Bioethanol Production by Enzymatic Hydrolysis from Different Lignocellulosic Sources
Abstract
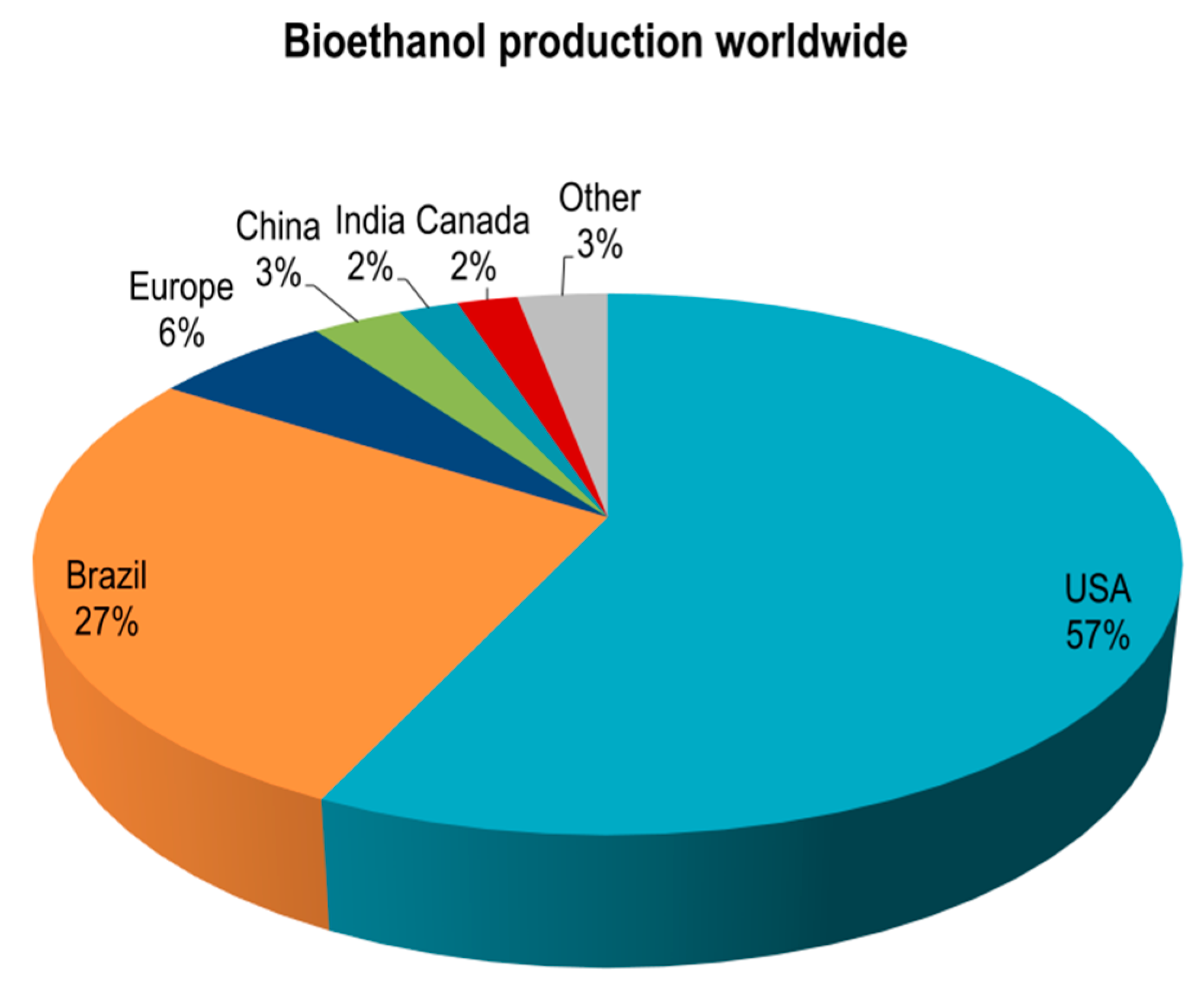
1. Introduction
2. Lignocellulosic Sources
2.1. Marine Algae
2.2. Agricultural Residues and Municipal Wastes
2.3. Forest Feedstocks
3. Lignocellulosic Biomass Composition
3.1. Cellulose
3.2. Hemicellulose
3.3. Lignin
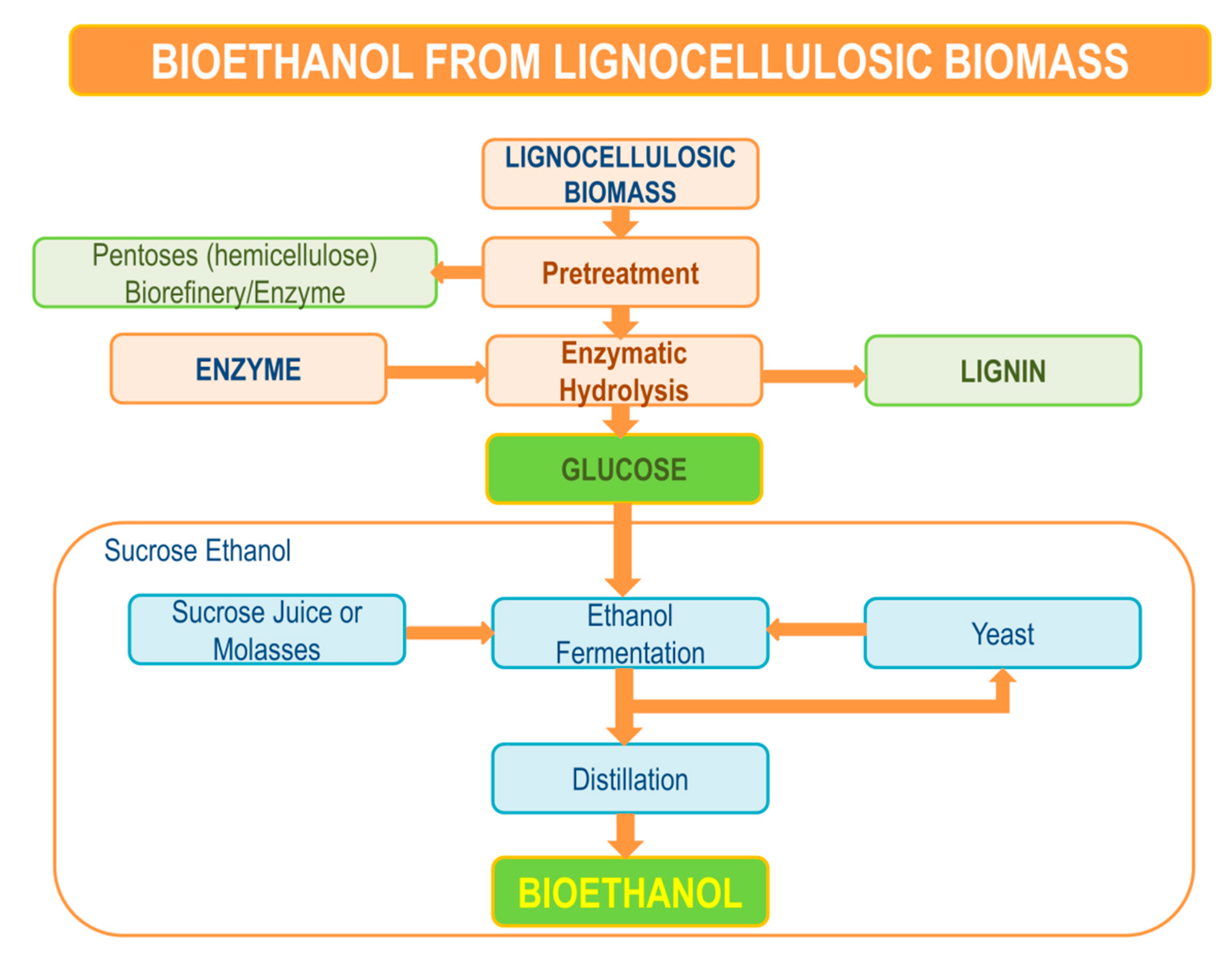
4. Pretreatment of Lignocellulosic Biomass
Cellulases and Hemicellulases
5. Enzymatic Hydrolysis
5.1. Factors Influencing Enzymatic Hydrolysis
5.1.1. Crystallinity of Cellulose
5.1.2. Particle Size of Lignocellulosic Biomass
5.1.3. Accessible Surface Area and Pore Volume of Lignocellulosic Biomass
5.2. Enzymatic Hydrolysis Pathway
5.2.1. From Marine Algae
5.2.2. From Agricultural Wastes and Residues
5.2.3. From Wood Feedstocks
6. Fermentation
7. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Menon, V.; Rao, M. Trends in Bioconversion of Lignocellulose: Biofuels, Platform Chemicals & Biorefinery Concept. Prog. Energy Combust. Sci. 2012, 38, 522–550. [Google Scholar] [CrossRef]
- Chandel, A.K.; Garlapati, V.K.; Singh, A.K.; Antunes, F.A.F.; da Silva, S.S. The Path Forward for Lignocellulose Biorefineries: Bottlenecks, Solutions, and Perspective on Commercialization. Bioresour. Technol. 2018, 264, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Alzagameem, A.; Khaldi-Hansen, B.E.; Büchner, D.; Larkins, M.; Kamm, B.; Witzleben, S.; Schulze, M. Lignocellulosic Biomass as Source for Lignin-Based Environmentally Benign Antioxidants. Molecules 2018, 23, 2664. [Google Scholar] [CrossRef] [PubMed]
- Limayem, A.; Ricke, S.C. Lignocellulosic Biomass for Bioethanol Production: Current Perspectives, Potential Issues and Future Prospects. Prog. Energy Combust. Sci. 2012, 38, 449–467. [Google Scholar] [CrossRef]
- Prasad, R.K.; Chatterjee, S.; Mazumder, P.B.; Gupta, S.K.; Sharma, S.; Vairale, M.G.; Datta, S.; Dwivedi, S.K.; Gupta, D.K. Bioethanol Production from Waste Lignocelluloses: A Review on Microbial Degradation Potential. Chemosphere 2019, 231, 588–606. [Google Scholar] [CrossRef] [PubMed]
- Soares, I.; Ferreira, P.; Hens, L. Energy and Environmental Challenges: Bringing Together Economics and Engineering (ICEE’17). Environ. Dev. Sustain. 2018, 20, 1–5. [Google Scholar] [CrossRef]
- Kim, J.; Sunagawa, M.; Kobayashi, S.; Shin, T.; Takayama, C. Developmental Localization of Calcitonin Gene-Related Peptide in Dorsal Sensory Axons and Ventral Motor Neurons of Mouse Cervical Spinal Cord. Neurosci. Res. 2016, 105, 42–48. [Google Scholar] [CrossRef]
- Yao, S.; Nie, S.; Yuan, Y.; Wang, S.; Qin, C. Efficient Extraction of Bagasse Hemicelluloses and Characterization of Solid Remainder. Bioresour. Technol. 2015, 185, 21–27. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, B.; Zhao, Z. Efficient Acid-Catalyzed Hydrolysis of Cellulose in Organic Electrolyte Solutions. Polym. Degrad. Stab. 2012, 97, 573–577. [Google Scholar] [CrossRef]
- Maitan-Alfenas, G.P.; Visser, E.M.; Guimarães, V.M. Enzymatic Hydrolysis of Lignocellulosic Biomass: Converting Food Waste in Valuable Products. Curr. Opin. Food Sci. 2015, 1, 44–49. [Google Scholar] [CrossRef]
- Saha, K.; Mashewari, U.; Sikder, J.; Chakraborty, S.; da Silva, S.S.; dos Santos, J.C. Membranes as a Tool to Support Biorefineries: Applications in Enzymatic Hydrolysis, Fermentation and Dehydration for Bioethanol Production. Renew. Sustain. Energy Rev. 2017, 74, 873–890. [Google Scholar] [CrossRef]
- Banerjee, S.; Mudliar, S.; Sen, R.; Giri, B.; Satpute, D.; Chakrabarti, T.; Pandey, R.A. Commercializing Lignocellulosic Bioethanol: Technology Bottlenecks and Possible Remedies. Biofuels Bioprod. Biorefining 2010, 4, 77–93. [Google Scholar] [CrossRef]
- Börjesson, J.; Peterson, R.; Tjerneld, F. Enhanced Enzymatic Conversion of Softwood Lignocellulose by Poly(Ethylene Glycol) Addition. Enzym. Microb. Technol. 2007, 40, 754–762. [Google Scholar] [CrossRef]
- Hou, Q.; Ju, M.; Li, W.; Liu, L.; Chen, Y.; Yang, Q. Pretreatment of Lignocellulosic Biomass with Ionic Liquids and Ionic Liquid-Based Solvent Systems. Molecules 2017, 22. [Google Scholar] [CrossRef]
- Balat, M. Production of Bioethanol from Lignocellulosic Materials via the Biochemical Pathway: A Review. Energy Convers. Manag. 2011, 52, 858–875. [Google Scholar] [CrossRef]
- Tabatabaei, M.; Aghbashlo, M. The Critical Role of Advanced Sustainability Assessment Tools in Enhancing the Real-World Application of Biofuels. Acta Innov. 2020, 67–73. [Google Scholar] [CrossRef]
- Holzleitner, M.; Moser, S.; Puschnigg, S. Evaluation of the Impact of the New Renewable Energy Directive 2018/2001 on Third-Party Access to District Heating Networks to Enforce the Feed-in of Industrial Waste Heat. Util. Policy 2020, 66, 101088. [Google Scholar] [CrossRef]
- Chiaramonti, D.; Goumas, T. Impacts on Industrial-Scale Market Deployment of Advanced Biofuels and Recycled Carbon Fuels from the EU Renewable Energy Directive II. Appl. Energy 2019, 251, 113351. [Google Scholar] [CrossRef]
- Naik, S.N.; Goud, V.V.; Rout, P.K.; Dalai, A.K. Production of First and Second Generation Biofuels: A Comprehensive Review. Renew. Sustain. Energy Rev. 2010, 14, 578–597. [Google Scholar] [CrossRef]
- Ullah, K.; Sharma, V.K.; Ahmad, M.; Lv, P.; Krahl, J.; Wang, Z. The Insight Views of Advanced Technologies and Its Application in Bio-Origin Fuel Synthesis from Lignocellulose Biomasses Waste, a Review. Renew. Sustain. Energy Rev. 2018, 82, 3992–4008. [Google Scholar] [CrossRef]
- Tahir, A.; Arshad, M.; Anum, F.; Abbas, M.; Javed, S.; Shahzad, M.I.; Ansari, A.R.; Bano, I.; Shah, F.A. Ecofuel future prospect and community impact. In Advances in Eco-Fuels for a Sustainable Environment; Azad, K., Ed.; Woodhead Publishing: Sawston, UK, 2019; Chapter 17; pp. 459–479. ISBN 978-0-08-102728-8. [Google Scholar]
- Saha, S.; Sharma, A.; Purkayastha, S.; Pandey, K.; Dhingra, S. Bio-plastics and Biofuel: Is it the Way in Future Development for End Users? In Plastics to Energy; Al-Salem, S.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2019; Chapter 14; pp. 365–376. ISBN 978-0-12-813140-4. [Google Scholar]
- Aro, E.-M. From First Generation Biofuels to Advanced Solar Biofuels. Ambio 2016, 45, 24–31. [Google Scholar] [CrossRef]
- Anu, A.; Kumar, A.; Jain, K.K.; Singh, B. Process Optimization for Chemical Pretreatment of Rice Straw for Bioethanol Production. Renew. Energy 2020, 156, 1233–1243. [Google Scholar] [CrossRef]
- Zhao, Y.; Damgaard, A.; Liu, S.; Chang, H.; Christensen, T.H. Bioethanol from Corn Stover—Integrated Environmental Impacts of Alternative Biotechnologies. Resour. Conserv. Recycl. 2020, 155, 104652. [Google Scholar] [CrossRef]
- Jugwanth, Y.; Sewsynker-Sukai, Y.; Gueguim Kana, E.B. Valorization of Sugarcane Bagasse for Bioethanol Production through Simultaneous Saccharification and Fermentation: Optimization and Kinetic Studies. Fuel 2020, 262, 116552. [Google Scholar] [CrossRef]
- Keshwani, D.R.; Cheng, J.J. Switchgrass for Bioethanol and Other Value-Added Applications: A Review. Bioresour. Technol. 2009, 100, 1515–1523. [Google Scholar] [CrossRef]
- Nanda, S.K.; Lin, W.-Y.; Lee, M.-Y.; Chen, R.-S. A Quantitative Classification of Essential and Parkinson’s Tremor Using Wavelet Transform and Artificial Neural Network on SEMG and Accelerometer Signals. In Proceedings of the IEEE International Conference on Networking, Sensing and Control, Taipei, Taiwan, 9–11 April 2015. [Google Scholar] [CrossRef]
- Kamyab, H.; Din, M.F.M.; Ponraj, M.; Keyvanfar, A.; Rezania, S.; Taib, S.M.; Majid, M.Z.A. Isolation and Screening of Microalgae from Agro-Industrial Wastewater (POME) for Biomass and Biodiesel Sources. Desalination Water Treat. 2016, 57, 29118–29125. [Google Scholar] [CrossRef]
- Phwan, C.K.; Ong, H.C.; Chen, W.-H.; Ling, T.C.; Ng, E.P.; Show, P.L. Overview: Comparison of Pretreatment Technologies and Fermentation Processes of Bioethanol from Microalgae. Energy Convers. Manag. 2018, 173, 81–94. [Google Scholar] [CrossRef]
- Keris-Sen, U.D.; Gurol, M.D. Using Ozone for Microalgal Cell Disruption to Improve Enzymatic Saccharification of Cellular Carbohydrates. Biomass Bioenergy 2017, 105, 59–65. [Google Scholar] [CrossRef]
- Guedes, A.C.; Amaro, H.M.; Sousa-Pinto, I.; Malcata, F.X. Algal spent biomass—A pool of applications. In Biofuels from Algae, 2nd ed.; Pandey, A., Chang, J.-S., Soccol, C.R., Lee, D.-J., Chisti, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 16; pp. 397–433. ISBN 978-0-444-64192-2. [Google Scholar]
- Suganya, T.; Varman, M.; Masjuki, H.H.; Renganathan, S. Macroalgae and Microalgae as a Potential Source for Commercial Applications along with Biofuels Production: A Biorefinery Approach. Renew. Sustain. Energy Rev. 2016, 55, 909–941. [Google Scholar] [CrossRef]
- Sudhakar, M.P.; Kumar, B.R.; Mathimani, T.; Arunkumar, K. A Review on Bioenergy and Bioactive Compounds from Microalgae and Macroalgae-Sustainable Energy Perspective. J. Clean. Prod. 2019, 228, 1320–1333. [Google Scholar] [CrossRef]
- Jiang, R.; Ingle, K.N.; Golberg, A. Macroalgae (Seaweed) for Liquid Transportation Biofuel Production: What Is Next? Algal Res. 2016, 14, 48–57. [Google Scholar] [CrossRef]
- Yuan, Y.; Macquarrie, D.J. Microwave Assisted Acid Hydrolysis of Brown Seaweed Ascophyllum Nodosum for Bioethanol Production and Characterization of Alga Residue. ACS Sustain. Chem. Eng. 2015, 3, 1359–1365. [Google Scholar] [CrossRef]
- Kumar, S.; Gupta, R.; Kumar, G.; Sahoo, D.; Kuhad, R.C. Bioethanol Production from Gracilaria Verrucosa, a Red Alga, in a Biorefinery Approach. Bioresour. Technol. 2013, 135, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Ryu, M.; Lee, E.Y. Saccharification of Alginate by Using Exolytic Oligoalginate Lyase from Marine Bacterium Sphingomonas Sp. MJ-3. J. Ind. Eng. Chem. 2011, 17, 853–858. [Google Scholar] [CrossRef]
- Adams, J.M.; Gallagher, J.A.; Donnison, I.S. Fermentation Study on Saccharina Latissima for Bioethanol Production Considering Variable Pre-Treatments. J. Appl. Phycol. 2008, 21, 569. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for Biodiesel Production and Other Applications: A Review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Chen, J.-J.; Li, Y.-R.; Lai, W.-L. Application of Experimental Design Methodology for Optimization of Biofuel Production from Microalgae. Biomass Bioenergy 2014, 64, 11–19. [Google Scholar] [CrossRef]
- Wargacki, A.J.; Leonard, E.; Win, M.N.; Regitsky, D.D.; Santos, C.N.S.; Kim, P.B.; Cooper, S.R.; Raisner, R.M.; Herman, A.; Sivitz, A.B.; et al. An Engineered Microbial Platform for Direct Biofuel Production from Brown Macroalgae. Science 2012, 335, 308–313. [Google Scholar] [CrossRef]
- Smachetti, M.E.S.; Rizza, L.S.; Coronel, C.D.; Nascimento, M.D.; Curatti, L. Microalgal Biomass as an Alternative Source of Sugars for the Production of Bioethanol. In Principles and Applications of Fermentation Technology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 351–386. ISBN 978-1-119-46038-1. [Google Scholar]
- Demirbaş, A. Bioethanol from Cellulosic Materials: A Renewable Motor Fuel from Biomass. Energy Sources 2005, 27, 327–337. [Google Scholar] [CrossRef]
- Kim, S.; Dale, B.E. Global Potential Bioethanol Production from Wasted Crops and Crops Residues. Biomass Bioenergy 2005, 29, 361–375. [Google Scholar] [CrossRef]
- Sarkar, N.; Ghosh, S.K.; Bannerjee, S.; Aikat, K. Bioethanol Production from Agricultural Wastes: An Overview. Renew. Energy 2012, 37, 19–27. [Google Scholar] [CrossRef]
- Nikku, M.; Deb, A.; Sermyagina, E.; Puro, L. Reactivity Characterization of Municipal Solid Waste and Biomass. Fuel 2019, 254, 115690. [Google Scholar] [CrossRef]
- Carević, I.; Baričević, A.; Štirmer, N.; Šantek Bajto, J. Correlation between Physical and Chemical Properties of Wood Biomass Ash and Cement Composites Performances. Constr. Build. Mater. 2020, 256, 119450. [Google Scholar] [CrossRef]
- Zabed, H.; Sahu, J.N.; Suely, A.; Boyce, A.N.; Faruq, G. Bioethanol Production from Renewable Sources: Current Perspectives and Technological Progress. Renew. Sustain. Energy Rev. 2017, 71, 475–501. [Google Scholar] [CrossRef]
- Stolarski, M.J.; Krzyżaniak, M.; Łuczyński, M.; Załuski, D.; Szczukowski, S.; Tworkowski, J.; Gołaszewski, J. Lignocellulosic Biomass from Short Rotation Woody Crops as a Feedstock for Second-Generation Bioethanol Production. Ind. Crop. Prod. 2015, 75, 66–75. [Google Scholar] [CrossRef]
- Ufodike, C.O.; Eze, V.O.; Ahmed, M.F.; Oluwalowo, A.; Park, J.G.; Okoli, O.I.; Wang, H. Evaluation of the Inter-Particle Interference of Cellulose and Lignin in Lignocellulosic Materials. Int. J. Biol. Macromol. 2020, 147, 762–767. [Google Scholar] [CrossRef]
- Zoghlami, A.; Paës, G. Lignocellulosic Biomass: Understanding Recalcitrance and Predicting Hydrolysis. Front. Chem. 2019, 7. [Google Scholar] [CrossRef]
- Deguchi, S.; Mukai, S.; Tsudome, M.; Horikoshi, K. Facile Generation of Fullerene Nanoparticles by Hand-Grinding. Adv. Mater. 2006, 18, 729–732. [Google Scholar] [CrossRef]
- Quiroz-Castañeda, R.E.; Folch-Mallol, J.L. Hydrolysis of Biomass Mediated by Cellulases for the Production of Sugars. Sustain. Degrad. Lignocellul. Biomass Tech. Appl. Commer. 2013. [Google Scholar] [CrossRef]
- Houfani, A.A.; Anders, N.; Spiess, A.C.; Baldrian, P.; Benallaoua, S. Insights from Enzymatic Degradation of Cellulose and Hemicellulose to Fermentable Sugars—A Review. Biomass Bioenergy 2020, 134, 105481. [Google Scholar] [CrossRef]
- Sacui, I.A.; Nieuwendaal, R.C.; Burnett, D.J.; Stranick, S.J.; Jorfi, M.; Weder, C.; Foster, E.J.; Olsson, R.T.; Gilman, J.W. Comparison of the Properties of Cellulose Nanocrystals and Cellulose Nanofibrils Isolated from Bacteria, Tunicate, and Wood Processed Using Acid, Enzymatic, Mechanical, and Oxidative Methods. ACS Appl. Mater. Interfaces 2014, 6, 6127–6138. [Google Scholar] [CrossRef] [PubMed]
- Uzyol, H.K.; Saçan, M.T. Bacterial Cellulose Production by Komagataeibacter Hansenii Using Algae-Based Glucose. Environ. Sci. Pollut. Res. Int. 2017, 24, 11154–11162. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.C. Hemicellulose Bioconversion. J. Ind. Microbiol. Biotechnol. 2003, 30, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic Biomass: A Sustainable Platform for the Production of Bio-Based Chemicals and Polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Auxenfans, T.; Crônier, D.; Chabbert, B.; Paës, G. Understanding the Structural and Chemical Changes of Plant Biomass Following Steam Explosion Pretreatment. Biotechnol. Biofuels 2017, 10, 36. [Google Scholar] [CrossRef]
- Bhalla, A.; Bischoff, K.M.; Sani, R.K. Highly Thermostable Xylanase Production from a Thermophilic Geobacillus Sp. Strain WSUCF1 Utilizing Lignocellulosic Biomass. Front. Bioeng. Biotechnol. 2015, 3, 1–8. [Google Scholar] [CrossRef]
- Agbor, V.B.; Cicek, N.; Sparling, R.; Berlin, A.; Levin, D.B. Biomass Pretreatment: Fundamentals toward Application. Biotechnol. Adv. 2011, 29, 675–685. [Google Scholar] [CrossRef]
- Santos, R.B.; Lee, J.M.; Jameel, H.; Chang, H.-M.; Lucia, L.A. Effects of Hardwood Structural and Chemical Characteristics on Enzymatic Hydrolysis for Biofuel Production. Bioresour. Technol. 2012, 110, 232–238. [Google Scholar] [CrossRef]
- Cheng, F.; Bayat, H.; Jena, U.; Brewer, C.E. Impact of Feedstock Composition on Pyrolysis of Low-Cost, Protein- and Lignin-Rich Biomass: A Review. J. Anal. Appl. Pyrolysis 2020, 147, 104780. [Google Scholar] [CrossRef]
- Silveira, M.H.L.; Morais, A.R.C.; da Costa Lopes, A.M.; Olekszyszen, D.N.; Bogel-Łukasik, R.; Andreaus, J.; Pereira Ramos, L. Current Pretreatment Technologies for the Development of Cellulosic Ethanol and Biorefineries. ChemSusChem 2015, 8, 3366–3390. [Google Scholar] [CrossRef]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for Pretreatment of Lignocellulosic Biomass for Efficient Hydrolysis and Biofuel Production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Karimi, K.; Taherzadeh, M.J. A Critical Review of Analytical Methods in Pretreatment of Lignocelluloses: Composition, Imaging, and Crystallinity. Bioresour. Technol. 2016, 200, 1008–1018. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Sun, S.; Cao, X.; Sun, R. The Role of Pretreatment in Improving the Enzymatic Hydrolysis of Lignocellulosic Materials. Bioresour. Technol. 2016, 199, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.R.; Jiao, S.; Lee, S.Y. Metabolic Engineering Strategies toward Production of Biofuels. Curr. Opin. Chem. Biol. 2020, 59, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.K.; Singhania, R.R.; Sim, S.J.; Pandey, A. Thermostable Cellulases: Current Status and Perspectives. Bioresour. Technol. 2019, 279, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.G.W.; Rodrigues, C.; Vandenberghe, L.P.d.S.; Woiciechowski, A.L.; Soccol, C.R. Current Advances in On-Site Cellulase Production and Application on Lignocellulosic Biomass Conversion to Biofuels: A Review. Biomass Bioenergy 2020, 132, 105419. [Google Scholar] [CrossRef]
- Srivastava, N.; Srivastava, M.; Mishra, P.K.; Gupta, V.K.; Molina, G.; Rodriguez-Couto, S.; Manikanta, A.; Ramteke, P.W. Applications of Fungal Cellulases in Biofuel Production: Advances and Limitations. Renew. Sustain. Energy Rev. 2018, 82, 2379–2386. [Google Scholar] [CrossRef]
- Xu, J.; Xiong, P.; He, B. Advances in Improving the Performance of Cellulase in Ionic Liquids for Lignocellulose Biorefinery. Bioresour. Technol. 2016, 200, 961–970. [Google Scholar] [CrossRef]
- Singh, S.; Madlala, A.M.; Prior, B.A. Thermomyces Lanuginosus: Properties of Strains and Their Hemicellulases. FEMS Microbiol. Rev. 2003, 27, 3–16. [Google Scholar] [CrossRef]
- Linton, S.M. Review: The Structure and Function of Cellulase (Endo-β-1,4-Glucanase) and Hemicellulase (β-1,3-Glucanase and Endo-β-1,4-Mannase) Enzymes in Invertebrates That Consume Materials Ranging from Microbes, Algae to Leaf Litter. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2020, 240, 110354. [Google Scholar] [CrossRef]
- Choudhary, J.; Singh, S.; Nain, L. Thermotolerant Fermenting Yeasts for Simultaneous Saccharification Fermentation of Lignocellulosic Biomass. Electron. J. Biotechnol. 2016, 21, 82–92. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Jagtap, S.S.; Bedekar, A.A.; Bhatia, R.K.; Patel, A.K.; Pant, D.; Rajesh Banu, J.; Rao, C.V.; Kim, Y.-G.; Yang, Y.-H. Recent Developments in Pretreatment Technologies on Lignocellulosic Biomass: Effect of Key Parameters, Technological Improvements, and Challenges. Bioresour. Technol. 2020, 300, 122724. [Google Scholar] [CrossRef] [PubMed]
- Spier, M.R.; Nogueira, A.; Alberti, A.; Gomes, T.A.; Dhillon, G.S. Potential Applications of Enzymes in Brewery and Winery. In Agro-Industrial Wastes as Feedstock for Enzyme Production; Dhillon, G.S., Kaur, S., Eds.; Academic Press: San Diego, CA, USA, 2016; Chapter 11; pp. 261–278. ISBN 978-0-12-802392-1. [Google Scholar]
- Servili, M.; Taticchi, A.; Esposto, S.; Sordini, B.; Urbani, S. Technological Aspects of Olive Oil Production. In Olive Germplasm—The Olive Cultivation, Table Olive and Olive Oil Industry in Italy; InTech: London, UK, 2012. [Google Scholar] [CrossRef]
- Li, H.; Wang, C.; Xiao, W.; Yang, Y.; Hu, P.; Dai, Y.; Jiang, Z. Dissecting the Effect of Polyethylene Glycol on the Enzymatic Hydrolysis of Diverse Lignocellulose. Int. J. Biol. Macromol. 2019, 131, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.-H.; Kadhum, H.J.; Murthy, G.S.; Dien, B.S.; Singh, V. High Solids Loading Biorefinery for the Production of Cellulosic Sugars from Bioenergy Sorghum. Bioresour. Technol. 2020, 318, 124051. [Google Scholar] [CrossRef] [PubMed]
- Zambare, V.P.; Christopher, L.P. Integrated Biorefinery Approach to Utilization of Pulp and Paper Mill Sludge for Value-Added Products. J. Clean. Prod. 2020, 274, 122791. [Google Scholar] [CrossRef]
- Tippkötter, N.; Duwe, A.-M.; Wiesen, S.; Sieker, T.; Ulber, R. Enzymatic Hydrolysis of Beech Wood Lignocellulose at High Solid Contents and Its Utilization as Substrate for the Production of Biobutanol and Dicarboxylic Acids. Bioresour. Technol. 2014, 167, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Ostadjoo, S.; Hammerer, F.; Dietrich, K.; Dumont, M.-J.; Friščić, T.; Auclair, K. Efficient Enzymatic Hydrolysis of Biomass Hemicellulose in the Absence of Bulk Water. Molecules 2019, 24, 4206. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, C.; Tong, S.; Cui, Z.; Liu, P. Enhanced Enzymatic Hydrolysis and Structural Features of Corn Stover by NaOH and Ozone Combined Pretreatment. Molecules 2018, 23, 1300. [Google Scholar] [CrossRef]
- Robak, K.; Balcerek, M. Current State-of-the-Art in Ethanol Production from Lignocellulosic Feedstocks. Microbiol. Res. 2020, 240, 126534. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Machado, E.M.S.; Carneiro, L.M.; Teixeira, J.A. Sugars Metabolism and Ethanol Production by Different Yeast Strains from Coffee Industry Wastes Hydrolysates. Appl. Energy 2012, 92, 763–768. [Google Scholar] [CrossRef]
- Wirawan, F.; Cheng, C.-L.; Lo, Y.-C.; Chen, C.-Y.; Chang, J.-S.; Leu, S.-Y.; Lee, D.-J. Continuous Cellulosic Bioethanol Co-Fermentation by Immobilized Zymomonas Mobilis and Suspended Pichia Stipitis in a Two-Stage Process. Appl. Energy 2020, 266, 114871. [Google Scholar] [CrossRef]
- Ling, Z.; Chen, S.; Zhang, X.; Xu, F. Exploring Crystalline-Structural Variations of Cellulose during Alkaline Pretreatment for Enhanced Enzymatic Hydrolysis. Bioresour. Technol. 2017, 224, 611–617. [Google Scholar] [CrossRef]
- Wada, M.; Ike, M.; Tokuyasu, K. Enzymatic Hydrolysis of Cellulose I Is Greatly Accelerated via Its Conversion to the Cellulose II Hydrate Form. Polym. Degrad. Stab. 2010, 95, 543–548. [Google Scholar] [CrossRef]
- Gu, H.; An, R.; Bao, J. Pretreatment Refining Leads to Constant Particle Size Distribution of Lignocellulose Biomass in Enzymatic Hydrolysis. Chem. Eng. J. 2018, 352, 198–205. [Google Scholar] [CrossRef]
- Kapoor, M.; Semwal, S.; Satlewal, A.; Christopher, J.; Gupta, R.P.; Kumar, R.; Puri, S.K.; Ramakumar, S.S.V. The Impact of Particle Size of Cellulosic Residue and Solid Loadings on Enzymatic Hydrolysis with a Mass Balance. Fuel 2019, 245, 514–520. [Google Scholar] [CrossRef]
- Li, X.; Xiong, N.; Wang, X.; Dai, X.; Guo, Y.; Dong, B. New Insight into Volatile Sulfur Compounds Conversion in Anaerobic Digestion of Excess Sludge: Influence of Free Ammonia Nitrogen and Thermal Hydrolysis Pretreatment. J. Clean. Prod. 2020, 277, 123366. [Google Scholar] [CrossRef]
- Wang, Z.; He, X.; Yan, L.; Wang, J.; Hu, X.; Sun, Q.; Zhang, H. Enhancing Enzymatic Hydrolysis of Corn Stover by Twin-Screw Extrusion Pretreatment. Ind. Crop. Prod. 2020, 143, 111960. [Google Scholar] [CrossRef]
- Lan, T.Q.; Wang, S.R.; Li, H.; Qin, Y.Y.; Yue, G.J. Effect of Lignin Isolated from P-Toluenesulfonic Acid Pretreatment Liquid of Sugarcane Bagasse on Enzymatic Hydrolysis of Cellulose and Cellulase Adsorption. Ind. Crop. Prod. 2020, 155, 112768. [Google Scholar] [CrossRef]
- Torr, K.M.; Love, K.T.; Simmons, B.A.; Hill, S.J. Structural Features Affecting the Enzymatic Digestibility of Pine Wood Pretreated with Ionic Liquids. Biotechnol. Bioeng. 2016, 113, 540–549. [Google Scholar] [CrossRef]
- Zhang, H.; Li, J.; Huang, G.; Yang, Z.; Han, L. Understanding the Synergistic Effect and the Main Factors Influencing the Enzymatic Hydrolyzability of Corn Stover at Low Enzyme Loading by Hydrothermal and/or Ultrafine Grinding Pretreatment. Bioresour. Technol. 2018, 264, 327–334. [Google Scholar] [CrossRef]
- Lu, M.; Li, J.; Han, L.; Xiao, W. An Aggregated Understanding of Cellulase Adsorption and Hydrolysis for Ball-Milled Cellulose. Bioresour. Technol. 2019, 273, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Herbaut, M.; Zoghlami, A.; Habrant, A.; Falourd, X.; Foucat, L.; Chabbert, B.; Paës, G. Multimodal Analysis of Pretreated Biomass Species Highlights Generic Markers of Lignocellulose Recalcitrance. Biotechnol. Biofuels 2018, 11, 52. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Foston, M.; Leisen, J.; DeMartini, J.; Wyman, C.E.; Ragauskas, A.J. Determination of Porosity of Lignocellulosic Biomass before and after Pretreatment by Using Simons’ Stain and NMR Techniques. Bioresour. Technol. 2013, 144, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Peciulyte, A.; Karlström, K.; Larsson, P.T.; Olsson, L. Impact of the Supramolecular Structure of Cellulose on the Efficiency of Enzymatic Hydrolysis. Biotechnol. Biofuels 2015, 8, 56. [Google Scholar] [CrossRef]
- Shokrkar, H.; Ebrahimi, S.; Zamani, M. Enzymatic Hydrolysis of Microalgal Cellulose for Bioethanol Production, Modeling and Sensitivity Analysis. Fuel 2018, 228, 30–38. [Google Scholar] [CrossRef]
- Onay, M. Bioethanol Production via Different Saccharification Strategies from H. Tetrachotoma ME03 Grown at Various Concentrations of Municipal Wastewater in a Flat-Photobioreactor. Fuel 2019, 239, 1315–1323. [Google Scholar] [CrossRef]
- Ngamsirisomsakul, M.; Reungsang, A.; Liao, Q.; Kongkeitkajorn, M.B. Enhanced Bio-Ethanol Production from Chlorella Sp. Biomass by Hydrothermal Pretreatment and Enzymatic Hydrolysis. Renew. Energy 2019, 141, 482–492. [Google Scholar] [CrossRef]
- Kumar, V.; Nanda, M.; Joshi, H.C.; Singh, A.; Sharma, S.; Verma, M. Production of Biodiesel and Bioethanol Using Algal Biomass Harvested from Fresh Water River. Renew. Energy 2018, 116, 606–612. [Google Scholar] [CrossRef]
- Rempel, A.; de Souza Sossella, F.; Margarites, A.C.; Astolfi, A.L.; Steinmetz, R.L.R.; Kunz, A.; Treichel, H.; Colla, L.M. Bioethanol from Spirulina Platensis Biomass and the Use of Residuals to Produce Biomethane: An Energy Efficient Approach. Bioresour. Technol. 2019, 288, 121588. [Google Scholar] [CrossRef]
- Sulfahri, M.; Mushlihah, S.; Husain, D.R.; Langford, A.; Tassakka, A.C.M.A.R. Fungal Pretreatment as a Sustainable and Low Cost Option for Bioethanol Production from Marine Algae. J. Clean. Prod. 2020, 265, 121763. [Google Scholar] [CrossRef]
- Qarri, A.; Israel, A. Seasonal Biomass Production, Fermentable Saccharification and Potential Ethanol Yields in the Marine Macroalga Ulva Sp. (Chlorophyta). Renew. Energy 2020, 145, 2101–2107. [Google Scholar] [CrossRef]
- Barbanera, M.; Lascaro, E.; Foschini, D.; Cotana, F.; Buratti, C. Optimization of Bioethanol Production from Steam Exploded Hornbeam Wood (Ostrya Carpinifolia) by Enzymatic Hydrolysis. Renew. Energy 2018, 124, 136–143. [Google Scholar] [CrossRef]
- Indira, D.; Das, B.; Bhawsar, H.; Moumita, S.; Johnson, E.M.; Balasubramanian, P.; Jayabalan, R. Investigation on the Production of Bioethanol from Black Tea Waste Biomass in the Seawater-Based System. Bioresour. Technol. Rep. 2018, 4, 209–213. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, J.; Cao, J.; Wang, Z. Pretreatment with Concurrent UV Photocatalysis and Alkaline H2O2 Enhanced the Enzymatic Hydrolysis of Sisal Waste. Bioresour. Technol. 2018, 267, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Talekar, S.; Patti, A.F.; Vijayraghavan, R.; Arora, A. An Integrated Green Biorefinery Approach towards Simultaneous Recovery of Pectin and Polyphenols Coupled with Bioethanol Production from Waste Pomegranate Peels. Bioresour. Technol. 2018, 266, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, A.B.; Ballesteros, I.; Ballesteros, M. The Potential of Agricultural Banana Waste for Bioethanol Production. Fuel 2018, 213, 176–185. [Google Scholar] [CrossRef]
- Vu, P.T.; Unpaprom, Y.; Ramaraj, R. Impact and Significance of Alkaline-Oxidant Pretreatment on the Enzymatic Digestibility of Sphenoclea Zeylanica for Bioethanol Production. Bioresour. Technol. 2018, 247, 125–130. [Google Scholar] [CrossRef]
- Boonchuay, P.; Techapun, C.; Leksawasdi, N.; Seesuriyachan, P.; Hanmoungjai, P.; Watanabe, M.; Takenaka, S.; Chaiyaso, T. An Integrated Process for Xylooligosaccharide and Bioethanol Production from Corncob. Bioresour. Technol. 2018, 256, 399–407. [Google Scholar] [CrossRef]
- Ben Atitallah, I.; Antonopoulou, G.; Ntaikou, I.; Alexandropoulou, M.; Nasri, M.; Mechichi, T.; Lyberatos, G. On the Evaluation of Different Saccharification Schemes for Enhanced Bioethanol Production from Potato Peels Waste via a Newly Isolated Yeast Strain of Wickerhamomyces Anomalus. Bioresour. Technol. 2019, 289, 121614. [Google Scholar] [CrossRef]
- Láinez, M.; Ruiz, H.A.; Arellano-Plaza, M.; Martínez-Hernández, S. Bioethanol Production from Enzymatic Hydrolysates of Agave Salmiana Leaves Comparing S. Cerevisiae and K. Marxianus. Renew. Energy 2019, 138, 1127–1133. [Google Scholar] [CrossRef]
- Subsamran, K.; Mahakhan, P.; Vichitphan, K.; Vichitphan, S.; Sawaengkaew, J. Potential Use of Vetiver Grass for Cellulolytic Enzyme Production and Bioethanol Production. Biocatal. Agric. Biotechnol. 2019, 17, 261–268. [Google Scholar] [CrossRef]
- Kotarska, K.; Dziemianowicz, W.; Świerczyńska, A. Study on the Sequential Combination of Bioethanol and Biogas Production from Corn Straw. Molecules 2019, 24, 4558. [Google Scholar] [CrossRef]
- Demiray, E.; Karatay, S.E.; Dönmez, G. Improvement of Bioethanol Production from Pomegranate Peels via Acidic Pretreatment and Enzymatic Hydrolysis. Environ. Sci. Pollut. Res. Int. 2019, 26, 29366–29378. [Google Scholar] [CrossRef]
- Coniglio, R.O.; Díaz, G.V.; Fonseca, M.I.; Castrillo, M.L.; Piccinni, F.E.; Villalba, L.L.; Campos, E.; Zapata, P.D. Enzymatic Hydrolysis of Barley Straw for Biofuel Industry Using a Novel Strain of Trametes Villosa from Paranaense Rainforest. Prep. Biochem. Biotechnol. 2020, 1–10. [Google Scholar] [CrossRef]
- Raja Sathendra, E.; Baskar, G.; Praveenkumar, R.; Gnansounou, E. Bioethanol Production from Palm Wood Using Trichoderma Reesei and Kluveromyces Marxianus. Bioresour. Technol. 2019, 271, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Cho, E.J.; Park, C.S.; Oh, C.H.; Park, B.-J.; Bae, H.-J. A Strategy for Sequential Fermentation by Saccharomyces Cerevisiae and Pichia Stipitis in Bioethanol Production from Hardwoods. Renew. Energy 2019, 139, 1281–1289. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, L.; Gleisner, R.; Zhu, J.Y. Co-Production of Bioethanol and Furfural from Poplar Wood via Low Temperature (≤90 °C) Acid Hydrotropic Fractionation (AHF). Fuel 2019, 254, 115572. [Google Scholar] [CrossRef]
- Ziegler-Devin, I.; Menana, Z.; Chrusciel, L.; Chalot, M.; Bert, V.; Brosse, N. Steam Explosion Pretreatment of Willow Grown on Phytomanaged Soils for Bioethanol Production. Ind. Crop. Prod. 2019, 140, 111722. [Google Scholar] [CrossRef]
- Raj, K.; Krishnan, C. Improved High Solid Loading Enzymatic Hydrolysis of Low-Temperature Aqueous Ammonia Soaked Sugarcane Bagasse Using Laccase-Mediator System and High Concentration Ethanol Production. Ind. Crop. Prod. 2019, 131, 32–40. [Google Scholar] [CrossRef]
- Assabjeu, A.C.; Noubissié, E.; Desobgo, S.C.Z.; Ali, A. Optimization of the Enzymatic Hydrolysis of Cellulose of Triplochiton Scleroxylon Sawdust in View of the Production of Bioethanol. Sci. Afr. 2020, 8, e00438. [Google Scholar] [CrossRef]
- Abdou Alio, M.; Tugui, O.-C.; Rusu, L.; Pons, A.; Vial, C. Hydrolysis and Fermentation Steps of a Pretreated Sawmill Mixed Feedstock for Bioethanol Production in a Wood Biorefinery. Bioresour. Technol. 2020, 310, 123412. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Zhao, D.; Khodadadi, M.; Len, C. Lignocellulosic Biomass for Bioethanol: Recent Advances, Technology Trends, and Barriers to Industrial Development. Curr. Opin. Green Sustain. Chem. 2020, 24, 56–60. [Google Scholar] [CrossRef]
- Mohd Azhar, S.H.; Abdulla, R.; Jambo, S.A.; Marbawi, H.; Gansau, J.A.; Mohd Faik, A.A.; Rodrigues, K.F. Yeasts in Sustainable Bioethanol Production: A Review. Biochem. Biophys. Rep. 2017, 10, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.M.; Mota, M.J.; Lopes, R.P.; Sousa, S.; Gomes, A.M.; Delgadillo, I.; Saraiva, J.A. Adaptation of Saccharomyces Cerevisiae to High Pressure (15, 25 and 35 MPa) to Enhance the Production of Bioethanol. Food Res. Int. 2019, 115, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Naghshbandi, M.P.; Tabatabaei, M.; Aghbashlo, M.; Gupta, V.K.; Sulaiman, A.; Karimi, K.; Moghimi, H.; Maleki, M. Progress toward Improving Ethanol Production through Decreased Glycerol Generation in Saccharomyces Cerevisiae by Metabolic and Genetic Engineering Approaches. Renew. Sustain. Energy Rev. 2019, 115, 109353. [Google Scholar] [CrossRef]
- Ko, J.K.; Lee, S.-M. Advances in Cellulosic Conversion to Fuels: Engineering Yeasts for Cellulosic Bioethanol and Biodiesel Production. Curr. Opin. Biotechnol. 2018, 50, 72–80. [Google Scholar] [CrossRef]
- Arora, R.; Behera, S.; Kumar, S. Bioprospecting Thermophilic/Thermotolerant Microbes for Production of Lignocellulosic Ethanol: A Future Perspective. Renew. Sustain. Energy Rev. 2015, 51, 699–717. [Google Scholar] [CrossRef]
- Gutiérrez-Antonio, C.; Gómez-Castro, F.I.; de Lira-Flores, J.A.; Hernández, S. A Review on the Production Processes of Renewable Jet Fuel. Renew. Sustain. Energy Rev. 2017, 79, 709–729. [Google Scholar] [CrossRef]
- Szambelan, K.; Nowak, J.; Szwengiel, A.; Jeleń, H.; Łukaszewski, G. Separate Hydrolysis and Fermentation and Simultaneous Saccharification and Fermentation Methods in Bioethanol Production and Formation of Volatile By-Products from Selected Corn Cultivars. Ind. Crop. Prod. 2018, 118, 355–361. [Google Scholar] [CrossRef]
- Szambelan, K.; Nowak, J.; Frankowski, J.; Szwengiel, A.; Jeleń, H.; Burczyk, H. The Comprehensive Analysis of Sorghum Cultivated in Poland for Energy Purposes: Separate Hydrolysis and Fermentation and Simultaneous Saccharification and Fermentation Methods and Their Impact on Bioethanol Effectiveness and Volatile by-Products from the Grain and the Energy Potential of Sorghum Straw. Bioresour. Technol. 2018, 250, 750–757. [Google Scholar] [CrossRef]
- Tavva, S.S.M.D.; Deshpande, A.; Durbha, S.R.; Palakollu, V.A.R.; Goparaju, A.U.; Yechuri, V.R.; Bandaru, V.R.; Muktinutalapati, V.S.R. Bioethanol Production through Separate Hydrolysis and Fermentation of Parthenium Hysterophorus Biomass. Renew. Energy 2016, 86, 1317–1323. [Google Scholar] [CrossRef]
- Chow, T.-J.; Su, H.-Y.; Tsai, T.-Y.; Chou, H.-H.; Lee, T.-M.; Chang, J.-S. Using Recombinant Cyanobacterium (Synechococcus Elongatus) with Increased Carbohydrate Productivity as Feedstock for Bioethanol Production via Separate Hydrolysis and Fermentation Process. Bioresour. Technol. 2015, 184, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Luiza Astolfi, A.; Rempel, A.; Cavanhi, V.A.F.; Alves, M.; Deamici, K.M.; Colla, L.M.; Costa, J.A.V. Simultaneous Saccharification and Fermentation of Spirulina Sp. and Corn Starch for the Production of Bioethanol and Obtaining Biopeptides with High Antioxidant Activity. Bioresour. Technol. 2020, 301, 122698. [Google Scholar] [CrossRef] [PubMed]
- Chohan, N.A.; Aruwajoye, G.S.; Sewsynker-Sukai, Y.; Gueguim Kana, E.B. Valorisation of Potato Peel Wastes for Bioethanol Production Using Simultaneous Saccharification and Fermentation: Process Optimization and Kinetic Assessment. Renew. Energy 2020, 146, 1031–1040. [Google Scholar] [CrossRef]




| Macroalgae | Sugar Composition |
|---|---|
| Phaeophyta (brown) | alginate cellulose mannitol fucoidin laminarin |
| Rhodophyta (red) | agar carrageenan cellulose lignin |
| Chlorophyta (green) | starch cellulose mannan ulvan |
| Microalgal Biomass | Pretreatment | Enzyme | Yield | Ref. |
|---|---|---|---|---|
| Mixed microalgae | enzymatic hydrolysis | cellulase from T. reesei | 57% glucose | [102] |
| Hindakia tetrachotoma ME03 | acidic, alkaline, enzymatic hydrolysis | β-glucosidase from E. coli, cellulase from A. niger, α-amylase from B. licheniformis, amyloglucosidase from A. niger | 92% sacchar. | [103] |
| Chlorella sp. | hydrothermal pretreatment | α-amylase, glucoamylase | 11 g/L of bioethanol | [104] |
| Microalgal biomass | acid hydrolysis | cellulase from T. reesei | 61% bioethanol | [105] |
| Spirulina platensis | none | α-amylase, amyloglucosidase | 80% polysacchar. | [106] |
| K. alvarezii, G. amansii | fungal | Cellic CTec2 | 38% ethanol | [107] |
| Ulva fasciata, Ulva rigida, Ulva ohnoi | none | cellulase, amyloglucosidase, α-amylase | 77% ethanol | [108] |
| Biomass | Pretreatment | Enzyme | Yield | Ref. |
|---|---|---|---|---|
| Palm wood | hydrothermal technique in conjunction with chemical method for removal of lignin | cellulase from T. reesei | 23g/L bioethanol yield | [122] |
Hardwood:
(Chionanthus retusus)
(Zelkova serrata),
(Acer palmatum)
(Castanea crenata)
(Robinia pseudoacacia) | hydrogen peroxide acetic acid pretreatment | cellulase (celluclast) | 81% ethanol yield | [123] |
| Poplar wood | acid hydrotrope | CTec3 cellulase | 68% bioethanol yield | [124] |
| Willow (Salix viminalis W) | steam explosion | cellulase from T. reesei | 65% ethanol yield | [125] |
| Sugacane bagasse | low temperature aqueous ammonia soaking | Cellic CTec2 cellulase | 91% ethanol yield | [126] |
| Sawdust from Ayous (Triplochiton scleroxylon) | Organosolv process | cellulase | 69% enzymatic hydrolysis yield | [127] |
| Sawmill mixed feedstock | microwave-assisted water/ethanol Organosolv pretreatment | Cellic CTec2 cellulase | 80% ethanol yield | [128] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasić, K.; Knez, Ž.; Leitgeb, M. Bioethanol Production by Enzymatic Hydrolysis from Different Lignocellulosic Sources. Molecules 2021, 26, 753. https://doi.org/10.3390/molecules26030753
Vasić K, Knez Ž, Leitgeb M. Bioethanol Production by Enzymatic Hydrolysis from Different Lignocellulosic Sources. Molecules. 2021; 26(3):753. https://doi.org/10.3390/molecules26030753
Chicago/Turabian StyleVasić, Katja, Željko Knez, and Maja Leitgeb. 2021. "Bioethanol Production by Enzymatic Hydrolysis from Different Lignocellulosic Sources" Molecules 26, no. 3: 753. https://doi.org/10.3390/molecules26030753
APA StyleVasić, K., Knez, Ž., & Leitgeb, M. (2021). Bioethanol Production by Enzymatic Hydrolysis from Different Lignocellulosic Sources. Molecules, 26(3), 753. https://doi.org/10.3390/molecules26030753








