A Ru-Complex Tethered to a N-Rich Covalent Triazine Framework for Tandem Aerobic Oxidation-Knoevenagel Condensation Reactions
Abstract
1. Introduction
2. Results and Discussion
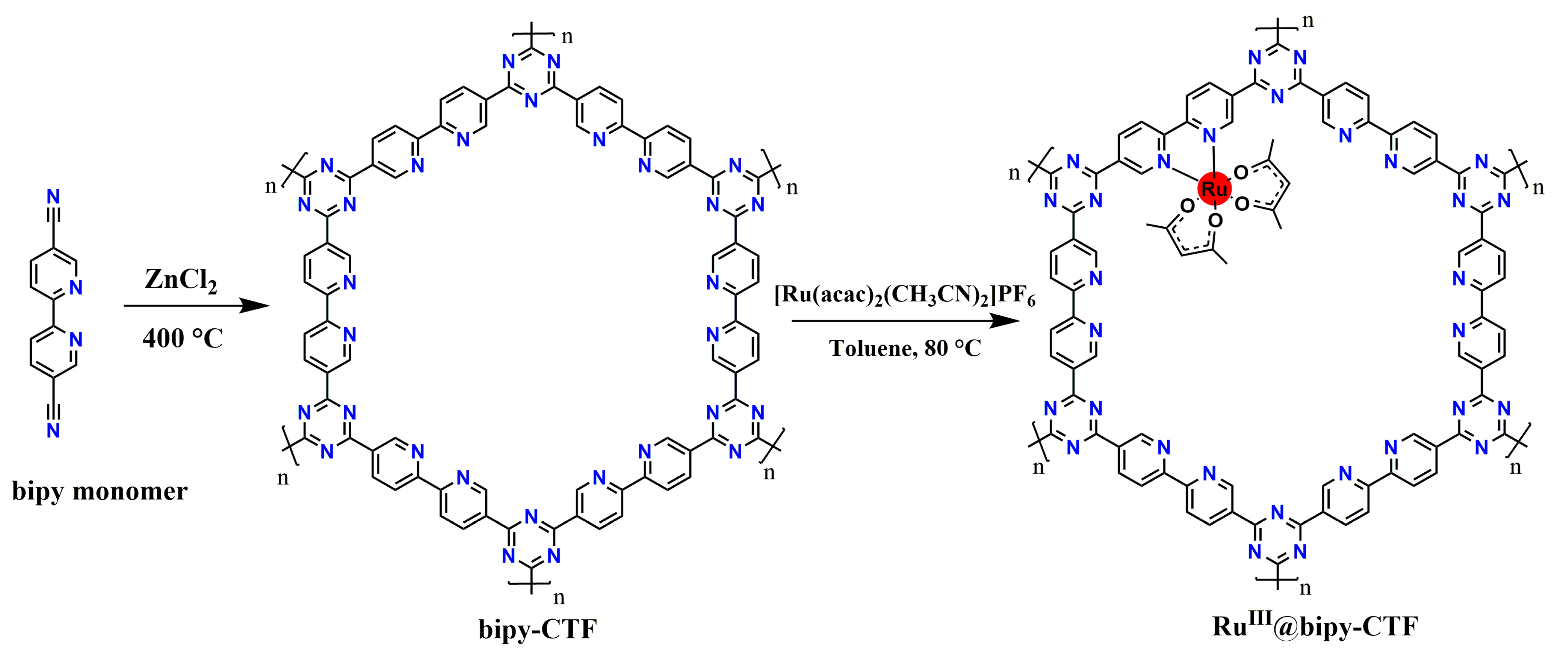
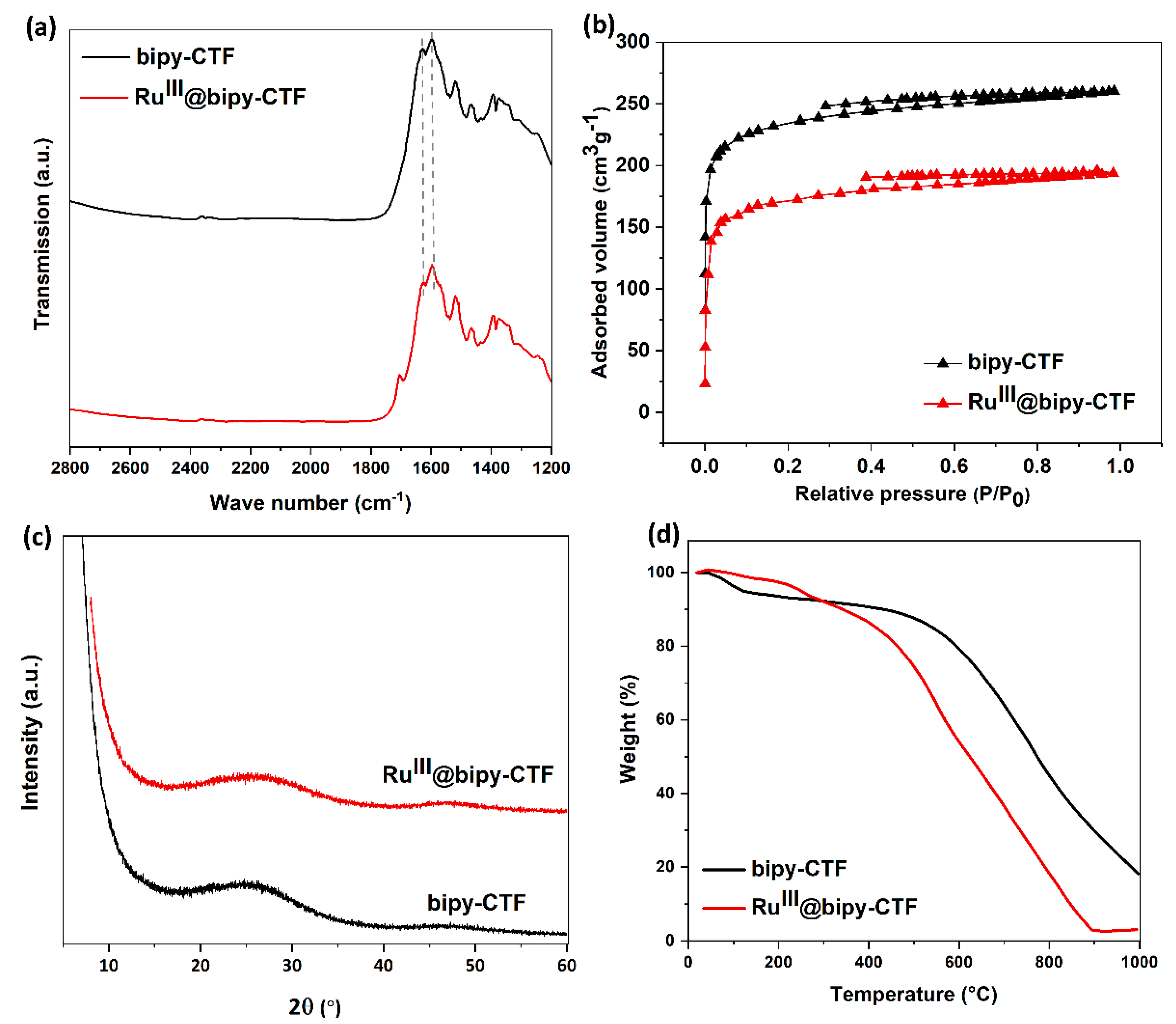
2.1. Synthesis and Characterization of the Modified Bipy-CTF with the Ru Complex (RuIII@bipy-CTF)
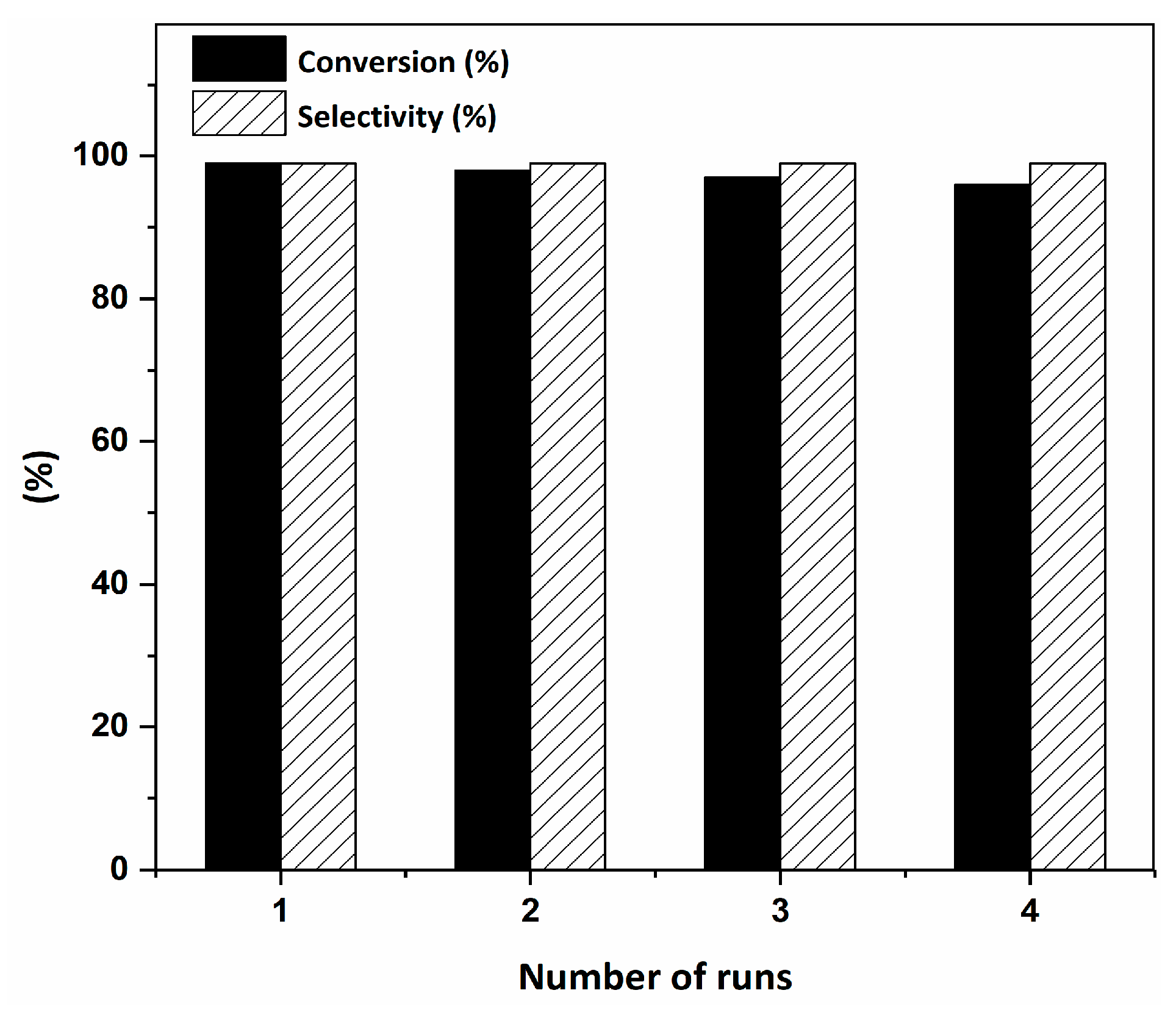
2.2. Catalytic Activity of the RuIII@bipy-CTF Catalyst in the Tandem Aerobic oxidation-Knoevenagel Condensation Reaction
3. Materials and Methods
3.1. Materials and Instrumentation
3.2. Synthesis of Bipy-CTFs and RuIII@bipy-CTF Materials
3.3. Catalytic Reactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liang, J.; Liang, Z.B.; Zou, R.Q.; Zhao, Y.L. Heterogeneous catalysis in zeolites, mesoporous silica, and metal-organic frameworks. Adv. Mater. 2017, 29, 1701139. [Google Scholar] [CrossRef] [PubMed]
- Lehman, S.E.; Larsen, S.C. Zeolite and mesoporous silica nanomaterials: Greener syntheses, environmental applications and biological toxicity. Environ. Sci. Nano. 2014, 1, 200–213. [Google Scholar] [CrossRef]
- Gopinath, K.P.; Vo, D.V.N.; Prakash, D.G.; Joseph, A.A.; Viswanathan, S.; Arun, J. Environmental applications of carbon-based materials: A review. Environ. Chem. Lett. 2020. [Google Scholar] [CrossRef]
- Huang, N.; Wang, K.C.; Drake, H.; Cai, P.Y.; Pang, J.D.; Li, J.L.; Che, S.; Huang, L.; Wang, Q.; Zhou, H.C. Tailor-made pyrazolide-based metal-organic frameworks for selective catalysis. J. Am. Chem Soc. 2018, 140, 6383–6390. [Google Scholar] [CrossRef]
- Fried, D.I.; Brieler, F.J.; Froba, M. Designing inorganic porous materials for enzyme adsorption and applications in biocatalysis. ChemCatChem 2013, 5, 862–884. [Google Scholar] [CrossRef]
- Rogge, S.M.J.; Bavykina, A.; Hajek, J.; Garcia, H.; Olivos-Suarez, A.I.; Sepulveda-Escribano, A.; Vimont, A.; Clet, G.; Bazin, P.; Kapteijn, F.; et al. Metal-organic and covalent organic frameworks as single-site catalysts. Chem Soc. Rev. 2017, 46, 3134–3184. [Google Scholar] [CrossRef]
- Thomas, A. Functional materials: From hard to soft porous frameworks. Angew. Chem. Int. Ed. 2010, 49, 8328–8344. [Google Scholar] [CrossRef]
- Geng, K.Y.; He, T.; Liu, R.Y.; Dalapati, S.; Tan, K.T.; Li, Z.P.; Tao, S.S.; Gong, Y.F.; Jiang, Q.H.; Jiang, D.L. Covalent organic frameworks: Design, synthesis, and functions. Chem. Rev. 2020, 120, 8814–8933. [Google Scholar] [CrossRef]
- Diercks, C.S.; Yaghi, O.M. The atom, the molecule, and the covalent organic framework. Science 2017, 355. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, P.; Antonietti, M.; Thomas, A. Porous, covalent triazine-based frameworks prepared by ionothermal synthesis. Angew. Chem. Int. Ed. 2008, 47, 3450–3453. [Google Scholar] [CrossRef] [PubMed]
- Artz, J. Covalent triazine-based frameworkstailor-made catalysts and catalyst supports for molecular and nanoparticulate species. ChemCatChem 2018, 10, 1753–1771. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, D.N.; Huang, D.L.; Zeng, G.M.; Xu, P.; Lai, C.; Chen, M.; Cheng, M.; Zhang, C.; Wang, Z.W. Covalent triazine frameworks for carbon dioxide capture. J. Mater. Chem. A 2019, 7, 22848–22870. [Google Scholar] [CrossRef]
- Chan-Thaw, C.E.; Villa, A.; Katekomol, P.; Su, D.S.; Thomas, A.; Prati, L. Covalent triazine framework as catalytic support for liquid phase reaction. Nano Lett. 2010, 10, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.H.; Zheng, D.S.; Liu, R.; Liu, G.H. Silica-supported molecular catalysts for tandem reactions. ChemCatChem 2018, 10, 1739–1752. [Google Scholar] [CrossRef]
- Cho, H.J.; Xu, B.J. Enabling selective tandem reactions via catalyst architecture engineering. Trends Chem. 2020, 2, 929–941. [Google Scholar] [CrossRef]
- Huang, Y.B.; Liang, J.; Wang, X.S.; Cao, R. Multifunctional metal-organic framework catalysts: Synergistic catalysis and tandem reactions. Chem Soc. Rev. 2017, 46, 126–157. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.S.; Lai, Y.H.; Yang, C.; Xu, D.Z. Iron-catalyzed one-pot oxidation/Knoevenagel condensation reaction using air as an oxidant. Appl. Organomet. Chem. 2019, 33, e4910. [Google Scholar] [CrossRef]
- Chen, C.; Yang, H.M.; Chen, J.Z.; Zhang, R.; Guo, L.; Gan, H.M.; Song, B.N.; Zhu, W.W.; Hua, L.; Hou, Z.S. One-pot tandem catalytic synthesis of alpha, beta-unsaturated nitriles from alcohol with nitriles in aqueous phase. Catal. Commun. 2014, 47, 49–53. [Google Scholar] [CrossRef]
- Yang, Z.W.; Kang, Q.X.; Quan, F.; Lei, Z.Q. Oxidation of alcohols using iodosylbenzene as oxidant catalyzed by ruthenium complexes under mild reaction conditions. J. Mol. Catal. A Chem. 2007, 261, 190–195. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Zhou, Q.; Ma, W.C.; Zhao, J.Q. Enantioselective oxidation of racemic secondary alcohols catalyzed by chiral Mn(III)-salen complex with sodium hypochlorite as oxidant. Catal. Commun. 2014, 45, 114–117. [Google Scholar] [CrossRef]
- Lou, J.D.; Xu, Z.N. Selective oxidation of primary alcohols with chromium trioxide under solvent free conditions. Tetrahedron Lett 2002, 43, 6095–6097. [Google Scholar] [CrossRef]
- Parmeggiani, C.; Matassini, C.; Cardona, F. A step forward towards sustainable aerobic alcohol oxidation: New and revised catalysts based on transition metals on solid supports. Green Chem. 2017, 19, 2030–2050. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Arends, I.W.C.E.; Ten Brink, G.J.; Dijksman, A. Green, catalytic oxidations of alcohols. Acc. Chem. Res. 2002, 35, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Piera, J.; Backvall, J.E. Catalytic oxidation of organic substrates by molecular oxygen and hydrogen peroxide by multistep electron transfer—A biomimetic approach. Angew. Chem. Int. Ed. 2008, 47, 3506–3523. [Google Scholar] [CrossRef]
- Lu, T.L.; Du, Z.T.; Liu, J.X.; Ma, H.; Xu, J. Aerobic oxidation of primary aliphatic alcohols over bismuth oxide supported platinum catalysts in water. Green Chem. 2013, 15, 2215–2221. [Google Scholar] [CrossRef]
- Abednatanzi, S.; Derakhshandeh, P.G.; Abbasi, A.; Van der Voort, P.; Leus, K. Direct synthesis of an Iridium(III) bipyridine metal-organic framework as a heterogeneous catalyst for aerobic alcohol oxidation. ChemCatChem 2016, 8, 3672–3679. [Google Scholar] [CrossRef]
- Zeng, X.M.; Chen, J.M.; Yoshimura, A.; Middleton, K.; Zhdankin, V.V. SiO2–supported RuCl3/3–(dichloroiodo)benzoic acid: Green catalytic system for the oxidation of alcohols and sulfides in water. RSC Adv. 2011, 1, 973–977. [Google Scholar] [CrossRef]
- Ganesamoorthy, S.; Tamizh, M.M.; Shanmugasundaram, K.; Karvembu, R. Immobilization of Ru(III) complex on silica: A heterogenized catalyst for selective oxidation of alcohols in water at room temperature. Tetrahedron Lett. 2013, 54, 7035–7039. [Google Scholar] [CrossRef]
- Clerick, S.; De Canck, E.; Hendrickx, K.; Van Speybroeck, V.; Van der Voort, P. Heterogeneous Ru(III) oxidation catalysts via ’click’ bidentate ligands on a periodic mesoporous organosilica support. Green Chem. 2016, 18, 6035–6045. [Google Scholar] [CrossRef]
- Ganesh Babu, S.; Krishnamoorthi, R.; Thiruneelakandan, R.; Karvembu, R. V2O5 Anchored RuO2: An efficient nanocatalyst for aerial oxidation of alcohols. Catal. Lett. 2014, 144, 1245–1252. [Google Scholar] [CrossRef]
- Wang, S.S.; Zhang, J.; Zhou, C.L.; Vo–Thanh, G.; Liu, Y. An ionic compound containing Ru(III)–complex cation and phosphotungstate anion as the efficient and recyclable catalyst for clean aerobic oxidation of alcohols. Catal. Commun. 2012, 28, 152–154. [Google Scholar] [CrossRef]
- Zahmakiran, M.; Özkar, S. Zeolite confined nanostructured dinuclear ruthenium clusters: Preparation, characterization and catalytic properties in the aerobic oxidation of alcohols under mild conditions. J. Mater. Chem. 2009, 19, 7112–7118. [Google Scholar] [CrossRef]
- Guo, H.J.; Liu, W.D.; Yin, G.C. Aerobic oxidation of alcohols to aldehydes and ketones using ruthenium(III)/Et3N catalyst. Appl Organomet. Chem. 2011, 25, 836–842. [Google Scholar] [CrossRef]
- Sodhi, R.K.; Paul, S.; Clark, J.H. A comparative study of different metal acetylacetonates covalently anchored onto amine functionalized silica: A study of the oxidation of aldehydes and alcohols to corresponding acids in water. Green Chem. 2012, 14, 1649–1656. [Google Scholar] [CrossRef]
- Hug, S.; Tauchert, M.E.; Li, S.; Pachmayr, U.E.; Lotsch, B.V. A functional triazine framework based on N–heterocyclic building blocks. J. Mater. Chem. 2012, 22, 13956–13964. [Google Scholar] [CrossRef]
- Bagherzadeh, M.; Karimi, H.; Amini, M. Immobilization of dioxomolybdenum(VI) Schiff base complex on graphene oxide nanosheets and its catalytic activity for oxidation of sulfides. J. Coord. Chem. 2017, 70, 2986–2998. [Google Scholar] [CrossRef]
- Osadchii, D.Y.; Olivos–Suarez, A.I.; Bavykina, A.V.; Gascon, J. Revisiting nitrogen species in covalent triazine frameworks. Langmuir 2017, 33, 14278–14285. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Huang, B.B.; Qiu, X.; Wang, X.; Luque, R.; Li, Y.W. Seed–mediated growth of MOF–encapsulated Pd@Ag core–shell nanoparticles: Toward advanced room temperature nanocatalysts. Chem. Sci. 2016, 7, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Asano, S.; Fujita, S.; Yoshida, H.; Arai, M. Nitrogen–doped, metal–free activated carbon catalysts for aerobic oxidation of alcohols. ACS Catal. 2015, 5, 2886–2894. [Google Scholar] [CrossRef]
- Abednatanzi, S.; Derakhshandeh, P.G.; Leus, K.; Vrielinck, H.; Callens, F.; Schmidt, J.; Savateev, A.; Van der Voort, P. Metal–free activation of molecular oxygen by covalent triazine frameworks for selective aerobic oxidation. Sci. Adv. 2020, 6, eaaz2310. [Google Scholar] [CrossRef]
- Abednatanzi, S.; Derakhshandeh, P.G.; Tack, P.; Muniz–Miranda, F.; Liu, Y.Y.; Everaert, J.; Meledina, M.; Vanden Bussche, F.; Vincze, L.; Stevens, C.V.; et al. Elucidating the promotional effect of a covalent triazine framework in aerobic oxidation. Appl. Catal. B Environ. 2020, 269, 118769. [Google Scholar] [CrossRef]
- Wang, J.S.; Jin, F.Z.; Ma, H.C.; Li, X.B.; Liu, M.Y.; Kan, J.L.; Chen, G.J.; Dong, Y.B. Au@Cu(II)–MOF: Highly efficient bifunctional heterogeneous catalyst for successive oxidation–condensation reactions. Inorg. Chem. 2016, 55, 6685–6691. [Google Scholar] [CrossRef]
- Qi, Y.; Luan, Y.; Peng, X.; Yang, M.; Hou, J.Y.; Wang, G. Design and synthesis of an Au@MIL–53(NH2) catalyst for a one–pot aerobic oxidation/knoevenagel condensation reaction. Eur. J. Inorg. Chem. 2015, 2015, 5099–5105. [Google Scholar] [CrossRef]
- Aryanejad, S.; Bagherzade, G.; Farrokhi, A. Efficient and recyclable novel Ni–based metal–organic framework nanostructure as catalyst for the cascade reaction of alcohol oxidation-Knoevenagel condensation. Appl. Organomet. Chem. 2018, 32, e3995. [Google Scholar] [CrossRef]
- Miao, Z.C.; Luan, Y.; Qi, C.; Ramella, D. The synthesis of a bifunctional copper metal organic framework and its application in the aerobic oxidation/Knoevenagel condensation sequential reaction. Dalton Trans. 2016, 45, 13917–13924. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Aguila, B.; Ma, S.Q. A bifunctional covalent organic framework as an efficient platform for cascade catalysis. Mater. Chem. Front. 2017, 1, 1310–1316. [Google Scholar] [CrossRef]
- Wu, J.Q.; Hua, W.M.; Yue, Y.H.; Gao, Z. A highly efficient bifunctional catalyst CoOx/tri–g–C(3)N(4)for one–pot aerobic oxidation-Knoevenagel condensation reaction. Catalysts 2020, 10, 712. [Google Scholar] [CrossRef]
- Liao, L.Y.; Kong, X.R.; Duan, X.F. Reductive couplings of 2–halopyridines without external ligand: Phosphine–free nickel–catalyzed synthesis of symmetrical and unsymmetrical 2,2’–bipyridines. J. Org. Chem. 2014, 79, 777–782. [Google Scholar] [CrossRef]
- Koiwa, T.; Masuda, Y.; Shono, J.; Kawamoto, Y.; Hoshino, Y.; Hashimoto, T.; Natarajan, K.; Shimizu, K. Synthesis, characterization, and detailed electrochemistry of binuclear ruthenium(III) complexes bridged by bisacetylacetonate. Crystal and molecular structures of [{Ru(acac)(2)}(2)(tae)] (acac=2,4–pentanedionate ion, tae=1,1,2,2–tetraacetylethanate dianion). Inorg. Chem. 2004, 43, 6215–6223. [Google Scholar]
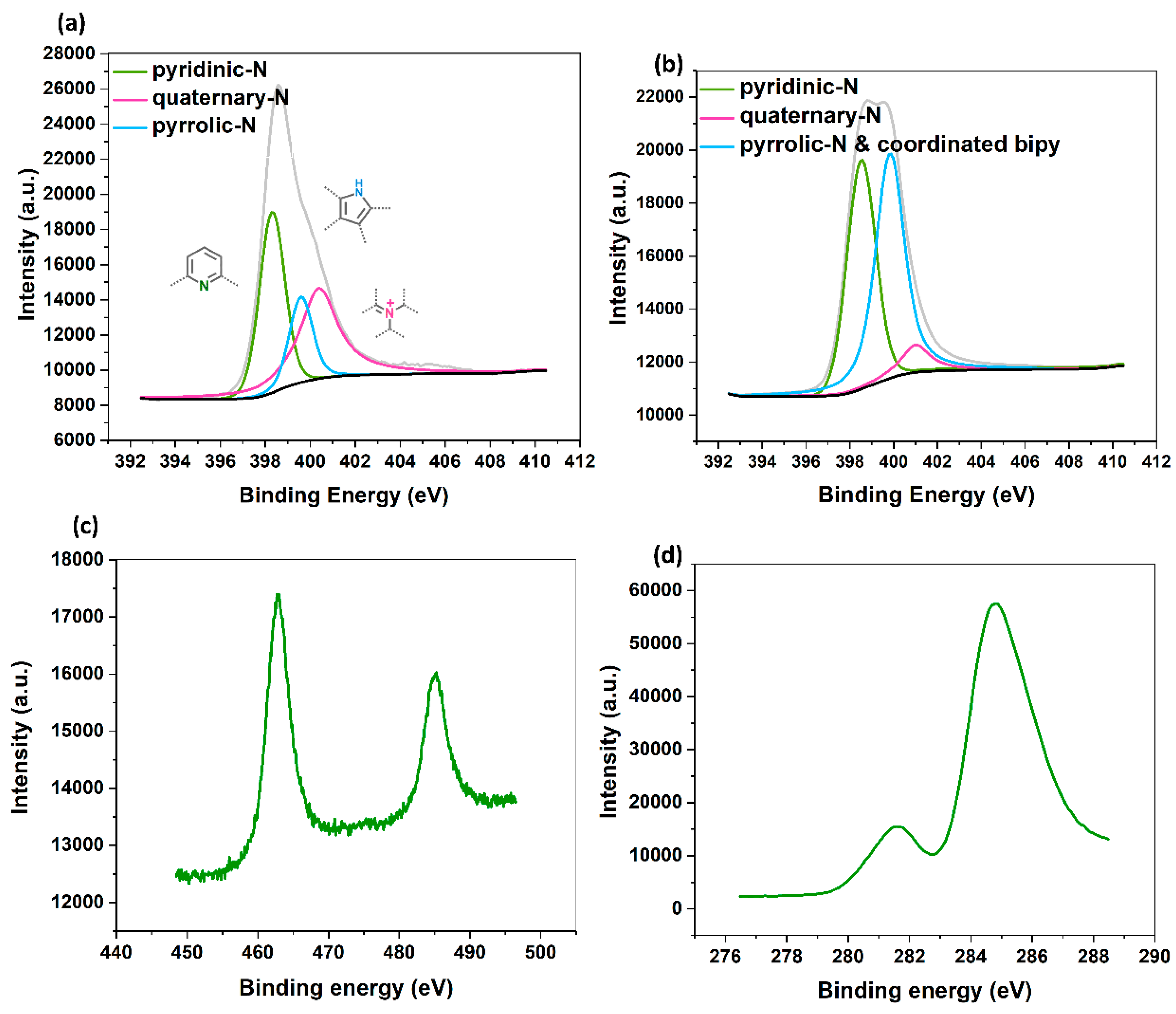
| Sample | C a (wt.%) | N a (wt.%) | C/N | Ru b (mmol g−1) |
|---|---|---|---|---|
| bipy-CTF | 58.92 | 20.27 | 2.9 | - |
| RuIII@bipy-CTF | 59.6 | 15.7 | 3.8 | 0.15 |
 | ||||
|---|---|---|---|---|
| Entry | Catalyst | Base | Conversion (%) | TON a |
| 1 | RuIII@bipy-CTF | No base | 37 | 37 |
| 2 | RuIII@bipy-CTF | Na2CO3 | 41 | 41 |
| 3 | RuIII@bipy-CTF | K2CO3 | 78 | 78 |
| 4 | RuIII@bipy-CTF | Cs2CO3 | 99 | 99 |
| 5 | RuIII@bipy-CTF b | Cs2CO3 | 64 | 267 |
| 6 | No catalyst | Cs2CO3 | <1 | - |
| 7 | RuIII@bipy-CTF c | Cs2CO3 | 3 | 3 |
| 8 | [Ru(acac)2(CH3CN)2]PF6 | Cs2CO3 | 54 | 54 |
| 9 | bipy-CTF d | Cs2CO3 | 39 | 39 |
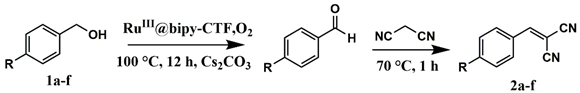 | |||
|---|---|---|---|
| Substrate | Product | Conversion of 1a–f (%) | Yield of 2a–f (%) |
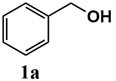 |  | 99 | 99 |
 | 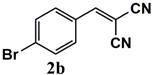 | 99 | 99 |
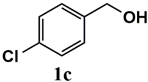 | 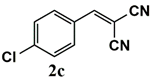 | 99 | 99 |
 |  | 97 | 97 |
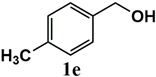 | 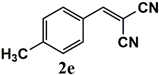 | 99 | 99 |
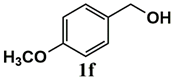 | 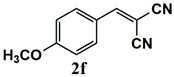 | 80 | 80 |
| Entry | Catalyst | Oxidant/Temp. (°C) | Time (h) a | Conv./Yield (%) | Ref |
|---|---|---|---|---|---|
| 1 | Au@Cu(II)-MOF | Air/110 | 15 + 7 | 99/99 | [42] |
| 2 | Au@MIL-53(NH2) | O2/100 | 13 | 99/99 | [43] |
| 3 | UoB-2 (Ni-MOF) | TBHP/65 | 1.5 | 94 | [44] |
| 4 | Cu3TATAT-3 MOF | O2, TEMPO/75 | 12 | 95/95 | [45] |
| 5 | Pd/COF-TaPa-Py | O2/80 | 4 + 1.5 | 98/98 | [46] |
| 6 | 5CoOx/tri-g-C3N4 | O2/80 | 6 | 96.4/96.4 | [47] |
| 7 | RuIII@bipy-CTF | O2/100 | 12 + 1 | 99/99 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watson, G.; Gohari Derakhshandeh, P.; Abednatanzi, S.; Schmidt, J.; Leus, K.; Van Der Voort, P. A Ru-Complex Tethered to a N-Rich Covalent Triazine Framework for Tandem Aerobic Oxidation-Knoevenagel Condensation Reactions. Molecules 2021, 26, 838. https://doi.org/10.3390/molecules26040838
Watson G, Gohari Derakhshandeh P, Abednatanzi S, Schmidt J, Leus K, Van Der Voort P. A Ru-Complex Tethered to a N-Rich Covalent Triazine Framework for Tandem Aerobic Oxidation-Knoevenagel Condensation Reactions. Molecules. 2021; 26(4):838. https://doi.org/10.3390/molecules26040838
Chicago/Turabian StyleWatson, Geert, Parviz Gohari Derakhshandeh, Sara Abednatanzi, Johannes Schmidt, Karen Leus, and Pascal Van Der Voort. 2021. "A Ru-Complex Tethered to a N-Rich Covalent Triazine Framework for Tandem Aerobic Oxidation-Knoevenagel Condensation Reactions" Molecules 26, no. 4: 838. https://doi.org/10.3390/molecules26040838
APA StyleWatson, G., Gohari Derakhshandeh, P., Abednatanzi, S., Schmidt, J., Leus, K., & Van Der Voort, P. (2021). A Ru-Complex Tethered to a N-Rich Covalent Triazine Framework for Tandem Aerobic Oxidation-Knoevenagel Condensation Reactions. Molecules, 26(4), 838. https://doi.org/10.3390/molecules26040838









