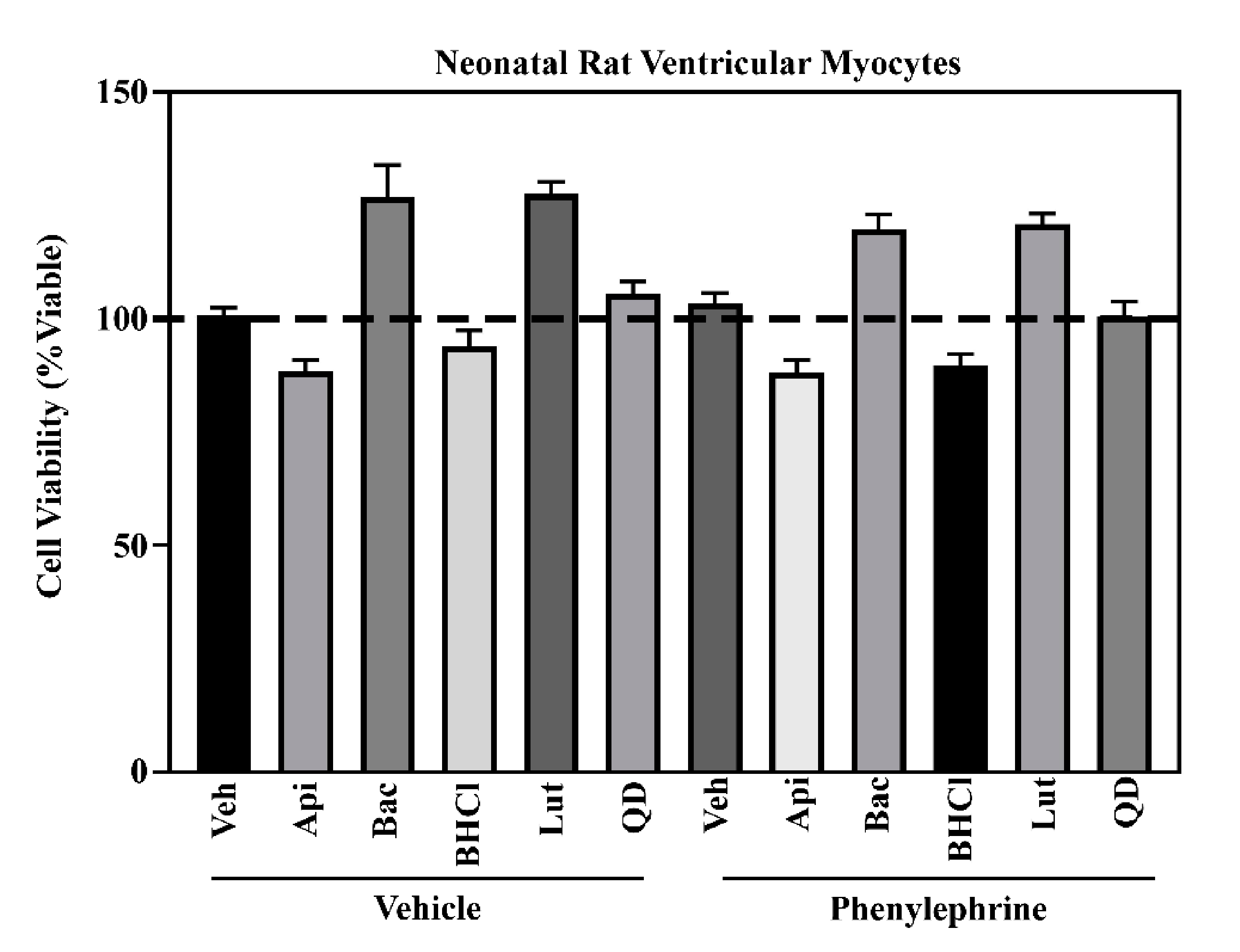

| Bioactive Food Compound | % Inhibition Cell Size | |
|---|---|---|
| PE | PMA | |
| Apigenin | 100 | 91 |
| Baicalein | 100 | 100 |
| Baicalin | N.I. | N.I. |
| Berberine Hydrochloride | 100 | 52 |
| Caffeic acid | N.I. | N.I. |
| Dihydromyricetin | N.I. | N.I. |
| Emodin | 100 | 78 |
| Epigallocatechin Gallate | 48 | 14 |
| Gossypol | Toxic | Toxic |
| Hematoxylin | N.I. | 76 |
| Indirubin | 38 | N.I. |
| Kaempferol | N.I. | 30 |
| Luteolin | 62 | 40 |
| Morin hydrate (Aurantica) | N.I. | 26 |
| Myricetin (Cannabiscetin) | Toxic | Toxic |
| Myricitrin (Myricitrine) | N.I. | 40 |
| Palmatine | 81 | N.I. |
| Quercetin dihydrate (Sophoretin) | 45 | 42 |
| Forward Primer | Reverse Primer | |
|---|---|---|
| ANP (Atrial Natriuretic Peptide) | GCCGGTAGAAGATGAGGTCAT’ | GCTTCCTCAGTCTGCTCACTCA |
| BNP (Brain Natriuretic Peptide) | GGTGCTGCCCCAGATGATT | CTGGAGACTGGCTAGGACTTC |
| IL-6 (Interleukin-6) | CTTCACAAGTCGGAGGCTTAAT | GCATCATCGCTGTTCATACAATC |
| IL-1α (Interleukin-1α) | CAGATGGGCTAACTAAGGGATAAG | AGAAGGTGCACAGTGAGATAAG |
| IL-1β (Interleukin-1β) | CTTCCTAAAGATGGCTGCACTA | CTGACTTGGCAGAGGACAAA |
| CXCL2 (CXC Chemokine Ligand 2) | GGCGGGACGACTGTTATTT | CACTGTGCCTTACAGAGAAGAC |
| CXCL6 (CXC Chemokine Ligand 6) | GCTACGCTGTGTTTGCTTAAC | GCAGGGATCACCTCCAAATTA |
| CCL3 (CC Chemokine Ligand 3) | GAGATTAGAGGCAGCAAGGAA | CTTGGCAGCAAACAGCTTATAG |
| CCL6 (CC Chemokine Ligand 6) | GGGCTCATACAAGATACGGTAAA | CATGGGATCTGTGAGGCATAG |
| 18S rRNA | GCCGCTAGAGGTGAAATTCTTA | CTTTCGCTCTGGTCCGTCTT |
| Phytochemical Class | Compound | Dietary Source |
|---|---|---|
| Flavonol | Quercetin | Teas, peppers, wines, onions, berries, apples |
| Flavone | Apigenin | Citrus, onions, celery, chamomile tea |
| Luteolin | Celery, parsley, broccoli, onions, carrots, peppers, cabbages, apples | |
| Baicalein | Scutellaria baicalensis | |
| Flavanolol | Dihydromyricetin | Ampelopsis grossedentata leaves and stems |
| Quinone | Emodin | Rhubarb, aloe vera, buckthorn, knotweed, fo-ti root, Cabbage, Beans |
| Alkaloid | Berberine Hydrochloride | Hydrastis canadensis, Coptis chinensis, Berberis aquifolium, Berberis vulgaris, Berberis aristata |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evans, L.; Shen, Y.; Bender, A.; Burnett, L.E.; Li, M.; Habibian, J.S.; Zhou, T.; Ferguson, B.S. Divergent and Overlapping Roles for Selected Phytochemicals in the Regulation of Pathological Cardiac Hypertrophy. Molecules 2021, 26, 1210. https://doi.org/10.3390/molecules26051210
Evans L, Shen Y, Bender A, Burnett LE, Li M, Habibian JS, Zhou T, Ferguson BS. Divergent and Overlapping Roles for Selected Phytochemicals in the Regulation of Pathological Cardiac Hypertrophy. Molecules. 2021; 26(5):1210. https://doi.org/10.3390/molecules26051210
Chicago/Turabian StyleEvans, Levi, Yiqui Shen, Abigail Bender, Leah E. Burnett, Musheng Li, Justine S. Habibian, Tong Zhou, and Bradley S. Ferguson. 2021. "Divergent and Overlapping Roles for Selected Phytochemicals in the Regulation of Pathological Cardiac Hypertrophy" Molecules 26, no. 5: 1210. https://doi.org/10.3390/molecules26051210
APA StyleEvans, L., Shen, Y., Bender, A., Burnett, L. E., Li, M., Habibian, J. S., Zhou, T., & Ferguson, B. S. (2021). Divergent and Overlapping Roles for Selected Phytochemicals in the Regulation of Pathological Cardiac Hypertrophy. Molecules, 26(5), 1210. https://doi.org/10.3390/molecules26051210







