Advances in Cryochemistry: Mechanisms, Reactions and Applications
Abstract
1. Introduction
2. The Mechanism of Accelerating Reactions Induced by Freezing
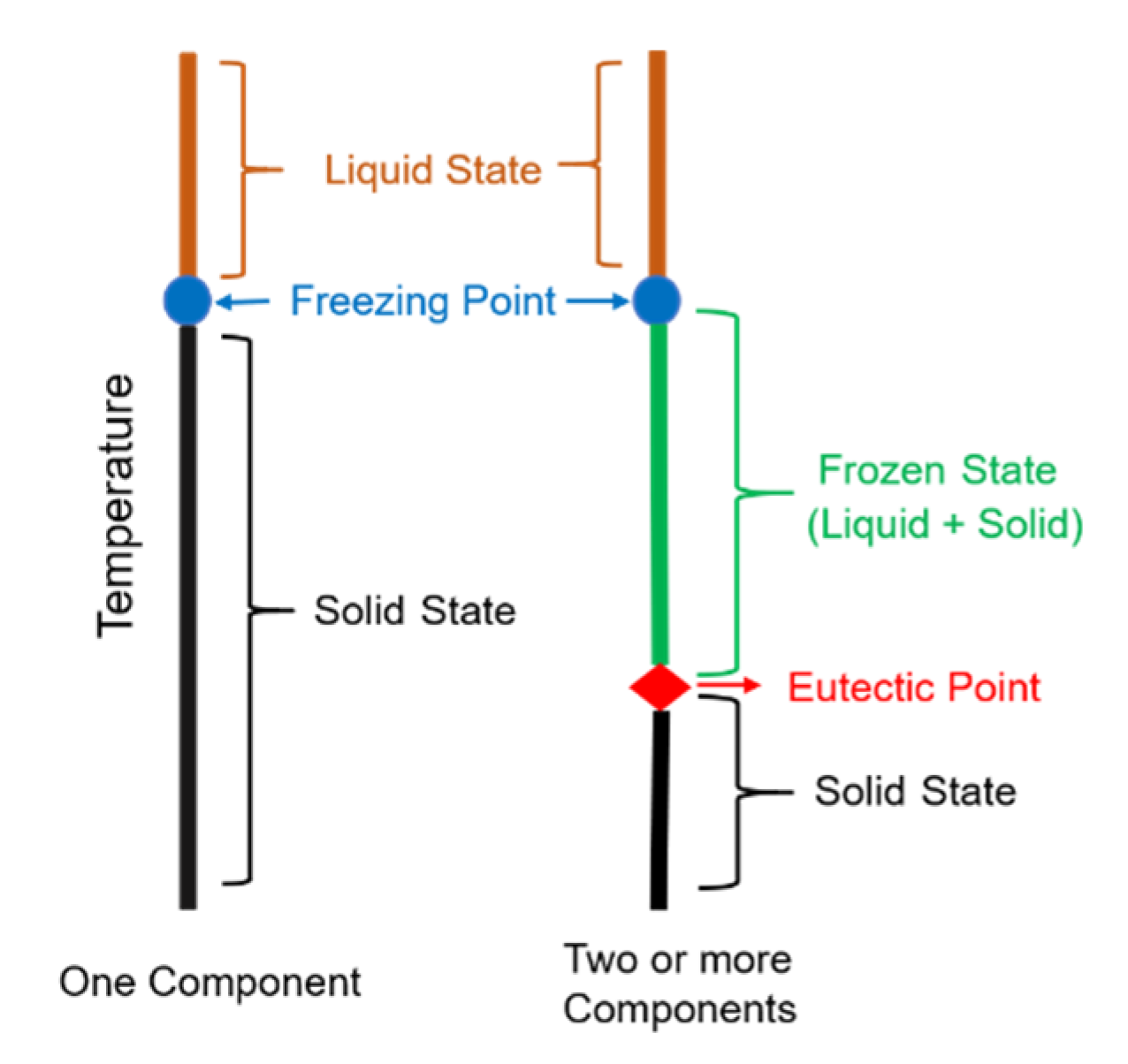
2.1. Freeze Concentration
2.2. Freezing-Potential
2.3. The Varies of pH Value Due to Neutralization of the Freezing Potential
2.4. Ice surface Catalysis
2.5. Chemical Equilibrium Shifts
2.6. The Cage Effect
2.7. Convection Effect
2.8. Temperature Differences
2.9. Conformational Changes
3. Kinetics of Reactions in Frozen Systems
- (1)
- Second- or higher-order reactions
- (2)
- Low total initial concentration and low reactant concentration
- (3)
- Small activation energy of the reaction
4. Various Reactions Accelerated in Frozen Systems
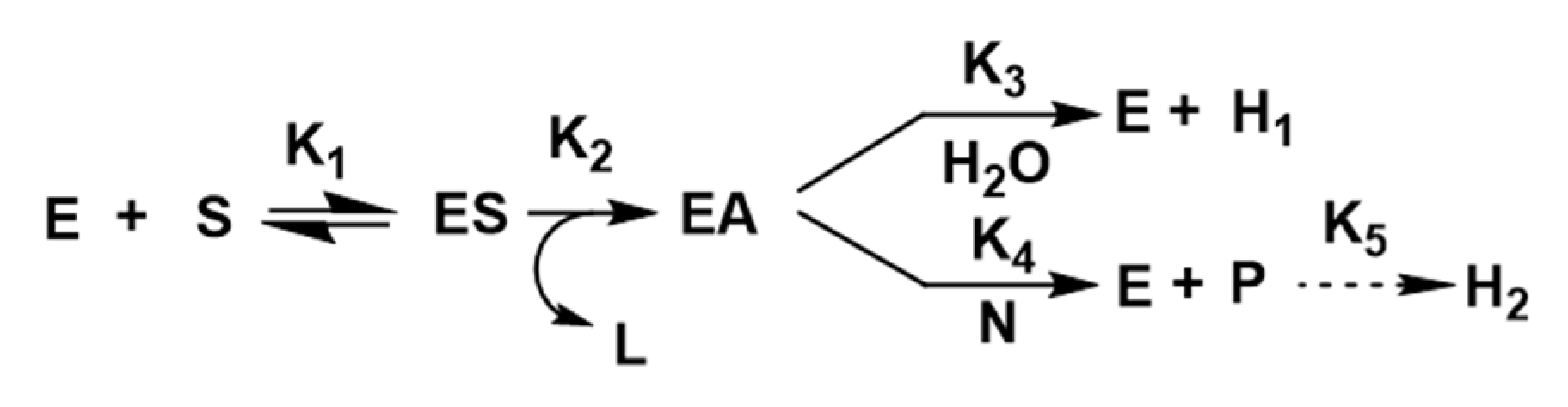
4.1. Hydrolysis Reactions
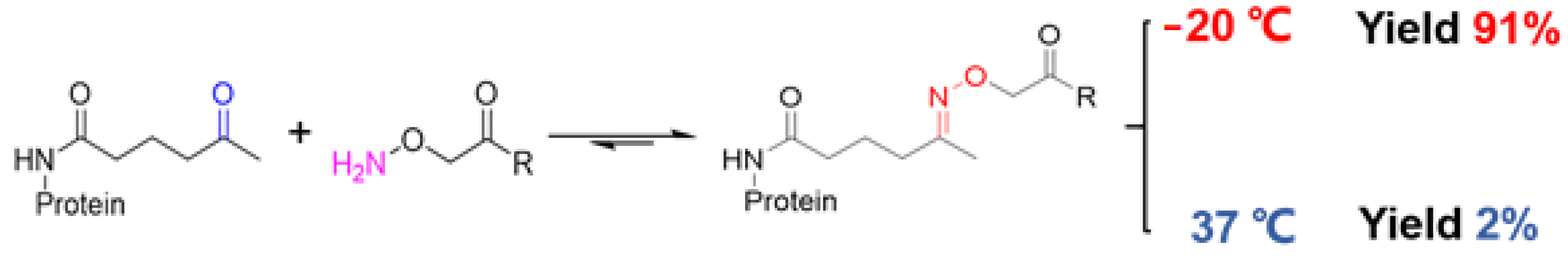
4.2. Oximation Reactions
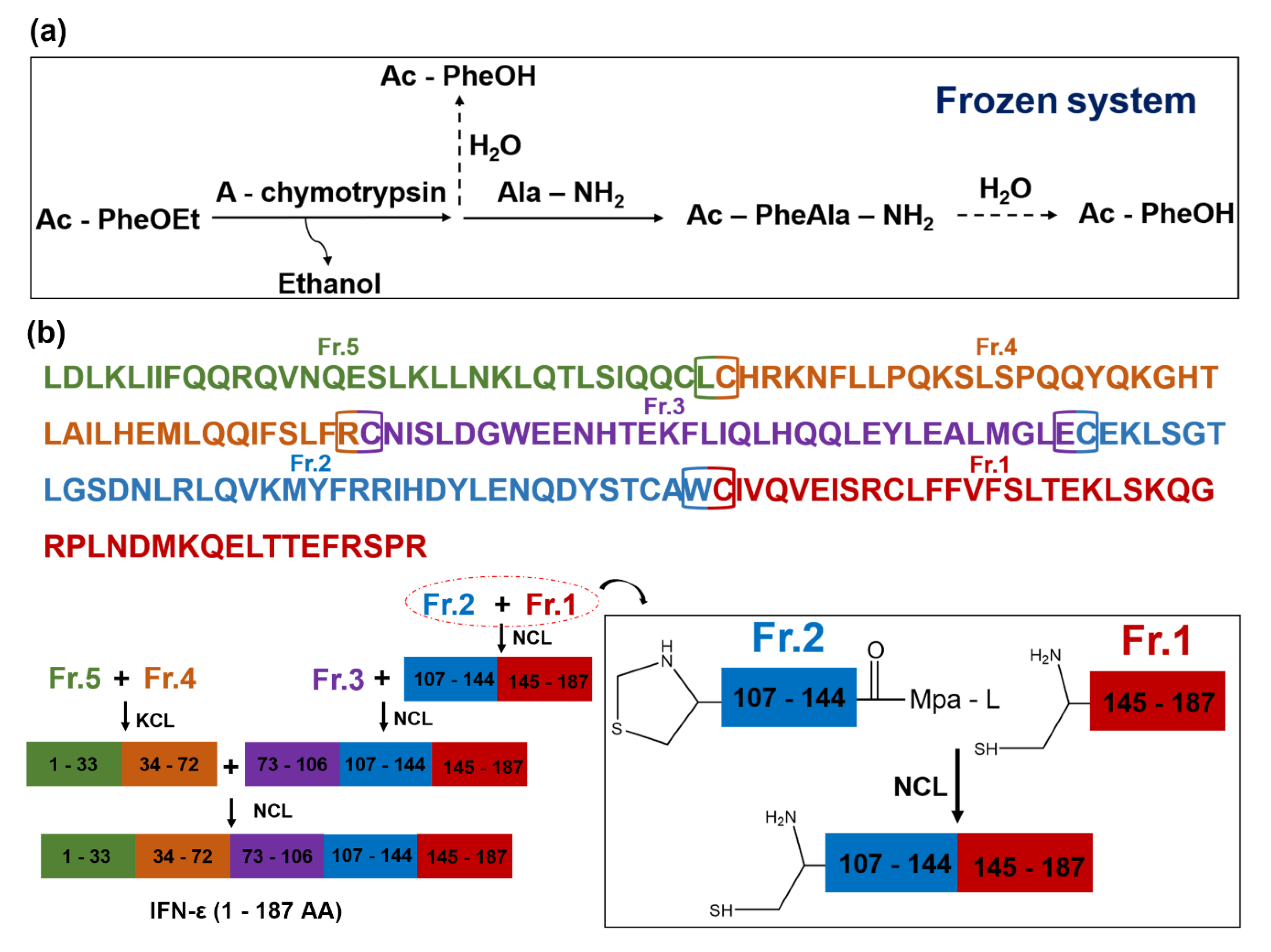
4.3. Peptide Synthesis
4.3.1. Enzyme-Catalyzed Synthesis of Peptides
4.3.2. Chemical Synthesis of Peptides
4.3.3. Conformational Changes of Peptides
4.4. Cryopolymerization Reactions
4.5. Other Reactions and Applications
4.6. Undesirable Reactions and Changes
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Murray, B.J.; O’sullivan, D.; Atkinson, J.D.; Webb, M.E. Ice nucleation by particles immersed in supercooled cloud droplets. Chem. Soc. Rev. 2012, 41, 6519–6554. [Google Scholar] [CrossRef] [PubMed]
- Knopf, D.A.; Alpert, P.A. A water activity based model of heterogeneous ice nucleation kinetics for freezing of water and aqueous solution droplets. Faraday Discuss. 2013, 165, 513–534. [Google Scholar] [CrossRef] [PubMed]
- Abbatt, J.P.D.; Thomas, J.L.; Abrahamsson, K.; Boxe, C.; Granfors, A.; Jones, A.E.; King, A.D.; Saiz-Lopez, A.; Shepson, P.B.; Sodeau, J.; et al. Halogen activation via interactions with environmental ice and snow in the polar lower troposphere and other regions. Atmos. Chem. Phys. 2012, 12, 6237–6271. [Google Scholar] [CrossRef]
- Jeong, D.; Kim, K.; Choi, W. Accelerated dissolution of iron oxides in ice. Atmos. Chem. Phys. 2012, 12, 11125–11133. [Google Scholar] [CrossRef]
- Jeong, D.; Kim, K.; Min, D.W.; Choi, W. Freezing-Enhanced Dissolution of Iron Oxides: Effects of Inorganic Acid Anions. Environ. Sci. Technol. 2015, 49, 12816–12822. [Google Scholar] [CrossRef]
- Singh, S.K.; Kolhe, P.; Mehta, A.P.; Chico, S.C.; Lary, A.L.; Huang, M. Frozen state storage instability of a monoclonal antibody: Aggregation as a consequence of trehalose crystallization and protein unfolding. Pharm. Res. 2011, 28, 873–885. [Google Scholar] [CrossRef]
- Kolhe, P.; Amend, E.K.; Singh, S. Impact of Freezing on pH of Buffered Solutions and Consequences for Monoclonal Antibody Aggregation. Biotechnol. Prog. 2010, 26, 727–733. [Google Scholar] [CrossRef]
- Grant, N.H.; Clark, D.E.; Alburn, H.E. Imidazole- and base-catalyzed hydrolysis of penicillin in frozen systems. J. Am. Chem. Soc. 1961, 83, 4476–4477. [Google Scholar] [CrossRef]
- Bruice, T.C.; Butler, A.R. Catalysis in Water and Ice. II.1 The Reaction of Thiolactones with Morpholine in Frozen Systems. J. Am. Chem. Soc. 1964, 86, 4104–4108. [Google Scholar] [CrossRef]
- Alburn, H.E.; Grant, N.H. Reactions in Frozen Systems. II.1 Enhanced Hydroxylaminolysis of Simple Amides. J. Am. Chem. Soc. 1965, 87, 4174–4177. [Google Scholar] [CrossRef]
- Pincock, R.E.; Kiovsky, T.E. Reactions in Frozen Solutions. III. Methyl Iodide with Triethylamine in Frozen Benzene Solutions. J. Am. Chem. Soc. 1966, 88. [Google Scholar] [CrossRef]
- Kiovsky, T.E.; Pincock, R.E. Demonstration of a reaction in frozen aqueous solutions. J. Chem. Educ. 1966, 43, 361–362. [Google Scholar] [CrossRef]
- Pincock, R.E. Reactions in frozen systems. Acc. Chem. Res. 1969, 2, 97–103. [Google Scholar] [CrossRef]
- Bogdan, A.; Molina, M.J.; Tenhu, H.; Bertel, E.; Bogdan, N.; Loerting, T. Visualization of Freezing Process in situ upon Cooling and Warming of Aqueous Solutions. Sci. Rep. 2014, 4, 1–6. [Google Scholar] [CrossRef]
- Bogdan, A.; Molina, M.J.; Tenhu, H. Freezing and glass transitions upon cooling and warming and ice/freeze-concentration-solution morphology of emulsified aqueous citric acid. Eur. J. Pharm. Biopharm. 2016, 109, 49–60. [Google Scholar] [CrossRef]
- Hänsler, M.; Jakubke, H.-D. Nonconventional Protease Catalysis in Frozen Aqueous Solutions. J. Pept. Sci. 1996, 2, 279–289. [Google Scholar] [CrossRef]
- Pincock, R.E.; Kiovsky, T.E. Reactions in Frozen Solutions. VI.1 The Reaction of Ethylene Chlorohydrin with Hydroxyl Ion in Ice. J. Am. Chem. Soc. 1966, 88, 4455–4459. [Google Scholar] [CrossRef]
- O’concubhair, R.; Sodeau, J.R. The Effect of Freezing on Reactions with Environmental Impact. Acc. Chem. Res. 2013, 46, 2716–2724. [Google Scholar] [CrossRef]
- Yang, Y.H.; Di, B.; Yang, D.S. The discovery of a freezing-induced peptide ligation during the total chemical synthesis of human interferon-ε. Org. Biomol. Chem. 2018, 16, 5097–5101. [Google Scholar] [CrossRef]
- Takenaka, N.; Ueda, A.; Daimon, T.; Bandow, H.; Dohmaru, T.; Maeda, Y. Acceleration mechanism of chemical reaction by freezing: The reaction of nitrous acid with dissolved oxygen. J. Phys. Chem. 1996, 100, 13874–13884. [Google Scholar] [CrossRef]
- Arakaki, T.; Shibata, M.; Miyake, T.; Hirakawa, T.; Sakugawa, H. Enhanced formation of formate by freezing in solutions of hydrated formaldehyde-metal-hydrogen peroxide. Geochem. J. 2004, 38, 383–388. [Google Scholar] [CrossRef]
- Champion, D.; Simatos, D.; Kalogianni, E.P.; Cayot, P.; Le Meste, M. Ascorbic acid oxidation in sucrose aqueous model systems at subzero temperatures. J. Agric. Food Chem. 2004, 52, 3399–3404. [Google Scholar] [CrossRef] [PubMed]
- O’sullivan, D.; Sodeau, J.R. Freeze-Induced Reactions: Formation of Iodine-Bromine Interhalogen Species from Aqueous Halide Ion Solutions. J. Phys. Chem. A 2010, 114, 12208–12215. [Google Scholar] [CrossRef] [PubMed]
- O’driscoll, P.; Minogue, N.; Takenaka, N.; Sodeau, J. Release of nitric oxide and iodine to the atmosphere from the freezing of sea-salt aerosol components. J. Phys. Chem. A 2008, 112, 1677–1682. [Google Scholar] [CrossRef]
- Takenaka, N.; Furuya, S.; Sato, K.; Bandow, H.; Maeda, Y.; Furukawa, Y. Rapid reaction of sulfide with hydrogen peroxide and formation of different final products by freezing compared to those in solution. Int. J. Chem. Kinet. 2003, 35, 198–205. [Google Scholar] [CrossRef]
- Beukers, R.; Ijlstra, J.; Berends, W. The effects of ultraviolet light on some components of the nucleic acids: III. Apurinic acid. Red. Trav. Chim. Pays-Bas 1959, 78, 247–251. [Google Scholar] [CrossRef]
- Beukers, R.; Ijlstra, J.; Berends, W. The effect of ultraviolet light on some components of the nucleic acids. VI The origin of the U.V. sensitivity of deoxyribonucleic acid. Red. Trav. Chim. Pays-Bas 1960, 79, 101–104. [Google Scholar] [CrossRef]
- Ray, D.; Malongwe, J.K.; Klan, P. Rate acceleration of the heterogeneous reaction of ozone with a model alkene at the air-ice interface at low temperatures. Environ. Sci. Technol. 2013, 47, 6773–6780. [Google Scholar] [CrossRef]
- Kitada, K.; Suda, Y.; Takenaka, N. Acceleration and Reaction Mechanism of the N-Nitrosation Reaction of Dimethylamine with Nitrite in Ice. J. Phys. Chem. A 2017, 121, 5383–5388. [Google Scholar] [CrossRef]
- Heger, D.; Klanova, J.; Klan, P. Enhanced protonation of cresol red in acidic aqueous solutions caused by freezing. J. Phys. Chem. B 2006, 110, 1277–1287. [Google Scholar] [CrossRef]
- Choi, Y.; Yoon, H.I.; Lee, C.; Vetrakova, L.; Heger, D.; Kim, K.; Kim, J. Activation of Periodate by Freezing for the Degradation of Aqueous Organic Pollutants. Environ. Sci. Technol. 2018, 52, 5378–5385. [Google Scholar] [CrossRef] [PubMed]
- Workman, E.J.; Reynolds, S.E. Electrical Phenomena Occurring during the Freezing of Dilute Aqueous Solutions and Their Possible Relationship to Thunderstorm Electricity. Phys. Rev. 1950, 78, 254–259. [Google Scholar] [CrossRef]
- Cobb, A.W.; Gross, G.W. Interfacial Electrical Effects Observed during the Freezing of Dilute Electrolytes in Water. J. Electrochem. Soc. 1969, 116, 796–804. [Google Scholar] [CrossRef]
- Watanabe, H.; Otsuka, T.; Harada, M.; Okada, T. Imbalance between Anion and Cation Distribution at Ice Interface with Liquid Phase in Frozen Electrolyte As Evaluated by Fluorometric Measurements of pH. J. Phys. Chem. C 2014, 118, 15723–15731. [Google Scholar] [CrossRef]
- Cheng, J.; Soetjipto, C.; Hoffmann, M.R.; Colussi, A.J. Confocal Fluorescence Microscopy of the Morphology and Composition of Interstitial Fluids in Freezing Electrolyte Solutions. J. Phys. Chem. Lett. 2009, 1, 374–378. [Google Scholar] [CrossRef]
- Ju, J.; Kim, J.; Vetráková, Ľ.; Seo, J.; Heger, D.; Lee, C.; Yoon, H. Accelerated redox reaction between chromate and phenolic pollutants during freezing. J. Hazard. Mater. 2017, 329, 330–338. [Google Scholar] [CrossRef]
- Takenaka, N.; Tanaka, M.; Okitsu, K.; Bandow, H. Rise in the pH of an Unfrozen Solution in Ice Due to the Presence of NaCl and Promotion of Decomposition of Gallic Acids Owing to a Change in the pH. J. Phys. Chem. A 2006, 110, 10628–10632. [Google Scholar] [CrossRef]
- Murase, N.; Franks, F. Salt precipitation during the freeze-concentration of phosphate buffer solutions. Biophys. Chem. 1989, 34, 293–300. [Google Scholar] [CrossRef]
- Pikal-Cleland, K.A.; Rodriguez-Hornedo, N.; Amidon, G.L.; Carpenter, J.F. Protein denaturation during freezing and thawing in phosphate buffer systems: Monomeric and tetrameric beta-galactosidase. Arch. Biochem. Biophys. 2000, 384, 398–406. [Google Scholar] [CrossRef]
- Pikal-Cleland, K.A.; Cleland, J.L.; Anchordoquy, T.J.; Carpenter, J.F. Effect of glycine on pH changes and protein stability during freeze–thawing in phosphate buffer systems. J. Pharm. Sci. 2002, 91, 1969–1979. [Google Scholar] [CrossRef]
- Sundaramurthi, P.; Shalaev, E.; Suryanarayanan, R. “pH Swing” in Frozen Solutions—Consequence of Sequential Crystallization of Buffer Components. J. Phys. Chem. Lett. 2009, 1, 265–268. [Google Scholar] [CrossRef]
- Bartels-Rausch, T.; Jacobi, H.W.; Kahan, T.F.; Thomas, J.L.; Thomson, E.S.; Abbatt, J.P.D. A review of air-ice chemical and physical interactions (AICI): Liquids, quasi-liquids, and solids in snow. Atmos. Chem. Phys. 2014, 14, 1587–1633. [Google Scholar] [CrossRef]
- Park, S.C.; Moon, E.S.; Kang, H. Some fundamental properties and reactions of ice surfaces at low temperatures. Phys. Chem. Chem. Phys. 2010, 12, 12000–12011. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.R.; Thomson, E.S.; Papagiannakopoulos, P.; Johansson, S.M.; Pettersson, J.B.C. Water Accommodation on Ice and Organic Surfaces: Insights from Environmental Molecular Beam Experiments. J. Phys. Chem. B 2014, 118, 13378–13386. [Google Scholar] [CrossRef] [PubMed]
- Boxe, C.S.; Saiz-Lopez, A. Multiphase modeling of nitrate photochemistry in the quasi-liquid layer (QLL): Implications for NOx release from the Arctic and coastal Antarctic snowpack. Atmos. Chem. Phys. 2008, 8, 4855–4864. [Google Scholar] [CrossRef]
- Dubowski, Y.; Colussi, A.J.; Hoffmann, M.R. Nitrogen Dioxide Release in the 302 nm Band Photolysis of Spray-Frozen Aqueous Nitrate Solutions. Atmospheric Implications. J. Phys. Chem. A 2001, 105, 4928–4932. [Google Scholar] [CrossRef]
- Dubowski, Y.; Colussi, A.J.; Boxe, C.; Hoffmann, M.R. Monotonic increase of nitrite yields in the photolysis of nitrate in ice and water between 238 and 294 K. J. Phys. Chem. 2002, 106, 6967–6971. [Google Scholar] [CrossRef]
- Kim, K.; Choi, W.; Hoffmann, M.R.; Yoon, H.-I.; Park, B.-K. Photoreductive Dissolution of Iron Oxides Trapped in Ice and Its Environmental Implications. Environ. Sci. Technol. 2010, 44, 4142–4148. [Google Scholar] [CrossRef]
- Menacherry, S.P.M.; Kim, K.; Lee, W.; Choi, C.H.; Choi, W. Ligand-Specific Dissolution of Iron Oxides in Frozen Solutions. Environ. Sci. Technol. 2018, 52, 13766–13773. [Google Scholar] [CrossRef]
- Anzo, K.; Harada, M.; Okada, T. Enhanced Kinetics of Pseudo First-Order Hydrolysis in Liquid Phase Coexistent with Ice. J. Phys. Chem. A 2013, 117, 10619–10625. [Google Scholar] [CrossRef]
- Tasaki, Y.; Okada, T. Up to 4 orders of magnitude enhancement of crown ether complexation in an aqueous phase coexistent with ice. J. Am. Chem. Soc. 2012, 134, 6128–6131. [Google Scholar] [CrossRef] [PubMed]
- Newberg, J.T. Equilibrium shifts upon freezing. Fluid Phase Equilibr. 2018, 478, 82–89. [Google Scholar] [CrossRef]
- Okada, T. Micro- and Nano-Liquid Phases Coexistent with Ice as Separation and Reaction Media. Chem. Rec. 2017, 17, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Ruzicka, R.; Baráková, L.; Klán, P. Photodecarbonylation of Dibenzyl Ketones and Trapping of Radical Intermediates by Copper(II) Chloride in Frozen Aqueous Solutions. J. Phys. Chem. B 2005, 109, 9346–9353. [Google Scholar] [CrossRef] [PubMed]
- Klánová, J.; Klán, P.; Heger, D.; Holoubek, I. Comparison of the effects of UV, H2O2/UV and gamma-irradiation processes on frozen and liquid water solutions of monochlorophenols. Photochem. Photobiol. Sci. 2003, 2, 1023–1031. [Google Scholar] [CrossRef]
- Lu, J.; Wang, H.; Tian, Z.; Hou, Y.; Lu, H. Cryopolymerization of 1,2-Dithiolanes for the Facile and Reversible Grafting-from Synthesis of Protein–Polydisulfide Conjugates. J. Am. Chem. Soc. 2020, 142, 1217–1221. [Google Scholar] [CrossRef]
- Takenaka, N.; Bandow, H. Chemical Kinetics of Reactions in the Unfrozen Solution of Ice. J. Phys. Chem. A 2007, 111, 8780–8786. [Google Scholar] [CrossRef]
- Gudipati, M.S. Matrix-Isolation in Cryogenic Water-Ices: Facile Generation, Storage, and Optical Spectroscopy of Aromatic Radical Cations. J. Phys. Chem. A 2004, 108, 4412–4419. [Google Scholar] [CrossRef]
- Sergeev, G.B.; Batyuk, V.A. Reactions in Frozen Multicomponent Systems. Russ. Chem. Revs. 1976, 45, 391–408. [Google Scholar] [CrossRef]
- Lozinsky, V.I. Cryogels on the basis of natural and synthetic polymers: Preparation, properties and application. Russ. Chem. Revs. 2002, 71, 489–511. [Google Scholar] [CrossRef]
- Bruice, T.C.; Butler, A.R. Catalysis in Water and Ice. A Comparison of the Kinetics of Hydrolysis of Acetic Anhydride, β-Propiolactone, and p-Nitrophenyl Acetate and the Dehydration of 5-Hydro-6-hydroxydeoxyuridine in Water and Ice. J. Am. Chem. Soc. 1964, 86, 313–319. [Google Scholar] [CrossRef]
- Crisalli, P.; Kool, E.T. Water-Soluble Organocatalysts for Hydrazone and Oxime Formation. J. Org. Chem. 2013, 78, 1184–1189. [Google Scholar] [CrossRef] [PubMed]
- Rashidian, M.; Mahmoodi, M.M.; Shah, R.; Dozier, J.K.; Wagner, C.R.; Distefano, M.D. A Highly Efficient Catalyst for Oxime Ligation and Hydrazone-Oxime Exchange Suitable for Bioconjugation. Bioconjug. Chem. 2013, 24, 333–342. [Google Scholar] [CrossRef]
- Wendeler, M.; Grinberg, L.; Wang, X.; Dawson, P.E.; Baca, M. Enhanced Catalysis of Oxime-Based Bioconjugations by Substituted Anilines. Bioconjug. Chem. 2014, 25, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Agten, S.M.; Suylen, D.P.L.; Hackeng, T.M. Oxime Catalysis by Freezing. Bioconjug. Chem. 2016, 27, 42–46. [Google Scholar] [CrossRef]
- Pawlas, J.; Nuijens, T.; Persson, J.; Svensson, T.; Schmidt, M.; Toplak, A.; Nillsson, M.; Rasmussen, J.H. Sustainable, cost-efficient manufacturing of therapeutic peptides using chemo-enzymatic peptide synthesis (CEPS). Green Chem. 2019, 21, 6451–6467. [Google Scholar] [CrossRef]
- Schmidt, M.; Toplak, A.; Quaedflieg, P.J.; Nuijens, T. Enzyme-mediated ligation technologies for peptides and proteins. Curr. Opin. Chem. Biol. 2017, 38, 1–7. [Google Scholar] [CrossRef]
- Lombard, C.; Saulnier, J.; Wallach, J.M. Recent trends in protease-catalyzed peptide synthesis. Protein Pept. Lett. 2005, 12, 621–629. [Google Scholar] [CrossRef]
- Jönsson, Å.; Adlercreutz, P.; Mattiasson, B. Effects of subzero temperatures on the kinetics of protease catalyzed dipeptide synthesis in organic media. Biotechnol. Bioeng. 1995, 46, 429–436. [Google Scholar] [CrossRef]
- Narai-Kanayama, A.; Hanaishi, T.; Aso, K. alpha-Chymotrypsin-catalyzed synthesis of poly-l-cysteine in a frozen aqueous solution. J. Biotechnol. 2012, 157, 428–436. [Google Scholar] [CrossRef]
- Schuster, M.; Aaviksaar, A.; Haga, M.; Ullmann, U.; Jakubke, H.D. Protease-catalyzed peptide synthesis in frozen aqueous systems: The “freeze-concentration model”. Biomed. Biochim. Acta 1991, 50, S84–S89. [Google Scholar] [PubMed]
- Haensler, M.; Arnold, K. Investigation of the effect of freezing on protease-catalyzed peptide synthesis using cryoprotectants and frozen organic solvent. Biol. Chem. 2000, 381, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Sletten, E.T.; Nuño, M.; Guthrie, D.; Seeberger, P.H. Real-time monitoring of solid-phase peptide synthesis using a variable bed flow reactor. Chem. Commun. 2019, 55, 14598–14601. [Google Scholar] [CrossRef]
- Han, F.P.; Guo, Y.; Ye, T. Total synthesis of antiallergic bicyclic peptide seongsanamide A. Org. Chem. Front. 2020, 7, 1658–1662. [Google Scholar] [CrossRef]
- Roesner, S.; Beadle, J.D.; Tam, L.K.B.; Wilkening, I.; Clarkson, G.J.; Raubo, P.; Shipman, M. Development of oxetane modified building blocks for peptide synthesis. Org. Biomol. Chem. 2020, 18, 5400–5405. [Google Scholar] [CrossRef]
- Sun, P.C.; Tang, W.L.; Huang, Y.; Hu, B.H. Improved Fmoc Solid-Phase Peptide Synthesis of Oxytocin with High Bioactivity. Synlett 2017, 28, 1780–1784. [Google Scholar] [CrossRef]
- Kumar, A.; Alhassan, M.; Lopez, J.; Albericio, F.; De La Torre, B.G. N-Butylpyrrolidinone for Solid-Phase Peptide Synthesis is Environmentally Friendlier and Synthetically Better than DMF. Chemsuschem 2020, 13, 5288–5294. [Google Scholar] [CrossRef]
- Ohkawachi, K.; Kobayashi, D.; Morimoto, K.; Shigenaga, A.; Denda, M.; Yamatsugu, K.; Kanai, M.; Otaka, A. Sulfanylmethyldimethylaminopyridine as a Useful Thiol Additive for Ligation Chemistry in Peptide/Protein Synthesis. Org. Lett. 2020, 22, 5289–5293. [Google Scholar] [CrossRef]
- Raheem, S.J.; Schmidt, B.W.; Solomon, V.R.; Salih, A.K.; Price, E.W. Ultrasonic-Assisted Solid-Phase Peptide Synthesis of DOTA-TATE and DOTA-linker-TATE Derivatives as a Simple and Low-Cost Method for the Facile Synthesis of Chelator-Peptide Conjugates. Bioconjug. Chem. 2020. [Google Scholar] [CrossRef]
- Vajda, T.; Szokan, G.; Hollosi, M. Cryochemistry: Freezing effect on peptide coupling in different organic solutions. J. Pept. Sci. 1998, 4, 300–304. [Google Scholar] [CrossRef]
- Dawson, P.; Muir, T.; Clark-Lewis, I.; Kent, S. Synthesis of Proteins by Native Chemical Ligation. Science 1994, 266, 776–779. [Google Scholar] [CrossRef] [PubMed]
- Taborsky, G. Protein Alterations at Low Temperatures: An Overview. Adv. Chem. 1979, 180, 1–26. [Google Scholar] [CrossRef]
- Kanavarioti, A.; Monnard, P.-A.; Deamer, D.W. Eutectic Phases in Ice Facilitate Nonenzymatic Nucleic Acid Synthesis. Astrobiology 2001, 1, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Attwater, J.; Wochner, A.; Pinheiro, V.B.; Coulson, A.; Holliger, P. Ice as a protocellular medium for RNA replication. Nat. Commun. 2010, 1, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lie, L.; Biliya, S.; Vannberg, F.; Wartell, R.M. Ligation of RNA Oligomers by the Schistosoma mansoni Hammerhead Ribozyme in Frozen Solution. J. Mol. Evol. 2016, 82, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Hol, W.G.J.; Van Duijnen, P.T.; Berendsen, H.J.C. The alpha-helix dipole and the properties of proteins. Nature 1978, 273, 443–446. [Google Scholar] [CrossRef]
- Horovitz, A.; Matthews, J.M.; Fersht, A.R. Alpha-helix stability in proteins. II. Factors that influence stability at an internal position. J. Mol. Biol. 1992, 227, 560–568. [Google Scholar] [CrossRef]
- Serrano, L.; Sancho, J.; Hirshberg, M.; Fersht, A.R. Alpha-helix stability in proteins. I. Empirical correlations concerning substitution of side-chains at the N and C-caps and the replacement of alanine by glycine or serine at solvent-exposed surfaces. J. Mol. Biol. 1992, 227, 544–559. [Google Scholar] [CrossRef]
- Miick, S.M.; Millhauser, G.L. Rotational diffusion and intermolecular collisions of a spin labeled alpha-helical peptide determined by electron spin echo spectroscopy. Biophys. J. 1992, 63, 917–925. [Google Scholar] [CrossRef][Green Version]
- Bock, J.R.; Gough, D.A. Predicting protein-protein interactions from primary structure. Bioinformatics 2001, 17, 455–460. [Google Scholar] [CrossRef]
- Brown, J.E.; Klee, W.A. Helix-coil transition of the isolated amino terminus of ribonuclease. Biochemistry 1971, 10, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Marqusee, S.; Robbins, V.H.; Baldwin, R.L. Unusually Stable Helix Formation in Short Alanine-Based Peptides. P. Natl. Acad. Sci. USA 1989, 86, 5286–5290. [Google Scholar] [CrossRef] [PubMed]
- Bradley, E.K.; Thomason, J.F.; Cohen, F.E.; Kosen, P.A.; Kuntz, I.D. Studies of synthetic helical peptides using circular dichroism and nuclear magnetic resonance. J. Mol. Biol. 1990, 215, 607–622. [Google Scholar] [CrossRef]
- Ebneth, A.; Adermann, K.; Wolfes, H. Does a synthetic peptide containing the leucine-zipper domain of c-myb form an alpha-helical structure in solution? Febs. Lett. 1994, 337, 265–268. [Google Scholar] [CrossRef]
- Haymet, A.D.J.; Ward, L.G.; Harding, M.M. Winter Flounder “Antifreeze” Proteins: Synthesis and Ice Growth Inhibition of Analogues that Probe the Relative Importance of Hydrophobic and Hydrogen-Bonding Interactions. J. Am. Chem. Soc. 1999, 30, 941–948. [Google Scholar] [CrossRef]
- Deprez, P.; Doss-Pepe, E.; Brodsky, B.; Inestrosa, N.C. Interaction of the collagen-like tail of asymmetric acetylcholinesterase with heparin depends on triple-helical conformation, sequence and stability. Biochem. J. 2000, 350, 283–290. [Google Scholar] [CrossRef]
- Holtzer, M.E.; Mints, L.; Angeletti, R.H.; D’ Avignon, D.A.; Holtzer, A. CD and (13)C(alpha)-NMR studies of folding equilibria in a two-stranded coiled coil formed by residues 190-254 of alpha-tropomyosin. Biopolymers 2001, 59, 257–265. [Google Scholar] [CrossRef]
- Nakagawa, K.; Yamada, Y.; Fujiwara, K.; Ikeguchi, M. Interactions Responsible for Secondary Structure Formation during Folding of Equine β-Lactoglobulin. J. Mol. Biol. 2007, 367, 1205–1214. [Google Scholar] [CrossRef]
- Mikelis, C.; Lamprou, M.; Koutsioumpa, M.; Koutsioubas, A.G.; Spyranti, Z.; Zompra, A.A.; Spyroulias, G.A.; Cordopatis, P.; Courty, J. A peptide corresponding to the C-terminal region of pleiotrophin inhibits angiogenesis in vivo and in vitro. J. Cell. Biochem. 2011, 112, 1532–1543. [Google Scholar] [CrossRef]
- Sun, X.L.; He, W.D.; Pan, T.T.; Ding, Z.L.; Zhang, Y.J. RAFT cryopolymerizations of acrylamides and acrylates in dioxane at −5 °C. Polymer 2010, 51, 110–114. [Google Scholar] [CrossRef]
- Haleem, A.; Wang, J.Y.; Li, H.J.; Hu, C.S.; Li, X.C.; He, W.D. Macroporous Oil-Sorbents with a High Absorption Capacity and High-Temperature Tolerance Prepared through Cryo-Polymerization. Polymers 2019, 11, 1620. [Google Scholar] [CrossRef] [PubMed]
- Petrov, P.D.; Tsvetanov, C.B. Cryogels via UV Irradiation. In Polymeric Cryogels; Springer: New York City, NY, USA, 2014; Volume 263, pp. 199–222. [Google Scholar]
- Shlyakhtin, O.A. Inorganic Cryogels. In Polymeric Cryogels; Springer: New York City, NY, USA, 2014; Volume 263, pp. 223–244. [Google Scholar]
- Lozinsky, V.I. Cryostructuring of Polymeric Systems. 55. Retrospective View on the More than 40 Years of Studies Performed in the A.N.Nesmeyanov Institute of Organoelement Compounds with Respect of the Cryostructuring Processes in Polymeric Systems. Gels 2020, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Lozinsky, V.I. Cryotropic gelation of poly(vinyl alcohol) solutions. Russ. Chem. Revs. 1998, 67, 573–586. [Google Scholar] [CrossRef]
- Wan, W.; Bannerman, A.D.; Yang, L.; Mak, H. Poly(Vinyl Alcohol) Cryogels for Biomedical Applications. In Polymeric Cryogels; Springer: New York City, NY, USA, 2014; Volume 263, pp. 283–321. [Google Scholar]
- Lozinsky, V.I.; Morozova, S.A.; Vainerman, E.S.; Titova, E.F.; Shtil’man, M.I.; Belavtseva, E.M.; Rogozhin, S.V. Study of cryostructurization of polymer systems. VIII. Characteristic features of the formation of crosslinked poly(acryl amide) cryogels under different thermal conditions. Acta Polym. 1989, 40, 8–15. [Google Scholar] [CrossRef]
- Sahiner, N.; Demirci, S. Conducting semi-interpenetrating polymeric composites via the preparation of poly(aniline), poly(thiophene), and poly(pyrrole) polymers within superporous poly(acrylic acid) cryogels. React. Funct. Polym. 2016, 105, 60–65. [Google Scholar] [CrossRef]
- Deng, Z.; Guo, Y.; Ma, P.X.; Guo, B. Rapid thermal responsive conductive hybrid cryogels with shape memory properties, photothermal properties and pressure dependent conductivity. J. Colloid Interf. Sci. 2018, 526, 281–294. [Google Scholar] [CrossRef]
- Kirsebom, H.; Elowsson, L.; Berillo, D.; Cozzi, S.; Inci, I.; Piskin, E.; Galaev, I.Y. Enzyme-catalyzed crosslinking in a partly frozen state: A new way to produce supermacroporous protein structures. Macromol. Biosci. 2013, 13, 67–76. [Google Scholar] [CrossRef]
- Maeng, S.K.; Cho, K.; Jeong, B.; Lee, J.; Lee, Y.; Lee, C.; Choi, K.J.; Hong, S.W. Substrate-immobilized electrospun TiO2 nanofibers for photocatalytic degradation of pharmaceuticals: The effects of pH and dissolved organic matter characteristics. Water Res. 2015, 86, 25–34. [Google Scholar] [CrossRef]
- Ramasundaram, S.; Seid, M.G.; Lee, W.; Kim, C.U.; Kim, E.J.; Hong, S.W.; Choi, K.J. Preparation, characterization, and application of TiO2-patterned polyimide film as a photocatalyst for oxidation of organic contaminants. J. Hazard. Mater. 2017, 340, 300–308. [Google Scholar] [CrossRef]
- Radke, M.; Lauwigi, C.; Heinkele, G.; Murdter, T.E.; Letzel, M. Fate of the Antibiotic Sulfamethoxazole and Its Two Major Human Metabolites in a Water Sediment Test. Environ. Sci. Technol. 2009, 43, 3135–3141. [Google Scholar] [CrossRef]
- Nödler, K.; Licha, T.; Barbieri, M.; Pérez, S. Evidence for the microbially mediated abiotic formation of reversible and non-reversible sulfamethoxazole transformation products during denitrification. Water Res. 2012, 46, 2131–2139. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.Q.; Xiao, Y.Y.; Wu, D.; Zhu, W.Y.; Zhou, Y. Nitrite-driven abiotic transformation of sulfonamide micropollutants during freezing process. Chem. Eng. J. 2017, 327, 1128–1134. [Google Scholar] [CrossRef]
- Thorat, A.A.; Munjal, B.; Geders, T.W.; Suryanarayanan, R. Freezing -induced protein aggregation-Role of pH shift and potential mitigation strategies. J. Control Release 2020, 323, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Krauskova, L.; Prochazkova, J.; Klaskova, M.; Filipova, L.; Chaloupkova, R.; Maly, S.; Heger, D. Suppression of protein inactivation during freezing by minimizing pH changes using ionic cryoprotectants. Int. J. Pharm. 2016, 509, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Shalaev, E.; Soper, A.K. Water in a Soft Confinement: Structure of Water in Amorphous Sorbitol. J. Phys. Chem. B 2016, 120, 7289–7296. [Google Scholar] [CrossRef] [PubMed]
- Shalaev, E.; Soper, A.; Zeitler, J.A.; Ohtake, S.; Roberts, C.J.; Pikal, M.J.; Wu, K. Freezing of Aqueous Solutions and Chemical Stability of Amorphous Pharmaceuticals: Water Clusters Hypothesis. J. Pharm. Sci. 2019, 108, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Sumerlin, B.S. Proteins as Initiators of Controlled Radical Polymerization: Grafting-from via ATRP and RAFT. ACS Macro Lett. 2011, 1, 141–145. [Google Scholar] [CrossRef]
- Isarov, S.A.; Pokorski, J.K. Protein ROMP: Aqueous Graft-from Ring-Opening Metathesis Polymerization. ACS Macro Lett. 2015, 4, 969–973. [Google Scholar] [CrossRef]


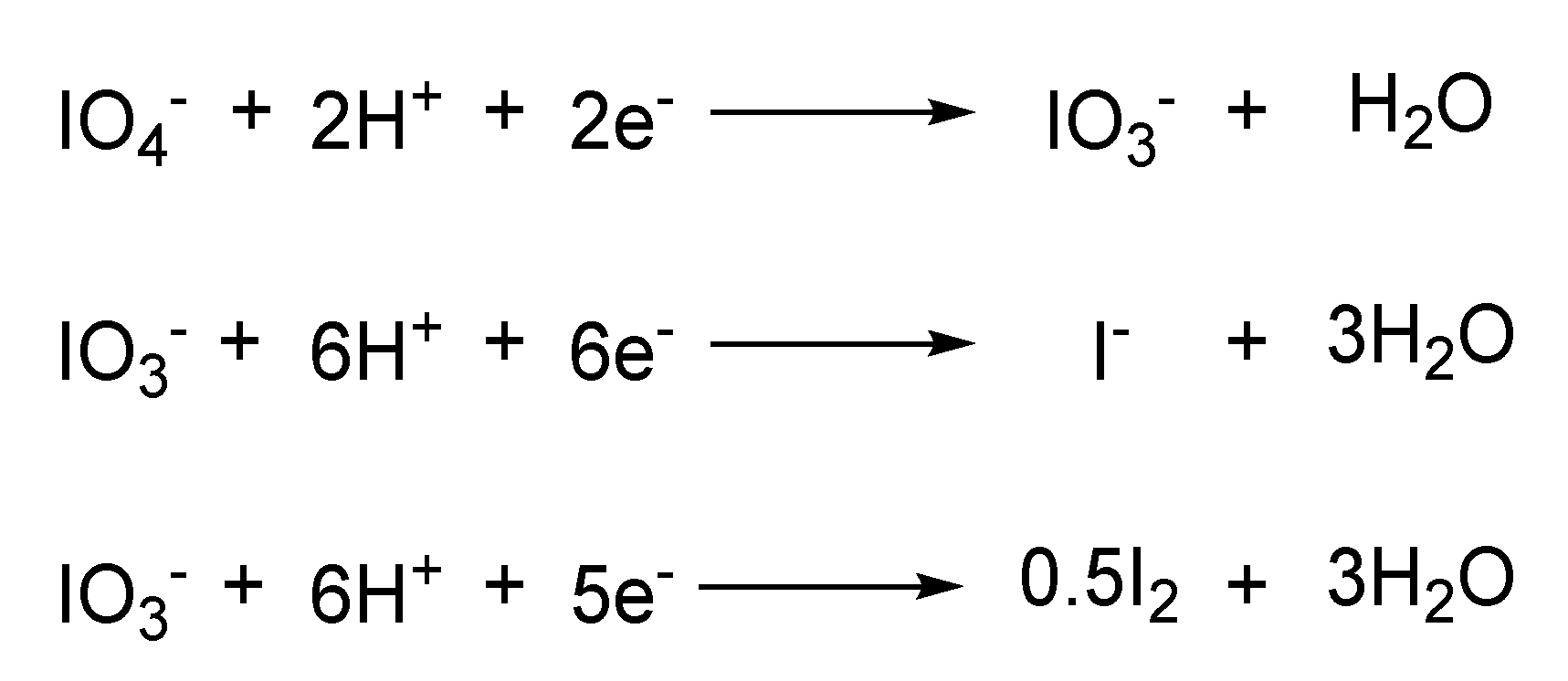
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, L.-Y.; Dai, Z.; Di, B.; Xu, L.-L. Advances in Cryochemistry: Mechanisms, Reactions and Applications. Molecules 2021, 26, 750. https://doi.org/10.3390/molecules26030750
An L-Y, Dai Z, Di B, Xu L-L. Advances in Cryochemistry: Mechanisms, Reactions and Applications. Molecules. 2021; 26(3):750. https://doi.org/10.3390/molecules26030750
Chicago/Turabian StyleAn, Lu-Yan, Zhen Dai, Bin Di, and Li-Li Xu. 2021. "Advances in Cryochemistry: Mechanisms, Reactions and Applications" Molecules 26, no. 3: 750. https://doi.org/10.3390/molecules26030750
APA StyleAn, L.-Y., Dai, Z., Di, B., & Xu, L.-L. (2021). Advances in Cryochemistry: Mechanisms, Reactions and Applications. Molecules, 26(3), 750. https://doi.org/10.3390/molecules26030750





