3.1. Analysis of Structural and Morphological Features of Nanoparticles
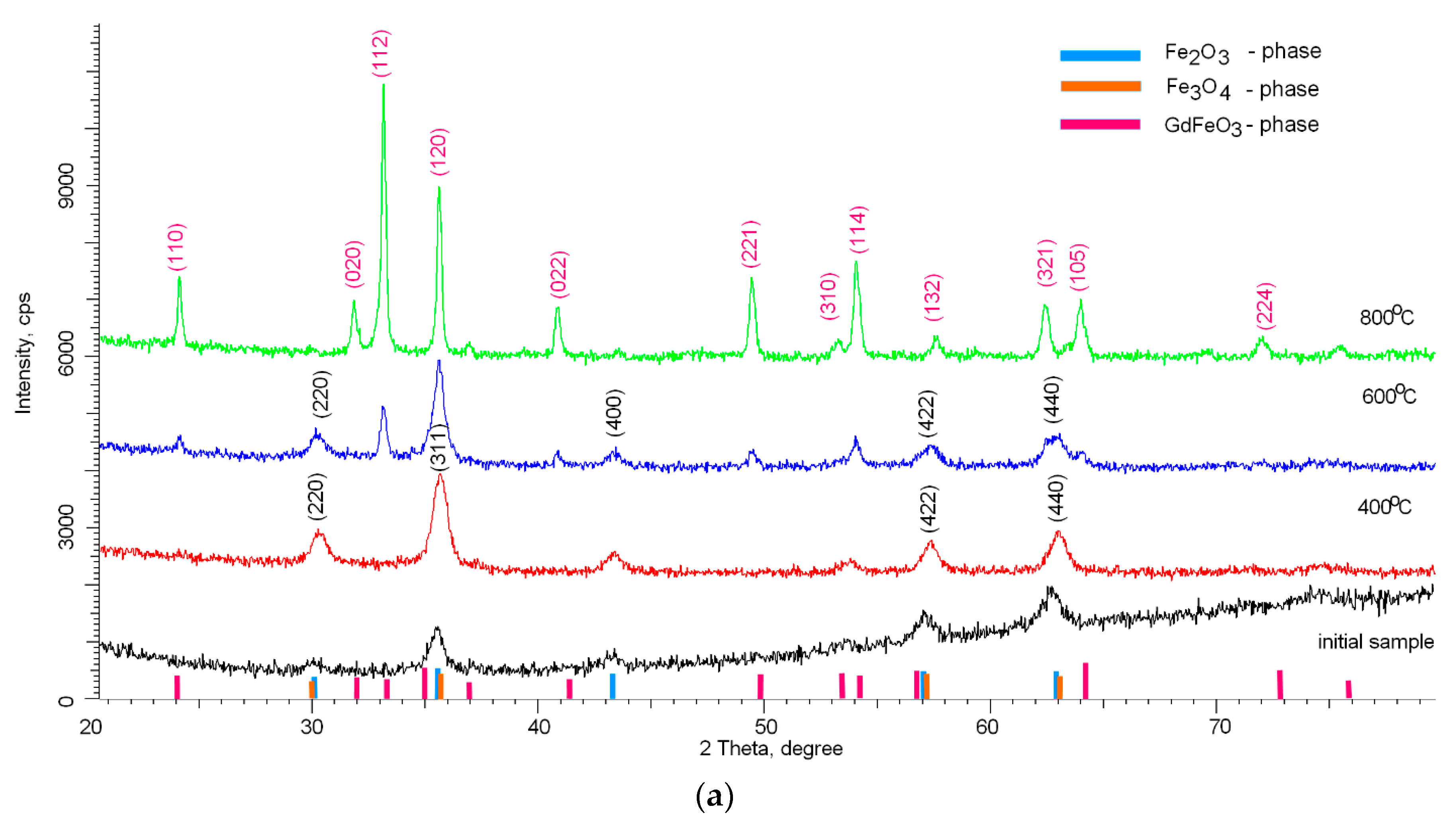
Figure 1 shows X-ray diffraction patterns reflecting the dynamics of phase transformations as a result of thermal annealing of nanoparticles. As a comparison, the diffraction pattern of the initial nanoparticles before annealing is shown, which clearly demonstrates the highly disordered amorphous structure of nanoparticles, the deformation of which is caused by the presence of a mixture of two phases in the composition of Fe
2O
3 and Fe
3O
4. As is known, the precise determination of the Fe
2O
3 and Fe
3O
4 phases using the X-ray diffraction method is difficult due to the close similarity of the structural features. A more accurate determination of the phase composition of iron-containing nanoparticles is possible using the method of Mössbauer spectroscopy, which makes it possible to determine the values of hyperfine magnetic fields with high accuracy, which are different for these phases. A preliminary analysis of the initial iron-containing nanoparticles showed that in the case of the initial nanoparticles, the values of hyperfine magnetic fields are 480–485 kOe, which are characteristic of the magnetite phase, while for annealed samples, the magnitude of the hyperfine magnetic fields exceeds 510–512 kOe, which is characteristic of partially disordered phases of hematite Fe
2O
3. The values obtained are also in good agreement with the previously presented data on phase transformations in [
22].
As can be seen from the presented data, in the case of thermal annealing at 400 °C, the main phase is the Fe
2O
3 phase with the crystal lattice parameter a = 8.3249 Å. The absence of peaks characteristic of phases containing gadolinium or gadolinium oxide may be due to the fact that during mechanical grinding, gadolinium remains in an amorphous state and does not have a clearly pronounced crystalline phase. In the case of annealing at temperatures of 600 °С, the diffraction pattern show the appearance of new peaks with angular positions 2θ = 24.19°, 33.18°, 40.89°, 49.31°, having a different shape and width than the peaks characteristic of Fe
2O
3, which indicates the appearance of a new phase in the structure of nanoparticles. An increase in the crystal lattice parameters for the Fe
2O
3 phase a = 8.3461 Å may be associated with the partial replacement of iron atoms with gadolinium atoms, which leads to the formation of a new phase. Applying the Rietveld method for a full-profile analysis of the position of diffraction peaks and their width [
24], it was found that new peaks are characteristic of the orthorhombic phase of GdFeO
3 (PDF#00-059-0665). In this case, an increase in the annealing temperature leads to ordering and dominance of the GdFeO
3 phase in the structure of nanoparticles. The crystal lattice parameters for the GdFeO3 phase are а = 5.3342 Å, b = 5.5946 Å, c = 7.6071 Å.
Additionally, the formation of the GdFeO
3 phase leads to an enlargement of the crystallite sizes determined using the Williamson–Hall method [
25]. For nanoparticles annealed at 400 °C, the average crystallite size is 15–17 nm. For samples annealed at a temperature of 600 °C, the size is 20–23 nm, while for the analysis of the peaks characteristic of the GdFeO
3 phase, the crystallite size was 33–35 nm. For samples annealed at 800 °C, the crystallite size was 35–40 nm.
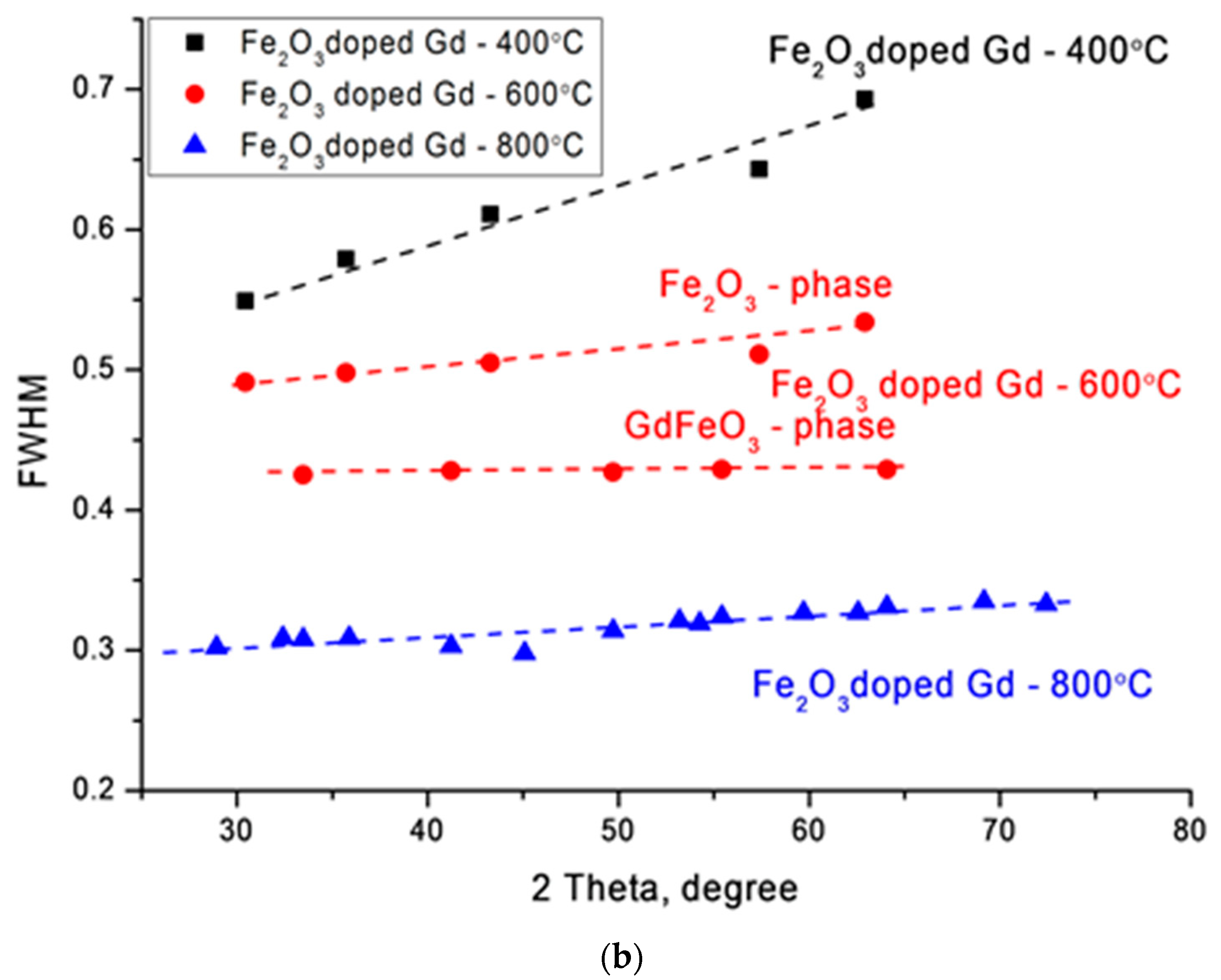
Figure 1b shows the changes in the FWHM values by the Williamson–Hall method, which reflect the change in the degree of structural changes, as well as grain sizes. In the case of nanoparticles annealed at a temperature of 400 °C, a large slope angle indicates a distortion of the crystal structure. In the case of nanoparticles annealed at a temperature of 600 °C, a change in the FWHM value is observed and is divided into two curves, which correspond to two phases Fe
2O
3 and GdFeO
3. In the case of nanoparticles annealed at 800 °C, the slope of the curve indicates a small contribution of deformations and distortions in the structure of nanoparticles, which indicates an increase in the degree of structural ordering in nanoparticles.
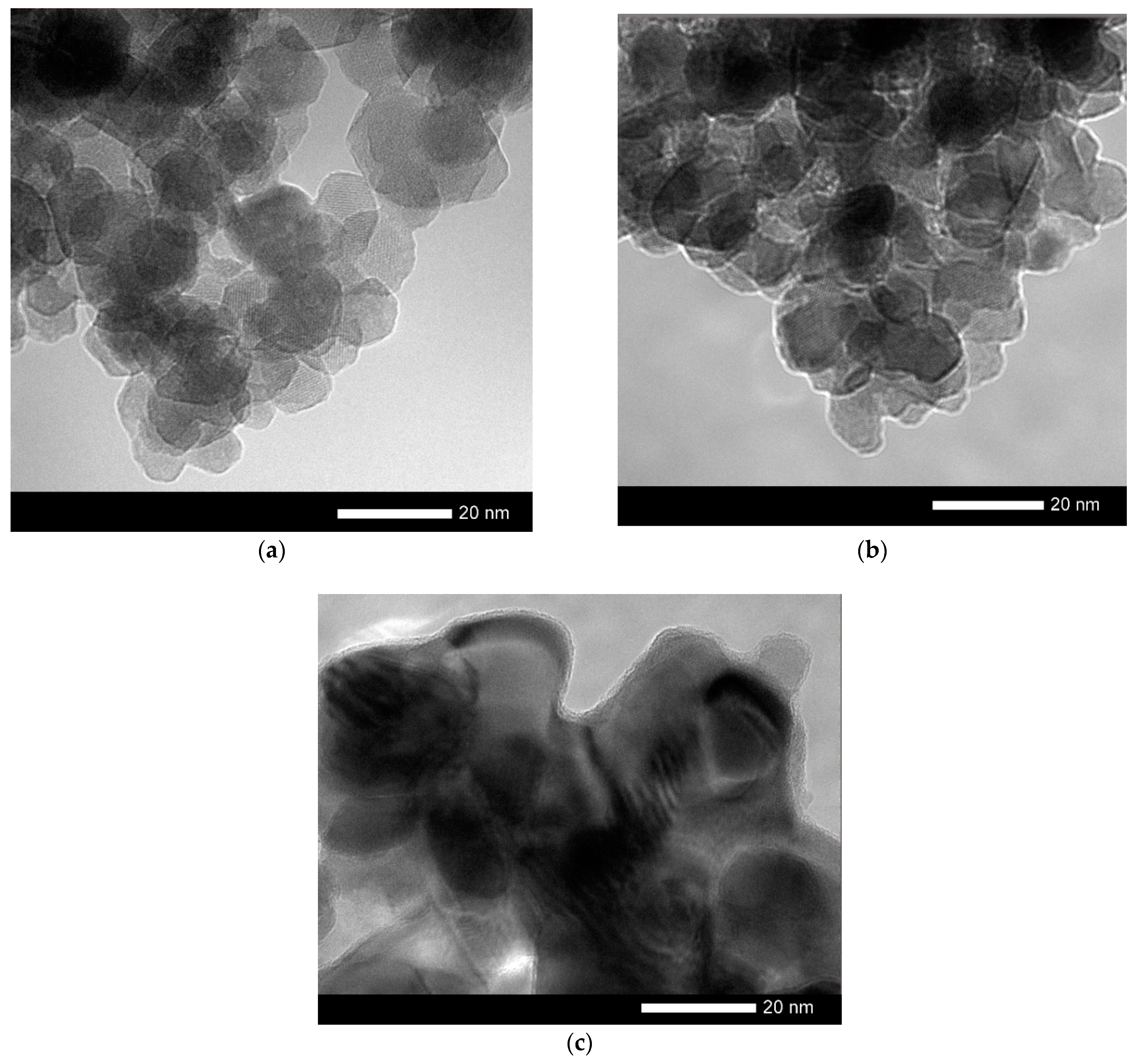
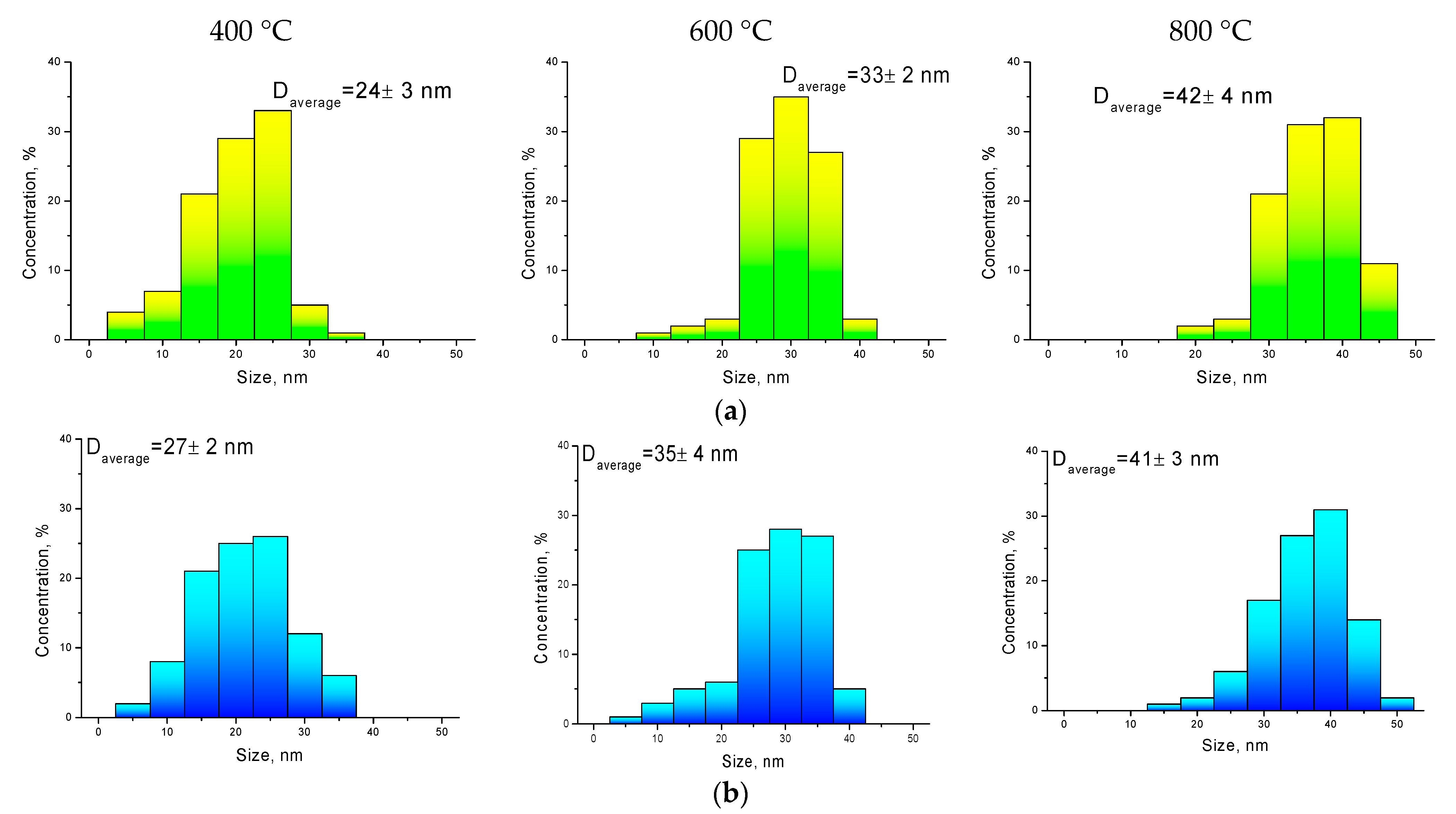
The change in the shape and size of nanoparticles is also confirmed by the data obtained using the method of transmission electron microscopy, presented in
Figure 2.
Changing the annealing conditions leads first to the ordering of nanoparticles and a change in their shape, and then to their enlargement at 800 °C. For nanoparticles obtained at an annealing temperature of 800 °C, the formation of inclusions characteristic of the residual Fe
2O
3 phase is observed in the structure, as well as a thin amorphous-like porous interlayer on the surface, the size of which is no more than 1 nm (see data in
Figure 2).
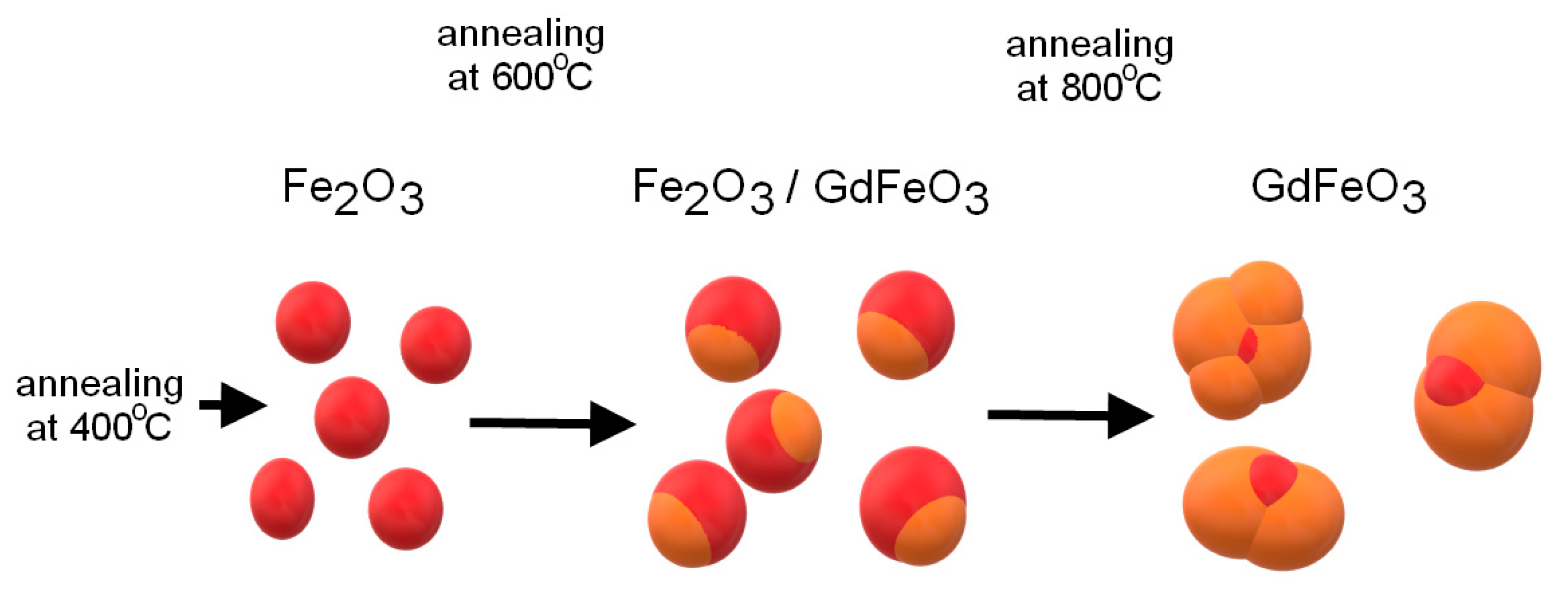
Thus, during the study, the kinetics of phase transformations was determined depending on the annealing temperature, which can be represented by the following scheme (see
Figure 3). According to the presented scheme, as well as the transmission electron microscopy data, the synthesized nanoparticles are a “core-shell” structure at an annealing temperature of 600 °C and single-phase spherical particles at a temperature of 800 °C.
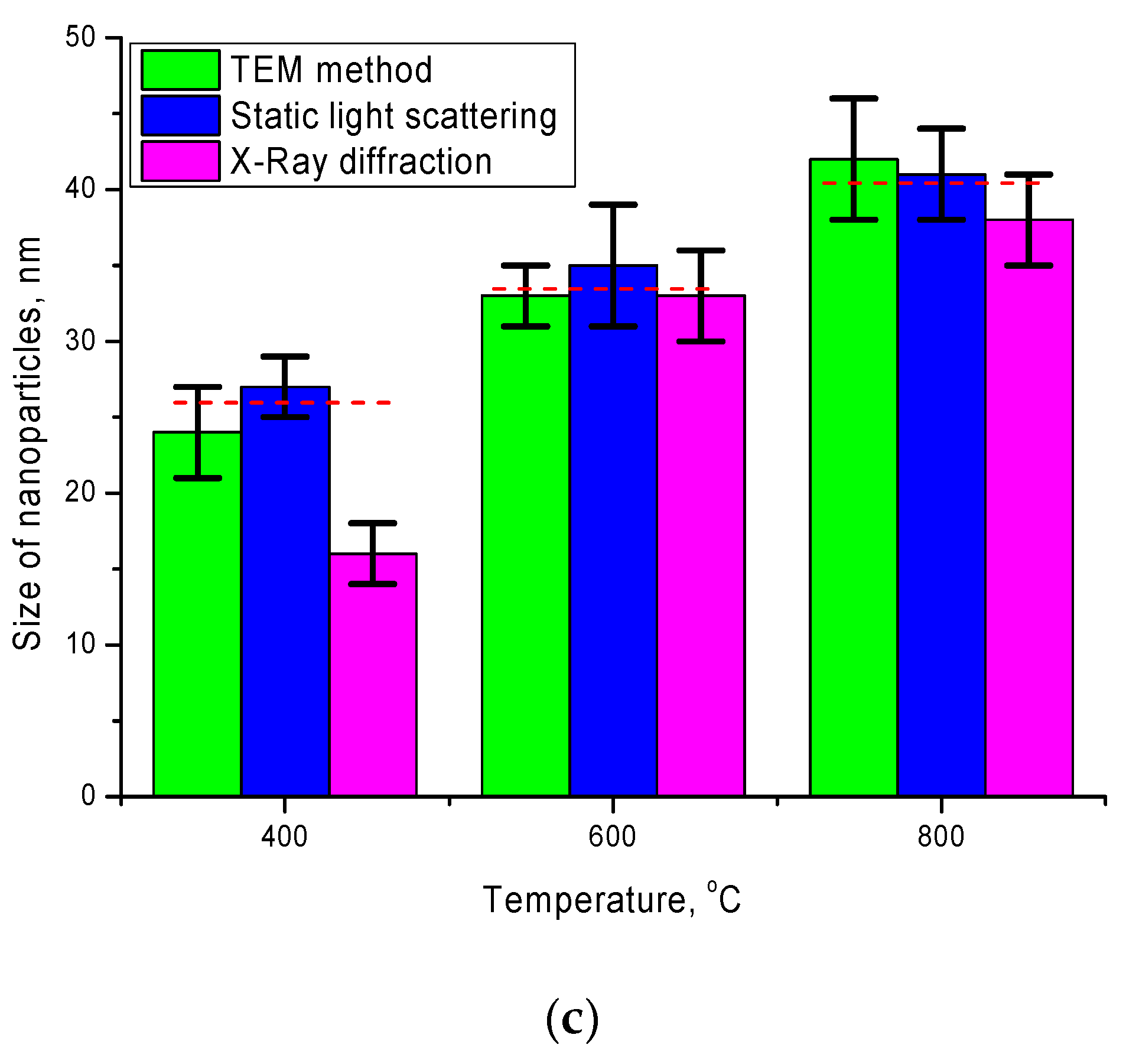
Figure 4 presents comparative diagrams of nanoparticle sizes determined using various methods: transmission electron microscopy and static light scattering.
As can be seen from the data presented, the size diagrams obtained by different methods correlate with each other and have good agreement in the case of annealed samples at temperatures of 600–800 °C. An insignificant deviation of the particle sizes determined using the X-ray diffraction method in the case of annealed nanoparticles at a temperature of 400 °C may be due to an unformed structure, as well as a strong distortion of the structure.
3.2. Research on Corrosion Properties and Hyperthermia
One of the key conditions for the applicability of nanoparticles as targeted drug delivery or biomedical purposes is their resistance to degradation and the rate of corrosion in various media. Knowing the rate of oxidation and phase degradation allows prediction of the time and area of application, as well as assessment of the consequences that nanoparticles may have for the body. In this case, as a rule, for iron-containing nanostructures, degradation mechanisms are associated with oxidation processes and phase transformations, which can lead to a deterioration in both structural and magnetic properties. These changes are primarily associated with the appearance of regions of structural disordering, a change in valence, the breaking of chemical bonds, and the appearance of a large number of oxygen vacancies in the structure. In the case of the use of nanoparticles in biomedicine, the main conditions for use in liquid media are acidity and temperature of the medium. As a rule, the modeling of the organism’s environment is carried out using an isotonic PBS solution, which makes it possible to closely model the environment of the human organism with pH = 7.4. The PBS solution contains salts of sodium chloride, sodium hydrogen phosphate, potassium chloride and potassium dihydrogen phosphate. The temperature of the environment also plays a significant role in the degradation process. Thus, for example, in [
26], it was shown that an increase in the temperature of the medium leads to a significant acceleration of the degradation processes of iron-containing nanoparticles. The maximum approximate temperature of the environment, typical for the hyperthermal application of nanoparticles, is 42–43 °C, which allows the killing of cancer cells without affecting healthy ones.
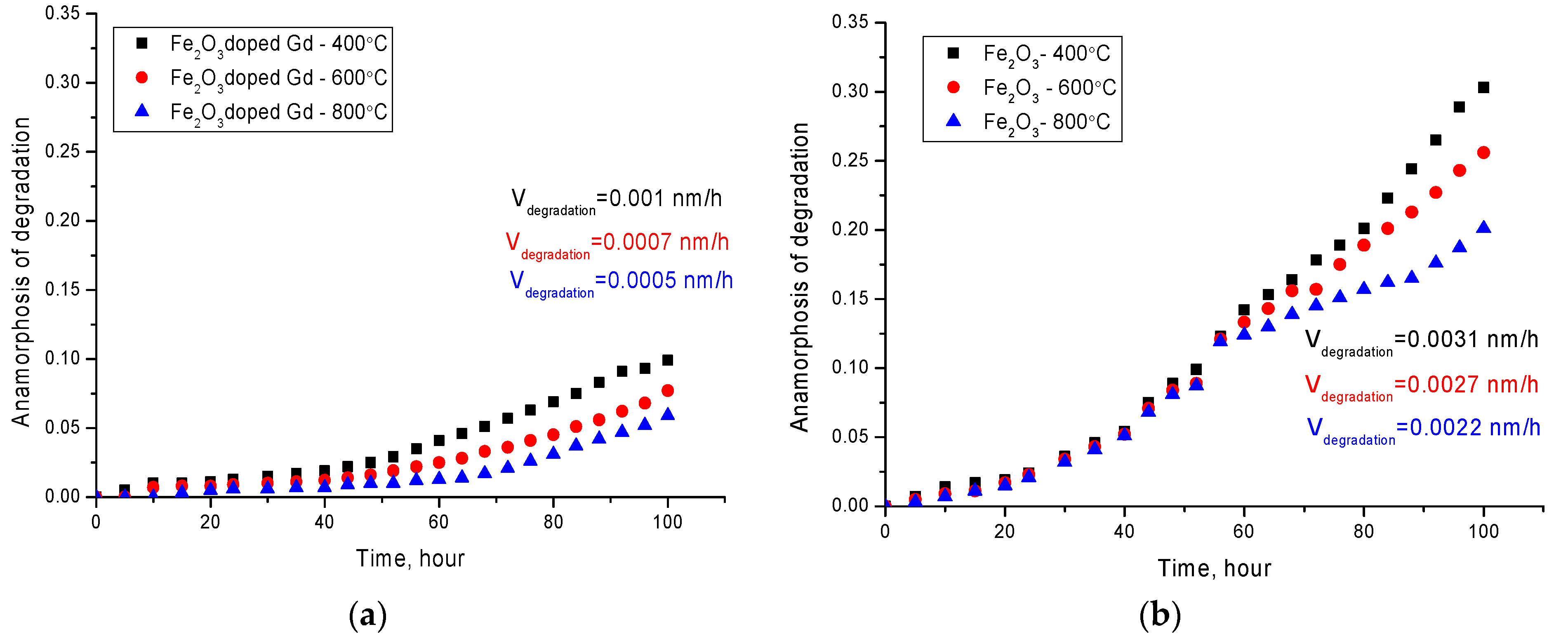
Figure 5 shows the results of the anamorphosis kinetics of the synthesized nanoparticles degradation for 100 h in a PBS solution at 42 ± 1 °С. To compare the effect of the formation of a shell from gadolinium on the rate of degradation of nanoparticles, additional studies of the kinetics of degradation of iron-containing particles were carried out. Nanoparticles were obtained according to the proposed method, except for the second stage of coating with gadolinium, and were also annealed in a given temperature range under the same conditions as particles with gadolinium. A more detailed presentation of the effect of thermal annealing on phase transformations and changes in the structural parameters of Fe
3O
4/Fe
2O
3 nanoparticles, as well as the degree of resistance to degradation, is shown in [
22,
23]. The authors of these works have found that thermal annealing at temperatures above 400 °C leads to an increase in the degree of structural ordering, as well as to a change in the phase composition of nanoparticles, followed by the dominance of the Fe
2O
3 hematite phase in the structure at temperatures above 600 °C. In addition, an increase in the annealing temperature led not only to a change in the phase composition and ordering of the values of the hyperfine magnetic field, but also to an increase in the size of nanoparticles, followed by a change in their shape from spherical to hexahedral or rhombic.
The assessment of the degree of degradation was carried out by plotting anamorphous or kinetic curves of degradation, which are graphs of the dependence of changes in the elemental and phase composition over time while in the medium. Determination of the order of the kinetic curve of degradation makes it possible to determine the kinetics and rate of the oxidation and destruction reaction. The data for plotting anamorphosis were obtained by analyzing the changes in the elemental and phase composition of the studied nanoparticles over time. The phase composition was estimated using the X-ray diffraction method, by taking diffractograms after each cycle, to determine the contribution of impurity phases to the change in the phase composition. The identification of impurity phases was carried out using the PDF2 database and the Rietveld method, which makes it possible to determine the contributions of phase inclusions with high accuracy. As impurity phases, FeO and FeOH phases, characteristic of oxidation of iron, were considered. Dynamics of elemental ratio change were carried out using energy dispersion analysis method, by determination of Fe, Gd, O component concentration change and comparison of the obtained data with initial samples.
As can be seen from the presented data, a two-stage nature of the degradation of nanoparticles is observed. The first stage is in the range of 0–45 h and 0–60 h for nanoparticles annealed at temperatures of 400 °C and 600–800 °C, respectively. This stage is characterized by small changes in the elemental and phase composition of nanoparticles, and indicates a high degree of stability of nanoparticles to oxidation in comparison with nanoparticles without gadolinium. At the same time, in contrast to nanoparticles with gadolinium, for Fe2O3 nanoparticles, the processes of degradation and oxidation occur at significantly intensities, and the degradation rate increases by a factor of 3–4 compared to the rate of degradation of Fe2O3 + Gd2O3 particles under similar conditions. Moreover, in both cases, a decrease in the degradation rate is observed with an increase in the annealing temperature, which leads to a change in the phase composition of nanoparticles.
It should be noted that the main changes associated with corrosion are due to the appearance in the structure of oxide and hydroxide phases of iron, with a highly disordered structure, which is almost amorphous. The second stage is characterized by a more intense increase in structural and phase changes, which indicates an acceleration of the processes of degradation and oxidation of nanoparticles.
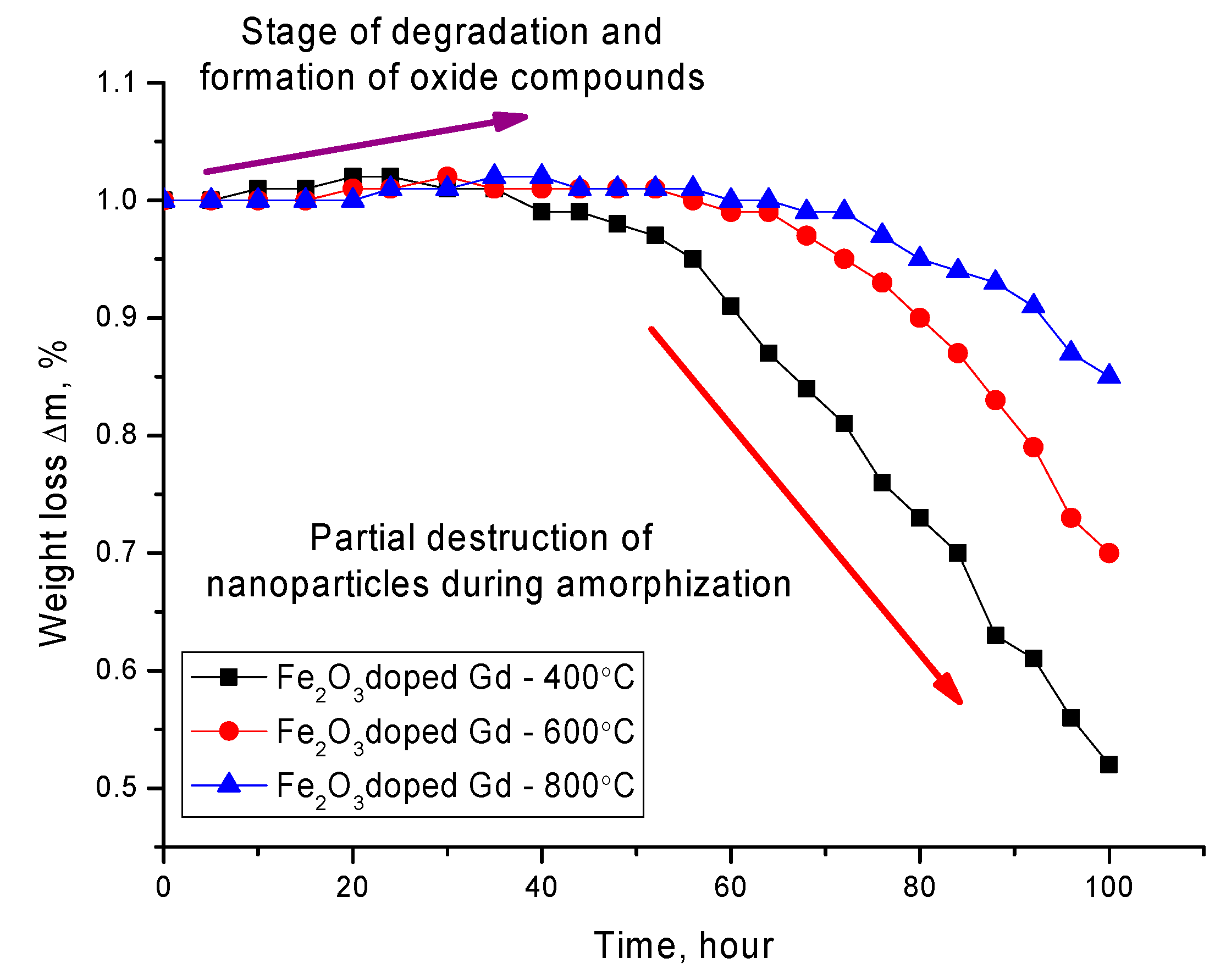
Figure 6 shows the data on the change in the mass of nanoparticles, as well as its loss as a result of degradation. Mass loss was calculated by determining the mass of nanoparticles depending on the residence time in the medium. The degradation experiment was carried out in several parallels. When a predetermined time interval was reached, the nanoparticles were captured by a magnet, and the solution was poured, after which the particles were dried and weighed using an analytical balance. The mass loss of nanoparticles during the experiment was determined from the change in mass before and after a certain time interval.
The general nature of changes in the dynamics of changes in the mass of nanoparticles can be divided into two stages. The first stage is characterized by a positive increase in the mass of nanoparticles, which is due to the formation of oxide inclusions on the surface of nanoparticles and the introduction of oxygen into the structure of nanoparticles. This stage is characterized by a low rate of degradation of nanoparticles. The second stage is typical for the loss of mass of nanoparticles, which is due to partial amorphization and degradation with subsequent destruction of nanoparticles or their fragmentation as a result of amorphization. A decrease in the mass loss, as well as the degradation rate for nanoparticles annealed at a temperature of 600–800 °C, is due to a change in the phase composition, as well as an increase in the degree of structural ordering as a result of annealing. The formation of the GdFeO3 phase leads to an increase in the degree of corrosion resistance, as well as to the processes of oxidation of nanoparticles in the medium. A similar situation is observed for unmodified nanoparticles, for which thermal annealing also leads to an increase in the degree of stability and a decrease in the rate of degradation.
In addition to resistance to degradation, an important property of the applicability of magnetic nanoparticles in biomedicine is the time required for processing in order to heat the nanoparticles to a temperature that will initiate the processes of local destruction of cancerous tumors using an alternating magnetic field. In this case, the time spent on heating plays an important role in determining the released thermal energy and the specific absorption coefficient.
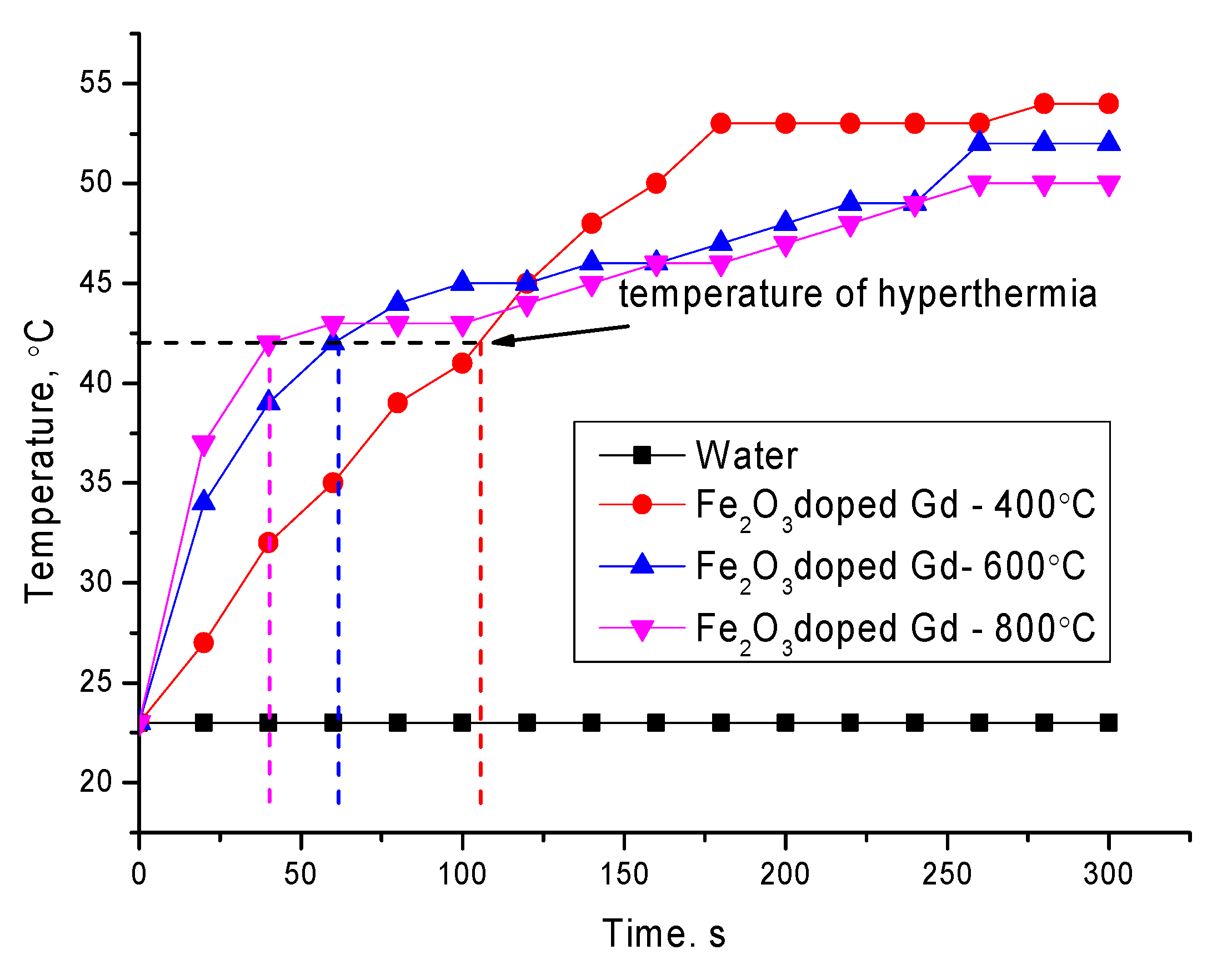
Figure 7 shows the dynamics of changes in the heating rate of aqueous solutions of the studied nanoparticles with a volume of 10 mg/mL in an alternating magnetic field at a frequency of 320 kHz. As a comparison, the curve of an aqueous solution without nanoparticles is shown, which shows the absence of the effect of the action of an alternating current magnetic field on the aqueous solution and its heating.
The heating rate to a hyperthermia temperature of 42–45 °C, which is characteristic of local death of tumor tissues without damaging healthy tissues for the studied nanoparticles, was 0.4 °С/s, 0.71 °С/s and 1.05 °С/s for Fe
2O
3 doped Gd-400 °С, Fe
2O
3 doped Gd-600 °С and Fe
2O
3 doped Gd-800 °С, respectively. At the same time, the formation of the GdFeO
3 phase in the structure of nanoparticles leads to a decrease in the solution temperature in comparison with the rest of the samples. The specific absorption rate (SAR) was calculated according to Formula (1) [
27]:
where
M is the mass of the solution, m
nanoparticles is the mass of nanoparticles,
Сwater is the specific heat capacity of the solution, and ∆
T/
t∆ is the slope of the temperature curve.
SAR data are presented in the diagram. The measurements were carried out at a magnetic field amplitude of 210 Oe and a frequency of 320 kHz. The results of the
SAR change are shown in
Figure 8а.
A rapid heating rate is associated with a change in the phase composition and the formation of the GdFeO3 phase. As is known from the literature, the formation of structures containing gadolinium leads to an increase in the heating rate, as well as an increase in the specific absorption. However, as it was found in the course of studies of phase and structural transformations, an increase in the annealing temperature leads to an increase in the particle size, which also affects the magnetic characteristics. According to the measurement data, the values of ultrafine magnetic parameters determined using the method of Mössbauer spectroscopy change from 480 kOe for the initial nanoparticles, which corresponds to a highly disordered mixture of Fe3O4/Fe2O3 phases, to 510–512 kOe for samples annealed at 800 °C. A change in the phase composition, as well as the ordering of the magnetic and structural properties, leads to a change in the behavior of the hyperthermal characteristics. At the same time the substitution of Gd atoms for Fe atoms at the sites of the crystal lattice with the subsequent formation of the GdFeO3 phase leads to a change in the energy barriers to the relaxation of magnetic moments and, hence, in the heating rate. At the same time, a change in the magnetic properties, as well as the size of particles, leads to their aggregation upon application of a magnetic field, which leads to a change in the dynamics and rate of heating of nanoparticles.
An important characteristic for determining the effectiveness of the use of nanoparticles in hyperthermia are the values of intrinsic loss power (ILP), which characterize the dissipated thermal power of magnetic nanoparticles, and also allow comparison of the results obtained in different laboratory conditions.
Intrinsic loss power were determined using Formula (2) [
28]:
where
H is the applied field strength,
f—Frequency.
Figure 8b shows a diagram of the change in the value of intrinsic loss power depending on the type of nanoparticles.
As can be seen from the presented data, a change in the phase composition of the synthesized nanoparticles as a result of heat treatment leads to a significant increase in the SAR value, which, for nanoparticles with the GdFeO
3 phase, exceeds by 2.6 times the analogous value for nanoparticles obtained at an annealing temperature of 400 °C. Thus, it was established that a change in the phase composition leads to an increase in the specific absorption value, which indicates a large amount of heat released per unit time in the local area. Moreover, an increase in the specific absorption value leads to an increase in the intrinsic loss power value. Accordingly, the obtained values of intrinsic loss power are in good agreement with the literature data for similar values of magnetic iron-containing nanoparticles used in hyperthermia [
29,
30,
31]. In addition, the change in SAR and ILP values can also be associated with an increase in the size of nanoparticles upon thermal annealing and subsequent phase transformation, which was shown in [
31].