One-Step Synthesis of Magnetic Nanocomposite with Embedded Biologically Active Substance
Abstract
1. Introduction
2. Results and Discussion
2.1. Sample Preparation
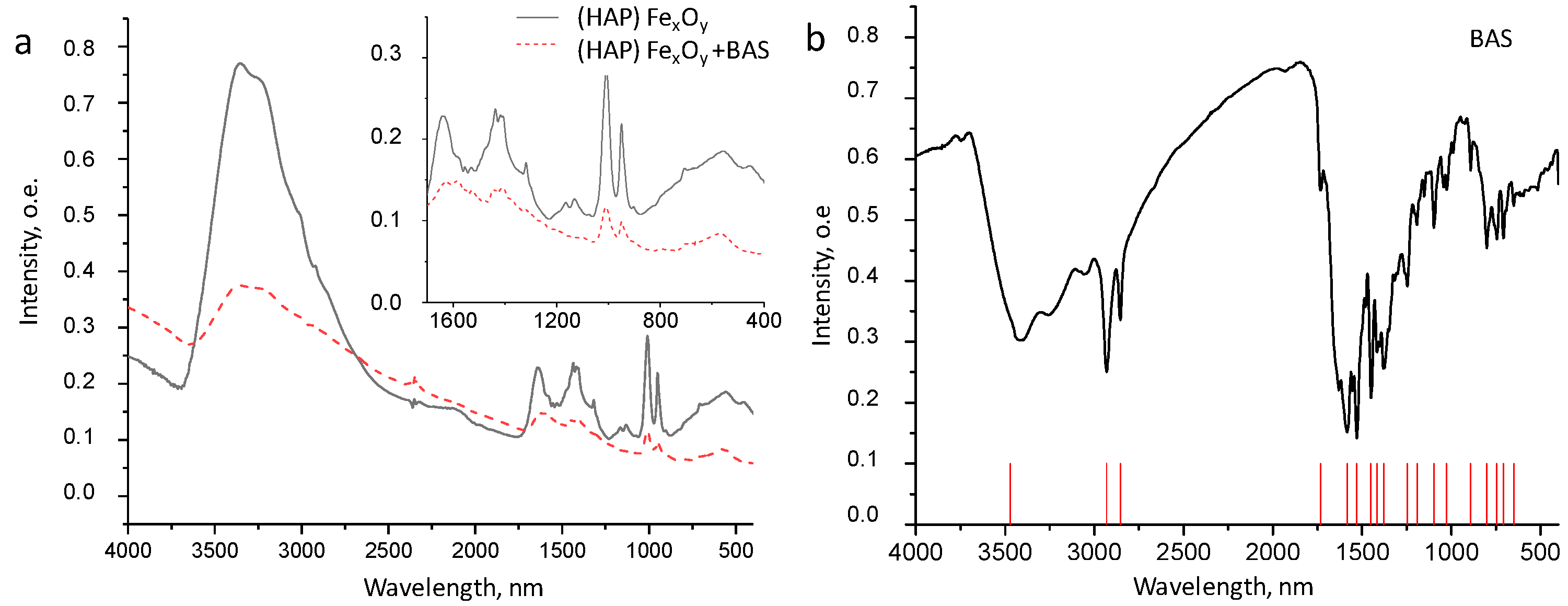
2.1.1. Formation of Composite (HAP)FexOy
2.1.2. Formation of Composite (HAP)FexOy + BAS
2.2. Morphology
2.3. Composition
2.4. Structure
2.5. Magnetic Properties
2.6. Drug Release Kinetics
2.7. Cytotoxicity
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kozlovskiy, A.; Zdorovets, M.; Kadyrzhanov, K.; Korolkov, I.; Rusakov, V.; Nikolaevich, L.; Fesenko, O.; Budnyk, O.; Yakimchuk, D.; Shumskaya, A. FeCo nanotubes: Possible tool for targeted delivery of drugs and proteins. Appl. Nanosci. 2018, 9, 1091–1099. [Google Scholar] [CrossRef]
- Pessan, J.P.; Paula, A.; Vieira, M.; Maria, T.; De Lima, T.; Carlos, A.; Delbem, B. Iron Oxide Nanoparticles for Biomedical Applications: A Perspective on Synthesis, Drugs, Antimicrobial Activity and Toxicity. Antibiotics 2018, 7, 46. [Google Scholar] [CrossRef]
- Tartaj, P.; Morales, P.; Veintemillas-verdaguer, S.; Gonz, T. The preparation of magnetic nanoparticles for applications in biomedicine. Appl. Phys. 2003, 36, R182. [Google Scholar] [CrossRef]
- Sun, S.; Chao, W.; Zhu, Z. Magnetic iron oxide nanoparticles: Synthesis and surface coating techniques for biomedical applications. Chin. Phys. B 2014, 23, 1–19. [Google Scholar] [CrossRef]
- Moraes Silva, S.; Tavallaie, R.; Sandiford, L.; Tilley, R.D.; Gooding, J.J. Gold coated magnetic nanoparticles: From preparation to surface modification for analytical and biomedical applications. Chem. Commun. 2016, 52, 7528–7540. [Google Scholar] [CrossRef]
- Tishkevich, D.I.; Korolkov, I.V.; Kozlovskiy, A.L.; Anisovich, M.; Vinnik, D.A. Immobilization of boron-rich compound on Fe 3 O 4 nanoparticles: Stability and cytotoxicity. J. Alloys Compd. 2019, 797, 573–581. [Google Scholar] [CrossRef]
- Kozlovskiy, A.L.; Ermekova, A.E.; Korolkov, I.V.; Chudoba, D.; Jazdzewska, M. Study of phase transformations, structural, corrosion properties and cytotoxicity of magnetite-based nanoparticles. Vacuum 2019, 163, 236–247. [Google Scholar] [CrossRef]
- Mango, L. Theranostics: A Unique Concept to Nuclear Medicine. Height. J. Cancer Sci. Res. 2017, 1, 001–004. [Google Scholar] [CrossRef][Green Version]
- Orlova, M.; Nikolaev, A.; Trofimova, T.; Orlov, A.; Severin, A.; Kalmykov, S. Hydroxyapatite and porphyrin-fullerene nanoparticles for diagnostic and therapeutic delivery of paramagnetic ions and radionuclides. Vestn. Rgmu. 2018, 6, 94–102. [Google Scholar] [CrossRef]
- Panseri, S.; Cunha, C.; Alessandro, T.D.; Sandri, M.; Russo, A.; Giavaresi, G.; Marcacci, M.; Hung, C.T.; Tampieri, A. Magnetic Hydroxyapatite Bone Substitutes to Enhance Tissue Regeneration: Evaluation In Vitro Using Osteoblast-Like Cells and In Vivo in a Bone Defect. PLoS ONE 2012, 7, e38710. [Google Scholar] [CrossRef]
- Yu, W.; Sun, T.W.; Ding, Z.; Qi, C.; Zhao, H.; Chen, F.; Shi, Z.; Zhu, Y.J.; Chen, D.; He, Y. Copper-doped mesoporous hydroxyapatite microspheres synthesized by a microwave-hydrothermal method using creatine phosphate as an organic phosphorus source: Application in drug delivery and enhanced bone regeneration. J. Mater. Chem. B 2017, 5, 1039–1052. [Google Scholar] [CrossRef]
- Musskaya, O.N.; Krut’ko, V.K.; Kulak, A.I.; Filatov, S.A.; Batyrev, E.V.; Safronova, T.V. Calcium Phosphate Compositions with Polyvinyl Alcohol for 3D Printing. Inorg. Mater. Appl. Res. 2020, 11, 192–197. [Google Scholar] [CrossRef]
- Mondal, S.; Manivasagan, P.; Bharathiraja, S.; Santha, M.; Kim, H.H.; Seo, H.; Lee, K.D.; Oh, J. Magnetic hydroxyapatite: A promising multifunctional platform for nanomedicine application. Int. J. Nanomed. 2017, I 2, 8389–8410. [Google Scholar] [CrossRef]
- Yang, C.-T.; Li, K.-Y.; Meng, F.-Q.; Lin, J.-F.; Young, I.-C.; Ivkov, R.; Lin, F.-H. ROS-induced HepG2 Cell Death from hyperthermia using Magnetic Hydroxyapatite Nanoparticles. Nanotechnology 2018, 29, 375101. [Google Scholar] [CrossRef]
- Rakshit, M.; Gautam, A.; Toh, L.Z.; Lee, Y.S.; Lai, H.Y.; Wong, T.T.; Ng, K.W. Hydroxyapatite particles induced modulation of collagen expression and secretion in primary human dermal fibroblasts. Int. J. Nanomed. 2020, 15, 4943–4956. [Google Scholar] [CrossRef]
- Seal, B.L.; Otero, T.C.; Panitch, A. Polymeric biomaterials for tissue and organ regeneration. Mater. Sci. Eng. R Rep. 2001, 34, 147–230. [Google Scholar] [CrossRef]
- Govindan, B.; Latha, B.S.; Nagamony, P.; Ahmed, F. Designed Synthesis of Nanostructured Magnetic Hydroxyapatite Based Drug Nanocarrier for Anti-Cancer Drug Delivery toward the Treatment of Human Epidermoid Carcinoma. Nanomaterials 2017, 7, 138. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Lin, F. Evaluation of magnetic-hydroxyapatite nanoparticles for gene delivery carrier. Biomed. Eng. Appl. Basis Commun. 2010, 22, 33–39. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, Y.; Wu, J.; Sun, J.; Liao, X.; Teng, Z.; Lu, G. Facile synthesis of biodegradable flower-like hydroxyapatite for drug and gene delivery. J. Colloid Interface Sci. 2020, 570, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Islam, R.A.; Al-Busaidi, H.; Zaman, R.; Abidin, S.A.Z.; Othman, I.; Chowdhury, E.H. Carbonate apatite and hydroxyapatite formulated with minimal ingredients to deliver SiRNA into breast cancer cells in vitro and in vivo. J. Funct. Biomater. 2020, 11, 63. [Google Scholar] [CrossRef]
- Liao, Y.; Li, H.; Shu, R.; Chen, H.; Zhao, L.; Song, Z.; Zhou, W. Mesoporous Hydroxyapatite/Chitosan Loaded with Recombinant-Human Amelogenin Could Enhance Antibacterial Effect and Promote Periodontal Regeneration. Front. Cell. Infect. Microbiol. 2020, 10, 180. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Sun, H.; Lu, Y.; Zhao, F.; Liu, C.; Wang, Q.; Zheng, C.; Lu, R.; Song, K. Strontium/Chitosan/Hydroxyapatite/Norcantharidin Composite That Inhibits Osteosarcoma and Promotes Osteogenesis in Vitro. Biomed. Res. Int. 2020, 2020, 9825073. [Google Scholar] [CrossRef]
- Gomes, D.S.; Santos, A.M.C.; Neves, G.A.; Menezes, R.R.; Grande, C.; Grande, C. A brief review on hydroxyapatite production and use in biomedicine (Uma breve revisão sobre a obtenção de hidroxiapatita e aplicação na biomedicina). Cerâmica 2019, 65, 282–302. [Google Scholar] [CrossRef]
- Ziarani, G.M.; Malmir, M.; Lashgari, N.; Badiei, A. The role of hollow magnetic nanoparticles in drug delivery. RSC Adv. 2019, 9, 25094–25106. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, D.; Shang, P. Review article A review of magnet systems for targeted drug delivery. J. Control. Release 2019, 302, 90–104. [Google Scholar] [CrossRef]
- Price, P.M.; Mahmoud, W.E.; Al-ghamdi, A.A.; Bronstein, L.M. Magnetic Drug Delivery: Where the Field Is Going. Front. Chem. 2018, 6, 1–7. [Google Scholar] [CrossRef]
- Ignatovich, J.V.; Sinyutich, Y.V.; Gusak, K.N.; Koroleva, E.V. Synthesis of N-[2(3,4) -aminophenyl]-4-({4-methyl-3-[4-(pyridin-3-yl) pyrimidin-2-ylamino] phenyl} aminomethyl) benzamides. Org. Chem. J. 2015, 51, 1479–1482. (In Russian) [Google Scholar] [CrossRef]
- Eryomin, A.N.; Pietkievich, A.V.; Abakshonok, A.V.; Siniutsich, Y.V. Associates of Thioalkyl Derivatives of 2-Arylaminopyrimidine with Hydroxyapatite-Based Nanocomposites. Russ. J. Gen. Chem. 2016, 86, 1886–1895. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Zhang, Z.; Xu, L.; Li, P. Study of the glass transition temperature and the mechanical properties of PET / modified silica nanocomposite by molecular dynamics simulation. Eur. Polym. J. 2016, 75, 36–45. [Google Scholar] [CrossRef]
- Ignatovich, Z.V.; Koroleva, E.V. Synthesis of Functionalized Amides of 2-(Arylamino) pyrimidine Series. Russ. J. Org. Chem. 2017, 53, 251–257. [Google Scholar] [CrossRef]
- Koroleva, E.V.; Agabekov, V.E. Microencapsulation of Imatinib Methanesulfonate microencapsulation of imatinib methanesulfonate. Pharm. Chem. J. 2017, 51, 486–490. [Google Scholar] [CrossRef]
- Scialla, S.; Palazzo, B.; Barca, A.; Fiore, A.; Monteduro, A.G.; Sannino, A.; Gervaso, F.; Fiore, A.; Monteduro, A.G.; Maruccio, G. Simplified preparation and characterization of magnetic hydroxyapatite-based nanocomposites. Mater. Sci. Eng. C 2017, 76, 1166–1174. [Google Scholar] [CrossRef]
- Horváth, B.; Rigó, M.; Guba, S.; Szalai, I.; Barabás, R. Magnetic field response of aqueous hydroxyapatite based. Heliyon 2019, e01507. [Google Scholar] [CrossRef] [PubMed]
- Blue, R.; Ions, N. Preparation of Chitosan Coated Magnetic Hydroxyapatite Nanoparticles and Preparation of Chitosan Coated Magnetic Hydroxyapatite Nanoparticles and Application for Adsorption of Reactive Blue 19 and Ni2+ Ions. Sci. World J. 2015, 2, 1–10. [Google Scholar] [CrossRef]
- Poinern, G.E.; Brundavanam, R.K.; Mondinos, N.; Jiang, Z.-T. Synthesis and characterisation of nanohydroxyapatite using an ultrasound assisted method. Ultrason. Sonochem. 2009, 16, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.; Gregor, M.; Wolf-brandstetter, C.; Kost, J. Pyroelectric, Piezoelectric, and Photoeffects in Hydroxyapatite Thin Films on Silicon. Appl. Phys. Lett. 2011, 98, 1–5. [Google Scholar] [CrossRef]
- Boekelheide, Z.; Miller, J.T.; Grüttner, C.; Dennis, C.L. The effects of intraparticle structure and interparticle interactions on the magnetic hysteresis loop of magnetic nanoparticles. J. Appl. Phys. 2019, 126, 043903. [Google Scholar] [CrossRef]






| Sample | Mass Content, % | |||||
|---|---|---|---|---|---|---|
| O | P | Ca | Fe | C | N | |
| (HAP)FexOy | 30 | 1.22 | 0.28 | 68.48 | – | – |
| (HAP)FexOy + BAS | 24.12 | 1.26 | 0.29 | 61.74 | 11.1 | 1.8 |
| Composite Type | Phase of FexOy | (hkl) | 2θ, ° | d, Å | L, nm | Lattice Parameter, Å | Degree of Crystallinity, % |
|---|---|---|---|---|---|---|---|
| (HAP)FexOy | γ-Fe2O3/Fe3O4 Cubic P4332(212) | 221 | 26.371 | 3.37697 | 45.37 | a = 8.37178, V = 586.75 | 64.3 |
| 220 | 30.193 | 2.95762 | 19.78 | ||||
| 311 | 35.544 | 2.52367 | 14.09 | ||||
| 320 | 38.315 | 2.34728 | 44.86 | ||||
| 400 | 42.997 | 2.10190 | 11.55 | ||||
| 511 | 56.852 | 1.61819 | 12.52 | ||||
| 440 | 62.777 | 1.47897 | 11.85 | ||||
| (HAP)FexOy + BAS | γ-Fe2O3/Fe3O4 + BAS Cubic P4332(212) | 221 | 27.040 | 3.29494 | 72.15 | a = 8.36176, V = 584.65 | 67.2 |
| 220 | 30.193 | 2.95762 | 12.95 | ||||
| 311 | 35.544 | 2.52367 | 12.02 | ||||
| 320 | - | - | - | ||||
| 400 | 43.188 | 2.09304 | 17.08 | ||||
| 311 | 46.724 | 1.94257 | 15.97 | ||||
| 422 | 53.604 | 1.70834 | 9.97 | ||||
| 511 | 57.330 | 1.60583 | 13.54 | ||||
| 440 | 62.586 | 1.48303 | 12.35 |
| Composite Type | Coercivity Hc, Oe | Mr/Ms | Ms, emu/g |
|---|---|---|---|
| (HAP)FexOy | 10 | 0.012 | 16.5 |
| (HAP)FexOy + BAS | 8 | 0.0052 | 8.4 |
| Composite Type | Concentration, mg/mL | Cell Death, % |
|---|---|---|
| (HAP)FexOy | 1.00 | 19.60 |
| 0.50 | 17.92 | |
| 0.10 | −2.11 | |
| 0.02 | −11.35 | |
| (HAP)FexOy + BAS | 1.00 | 27.93 |
| 0.50 | 23.90 | |
| 0.10 | 9.74 | |
| 0.02 | 0.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ignatovich, Z.; Novik, K.; Abakshonok, A.; Koroleva, E.; Beklemisheva, A.; Panina, L.; Kaniukov, E.; Anisovich, M.; Shumskaya, A. One-Step Synthesis of Magnetic Nanocomposite with Embedded Biologically Active Substance. Molecules 2021, 26, 937. https://doi.org/10.3390/molecules26040937
Ignatovich Z, Novik K, Abakshonok A, Koroleva E, Beklemisheva A, Panina L, Kaniukov E, Anisovich M, Shumskaya A. One-Step Synthesis of Magnetic Nanocomposite with Embedded Biologically Active Substance. Molecules. 2021; 26(4):937. https://doi.org/10.3390/molecules26040937
Chicago/Turabian StyleIgnatovich, Zhanna, Khristina Novik, Anna Abakshonok, Elena Koroleva, Anna Beklemisheva, Larisa Panina, Egor Kaniukov, Marina Anisovich, and Alena Shumskaya. 2021. "One-Step Synthesis of Magnetic Nanocomposite with Embedded Biologically Active Substance" Molecules 26, no. 4: 937. https://doi.org/10.3390/molecules26040937
APA StyleIgnatovich, Z., Novik, K., Abakshonok, A., Koroleva, E., Beklemisheva, A., Panina, L., Kaniukov, E., Anisovich, M., & Shumskaya, A. (2021). One-Step Synthesis of Magnetic Nanocomposite with Embedded Biologically Active Substance. Molecules, 26(4), 937. https://doi.org/10.3390/molecules26040937







