Efficient Regioselective Synthesis of Novel Water-Soluble 2H,3H-[1,4]thiazino[2,3,4-ij]quinolin-4-ium Derivatives by Annulation Reactions of 8-quinolinesulfenyl Halides
Abstract
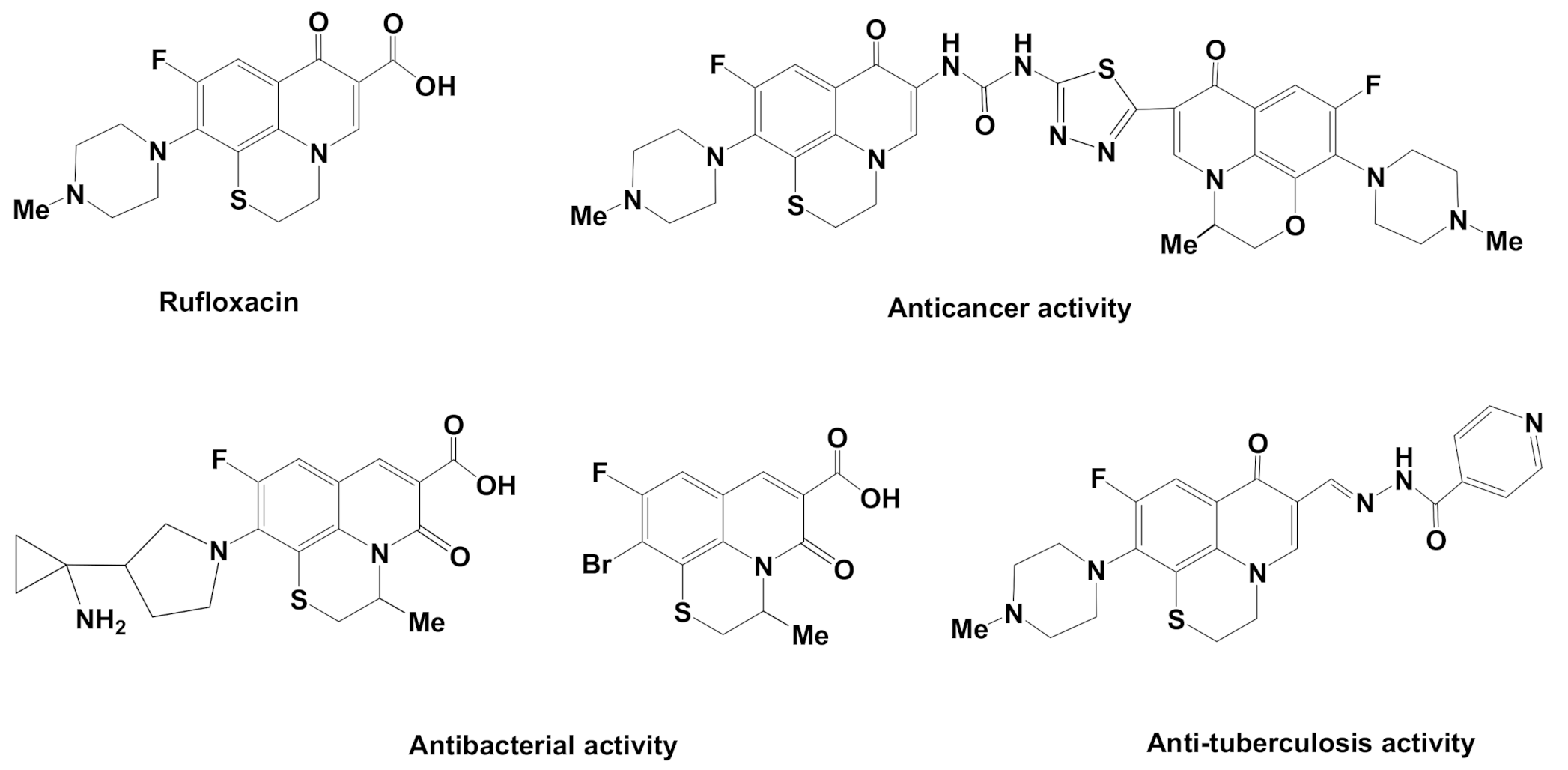
1. Introduction
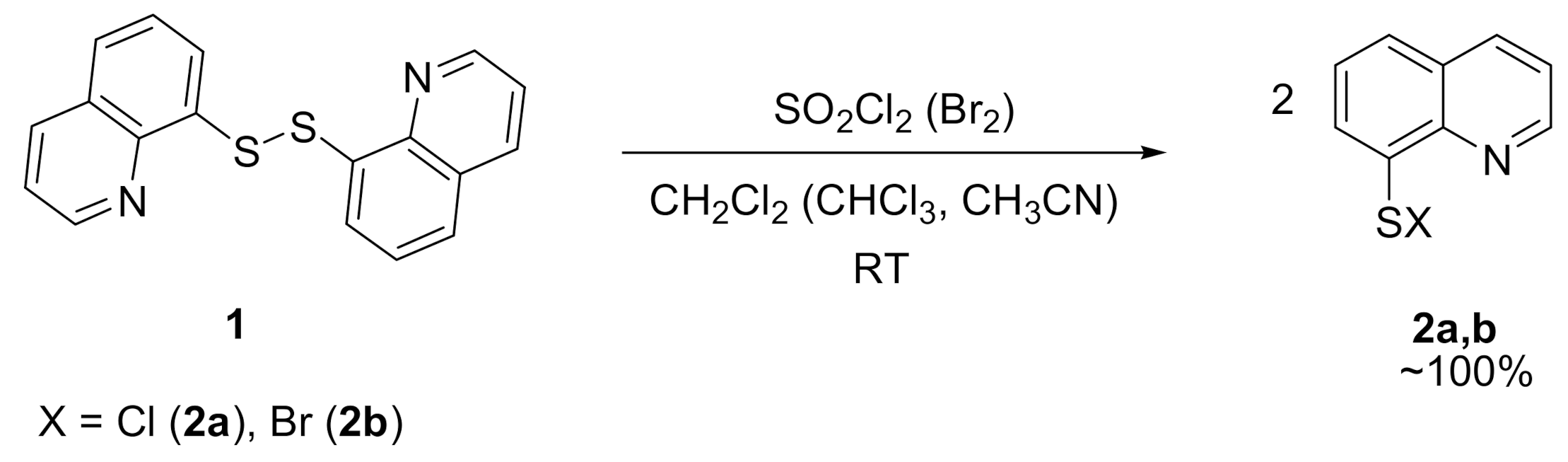
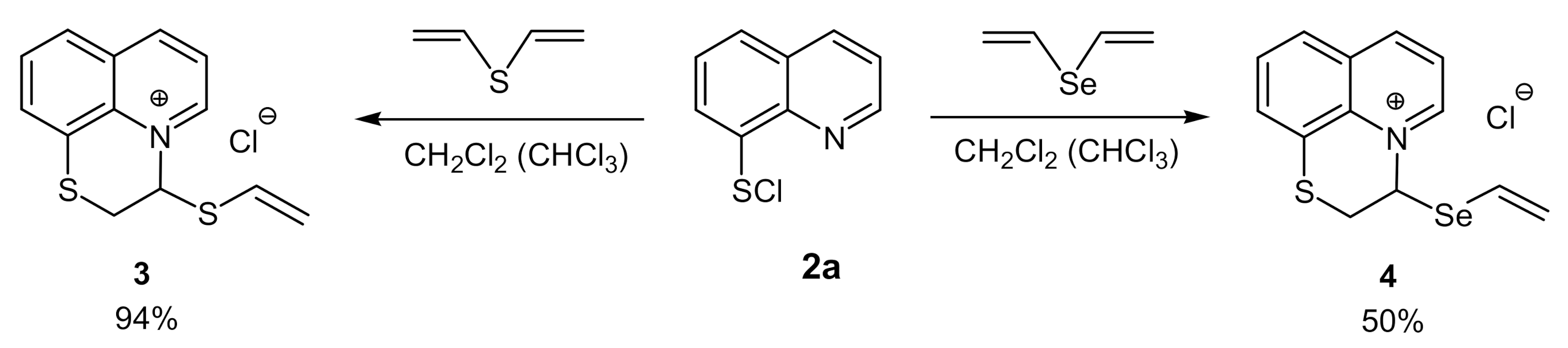
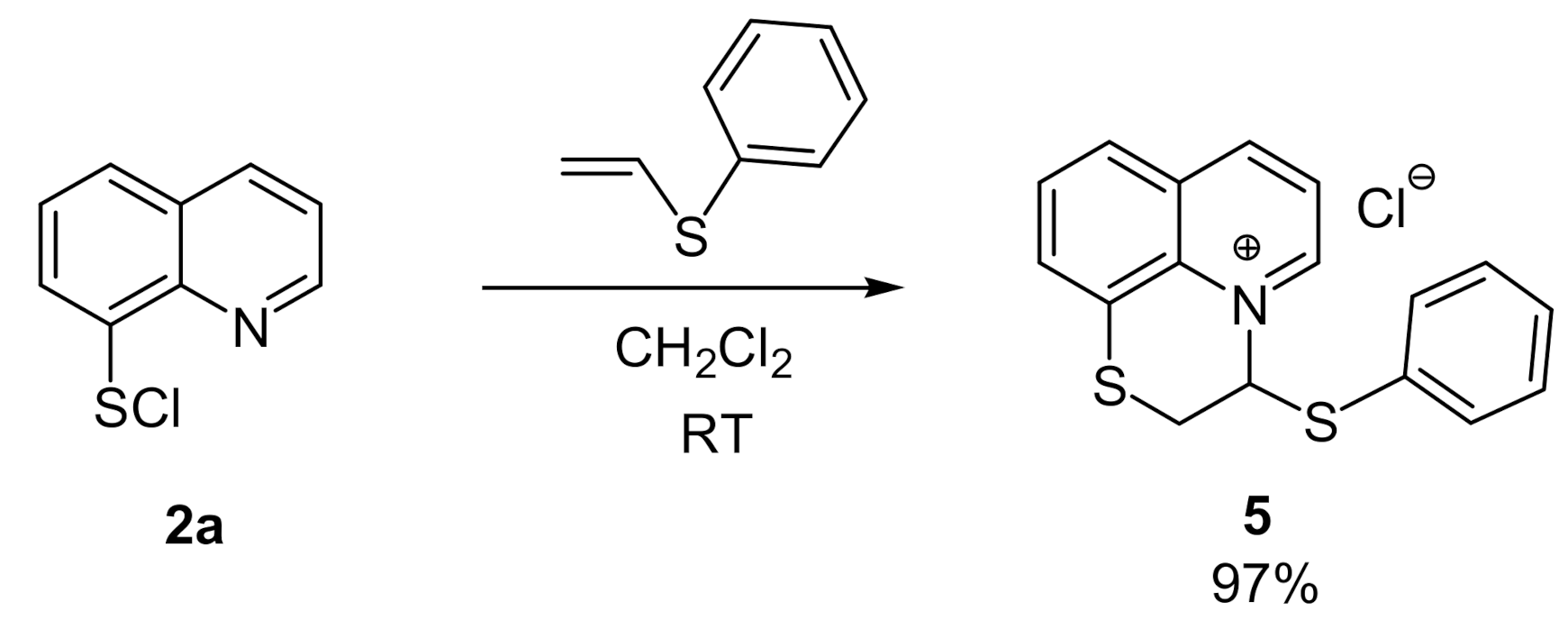
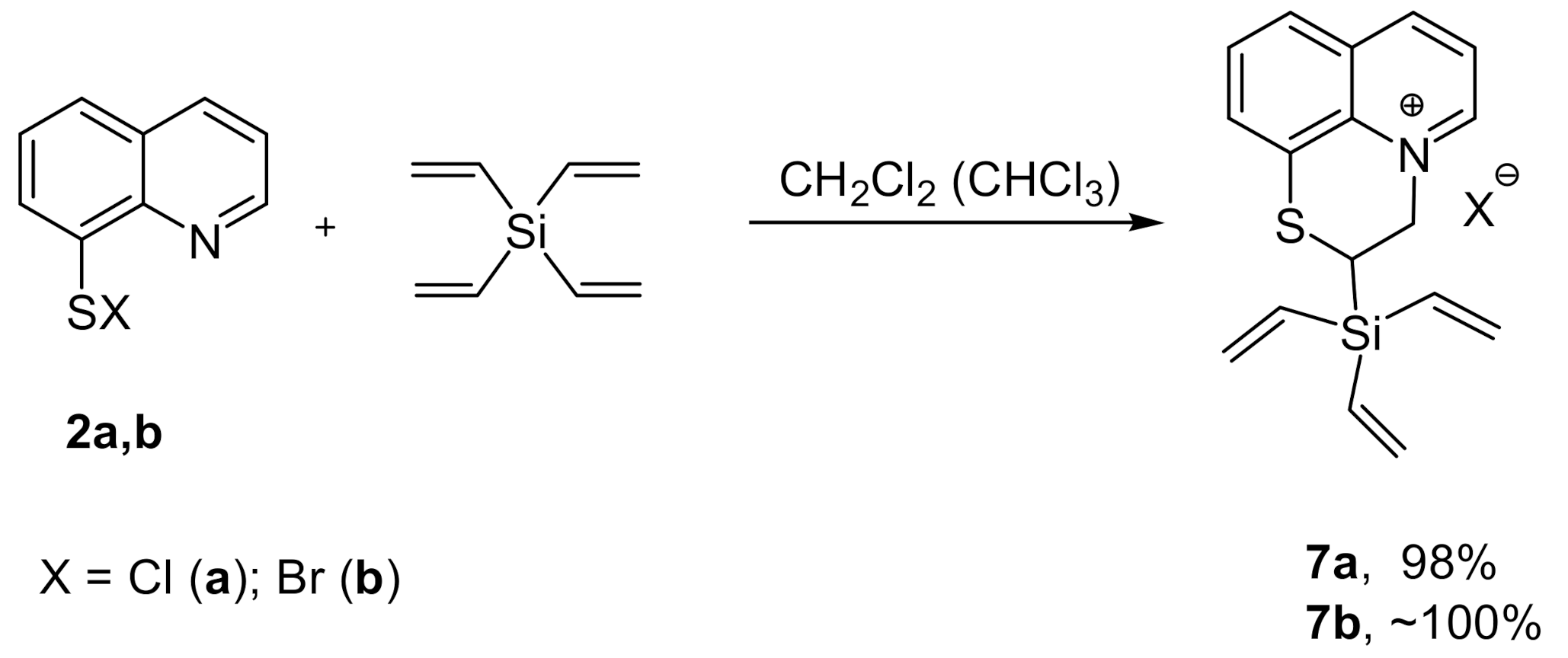
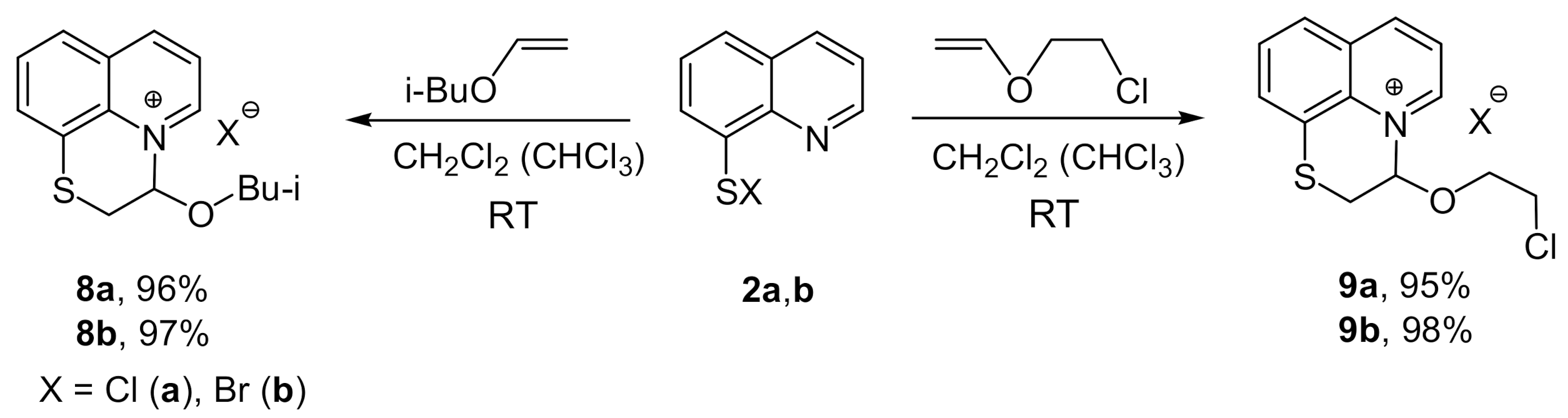
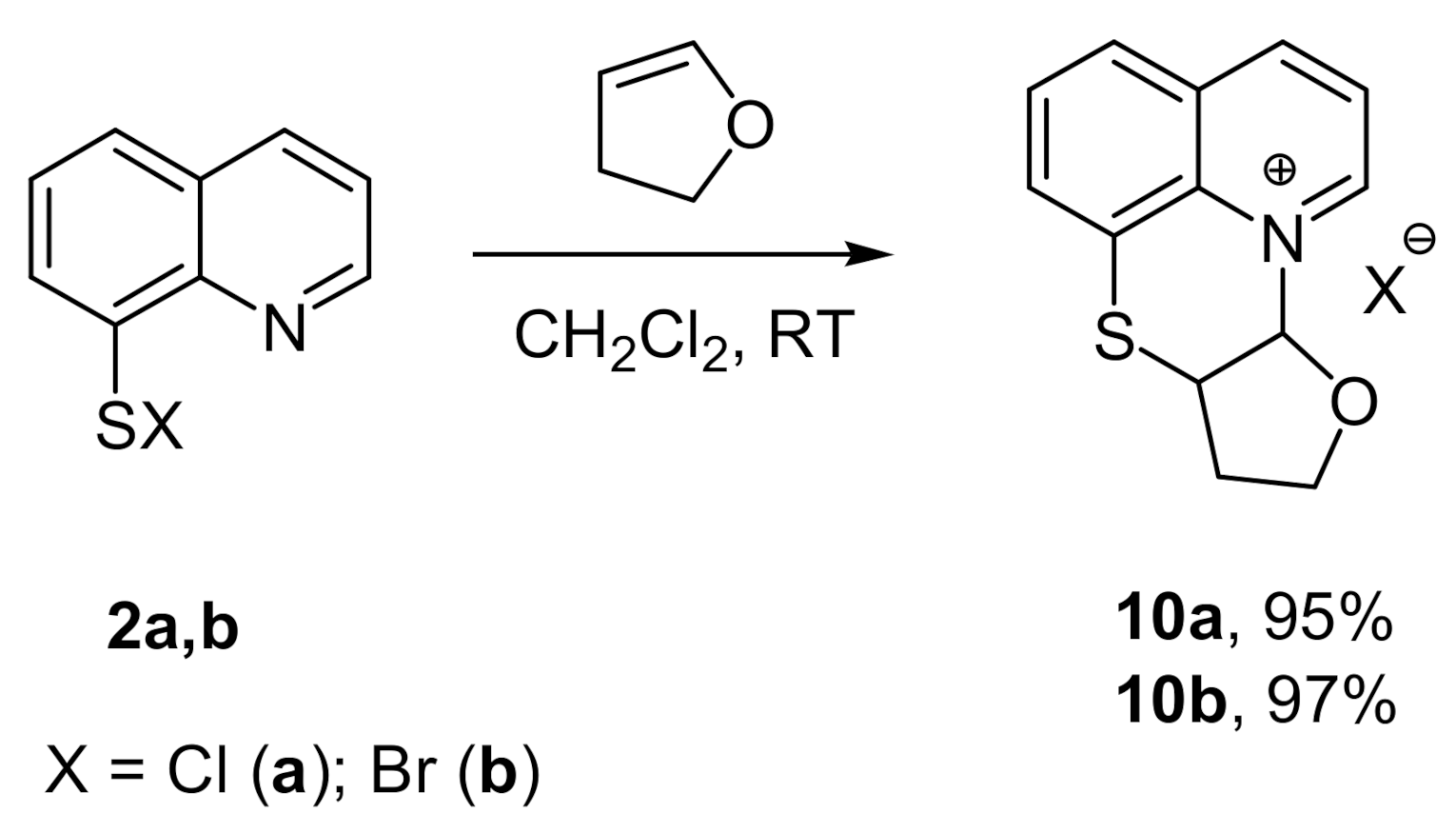
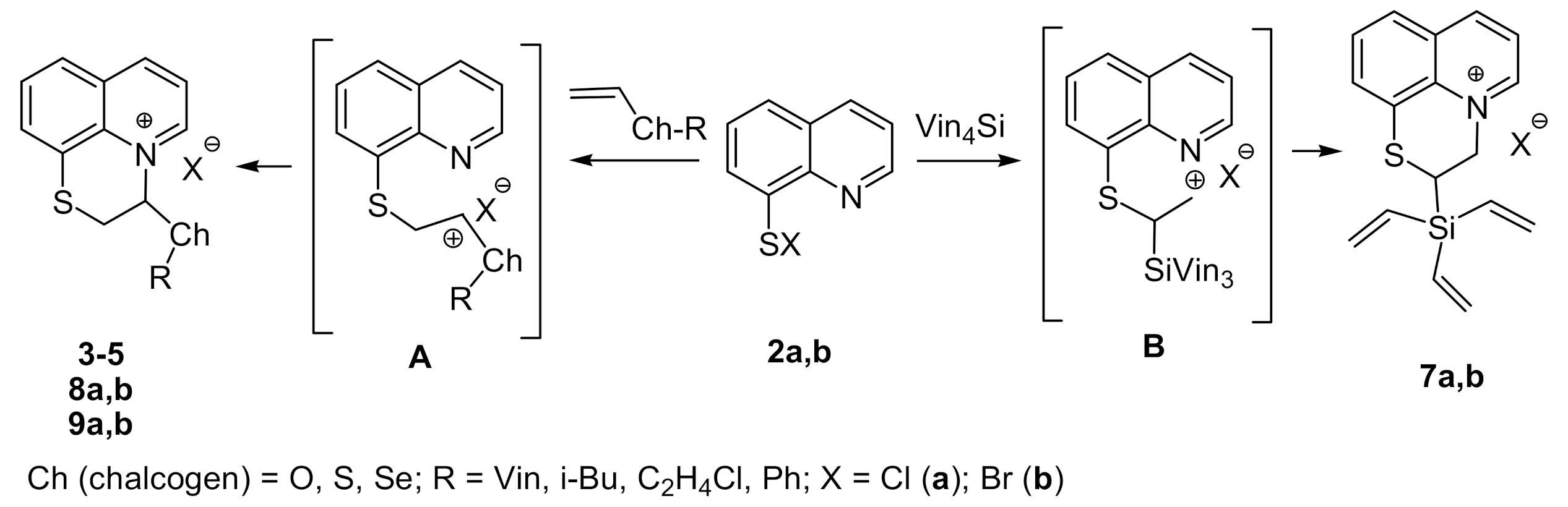
2. Results and Discussion
3. Experimental Section
3.1. General Information
3.2. Synthesis of Compounds 3–10a,b
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Weyesa, A.; Mulugeta, E. Recent advances in the synthesis of biologically and pharmaceutically active quinoline and its analogues: A review. RSC Adv. 2020, 10, 20784–20793. [Google Scholar] [CrossRef]
- Chung, P.-Y.; Bian, Z.-X.; Pun, H.-Y.; Chan, D.; Chan, A.S.-C.; Chui, C.-H.; Tang, J.C.-O.; Lam, K.-H. Recent advances in research of natural and synthetic bioactive quinolines. Future Med. Chem. 2015, 7, 947–967. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Bawa, S.; Gupta, H. Biological activities of quinoline derivatives. Mini Rev. Med. Chem. 2009, 9, 1648–1654. [Google Scholar] [CrossRef]
- Raut, K.; Thombare, R.; Zagade, P.; Kumbhar, N. Different biological activities of quinoline. World J. Pharm. Res. 2020, 9, 674–689. [Google Scholar]
- Abass, M.; Alzandi, A.R.A.; Hassan, M.M.; Mohamed, N. Recent Advances on Diversity Oriented Heterocycle Synthesis of Fused Quinolines and Its Biological Evaluation. Polycycl. Arom. Comp. 2020, 1710856. [Google Scholar] [CrossRef]
- Shiro, T.; Fukaya, T.; Tobe, M. The chemistry and biological activity of heterocycle-fused quinolinone derivatives: A review. Eur. J. Med. Chem. 2015, 97, 397–408. [Google Scholar] [CrossRef] [PubMed]
- McKee, D.L.; Sternberg, A.; Stange, U.; Laufer, S.; Naujokat, C. Candidate drugs against SARS-CoV-2 and COVID-19. Pharmacol. Res. 2020, 157, 104859. [Google Scholar] [CrossRef]
- Feng, M.; Tang, B.; Liang, S.H.; Jiang, X. Sulfur Containing Scaffolds in Drugs: Synthesis and Application in Medicinal Chemistry. Curr. Top. Med. Chem. 2016, 16, 1200–1216. [Google Scholar] [CrossRef]
- Good, J.A.D.; Kulen, A.M.; Almqvist, K.F.; Cairns, A.G.; Ponten, J.F. 2,3-dihydrothiazolo[3,2-a]pyridin-5-one derivatives, intermediates thereof, and their use as antibacerial agents. Chem. Abstr. 2016, 164, 602146. [Google Scholar]
- Shi, F.; Li, C.; Xia, M.; Miao, K.; Zhao, Y.; Tu, S.; Zheng, W.; Zhang, G.; Ma, N. Green chemoselective synthesis of thiazolo[3,2-a]pyridine derivatives and evaluation of their antioxidant and cytotoxic activities. Bioorg. Med. Chem. Lett. 2009, 19, 5565–5568. [Google Scholar] [CrossRef]
- Manfroni, G.; Meschini, F.; Barreca, M.L.; Leyssen, P.; Samuele, A.; Iraci, N.; Sabatini, S.; Massari, S.; Maga, G.; Neyts, J.; et al. Pyridobenzothiazole derivatives as new chemotype targeting the HCV NS5B polymerase. Bioorg. Med. Chem. 2012, 20, 866–876. [Google Scholar] [CrossRef]
- Li, S.; Huang, Q.; Liu, Y.J.; Zhang, X.L.; Liu, S.; He, C.; Gong, P. Design, synthesis and antitumor activity of bisquinoline derivatives connected by 4-oxy-3-fluoroaniline moiety. Eur. J. Med. Chem. 2013, 64, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.S.; Liaqat, S.; Girgis, A.S.; Samir, A.; Hall, C.D.; Katritzky, A.R. Novel antibacterial active quinolone-fluoroquinolone conjugates and 2D-QSAR studies. Bioorg. Med. Chem. Lett. 2015, 25, 3816–3821. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Fan, Y.L.; Zhao, F.; Ren, Q.C.; Wu, X.; Chang, L.; Gao, F. Quinolone derivatives and their activities against methicillin-resistant Staphylococcus aureus (MRSA). Eur. J. Med. Chem. 2018, 157, 1081–1095. [Google Scholar] [CrossRef]
- Hu, G.; Liang, J.; Liu, J.; Zhang, H. Preparation of propenone derivative of N-demethylated Rufloxacin as antitumor drugs. Chem. Abstr. 2020, 173, 407358. [Google Scholar]
- Cen, S.; Yang, L.; Li, X.; Hu, G. Preparation method of Rufloxacin-containing bis-fluoroquinolone oxadiazole urea derivative applied to antitumor drug. Chem. Abstr. 2019, 171, 203187. [Google Scholar]
- Fukuda, Y.; Seto, S.; Tanioka, A.; Ikeda, M. Preparation of pyridobenzothiazine derivatives having tachykinin antagonism, in particular, substance P receptor antagonism. Chem. Abstr. 2000, 132, 151827. [Google Scholar]
- Hou, L.; Du, L.; Li, Y.; Hu, G.; Sun, J.; Zhang, C.; Shen, R.; Wang, N. Preparation of fluoroquinolone 1,3,4-thiadiazole urea rufloxacin derivatives useful for the treatment of cancer. Chem. Abstr. 2019, 171, 467857. [Google Scholar]
- Lapointe, G.; Mergo, W.; Moser, H.E.; Rivkin, A.; Skepper, C.K.; Williams, S.L. Preparation of tricyclic 2-quinolinones as antibacterials. Chem. Abstr. 2018, 169, 515481. [Google Scholar]
- Hu, G.; Wang, G.; Jing, Y. 3-[[2-(4-Pyridinylcarbonyl)hydrazinylidene]methyl]-7-fluoroquinolone derivatives useful in the treatment of tuberculosis. Chem. Abstr. 2012, 158, 131756. [Google Scholar]
- Musalov, M.V.; Yakimov, V.A.; Potapov, V.A.; Amosova, S.V.; Borodina, T.N.; Zinchenko, S.V. A novel methodology for the synthesis of condensed selenium heterocycles based on the annulation and annulation–methoxylation reactions of selenium dihalides. New J. Chem. 2019, 43, 18476–18483. [Google Scholar] [CrossRef]
- Musalov, M.V.; Potapov, V.A. Selenium dihalides: New possibilities for the synthesis of selenium-containing heterocycles. Chem. Heterocycl. Compd. 2017, 53, 150–152. [Google Scholar] [CrossRef]
- Accurso, A.A.; Cho, S.-H.; Amin, A.; Potapov, V.A.; Amosova, S.V.; Finn, M.G. Thia-, Aza-, and Selena[3.3.1]bicyclononane Dichlorides: Rates vs. Internal Nucleophile in Anchimeric Assistance. J. Org. Chem. 2011, 76, 4392–4395. [Google Scholar] [CrossRef]
- Potapov, V.A.; Amosova, S.V.; Abramova, E.V.; Lyssenko, K.A.; Musalov, M.V.; Finn, M.G. Transannular Addition of Selenium Dichloride and Dibromide to 1,5-Cyclooctadiene: Synthesis of 2,6-Dihalo-9-selenabicyclo[3.3.1]nonanes and Their Complexes with Selenium Dihalides. New J. Chem. 2015, 39, 8055–8059. [Google Scholar] [CrossRef]
- Potapov, V.A.; Amosova, S.V.; Kashik, A.S. Reactions of selenium and tellurium metals with phenylacetylene in 3-phase catalytical systems. Tetrahedron Lett. 1989, 30, 613–616. [Google Scholar] [CrossRef]
- Potapov, V.A.; Volkova, K.A.; Penzik, M.V.; Albanov, A.I.; Amosova, S.V. Reaction of selenium dichloride with divinyl selenide. Russ. J. Org. Chem. 2008, 44, 1556–1557. [Google Scholar] [CrossRef]
- Potapov, V.A.; Malinovich, D.A.; Amosova, S.V.; Rusakov, Y.Y.; Bhasin, K.K. Reaction of 2-pyridylselenenyl bromide with divinyl selenide. Chem. Heterocycl. Comp. 2012, 48, 1129–1131. [Google Scholar] [CrossRef]
- Potapov, V.A.; Musalova, M.V.; Ishigeev, R.S.; Musalov, M.V.; Panov, V.A.; Khabibulina, A.G.; Amosova, S.V.; Bhasin, K.K. Efficient and selective syntheses of novel unsaturated chalcogen-containing pyridine derivatives. Tetrahedron Lett. 2016, 57, 5341–5343. [Google Scholar] [CrossRef]
- Potapov, V.A.; Ishigeev, R.S.; Amosova, S.V.; Borodina, T.N. Synthesis of a novel family of water-soluble 2H,3H-[1,3]thia- and -selenazolo[3,2-a]pyridin-4-ium heterocycles by annulation reactions. Tetrahedron Lett. 2019, 60, 475–479. [Google Scholar] [CrossRef]
- Potapov, V.A.; Ishigeev, R.S.; Amosova, S.V. Synthesis of 3-(2-oxopyrrolidin-1-yl)-2H,3H-[1,3]selenazolo[3,2-a]pyridin-4-ium chloride. Russ. J. Org. Chem. 2017, 53, 1604–1605. [Google Scholar] [CrossRef]
- Potapov, V.A.; Ishigeev, R.S.; Amosova, S.V. Regioselective Reaction of Pyridine-2-Sulfenyl Chloride with Isoeugenole. Russ. J. Org. Chem. 2018, 54, 1262–1263. [Google Scholar]
- Potapov, V.A.; Ishigeev, R.S.; Shkurchenko, I.V.; Zinchenko, S.V.; Amosova, S.V. Natural compounds and their structural analogs in regio- and stereoselective synthesis of new families of water-soluble 2H,3H-[1,3]thia- and -selenazolo[3,2-a]pyridin-4-ium heterocycles by annulation reactions. Molecules 2020, 25, 376. [Google Scholar] [CrossRef]
- Kim, D.G.; Vershinina, E.A.; Sharutin, V.V. Synthesis, transformations and halocyclization of 8-(prop-2-ynylsulfanyl)quinoline and 8-(2-bromoprop-2-enylsulfanyl)quinoline. J. Sulfur Chem. 2020, 41, 71–81. [Google Scholar] [CrossRef]
- Kim, D.G. Halocyclization of 8-allylthioquinoline. Chem. Heterocycl. Compd. 1997, 33, 989–991. [Google Scholar] [CrossRef]
- Batalov, V.I.; Kim, D.G.; Slepukhin, P.A. Heterocyclization of 8-(2-methyl-prop-2-enylsulfanyl)quinoline using electrophilic reagents. Chem. Heterocycl. Compd. 2013, 49, 1092–1096. [Google Scholar] [CrossRef]
- Borisov, A.V.; Goncharova, T.V.; Borisova, G.N.; Osmanov, V.K.; Matsulevich Zh., V. Polar cycloaddition of 8-quinolinesulfenyl chloride to styrene. Chem. Heterocycl. Compd. 2001, 37, 382–383. [Google Scholar] [CrossRef]
- Borisov, A.V.; Osmanov, V.K.; Borisova, G.N.; Matsulevich, Z.V.; Fukin, G.K. Synthesis of condensed sulfur- and nitrogen-containing heterocycles via polar cycloaddition of hetarenesulfenyl chlorides to a C-C multiple bond. Mendeleev Commun. 2009, 19, 49–51. [Google Scholar] [CrossRef]
- Borisov, A.V.; Belsky, V.K.; Goncharova, T.V.; Borisova, G.N.; Osmanov, V.K.; Matsulevich, Z.V.; Frolova, N.G.; Savin, E.D. Sulfenyl halides in the synthesis of heterocycles. Part 2. Cyclization in reactions of hetarenesulfenyl chlorides with 3,3-dimethyl-1-butene. Chem. Heterocycl. Compd. 2005, 41, 771–777. [Google Scholar] [CrossRef]
- Trofimov, B.A.; Amosova, S.V. Divinyl Sulfide: Synthesis, Properties and Applications. Sulfur Rep. 1984, 3, 323–393. [Google Scholar] [CrossRef]
- Gusarova, N.K.; Potapov, V.A.; Amosova, S.V.; Trofimov, B.A. Alkylvinyl Selenides from Acetylene, Elemental Selenium and Alkyl Halides. Zhurnal Org. Khimii 1983, 19, 2477–2480. (In Russian) [Google Scholar]
- Gusarova, N.K.; Trofimov, B.A.; Potapov, V.A.; Amosova, S.V.; Sinegovskaya, L.M. Reactions of Elemental Selenium with Acetylenes. 1. Identification of Products of Reaction of Elemental Selenium with Acetylene. Zhurnal Org. Khimii 1984, 20, 484–489. (In Russian) [Google Scholar]
- Potapov, V.A.; Amosova, S.V. Synthesis of vinylic selenides and tellurides by the addition of alkaneselenolate and alkanetellurolate anions to acetylenes. Phosphorus Sulfur Silicon Relat. Elem. 1993, 79, 277–280. [Google Scholar] [CrossRef]
- Potapov, V.A.; Gusarova, N.K.; Amosova, S.V.; Kashik, A.S.; Trofimov, B.A. Reactions of Chalcogen with Acetylenes. 2. Reaction of Selenium Metals with Acetylene in the HMPA and DMSO Media. Zhurnal Org. Khimii 1986, 22, 276–281. (In Russian) [Google Scholar]
- Gusarova, N.K.; Trofimov, B.A.; Tatarinova, A.A.; Potapov, V.A.; Gusarov, A.V.; Amosova, S.V.; Voronkov, M.G. Reactions of chalcogenes with acetylene. 4. Synthesis of divinyltelluride by the direct reaction of tellurium with acetylene. Zhurnal Org. Khimii 1989, 25, 39–45. (In Russian) [Google Scholar]
- Trofimov, B.A.; Gusarova, N.K.; Tatarinova, A.A.; Amosova, S.V.; Potapov, V.A.; Sinegovskaya, L.M.; Voronkov, M.G. Alkylvinyl tellurides from tellurium, acetylene and alkyl halides. Tetrahedron 1988, 44, 6739–6744. [Google Scholar] [CrossRef]
- Trofimov, B.A.; Gusarova, N.K.; Tatarinova, A.A.; Amosova, S.V. Reaction of divinyl telluride with bromine. Zhurnal Org. Khimii 1983, 19, 457–458. [Google Scholar]
- Potapov, V.A.; Musalov, M.V.; Amosova, S.V.; Musalova, M.V.; Penzik, M.V. Reaction of Selenium Dichloride with Divinyl Telluride. Russ. J. Org. Chem. 2011, 47, 950–951. [Google Scholar] [CrossRef]
- Cooke, F.; Moerck, R.; Schwindeman, J.; Magnus, P. Silicon in synthesis. 8. Vinyltrimethylsilane, a convenient ethylene equivalent for the synthesis of vinyl arylsulfides, vinyl arylsulfoxides, thiosilylketene acetals, and fused cyclopentenones. J. Org. Chem. 1980, 45, 1046–1053. [Google Scholar] [CrossRef]
- Wierschke, S.G.; Chandrasekhar, J.; Jorgensen, W.L. Magnitude and origin of the beta-silicon effect on carbenium ions. J. Am. Chem. Soc. 1985, 107, 1496–1500. [Google Scholar] [CrossRef]
- Koval’, I.V. Sulfenyl chlorides in organic synthesis. Russ. Chem. Rev. 1995, 64, 731–748. [Google Scholar] [CrossRef]
- Myshlyaeva, L.V.; Krasnoschekov, V.V. Analyticheskaya Khimiya Kremniya (Analytical Chemistry of Silicon); Nauka: Moscow, Russia, 1972; pp. 51–75. (In Russian) [Google Scholar]
- Trofimov, B.A.; Amosova, S.V. Divinil Sulfid i Ego Proizvodnye (Divinyl Sulfide and Its Derivatives); Nauka: Novisibirsk, Russia, 1983; p. 264. (In Russian) [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potapov, V.A.; Ishigeev, R.S.; Amosova, S.V. Efficient Regioselective Synthesis of Novel Water-Soluble 2H,3H-[1,4]thiazino[2,3,4-ij]quinolin-4-ium Derivatives by Annulation Reactions of 8-quinolinesulfenyl Halides. Molecules 2021, 26, 1116. https://doi.org/10.3390/molecules26041116
Potapov VA, Ishigeev RS, Amosova SV. Efficient Regioselective Synthesis of Novel Water-Soluble 2H,3H-[1,4]thiazino[2,3,4-ij]quinolin-4-ium Derivatives by Annulation Reactions of 8-quinolinesulfenyl Halides. Molecules. 2021; 26(4):1116. https://doi.org/10.3390/molecules26041116
Chicago/Turabian StylePotapov, Vladimir A., Roman S. Ishigeev, and Svetlana V. Amosova. 2021. "Efficient Regioselective Synthesis of Novel Water-Soluble 2H,3H-[1,4]thiazino[2,3,4-ij]quinolin-4-ium Derivatives by Annulation Reactions of 8-quinolinesulfenyl Halides" Molecules 26, no. 4: 1116. https://doi.org/10.3390/molecules26041116
APA StylePotapov, V. A., Ishigeev, R. S., & Amosova, S. V. (2021). Efficient Regioselective Synthesis of Novel Water-Soluble 2H,3H-[1,4]thiazino[2,3,4-ij]quinolin-4-ium Derivatives by Annulation Reactions of 8-quinolinesulfenyl Halides. Molecules, 26(4), 1116. https://doi.org/10.3390/molecules26041116






