Periphery-Fused Chiral A2B-Type Subporphyrin
Abstract
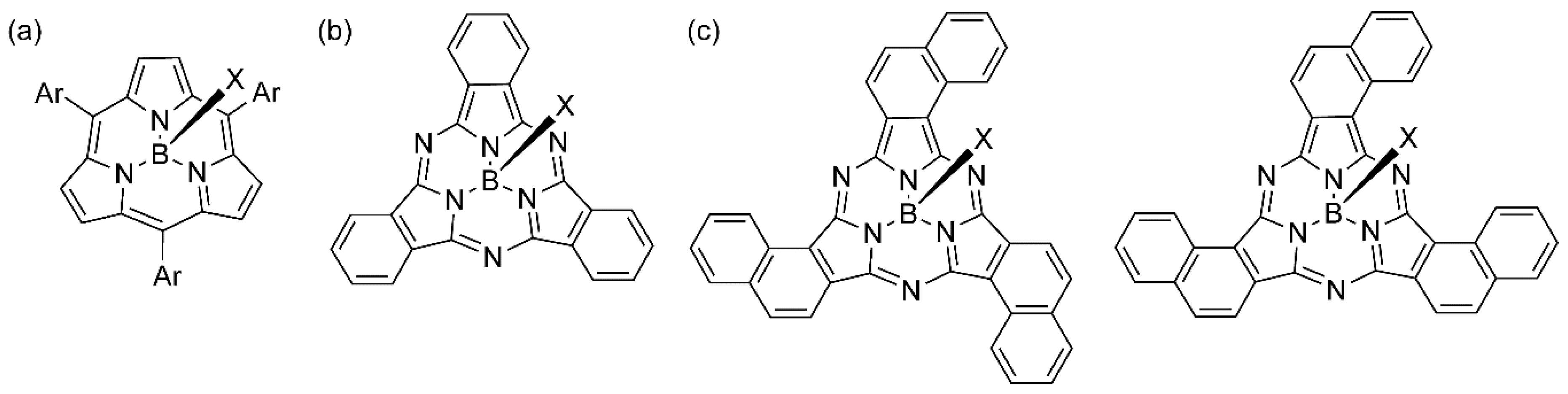
1. Introduction
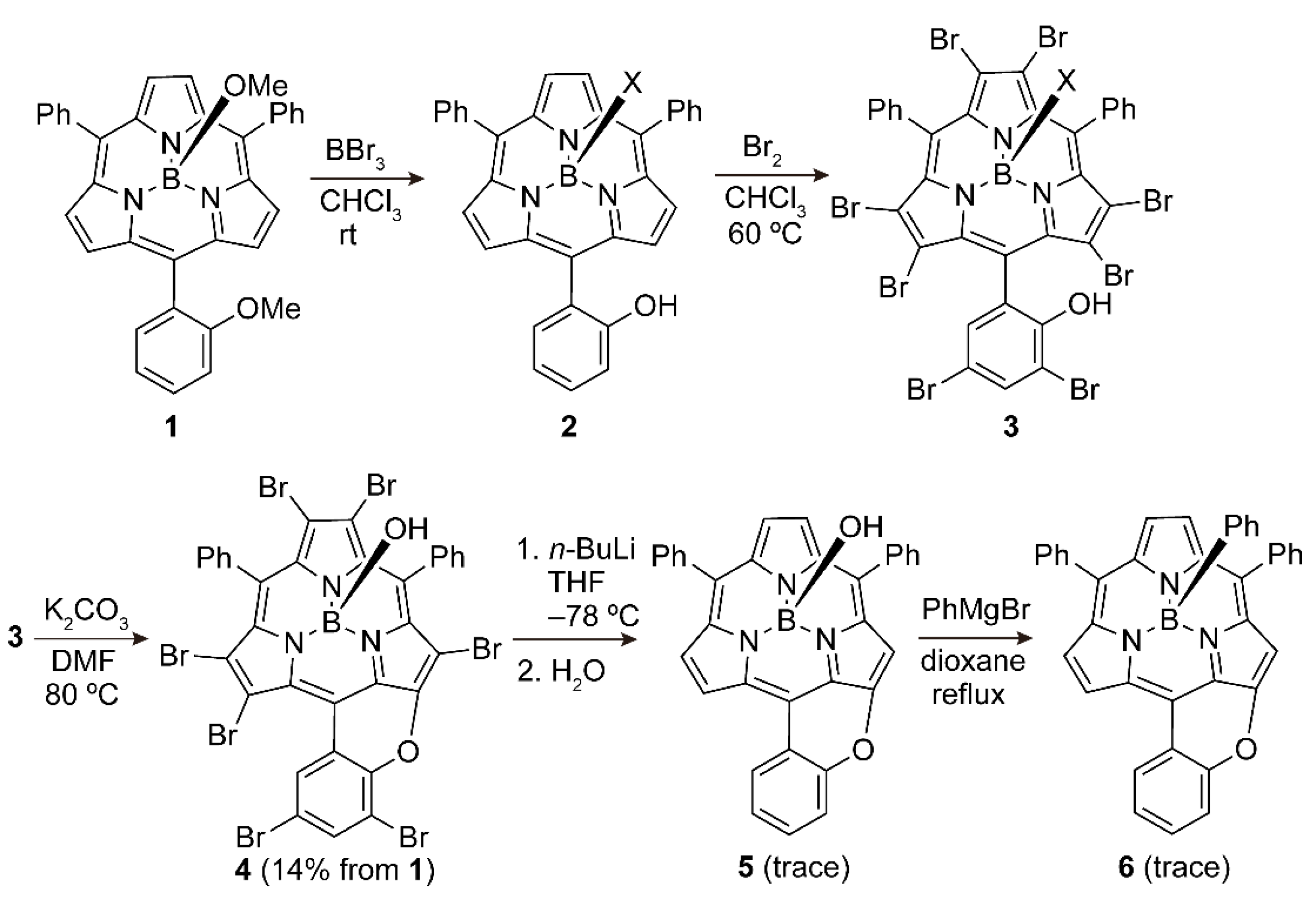
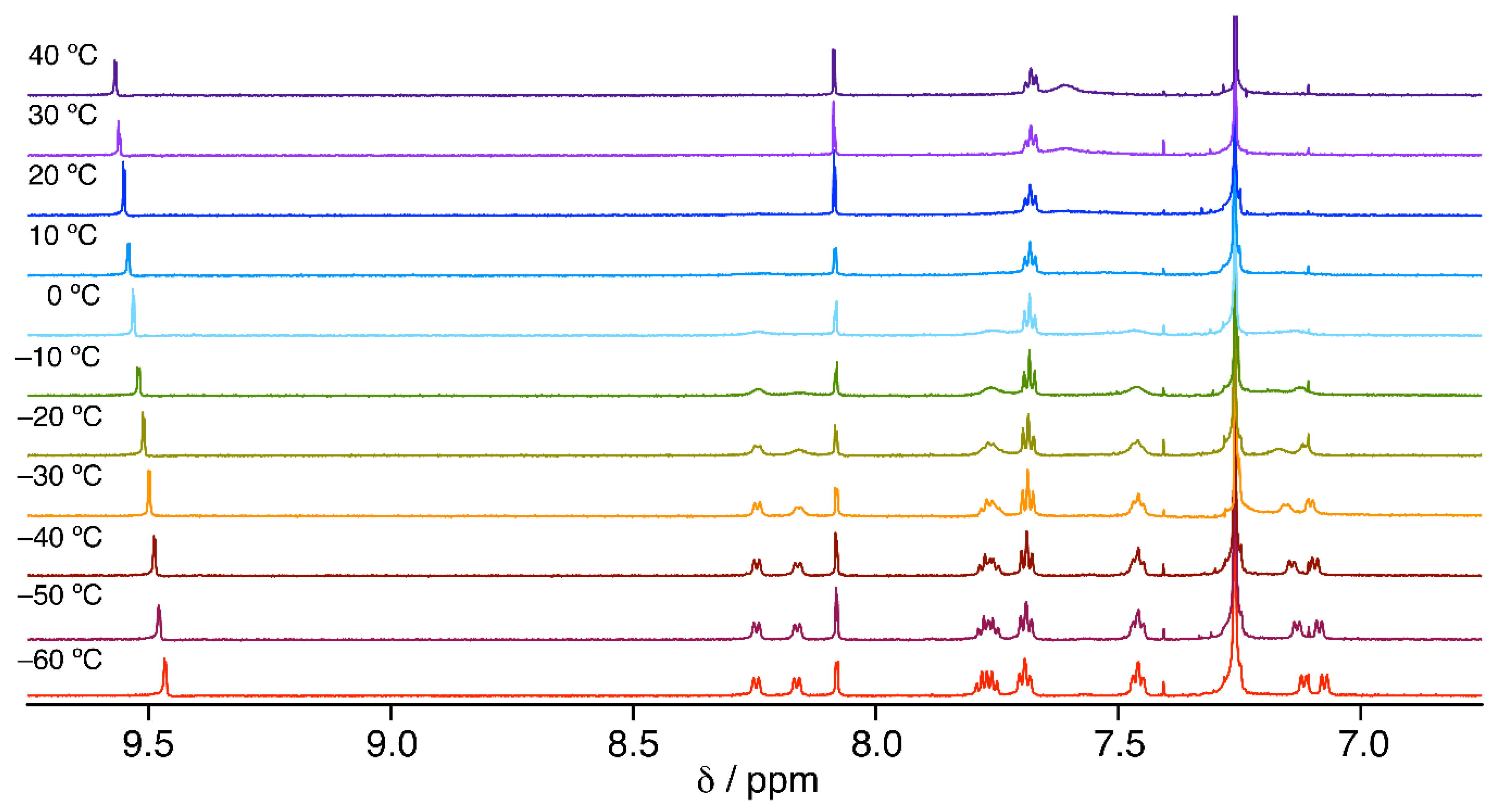
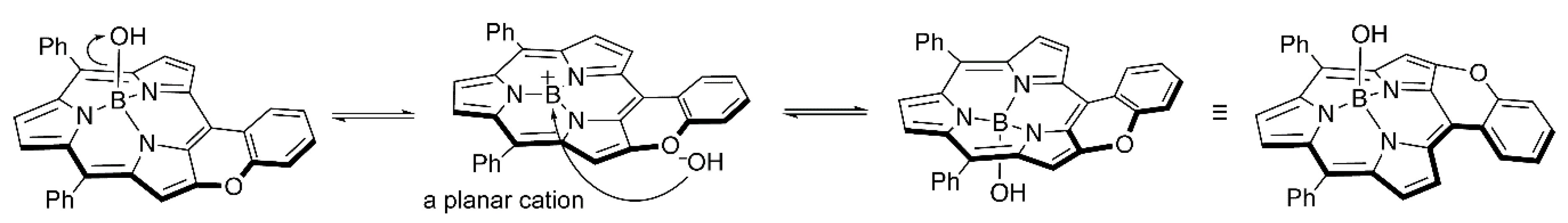
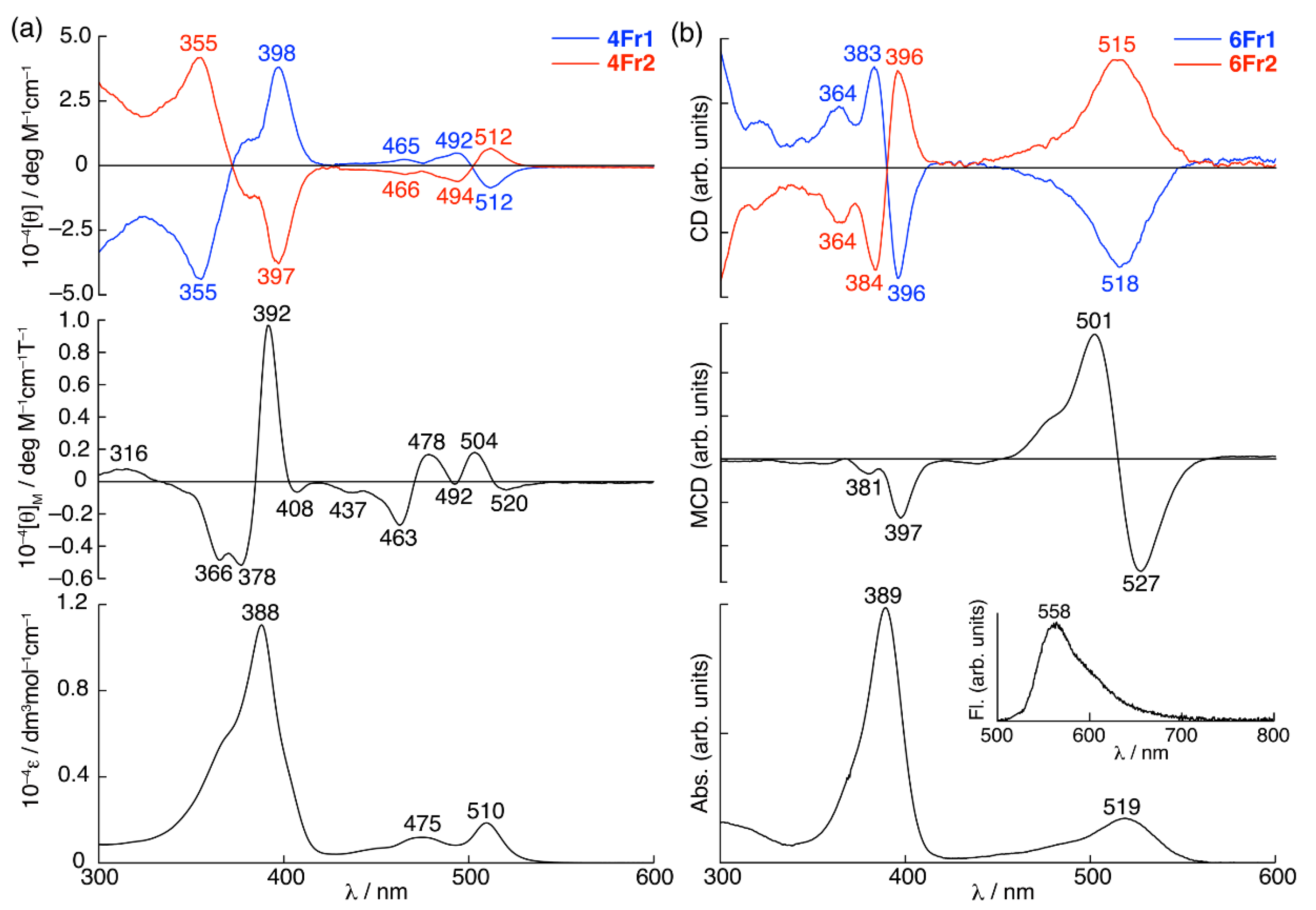
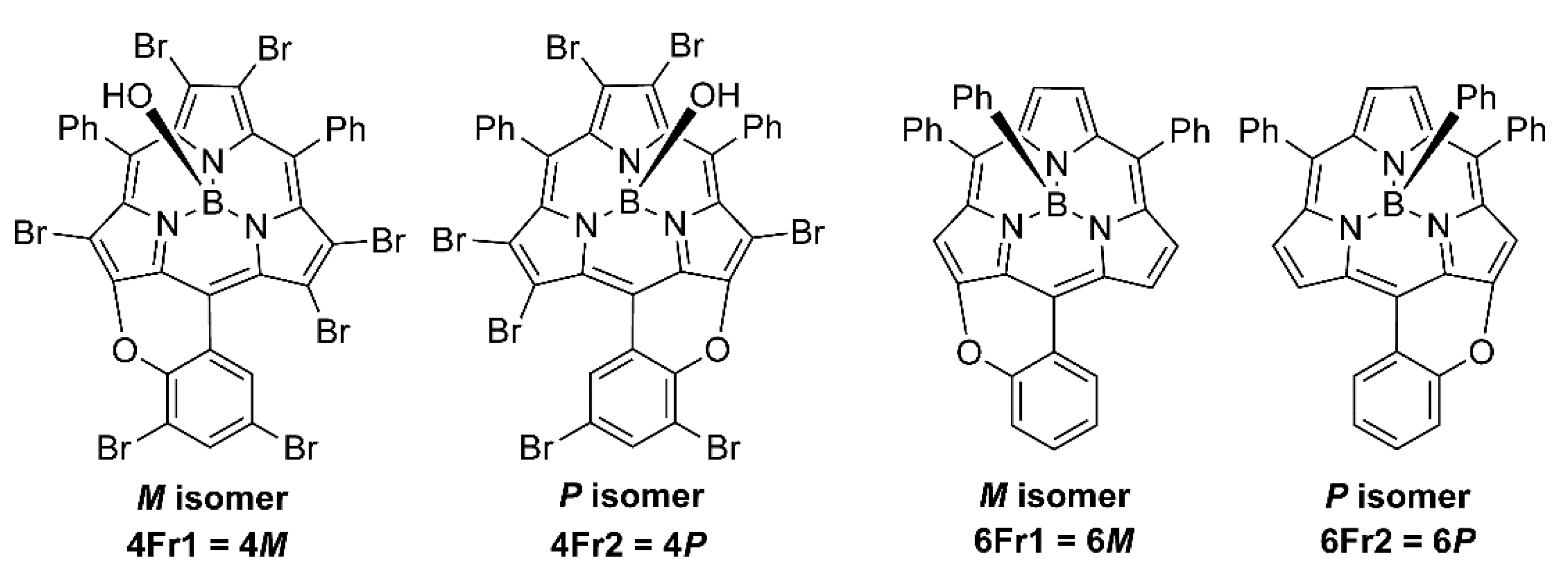
2. Results and Discussion
3. Materials and Methods
3.1. General Procedure
3.2. Theoretical Calculation Details
3.3. Synthetic Procedures
3.3.1. (5-(2-Hydroxyphenyl)-10,15-diphenylsubporphyrinato)boron(III) 2
3.3.2. (2,3,7,8,12,13-Hexabromo-5-(3,5-dibromo-2-hydroxyphenyl)-10,15-diphenylsubporphyrinato)boron(III) 3
3.3.3. Axially-Hydroxy-Substituted Pyran-Fused Perbromo-Subporphyrin 4
3.3.4. Axially-Hydroxy-Substituted Pyran-Fused Subporphyrin 5
3.3.5. Axially-Phenyl-Substituted Pyran-Fused Subporphyrin 6
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sessler, J.L.; Weghorn, S.J. Expanded, Contracted, and Isomeric Porphyrins; Pergamon Press: New York, NY, USA, 1997. [Google Scholar]
- Shimizu, S. Recent Advances in Subporphyrins and Triphyrin Analogues: Contracted Porphyrins Comprising Three Pyrrole Rings. Chem. Rev. 2017, 117, 2730–2784. [Google Scholar] [CrossRef]
- Meller, A.; Ossko, A. Triisindolo[1,2,3-Cd-1’,2’,3’-Gh-1’’,2’’,3’’-Kl][2,3a,5,6a,8,9a,9b]-Hena-Azaboraphenalene. Monatsh. Chem. 1972, 103, 150–155. [Google Scholar] [CrossRef]
- Inokuma, Y.; Kwon, J.H.; Ahn, T.K.; Yoon, M.C.; Kim, D.; Osuka, A. Tribenzosubporphines: Synthesis and Characterization. Angew. Chem. Int. Ed. 2006, 45, 961–964. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Takeuchi, Y.; Matsuda, A. meso-Aryl Subporphyrins. Angew. Chem. Int. Ed. 2007, 46, 758–760. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, Y.; Matsuda, A.; Kobayashi, N. Synthesis and Characterization of meso-Triarylsubporphyrins. J. Am. Chem. Soc. 2007, 129, 8271–8281. [Google Scholar] [CrossRef] [PubMed]
- Inokuma, Y.; Yoon, Z.S.; Kim, D.; Osuka, A. Meso-Aryl-Substituted Subporphyrins: Synthesis, Structures, and Large Substituent Effects on Their Electronic Properties. J. Am. Chem. Soc. 2007, 129, 4747–4761. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Shinokubo, H.; Sakurai, H. Figuration of bowl-shaped π-conjugated molecules: Properties and functions. Mater. Chem. Front. 2018, 2, 635–661. [Google Scholar] [CrossRef]
- Scott, L.T.; Hashemi, M.M.; Bratcher, M.S. Corannulene bowl-to-bowl inversion is rapid at room temperature. J. Am. Chem. Soc. 1992, 114, 1920–1921. [Google Scholar] [CrossRef]
- Borchardt, A.; Fuchicello, A.; Kilway, K.V.; Baldridge, K.K.; Siegel, J.S. Synthesis and dynamics of the corannulene nucleus. J. Am. Chem. Soc. 1992, 114, 1921–1923. [Google Scholar] [CrossRef]
- Sakurai, H.; Daiko, T.; Hirao, T. A Synthesis of Sumanene, a Fullerene Fragment. Science 2003, 301, 1878. [Google Scholar] [CrossRef]
- Yoshida, K.; Osuka, A. β,β-(1,4-Dithiino)subporphyrin Dimers Capturing Fullerenes with Large Association Constants. Chem. Eur. J. 2016, 22, 9396–9403. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Mori, H.; Tanaka, T.; Mori, T.; Osuka, A. ABC-Type meso-Triaryl-Substituted Subporphyrins. Eur. J. Org. Chem. 2014, 3997–4004. [Google Scholar] [CrossRef]
- Shimizu, S.; Miura, A.; Khene, S.; Nyokong, T.; Kobayashi, N. Chiral 1,2-Subnaphthalocyanines. J. Am. Chem. Soc. 2011, 133, 17322–17328. [Google Scholar] [CrossRef]
- Liang, X.; Shimizu, S.; Kobayashi, N. Sizeable red-shift of absorption and fluorescence of subporphyrazine induced by peripheral push and pull substitution. Chem. Commun. 2014, 50, 13781–13784. [Google Scholar] [CrossRef]
- Yoshida, K.; Copley, G.; Mori, H.; Osuka, A. Probing the Rotational Dynamics of meso-(2-Substituted)aryl Substituents in A2B-Type Subporphyrins. Chem. Eur. J. 2014, 20, 10065–10072. [Google Scholar] [CrossRef] [PubMed]
- Saga, S.; Hayashi, S.-y.; Yoshida, K.; Tsurumaki, E.; Kim, P.; Sung, Y.M.; Sung, J.; Tanaka, T.; Kim, D.; Osuka, A. Subporphyrins with an Axial B–C Bond. Chem. Eur. J. 2013, 19, 11158–11161. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, D.; Mori, H.; Kitano, M.; Cha, W.-Y.; Oh, J.; Tanaka, T.; Kim, D.; Osuka, A. Nucleophilic Aromatic Substitution Reactions of meso-Bromosubporphyrin: Synthesis of a Thiopyrane-Fused Subporphyrin. Chem. Eur. J. 2014, 20, 16194–16202. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, S.; Nakano, S.; Kojima, A.; Kobayashi, N. A Core-Expanded Subphthalocyanine Analogue with a Significantly Distorted Conjugated Surface and Unprecedented Properties. Angew. Chem. Int. Ed. 2014, 53, 2408–2412. [Google Scholar] [CrossRef] [PubMed]
- Tsurumaki, E.; Inokuma, Y.; Easwaramoorthi, S.; Lim, J.M.; Kim, D.; Osuka, A. Peripheral Hexabromination, Hexaphenylation, and Hexaethynylation of meso-Aryl-Substituted Subporphyrins. Chem. Eur. J. 2009, 15, 237–247. [Google Scholar] [CrossRef]
- Yoshida, K.; Osuka, A. Observation of Diastereomeric Interconversions of β-Sulfinylsubporphyrins as Evidence for Bowl Inversion. Chem. Eur. J. 2015, 21, 11727–11734. [Google Scholar] [CrossRef]
- Mack, J.; Stillman, M.J.; Kobayashi, N. Application of MCD spectroscopy to porphyrinoids. Coord. Chem. Rev. 2007, 251, 429–453. [Google Scholar] [CrossRef]
- Kobayashi, N.; Muranaka, A.; Mack, J. Circular Dichroism and Magnetic Circular Dichroism Spectroscopy for Organic Chemists; Royal Society of Chemistry: UK, 2012. [Google Scholar]
- Kaito, A.; Nozawa, T.; Yamamoto, T.; Hatano, M.; Orii, Y. LCAO MO SCF π-Electron Calculations on Magnetic Circular-Dichroism of Porphin, Protoporphyrin, and Porphyrin-A. Chem. Phys. Lett. 1977, 52, 154–160. [Google Scholar] [CrossRef]
- Tajiri, A.; Winkler, J. Magnetic Circular Dichroism and Molecular Orbital Studies on Condensed Thiadiazoles. Z. Naturforsch. A Phys. Sci. 1983, 38a, 1263–1269. [Google Scholar] [CrossRef][Green Version]
- Michl, J. Magnetic circular dichroism of cyclic π-electron systems. 1. Algebraic solution of the perimeter model for the A and B terms of high-symmetry systems with a (4N + 2)-electron [n]annulene perimeter. J. Am. Chem. Soc. 1978, 100, 6801–6811. [Google Scholar] [CrossRef]
- Michl, J. Electronic Structure of Aromatic π-Electron Systems as Reflected in their MCD Spectra. Pure Appl. Chem. 1980, 52, 1549–1563. [Google Scholar] [CrossRef][Green Version]
- Michl, J. Magnetic circular dichroism of aromatic molecules. Tetrahedron 1984, 40, 3845–3934. [Google Scholar] [CrossRef]
- Vancoillie, S.; Hendrickx, M.; Nguyen, M.T.; Pierloot, K.; Ceulemans, A.; Mack, J.; Kobayashi, N. Fourteen-Electron Ring Model and the Anomalous Magnetic Circular Dichroism of meso-Triarylsubporphyrins. J. Phys. Chem. A 2012, 116, 3960–3967. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian09; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]

| Compd. | No. | λa | fb | Major Contributions c |
|---|---|---|---|---|
| 4’ | 1 | 478 | 0.14 | H–1→L+1 (19%), H→L (77%) |
| 2 | 463 | 0.02 | H–1→L (38%), H→L+1 (59%) | |
| 4 | 389 | 0.13 | H–3→L (34%), H–2→L (11%), H–2→L+1 (19%), H–1→L (20%) | |
| 8 | 361 | 0.53 | H–3→L+1 (25%), H–1→L+1 (36%), H→L (12%) | |
| 6’ | 1 | 477 | 0.16 | H–1→L+1 (18%), H→L (80%) |
| 2 | 461 | 0.09 | H–1→L (28%), H→L+1 (70%) | |
| 3 | 365 | 0.61 | H–2→L+1 (12%), H–1→L (52%), H→L+1 (22%) | |
| 4 | 361 | 0.43 | H–2→L (14%), H–1→L+1 (56%), H→L (12%) |

| Compd. | No. | λa | μb | mc | Re | |
|---|---|---|---|---|---|---|
| (nm) | (°) | |||||
| 4’P | 1 | 478 | 3.77 | 3.82 | 90 | 0.92 |
| 2 | 463 | 1.27 | 1.03 | 90 | –0.09 | |
| 3 | 401 | 1.97 | 1.07 | 122 | –11.0 | |
| 4 | 389 | 3.29 | 6.00 | 88 | 6.70 | |
| 8 | 361 | 6.39 | 4.10 | 77 | 59.4 | |
| 6’P | 1 | 477 | 4.08 | 3.95 | 87 | 7.95 |
| 2 | 461 | 2.94 | 1.23 | 81 | 5.39 | |
| 3 | 365 | 6.87 | 1.78 | 78 | 26.4 | |
| 4 | 361 | 5.73 | 2.72 | 95 | –13.2 | |
| 6’P-90 | 1 | 453 | 3.02 | 4.40 | 81 | 19.8 |
| 2 | 442 | 1.93 | 1.27 | 90 | –0.10 | |
| 3 | 355 | 6.11 | 1.75 | 88 | 3.55 | |
| 4 | 352 | 5.44 | 3.71 | 85 | 18.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirokawa, S.; Kobayashi, N.; Shimizu, S. Periphery-Fused Chiral A2B-Type Subporphyrin. Molecules 2021, 26, 1140. https://doi.org/10.3390/molecules26041140
Hirokawa S, Kobayashi N, Shimizu S. Periphery-Fused Chiral A2B-Type Subporphyrin. Molecules. 2021; 26(4):1140. https://doi.org/10.3390/molecules26041140
Chicago/Turabian StyleHirokawa, Shoma, Nagao Kobayashi, and Soji Shimizu. 2021. "Periphery-Fused Chiral A2B-Type Subporphyrin" Molecules 26, no. 4: 1140. https://doi.org/10.3390/molecules26041140
APA StyleHirokawa, S., Kobayashi, N., & Shimizu, S. (2021). Periphery-Fused Chiral A2B-Type Subporphyrin. Molecules, 26(4), 1140. https://doi.org/10.3390/molecules26041140







