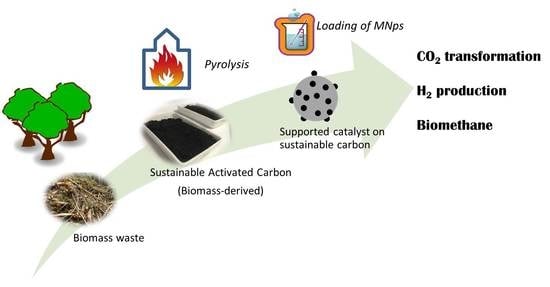
Sustainable Carbon as Efficient Support for Metal-Based Nanocatalyst: Applications in Energy Harvesting and Storage
Abstract
1. Introduction
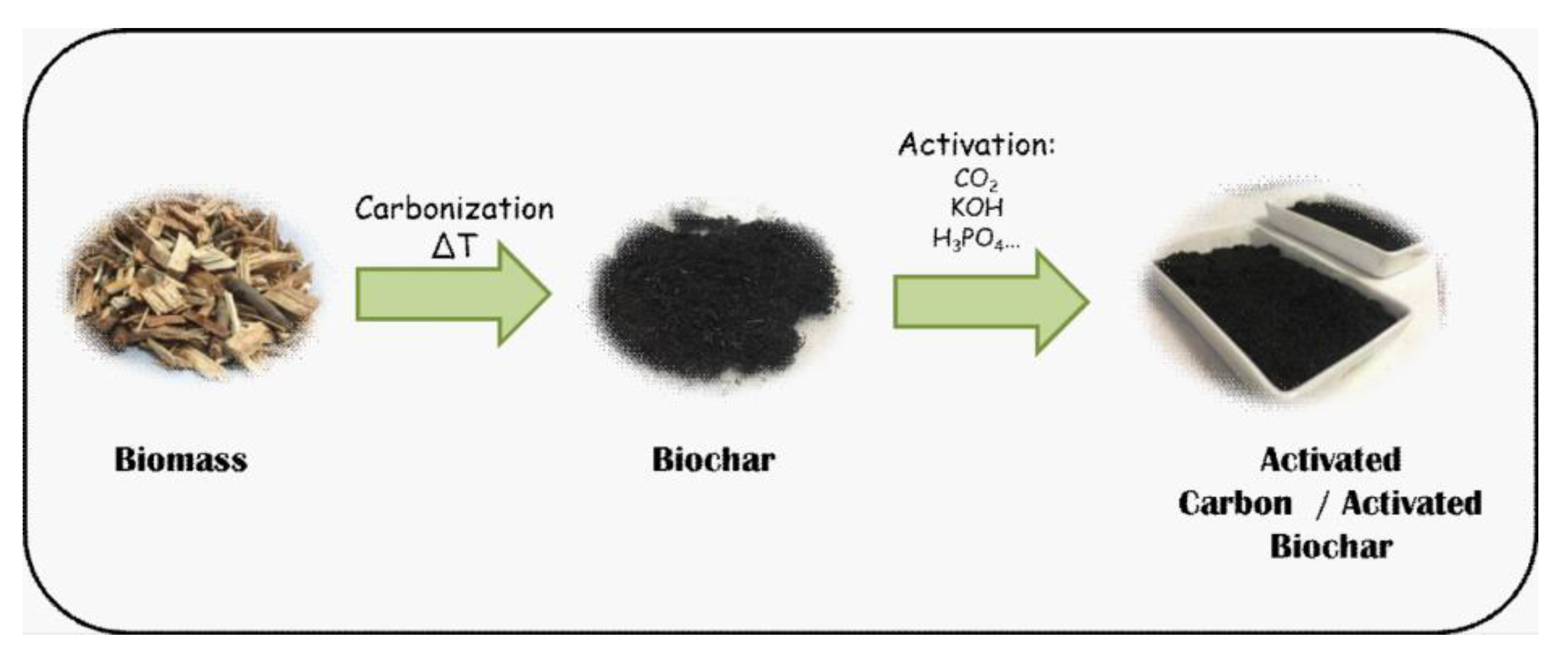
2. Sustainable Carbon-Based Materials
2.1. Synthesis of Sustainable Carbon-Based Materials
2.2. Properties of Biomass-Derived Carbons
Surface Area and Porosity
3. Biochar-Based Materials for Catalysis
3.1. Biochar-Based Materials as Catalyst Support for Energy Applications
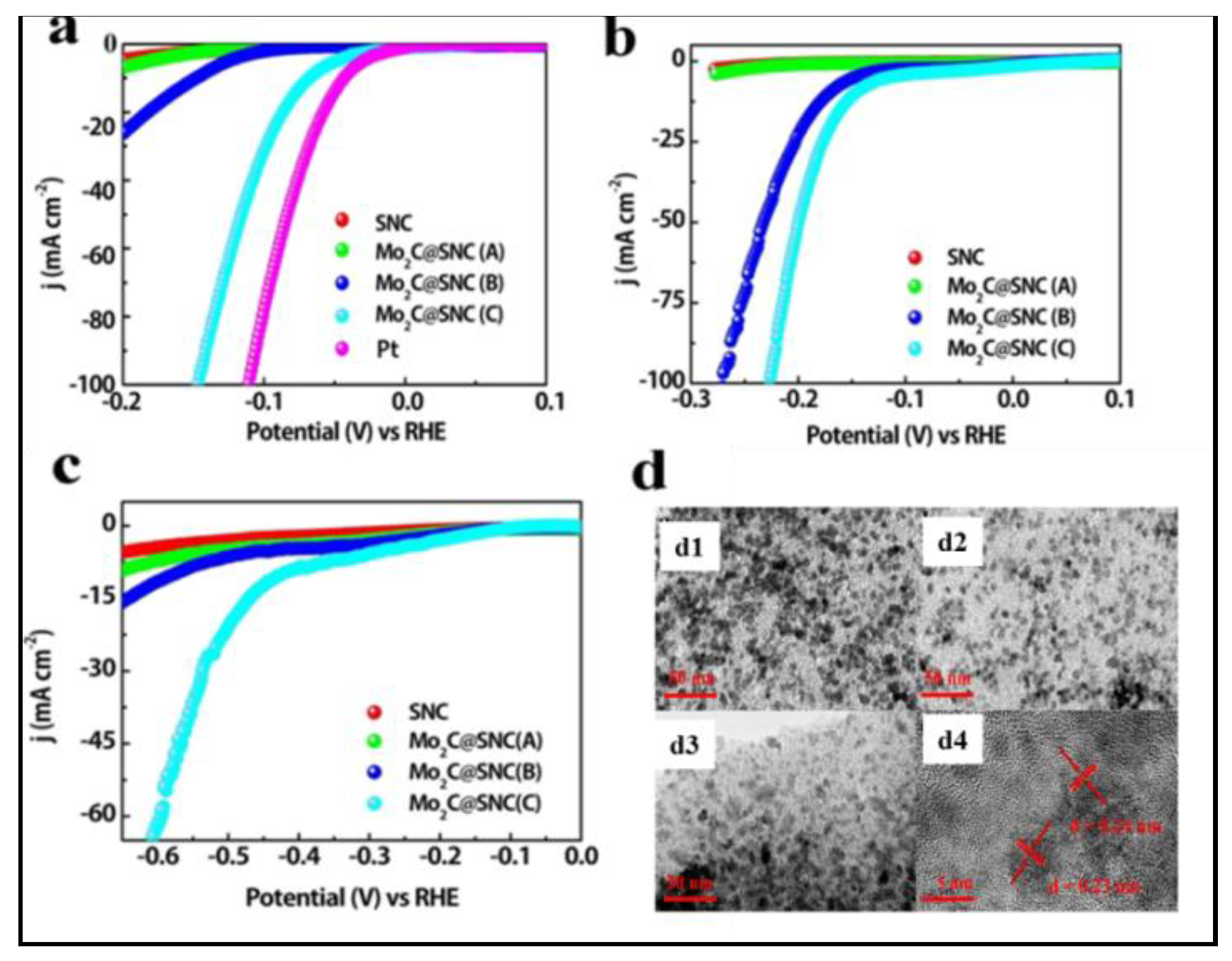
3.1.1. Biochar for Hydrogen Energy
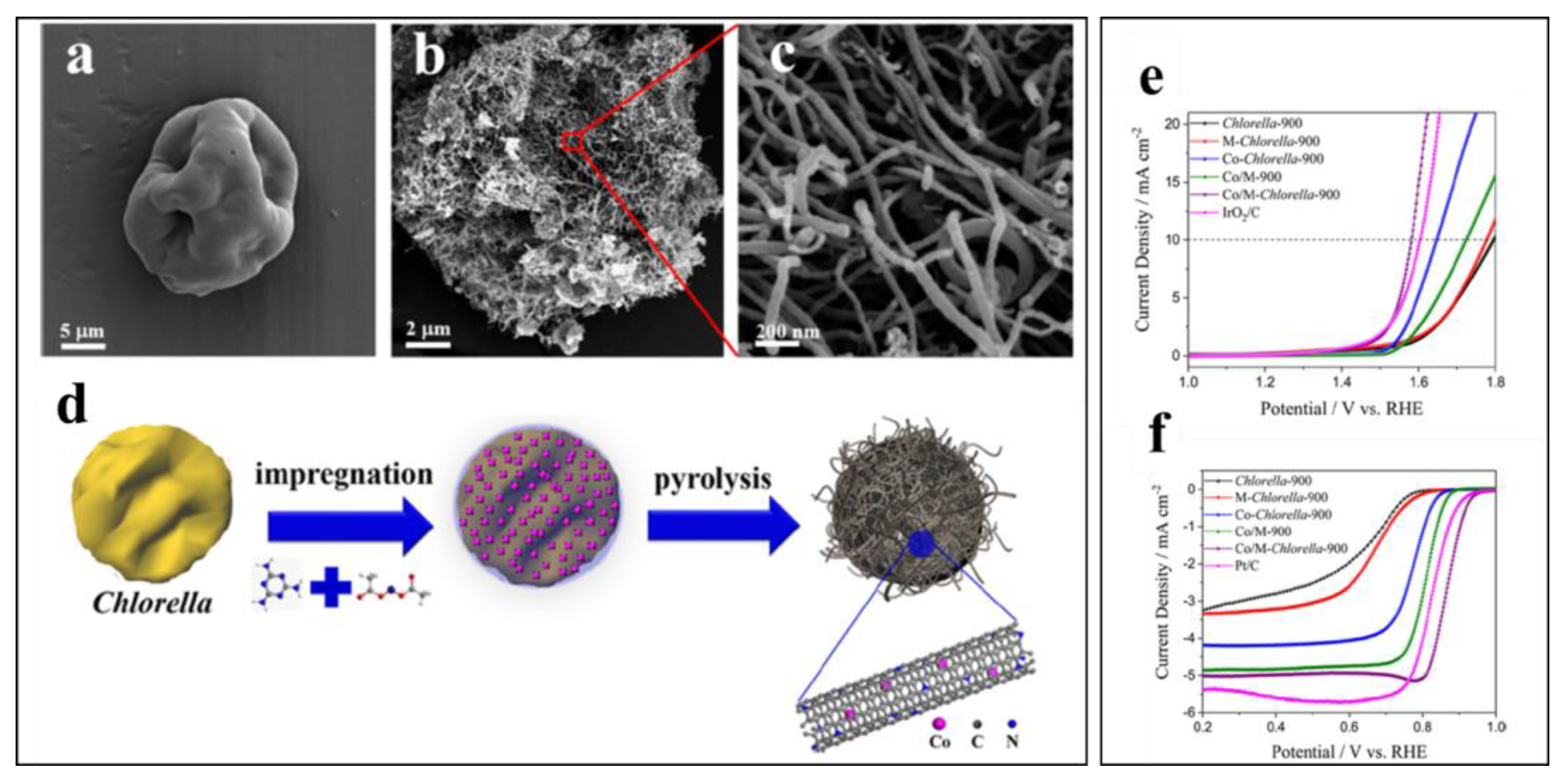
3.1.2. Biochar for Oxygen Electrocatalysis
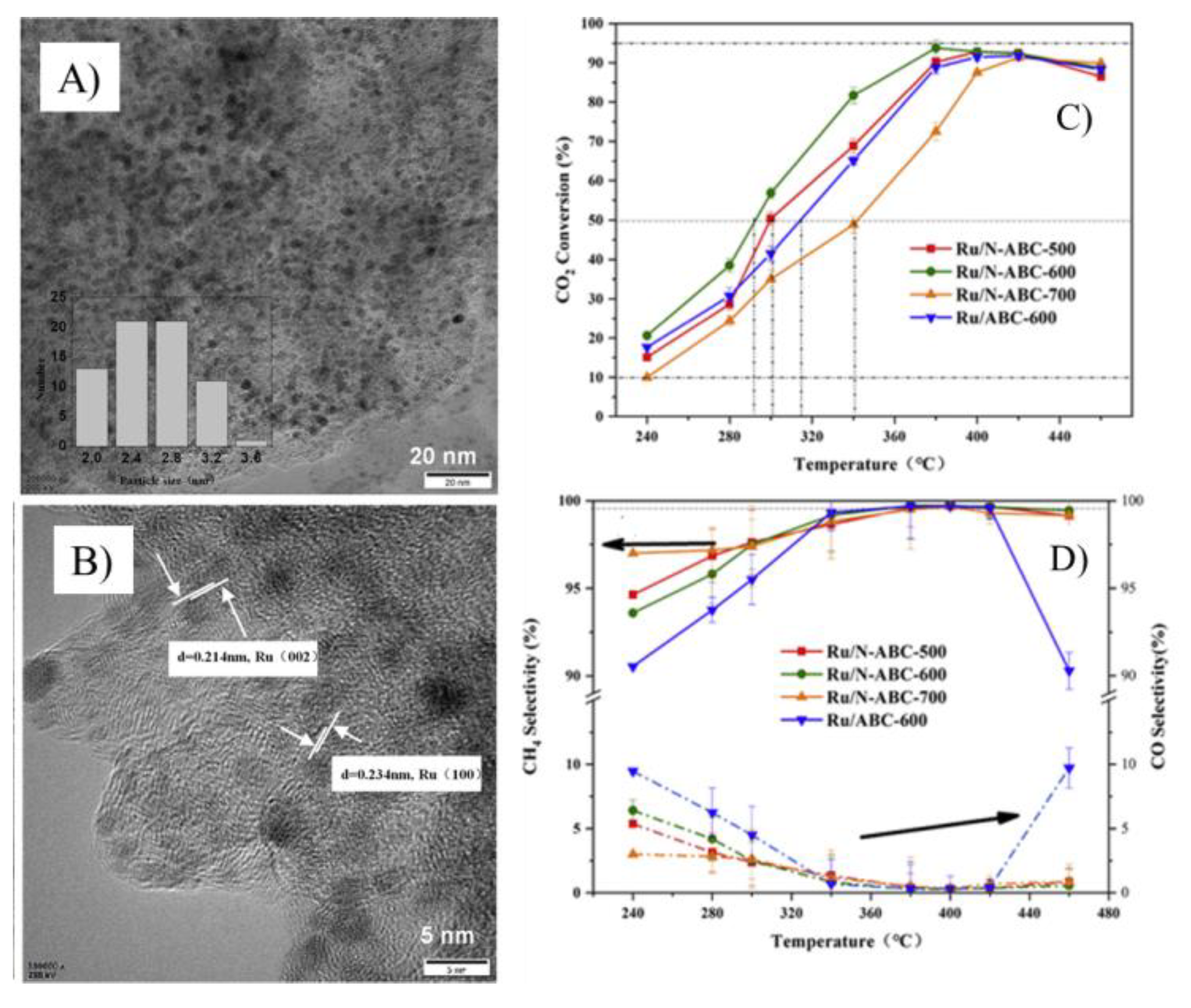
3.1.3. Biochar for Methanation of Carbon Dioxide
4. Conclusions and Future Perspectives
Funding
Conflicts of Interest
References
- A sustainable Bioeconomy for Europe: Strengthening the Connection between Economy, Society and the Environment. Updated Bioeconomy Strategy. Available online: https://ec.europa.eu/knowledge4policy/node/34337_es (accessed on 1 April 2020).
- Sharma, H.K.; Xu, C.; Qin, W. Biological Pretreatment of Lignocellulosic Biomass for Biofuels and Bioproducts: An Overview. Waste Biomass Valor 2019, 10, 235–251. [Google Scholar] [CrossRef]
- González-García, S.; Gullón, B.; Rivas, S.; Feijoo, G.; Moreira, M.T. Environmental performance of biomass refining into high-added value compounds. J. Clean. Prod. 2016, 120, 170–180. [Google Scholar] [CrossRef]
- Liu, W.-J.; Jiang, H.; Yu, H.-Q. Emerging applications of biochar-based materials for energy conversion. Energy. Environ. Sci. 2019, 12, 1751. [Google Scholar] [CrossRef]
- Maneerung, T.; Liew, J.; Dai, Y.; Kawi, S.; Chong, C.; Wang, C.-H. Activated carbon derived from carbon residue from biomass gasification and its application for dye adsorption: Kinetics, isotherms and thermodynamic studies. Bioresour. Technol. 2016, 200, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Wang, K.; Wu, L.; Yu, S.-H.; Antonietti, M.; Titirici, M.-M. Engineering carbon materials from the hydrothermal carbonization process of biomass. Adv. Mater. 2010, 22, 813–828. [Google Scholar] [CrossRef] [PubMed]
- Xiu, S.; Shahbazi, A.; Li, R. Characterization, Modification and Application of Biochar for Energy Storage and Catalysis: A Review. Trends Renew. Energy 2017, 3, 86–101. [Google Scholar] [CrossRef]
- Khezami, L.; Chetouani, A.; Taouk, B.; Capart, R. Production and characterisation of activated carbon from wood components in powder: Cellulose, lignin, xylan. Powder Technol. 2005, 157, 48–56. [Google Scholar] [CrossRef]
- Contescu, C.I.; Adhikari, S.P.; Gallego, N.C.; Evans, N.D.; Biss, B.E. Activated Carbons Derived from High-Temperature Pyrolysis of Lignocellulosic Biomass. J. Carbon Res. 2018, 4, 51. [Google Scholar] [CrossRef]
- Ioannidou, I.; Zabaniotou, A. Agricultural residues as precursors for activated carbon production—A review. Renew. Sustain. Energy Resour. 2007, 11, 1966–2005. [Google Scholar] [CrossRef]
- Namaalwa, J.; Sankhayan, P.L.; Hofstad, O. A dynamic bio-economic model for analyzing deforestation and degradation: An application to woodlands in Uganda. Forest Policy Econ. 2007, 9, 479–495. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties. Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.-H.; Kwon, E.E. Biochar as catalyst. Renew. Sust. Energy Rev. 2017, 77, 70–79. [Google Scholar] [CrossRef]
- Prati, L.; Bergna, D.; Villa, A.; Spontoni, P.; Bianchi, C.L.; Hu, T.; Romar, H.; Lassi, U. Carbons From second generation biomass as sustainable supports for catalytic systems. Cat. Tod. 2018, 301, 239–243. [Google Scholar] [CrossRef]
- Shen, Y.; Zhao, P.; Shao, Q. Porous silica and carbon derived materials from rice husk pyrolysis char. Microporous. Mesoporous. Mater. 2014, 188, 46–76. [Google Scholar] [CrossRef]
- Azargohar, R.; Dalai, A.K. Steam and KOH activation of biochar. Experimental and modeling studies. Microporous. Mesoporous. Mater. 2008, 110, 413–421. [Google Scholar] [CrossRef]
- Weber, K.; Quicker, P. Properties of biochar. Fuel 2018, 217, 240–261. [Google Scholar] [CrossRef]
- Lam, E.; Luong, J.H.T. Carbon materials as catalyst supports and catalysts in the transformation of biomass to fuels and chemicals. ACS Catal. 2014, 4, 3393–3410. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Jagiello, J.; Kenvin, J.; Celzard, A.; Fierro, V. Enhanced resolution of ultra micropore size determination of biochars and activated carbons by dual gas analysis using N2 and CO2 with 2DNLDFT adsorption models. Carbon 2019, 144, 206–215. [Google Scholar] [CrossRef]
- Jagiello, J.; Kenvin, J.; Ania, C.O.; Parra, J.B.; Celzard, A.; Fierro, V. Exploiting the adsorption of simple gases O2 and H2 with minimal quadrupole moments for the dual gas characterization of nanoporous carbons using 2D-NLDFT models. Carbon 2020, 160, 164–175. [Google Scholar] [CrossRef]
- Rodriguez-Reinoso, F.; Sepúlveda-Escribano, A. Carbon as catalyst support. In Carbon Materials for Catalysis; Serp, P., Fugueiredo, J.L., Eds.; John Wiley & Sons: New York, NY, USA, 2008; Chapter 5; pp. 131–155. [Google Scholar]
- Buaki-Sogó, M.; García, H.; Aprile, C. Imidazolium Based Silica Microreactors for the Efficient Conversion of Carbon Dioxide. Catal. Sci. Technol. 2015, 5, 1222–1230. [Google Scholar] [CrossRef]
- Buaki-Sogó, M.; Vivian, A.; Bivona, L.A.; García, H.; Gruttadauria, M.; Aprile, C. Imidazolium functionalized carbon nanotubes for the synthesis of cyclic carbonates. Reducing the gap between homogeneous and heterogeneous catalysis. Catal. Sci. Technol. 2016, 6, 8418–8427. [Google Scholar] [CrossRef]
- Somerville, M.; Jahanshahi, S. The effect of temperature and compression during pyrolysis on the density of charcoal made from Australian eucalypt wood. Renew. Energy 2015, 80, 471–478. [Google Scholar] [CrossRef]
- Brewer, C.E.; Chuang, V.J.; Masiello, C.A.; Gonnermann, H.; Gao, X.; Dugan, B.; Driver, L.E.; Panzacchi, P.; Zygourakis, K.; Davies, D.A.; et al. New approaches to measuring biochar density and porosity. Biomass Bioenergy 2014, 66, 176–185. [Google Scholar] [CrossRef]
- Anovitz, L.M.; Cole, D.R. Characterization and analysis of porosity and pore structure. Rev. Mineral. Geochem. 2015, 80, 61–164. [Google Scholar] [CrossRef]
- Wang, R.; Sang, S.; Zhu, D.; Liu, S.; Yu, K. Pore characteristics and controlling factors of the Lower Cambrian Hetang Formation shale in Northeast Jiangxi, China. Energ. Explor. Exploit. 2017, 1–23. [Google Scholar] [CrossRef]
- Pasel, J.; KaÈûner, P.; Montanari, B.; Gazzano, M.; Vaccari, A.; Makowski, W.; Lojewski, T.; Dziembaj, R.; Papp, H. Transition metal oxides supported on active carbons as low temperature catalysts for the selective catalytic reduction (SCR) of NO with NH3. Appl. Catal. B Environ. 1998, 18, 199–213. [Google Scholar] [CrossRef]
- Bazan, A.; Nowicki, P.; Półrolniczak, P.; Pietrzak, R. Thermal analysis of activated carbon obtained from residue after supercritical extraction of hops. J. Therm. Anal. Calorim. 2016, 125, 1199–1204. [Google Scholar] [CrossRef]
- Rodríguez-reinoso, F. The role of carbon materials in heterogeneous catalysis. Carbon 1998, 36, 159–175. [Google Scholar] [CrossRef]
- Liu, W.-J.; Jiang, H.; Yu, H.-Q. Development of biochar-based functional materials. Toward a sustainable platform carbon material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef]
- Umeyama, T.; Imahori, H. Photofunctional hybrid nanocarbon materials. J. Phys. Chem. C. 2013, 117, 3195–3209. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S. Advanced Photocatalytic Materials by Nanocarbon Hybrid Materials. In Nanocarbon Hybrids; Eder, D., Schlögl, R., Eds.; De Gruyter: Berlin, Germany, 2013. [Google Scholar]
- Nishihara, H.; Kyotani, T. Templated Nanocarbons for Energy Storage. Adv. Mater. 2012, 24, 4473–4498. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xiao, J.; Wang, H.; Shao, M. Carbon-Based Electrocatalysts for Hydrogen and Oxygen Evolution Reactions. ACS Catal. 2017, 7, 7855–7865. [Google Scholar] [CrossRef]
- Borenstein, A.; Hanna, O.; Attias, R.; Luski, S.; Brousse, T.; Aurbach, D. Carbon-based composite materials for supercapacitor electrodes. A review. J. Mater. Chem. A. 2017, 5, 12653–12672. [Google Scholar] [CrossRef]
- Dai, L.; Xue, Y.; Qu, L.; Choi, H.-J.; Baek, J.-B. Metal-Free Catalysts for Oxygen Reduction Reaction. Chem. Rev. 2015, 115, 4823–4892. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lv, W.; Tao, Y.; Yang, Q.-H. Towards superior volumetric performance. Design and preparation of novel carbon materials for energy storage. Energy Environ. Sci 2015, 8, 1390–1403. [Google Scholar] [CrossRef]
- Mian, M.M.; Liu, G. Recent progress in biochar-supported photocatalysts. Synthesis, role of biochar, and applications. RSC Adv. 2018, 8, 14237–14248. [Google Scholar] [CrossRef]
- Xiong, X.; Yu, I.K.M.; Cao, L.; Tsang, D.C.W.; Zhang, S.; Ok, Y.S. A review of biochar-based catalysts for chemical synthesis, biofuel production, and pollution control. Bioresour. Technol. 2017, 246, 254–270. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, J.; Meng, Y.; Aihemaiti, A.; Xu, Y.; Xiang, H.; Gao, Y.; Chen, X. Preparation, environmental application and prospect of biochar-supported metal nanoparticles: A review. J. Hazard. Mater. 2020, 388, 122026. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, Z.; Zhu, Y. Porous carbon-based materials for hydrogen storage. Advancement and challenges. J. Mater. Chem. A. 2013, 1, 9365–9381. [Google Scholar] [CrossRef]
- Back, C.-K.; Sandí, G.; Prakash, J.; Hranisavljevic, J. Hydrogen Sorption on Palladium-Doped Sepiolite-Derived Carbon Nanofibers. J. Phys. Chem. B. 2006, 110, 16225–16231. [Google Scholar] [CrossRef] [PubMed]
- Bhat, V.V.; Contescu, C.I.; Gallego, N.C. Kinetic effect of Pd additions on the hydrogen uptake of chemically-activated ultramicroporous carbon. Carbon 2010, 48, 2361–2364. [Google Scholar] [CrossRef]
- Cheon, Y.E.; Suh, M.P. Enhanced Hydrogen Storage by Palladium Nanoparticles Fabricated in a Redox-Active Metal–Organic Framework. Angew. Chem. Int. Ed. 2009, 48, 2899–2903. [Google Scholar] [CrossRef] [PubMed]
- Dufour, A.; Celzard, A.; Fierro, V.; Broust, F.; Courson, C.; Zoulalian, A.; Rouzaud, J.N. Catalytic conversion of methane over a biomass char for hydrogen production: Deactivation and regeneration by steam gasification. Appl. Catal. A General 2015, 490, 170–180. [Google Scholar] [CrossRef]
- Dufour, A.; Valin, S.; Castelli, P.; Thiery, S.; Boissonnet, G.; Zoulalian, A.; Glaud, P.-A. Mechanisms and Kinetics of Methane Thermal Conversion in a Syngas. Ind. Eng. Chem. Res. 2009, 48, 6564–6572. [Google Scholar] [CrossRef]
- Marshall, J. Solar energy: Springtime for the artificial leaf. Nature 2014, 510, 22–24. [Google Scholar] [CrossRef]
- Lin, Y.; Pan, Y.; Zhang, J. CoP nanorods decorated biomass derived N, P co-doped carbon flakes as an efficient hybrid catalyst for electrochemical hydrogen evolution. Electrochim. Acta 2017, 232, 561–569. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, M.; Yu, D.; Li, T.; Wan, M.; Zhu, H.; Du, J.; Yao, J. Functional materials from nature: Honeycomb-like carbon nanosheets derived from silk cocoon as excellent electrocatalysts for hydrogen evolution reaction. Electrochim. Acta 2016, 215, 223–230. [Google Scholar] [CrossRef]
- Cui, W.; Liu, Q.; Xing, Z.; Asiri, A.M.; Alamry, K.A.; Sun, X. MoP nanosheets supported on biomass-derived carbon flake: One-step facile preparation and application as a novel high-active electrocatalyst toward hydrogen evolution reaction. Appl. Catal. B. 2015, 164, 144–150. [Google Scholar] [CrossRef]
- Lai, F.; Miao, Y.-E.; Huang, Y.; Zhang, Y.; Liu, T. Nitrogen-Doped Carbon Nanofiber/Molybdenum Disulfide Nanocomposites Derived from Bacterial Cellulose for High-Efficiency Electrocatalytic Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces 2016, 8, 3558–3566. [Google Scholar] [CrossRef]
- Chen, W.-F.; Iyer, S.; Sasaki, K.; Wang, C.-H.; Zhu, Y.; Muckerman, J.T.; Fujita, E. Biomass-derived electrocatalytic composites for hydrogen evolution. Energy Environ. Sci. 2013, 6, 1818–1826. [Google Scholar] [CrossRef]
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrogen Energy 2013, 38, 4901. [Google Scholar] [CrossRef]
- Benck, J.D.; Chen, Z.; Kuritzky, L.Y.; Forman, A.J.; Jaramillo, T.F. Amorphous Molybdenum Sulfide Catalysts for Electrochemical Hydrogen Production: Insights into the Origin of their Catalytic Activity. ACS Catal. 2012, 2, 1916. [Google Scholar] [CrossRef]
- Kibsgaard, J.; Jaramillo, T.F. Molybdenum Phosphosulfide: An Active, Acid-Stable, Earth-Abundant Catalyst for the Hydrogen Evolution Reaction. Angew. Chem. Int. Ed. 2014, 53, 14433–14437. [Google Scholar] [CrossRef]
- Yuan, W.; Wang, X.; Zhong, X.; Li, C.M. CoP Nanoparticles in Situ Grown in Three-Dimensional Hierarchical Nanoporous Carbons as Superior Electrocatalysts for Hydrogen Evolution. ACS Appl. Mater. Interfaces 2016, 8, 20720–20729. [Google Scholar] [CrossRef] [PubMed]
- Abghoui, Y.; Skúlason, E. Hydrogen Evolution Reaction Catalyzed by Transition-Metal Nitrides. J. Phys. Chem. C. 2017, 121, 24036. [Google Scholar] [CrossRef]
- Humagain, G.; MacDougal, K.; MacInnis, J.; Lowe, J.M.; Coridan, R.H.; MacQuarrie, S.; Dasog, M. Highly Efficient, Biochar-Derived Molybdenum Carbide Hydrogen Evolution Electrocatalyst. Adv. Energy Mater. 2018, 8, 1801461. [Google Scholar] [CrossRef]
- Vrubel, H.; Hu, X. Molybdenum Boride and Carbide Catalyze Hydrogen Evolution in both Acidic and Basic Solutions. Angew. Chem. Int. Ed. 2012, 51, 12703–12706. [Google Scholar] [CrossRef]
- Miao, M.; Pan, J.; He, T.; Yan, Y.; Xia, B.Y.; Wang, X. Molybdenum Carbide-Based Electrocatalysts for Hydrogen Evolution Reaction. Chem. Eur. J. 2017, 23, 10947. [Google Scholar] [CrossRef]
- Popczun, E.J.; McKone, J.R.; Read, C.G.; Biacchi, A.J.; Wiltrout, A.M.; Lewis, N.S.; Schaak, R.E. Nanostructured nickel phosphide as an electrocatalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 2013, 135, 9267–9270. [Google Scholar] [CrossRef]
- Callejas, J.F.; McEnaney, J.M.; Read, C.G.; Crompton, J.C.; Biacchi, A.J.; Popczun, E.J.; Gordon, T.R.; Lewis, N.S.; Schaak, R.E. Electrocatalytic and photocatalytic hydrogen production from acidic and neutral-pH aqueous solutions using iron phosphide nanoparticles. ACS Nano 2014, 8, 11101–11107. [Google Scholar] [CrossRef]
- Tian, J.; Liu, Q.; Cheng, N.; Asiri, A.M.; Sun, X. Self-supported Cu3P nanowire arrays as an integrated high-performance three-dimensional cathode for generating hydrogen from water. Angew. Chem. Int. Ed. 2014, 53, 9577–9581. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-S.; Wang, Y.; Liu, C.-H.; Li, S.-L.; Wang, Y.-G.; Dong, L.-Z.; Dai, Z.-H.; Li, Y.-F.; Lan, Y.-Q. Highly Active and Stable Hydrogen Evolution Electrocatalysts Based on Molybdenum Compounds on Carbon Nanotube Graphene Hybrid Support. Nat. Commun. 2016, 7, 11204. [Google Scholar] [CrossRef] [PubMed]
- An, K.; Xu, X.; Liu, X.-X. Mo2C-Based Electrocatalyst with Biomass-Derived Sulfur and Nitrogen Co-Doped Carbon as a Matrix for Hydrogen Evolution and Organic Pollutant Removal. ACS Sustainable Chem. Eng. 2018, 6, 1446–1455. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, L.; Zhang, L.; Huang, Y.; Lu, H.; Fan, W.; Liu, T. Cotton Wool Derived Carbon Fiber Aerogel Supported Few-Layered MoSe2 Nanosheets As Efficient Electrocatalysts for Hydrogen Evolution. ACS Appl. Mater. Interfaces 2016, 8, 7077–7085. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.G.; Wang, X.; Jia, C.; Yang, J.; Wang, Q. Redox-Mediated ORR and OER Reactions: Redox Flow Lithium Oxygen Batteries Enabled with a Pair of Soluble Redox Catalysts. ACS Catal. 2016, 6, 6191–6197. [Google Scholar] [CrossRef]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-Doped Carbon Nanotube Arrays with High Electrocatalytic Activity for Oxygen Reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef]
- Shao, M.; Chang, Q.; Dodelet, J.-P.; Chenitz, R. Recent Advances in Electrocatalysts for Oxygen Reduction Reaction. Chem. Rev. 2016, 116, 3594–3657. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, X.; Dou, M.; Ji, J.; Wang, F. Biomass Derived N-Doped Porous Carbon Supported Single Fe Atoms as Superior Electrocatalysts for Oxygen Reduction. Small 2017, 13, 1604290. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zheng, L.; Deng, Y.; Liu, L.; Zeng, J.; Li, X.; Liao, S. Enhancement of Oxygen Reduction Performance of Biomass-Derived Carbon through Co-Doping with Early Transition Metal. J. Electrochem. Soc. 2018, 15, J3148–J3156. [Google Scholar] [CrossRef]
- Yang, L.; Zeng, X.; Wang, D.; Cao, D. Biomass-derived FeNi alloy and nitrogen-codoped porous carbons as highly efficient oxygen reduction and evolution bifunctional electrocatalysts for rechargeable Zn-air battery. Energy Storage Mater. 2018, 12, 277–283. [Google Scholar] [CrossRef]
- Liu, F.; Peng, H.; Qiao, X.; Fu, Z.; Huang, P.; Liao, S. High-performance doped carbon electrocatalyst derived from soybean biomass and promoted by zinc chloride. Int. J. Hydrogen Energy 2014, 39, 10128–10134. [Google Scholar] [CrossRef]
- Xiong, L.; Chen, J.-J.; Huang, Y.-X.; Li, W.-W.; Xie, J.-F.; Yu, H.-Q. An oxygen reduction catalyst derived from a robust Pd-reducing bacterium. Nano Energy 2015, 12, 33–42. [Google Scholar] [CrossRef]
- Wang, G.; Deng, Y.; Yu, J.; Zheng, L.; Du, L.; Song, H.; Liao, S. From Chlorella to Nest-like Framework Constructed with Doped Carbon Nanotubes: A Biomass-Derived, High-Performance, Bifunctional Oxygen Reduction/Evolution Catalyst. ACS Appl. Mater. Interfaces 2017, 9, 32168–32178. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Li, C.; Prajitno, H.; Yoo, J.; Patel, J.; Yang, Y.; Lim, S. Recent trend in thermal catalytic low temperature CO2 methanation. A critical review. Cat. Today 2020. [Google Scholar] [CrossRef]
- Ma, S.; Tan, Y.; Han, Y. Methanation of syngas over coral reef-like Ni/Al2O3 catalysts. J. Nat. Gas. Chem. 2011, 20, 435–442. [Google Scholar] [CrossRef]
- Gao, J.; Liu, Q.; Gu, F.; Liu, B.; Zhong, Z.; Su, F. Recent advances in methanation catalysts for the production of synthetic gas natural. RSC Adv. 2015, 5, 22759–22776. [Google Scholar] [CrossRef]
- Bailera, M.; Lisbona, P.; Romeo, L.M.; Espatolero, S. Power to Gas projects review: Lab, pilot and demo plants for storing renewable energy and CO2. Renew. Sust. Energy Mater. 2019, 69, 292–312. [Google Scholar] [CrossRef]
- Aryal, N.; Kvist, T.; Ammam, F.; Pant, D.; Ottosen, L.D.M. An overview of microbial biogas enrichment. Bioresour. Technol. 2018, 264, 359–369. [Google Scholar] [CrossRef]
- Thema, M.; Weidlich, T.; Horl, M.; Bellack, A.; Mors, F.; Hackl, F.; Kohlmayer, M.; Gleich, J.; Stabenau, C.; Trabold, T.; et al. Biological CO2-Methanation: An Approach to Standardization. Energies 2019, 12, 1670. [Google Scholar] [CrossRef]
- Thema, M.; Bauer, F.; Sterner, M. Power-to-Gas: Electrolysis and methanation status review. Renew. Sust. Energy Rev. 2019, 112, 775–787. [Google Scholar] [CrossRef]
- Mota, F.M.; Kim, D.H. From CO2 methanation to ambitious long-chain hydrocarbons. Alternative fuels paving the path to sustainability. Chem. Soc. Rev. 2019, 48, 205–259. [Google Scholar] [CrossRef] [PubMed]
- Variava, M.F.; Church, T.L.; Noorbehesht, N.; Harris, A.T.; Minett, A. Carbon-supported gas-cleaning catalysts enable syn gas methanation at atmospheric pressure. Cat. Sci. Technol. 2014, 5, 515–524. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Y.; Xiao, X.; Wang, W.; Wang, N.; Qian, W.; Chu, W. Regulation of Ni–CNT Interaction on Mn-Promoted Nickel Nanocatalysts Supported on Oxygenated CNTs for CO2 Selective Hydrogenation. ACS Appl. Mater. Interfaces 2018, 10, 41224–41236. [Google Scholar] [CrossRef]
- Swalus, C.; Jacquemin, M.; Poleunis, C.; Bertrand, P.; Ruiz, P. CO2 methanation on Rh/γ-Al2O3 catalyst at low temperature: “In situ” supply of hydrogen by Ni/activated carbon catalyst. Appl. Catal. B-Environ. 2012, 125, 41–50. [Google Scholar] [CrossRef]
- Roldán, L.; Marco, Y.; García-Bordejé, E. Origin of the Excellent Performance of Ru on Nitrogen-Doped Carbon Nanofibers for CO2 Hydrogenation to CH4. Chem. Sus. Chem. 2017, 10, 1139–1144. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, H.; Yin, Q.; Zhu, L.; Yin, S. Methanation of bio-syngas over a biochar supported catalyst. New J. Chem. 2014, 38, 4471–4477. [Google Scholar] [CrossRef]
- Zhu, L.; Yin, S.; Yin, Q.; Wang, H.; Wang, S. Biochar. A new promising catalyst support using methanation as a probe reaction. Energy Sci. Eng. 2015, 3, 126–134. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Zhu, L.; Li, Y.; Wang, K.; Qiu, K.; Tippayawong, N.; Aggarangsi, P.; Reubroycharoen, P.; Wang, S. Biomass derived N-doped biochar as efficient catalyst support for CO2 methanation. J. CO2 Utilization 2019, 34, 733–741. [Google Scholar] [CrossRef]
- Wang, W.; Duong-Viet, C.; Xu, Z.; Ba, H.; Tuci, G.; Giambastiani, G.; Liu, Y.; Truong-Huu, T.; Nhut, J.M.; Pham-Huu, C. CO2 methanation under dynamic operational mode using nickel nanoparticles decorated carbon felt (Ni/OCF) combined with inductive heating. Catal. Today 2019. [Google Scholar] [CrossRef]
- Pérez-Mayoral, E.; Calvino-Casilda, V.; Soriano, E. Metal-supported carbon-based materials. Opportunities and challenges in the synthesis of valuable products. Catal. Sci. Technol. 2016, 6, 1265–1291. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhu, Z.; Dong, S. Carbon-Based Nanostructures for Advanced Catalysis. Chem. Cat. Chem. 2015, 7, 2806–2815. [Google Scholar] [CrossRef]
- Yan, Q.; Wan, C.; Liu, J.; Gao, J.; Yu, F.; Zhang, J.; Cai, Z. Iron nanoparticles in situ encapsulated in biochar-based carbon as an effective catalyst for the conversion of biomass-derived syngas to liquid hydrocarbons. Green Chem. 2013, 15, 1631–1640. [Google Scholar] [CrossRef]
Sample Availability: Samples of the biomass-derived carbons are available from the authors. |




| Sample | Type of Isotherm (IUPAC) | BET Analysis | t-plot Analysis | ||
|---|---|---|---|---|---|
| SBET (m2/g) Experimental | Vmic (cm3/g) | Smic (m2/g) | Sext (m2/g) | ||
| Carbon Derived from Pruning Remains | Type IV (micro-mesoporous) | 639 | 0.19 | 399 | 239 |
| Carbon Derived from Sawdust | Type I (microporous) | 508 | 0.21 | 433 | 75 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buaki-Sogó, M.; Zubizarreta, L.; García-Pellicer, M.; Quijano-López, A. Sustainable Carbon as Efficient Support for Metal-Based Nanocatalyst: Applications in Energy Harvesting and Storage. Molecules 2020, 25, 3123. https://doi.org/10.3390/molecules25143123
Buaki-Sogó M, Zubizarreta L, García-Pellicer M, Quijano-López A. Sustainable Carbon as Efficient Support for Metal-Based Nanocatalyst: Applications in Energy Harvesting and Storage. Molecules. 2020; 25(14):3123. https://doi.org/10.3390/molecules25143123
Chicago/Turabian StyleBuaki-Sogó, Mireia, Leire Zubizarreta, Marta García-Pellicer, and Alfredo Quijano-López. 2020. "Sustainable Carbon as Efficient Support for Metal-Based Nanocatalyst: Applications in Energy Harvesting and Storage" Molecules 25, no. 14: 3123. https://doi.org/10.3390/molecules25143123
APA StyleBuaki-Sogó, M., Zubizarreta, L., García-Pellicer, M., & Quijano-López, A. (2020). Sustainable Carbon as Efficient Support for Metal-Based Nanocatalyst: Applications in Energy Harvesting and Storage. Molecules, 25(14), 3123. https://doi.org/10.3390/molecules25143123