Production of Bulk Chemicals with Biocatalysis: Drivers and Challenges Reflected in Recent Industrial Granted Patents (2015–2020)
Abstract
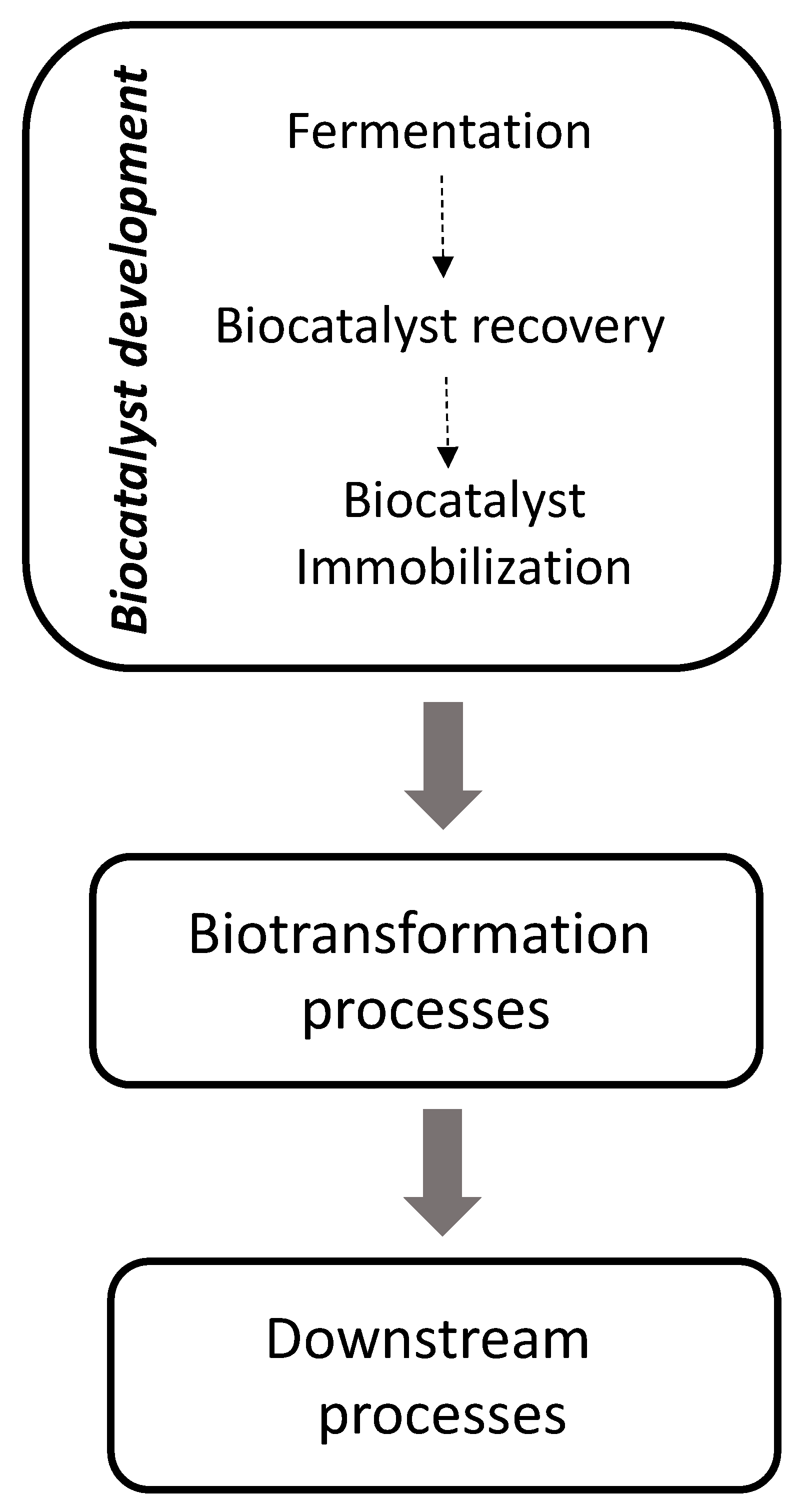
1. Motivation for Biocatalysis Applied to Bulk Chemicals
2. Recent Granted Patents Applying Biocatalysis for Biofuel Production
3. Recent Granted Patents Related to Biotransformations and Sugar Commodities
4. Recent Granted Patents Related to Bioprocesses for Ester Production
5. Biotransformations with Furans to Obtain Bulk Compounds
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bommarius, A.S.; Riebel-Bommarius, B.R. Biocatalysis Fundamentals and Applications; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2004. [Google Scholar]
- De Gonzalo, G.; Domínguez de María, P. (Eds.) Biocatalysis–An Industrial Perspective; Royal Society of Chemistry: Cambridge, UK, 2018. [Google Scholar]
- Schwarz, J.; Rosenthal, K.; Snajdrova, R.; Kittelmann, M.; Lütz, S. The development of biocatalysis as a tool for drug discovery. Chimia (Arau) 2020, 74, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Hönig, M.; Sondermann, P.; Turner, N.J.; Carreira, E.M. Enantioselective Chemo- and biocatalysis: Partners in retrosynthesis. Angew. Chem. Int. Ed. 2017, 56, 8942–8973. [Google Scholar] [CrossRef] [PubMed]
- Stoltz, B.M.; Wright, A.C.; Ebner D, C. Enantioselective oxidation of alcohols. Sci. Synth. 2017, 4, 569–585. [Google Scholar]
- Aranda, C.; Oksdath-Mansilla, G.; Bisogno, F.R.; De De Gonzalo, G. Deracemisation Processes Employing Organocatalysis and Enzyme Catalysis. Adv. Synth. Catal. 2020, 362, 1233–1257. [Google Scholar] [CrossRef]
- Martínez-Montero, L.; Schrittwieser, J.H.; Kroutil, W. Regioselective Biocatalytic Transformations Employing Transaminases and Tyrosine Phenol Lyases. Top. Catal. 2019, 62, 1208–1217. [Google Scholar] [CrossRef]
- Barbayianni, E.; Kokotos, G. Biocatalyzed Regio- and Chemoselective Ester Cleavage: Synthesis of Bioactive Molecules. ChemCatChem 2012, 4, 592–608. [Google Scholar] [CrossRef]
- Burton, S.G.; A Cowan, D.; Woodley, J.M. The search for the ideal biocatalyst. Nat. Biotechnol. 2002, 20, 37–45. [Google Scholar] [CrossRef]
- Wiltschi, B.; Cernava, T.; Dennig, A.; Casas, M.G.; Geier, M.; Gruber, S.; Haberbauer, M.; Heidinger, P.; Acero, E.H.; Kratzer, R.; et al. Enzymes revolutionize the bioproduction of value-added compounds: From enzyme discovery to special applications. Biotechnol. Adv. 2020, 40, 107520. [Google Scholar] [CrossRef]
- Tufvesson, P.; Fu, W.; Jensen, J.S.; Woodley, J.M. Process considerations for the scale-up and implementation of biocatalysis. Food Bioprod. Process. 2010, 88, 3–11. [Google Scholar] [CrossRef]
- Enzymes Market Size, Share & Trends Analysis Report By Application (Industrial Enzymes, Specialty Enzymes), By Product (Carbohydrase, Proteases, Lipases), By Source, By Region, And Segment Forecasts, 2020–2027. Available online: https://www.grandviewresearch.com/industry-analysis/enzymes-industry (accessed on 30 January 2021).
- Pellis, A.; Cantone, S.; Ebert, C.; Gardossi, L. Evolving biocatalysis to meet bioeconomy challenges and opportunities. New Biotechnol. 2018, 40, 154–169. [Google Scholar] [CrossRef]
- Zheng, C.C.; Wu, J.W.; Jin, Z.H.; Ye, Z.F.; Yang, S.; Sun, Y.Q.; Fei, H. Exogenous enzymes as functional additives in finfish aquaculture. Aquac. Nutr. 2020, 26, 213–224. [Google Scholar] [CrossRef]
- Al-Ghanayem, A.A.; Joseph, B. Current prospective in using cold-active enzymes as eco-friendly detergent additive. Appl. Microbiol. Biotechnol. 2020, 104, 2871–2882. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, M.; Gramegna, G.; Benedetti, M.; Mattei, B. Industrial Use of Cell Wall Degrading Enzymes: The Fine Line Between Production Strategy and Economic Feasibility. Front. Bioeng. Biotechnol. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Bolivar, J.M.; López-Gallego, F. Characterization and evaluation of immobilized enzymes for applications in flow reactors. Curr. Opin. Green Sustain. Chem. 2020, 25, 100349. [Google Scholar] [CrossRef]
- Wahab, R.A.; Elias, N.; Abdullah, F.; Ghoshal, S.K. On the taught new tricks of enzymes immobilization: An all-inclusive overview. React. Funct. Polym. 2020, 152, 104613. [Google Scholar] [CrossRef]
- Monteiro, R.R.; Dos Santos, J.C.; Alcántara, A.R.; Fernandez-Lafuente, R. Enzyme-Coated Micro-Crystals: An Almost Forgotten but Very Simple and Elegant Immobilization Strategy. Catalysts 2020, 10, 891. [Google Scholar] [CrossRef]
- Guajardo, N.; Ahumada, K.; De María, P.D. Immobilized lipase-CLEA aggregates encapsulated in lentikats® as robust biocatalysts for continuous processes in deep eutectic solvents. J. Biotechnol. 2020, 310, 97–102. [Google Scholar] [CrossRef]
- Guajardo, N.; De María, H.P.D. Continuous Biocatalysis in Environmentally-Friendly Media: A Triple Synergy for Future Sustainable Processes. ChemCatChem 2019, 11, 3128–3137. [Google Scholar] [CrossRef]
- Guajardo, N.; Schrebler, R.A.; De María, P.D. From batch to fed-batch and to continuous packed-bed reactors: Lipase-catalyzed esterifications in low viscous deep-eutectic-solvents with buffer as cosolvent. Bioresour. Technol. 2019, 273, 320–325. [Google Scholar] [CrossRef]
- Woodley, J.M. Towards the sustainable production of bulk-chemicals using biotechnology. New Biotechnol. 2020, 59, 59–64. [Google Scholar] [CrossRef]
- Guajardo, N.; De María, P.D.; Ahumada, K.; Schrebler, R.A.; Ramírez-Tagle, R.; Crespo, F.A.; Carlesi, C. Water as Cosolvent: Nonviscous Deep Eutectic Solvents for Efficient Lipase-Catalyzed Esterifications. ChemCatChem 2017, 9, 1393–1396. [Google Scholar] [CrossRef]
- Guajardo, N.; De María, P.D. Assessing biocatalysis using dihydrolevoglucosenone (Cyrene™) as versatile bio-based (co)solvent. Mol. Catal. 2020, 485, 110813. [Google Scholar] [CrossRef]
- De María, P.D. “Nonsolvent” Applications of Ionic Liquids in Biotransformations and Organocatalysis. Angew. Chem. Int. Ed. 2008, 47, 6960–6968. [Google Scholar] [CrossRef] [PubMed]
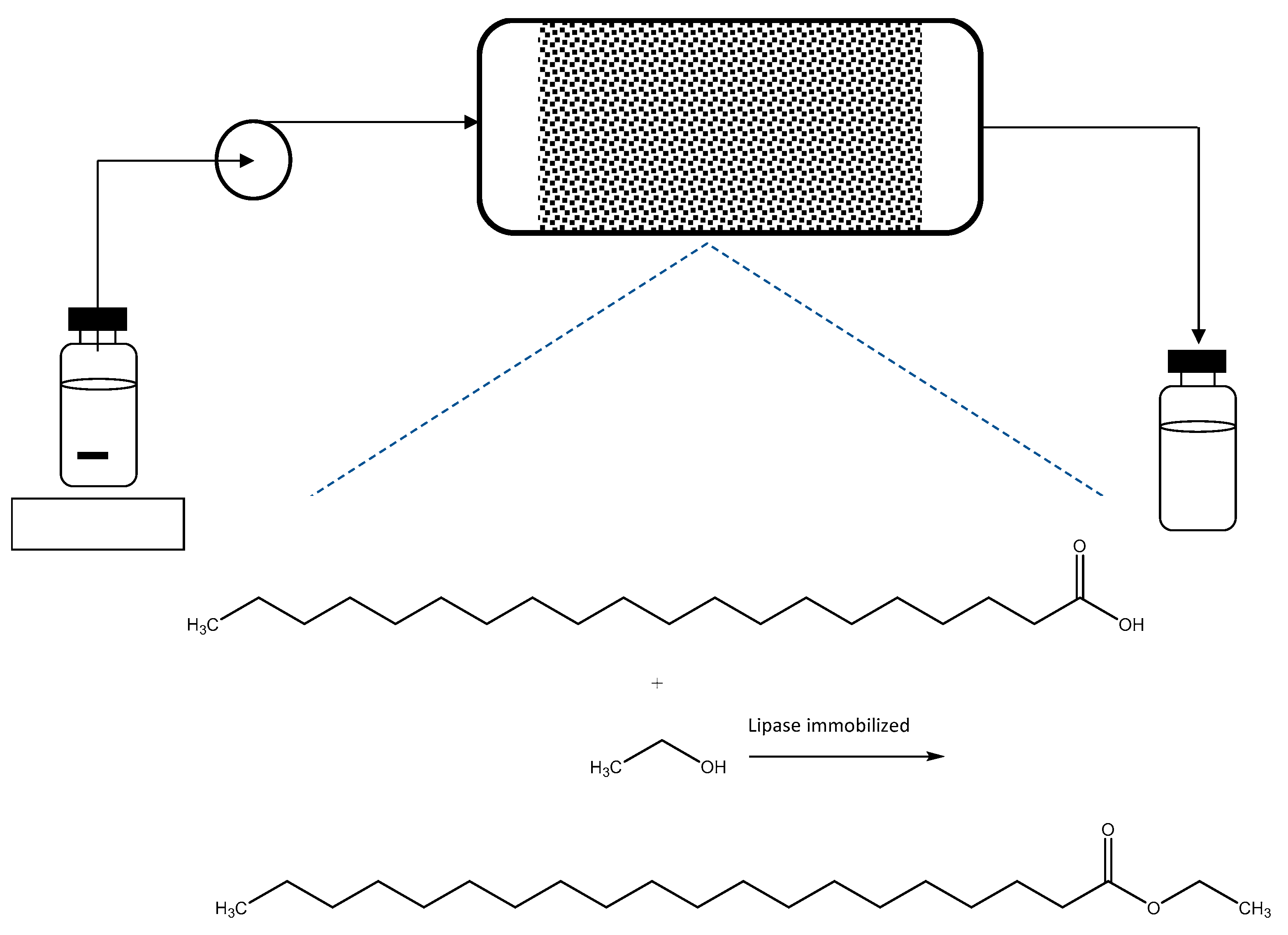
- Jianzhong, H.; Feng, Q.; Zhengyu, S.; Xinwei, Y.; Zianzhang, J.; Mignliang, Z. The Application of Carrier-Free Immobilization Rhizopus oryzae and Preparation Method Thereof and Production Biodiesel. CN Patent 104928279B, 15 June 2018. [Google Scholar]
- Zhenhong, Y.; Changlin, M.; Pengmei, L.; Xinshu, Z.; Zhongming, W.; Wen, L.; Huiwen, L.; Lingmei, Y.; Shuna, L. A Kind of Method of Carrier-Free Immobilized Lipase Catalysis for Preparing Biodiesel Oil under ion Liquid System. CN Patent 104911222B, 16 January 2018. [Google Scholar]
- Jing, G.; Li, M.; Yanjun, J. A Kind of Method of Static State Emulsion Method Immobilized Lipase Production Biodiesel. CN Patent 105274157B, 20 July 2018. [Google Scholar]
- Fei, W.; Qiyang, H.; Zhilin, L.; Xun, L.; Yu, Z. A Kind of Efficient Catalytic Prepares Biodiesel Tencel Bio-Based Immobilized Whole-cell Catalyst and Preparation Method Thereof. CN Patent 104818267B, 27 October 2017. [Google Scholar]
- Chung, S.M.; Jin, K.R. Method for Producing Fatty Acid, Glycerol or Unsaturated Fatty Acid Using Monoacylglycerol-Specific lipase AtMAGL6 or AtMAGL8 Protein. KR Patent 101730078B1, 25 April 2017. [Google Scholar]
- Guan-Chiun, L.; Ting-Chun, K. Methods for Producing Biodiesel by Recombinant Lipase. U.S. Patent 9970035B2, 15 May 2018. [Google Scholar]
- Yingti, J.; Ziaowei, Z.; Xin, P. Meal Kitchen Waste Oil System Biodiesel’s Transesterification Ware. CN Patent 206173356U, 17 May 2017. [Google Scholar]
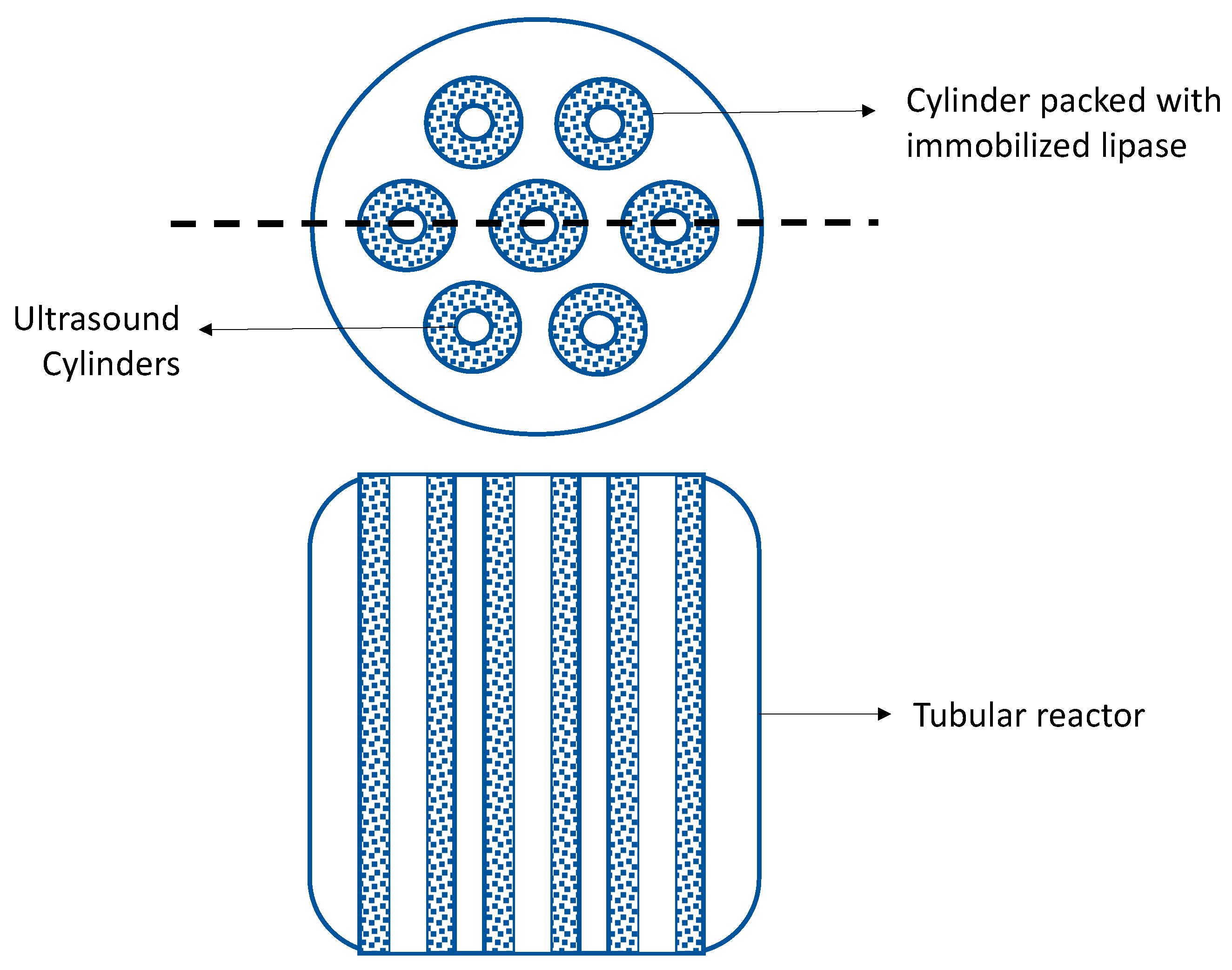
- Kailong, L.; Luole, Z. A Kind of Device and Method of Biodiesel Production by Bio-Enzymatic Method. CN Patent 105001996B, 28 September 2018. [Google Scholar]
- Cao, W.; Qiu, L.; Zhao, L. The Preparation Method of Biodiesel. CN Patent 105001922B, 24 August 2016. [Google Scholar]
- Fengsheng, Y.; Xiang, L. A Kind of Engine Oil Base Oil and Preparation Method Thereof. CN Patent 104804797B, 1 December 2017. [Google Scholar]
- Markets and markets. 2017. Available online: https://www.marketsandmarkets.com/Market-Reports/industrial-sugar-market-54437928.html (accessed on 30 January 2021).
- Lee, P.; Oh, H.; Kim, S.Y.; Kim, Y. Effects of d -allulose as a sucrose substitute on the physicochemical, textural, and sensorial properties of pound cakes. J. Food Process. Preserv. 2020, 44. [Google Scholar] [CrossRef]
- Wichelecki, D.J. Enzymatic Synthesis of D-Tagatose. U.S. Patent 10138506B2, 27 November 2018. [Google Scholar]
- Yanhe, M.; Yuanxia, S. The Preparation Method of Tagatose. CN Patent 106399427B, 13 November 2018. [Google Scholar]
- Maceachran, D.; Cunningham, D.S.; Blake, W.J.; Moura, M.E. Cell-Free Production of Sugars. U.S. Patent 10577635B2, 3 March 2020. [Google Scholar]
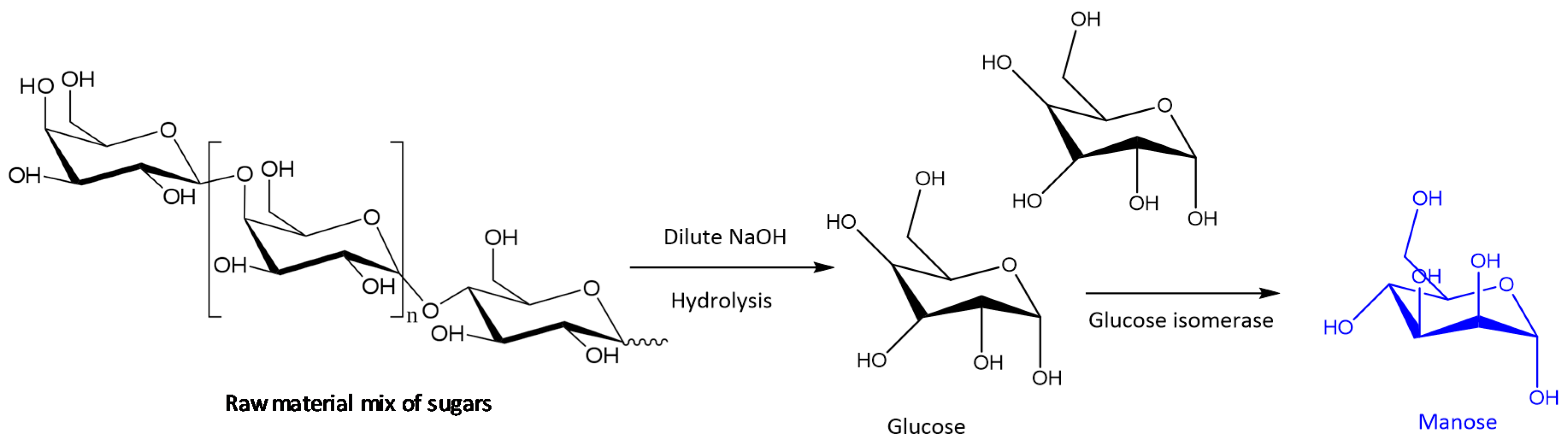
- Xiaoxue, L.; Hongtao, J.; Yongqiang, Z.; Chengliang, Z. A Kind of preparation METHOD of Mannose. CN Patent 105001273B, 29 June 2018. [Google Scholar]
- Jianqiang, L.; Jianqun, L. A Method of Producing D-Psicose. CN Patent 106480125B, 21 May 2019. [Google Scholar]
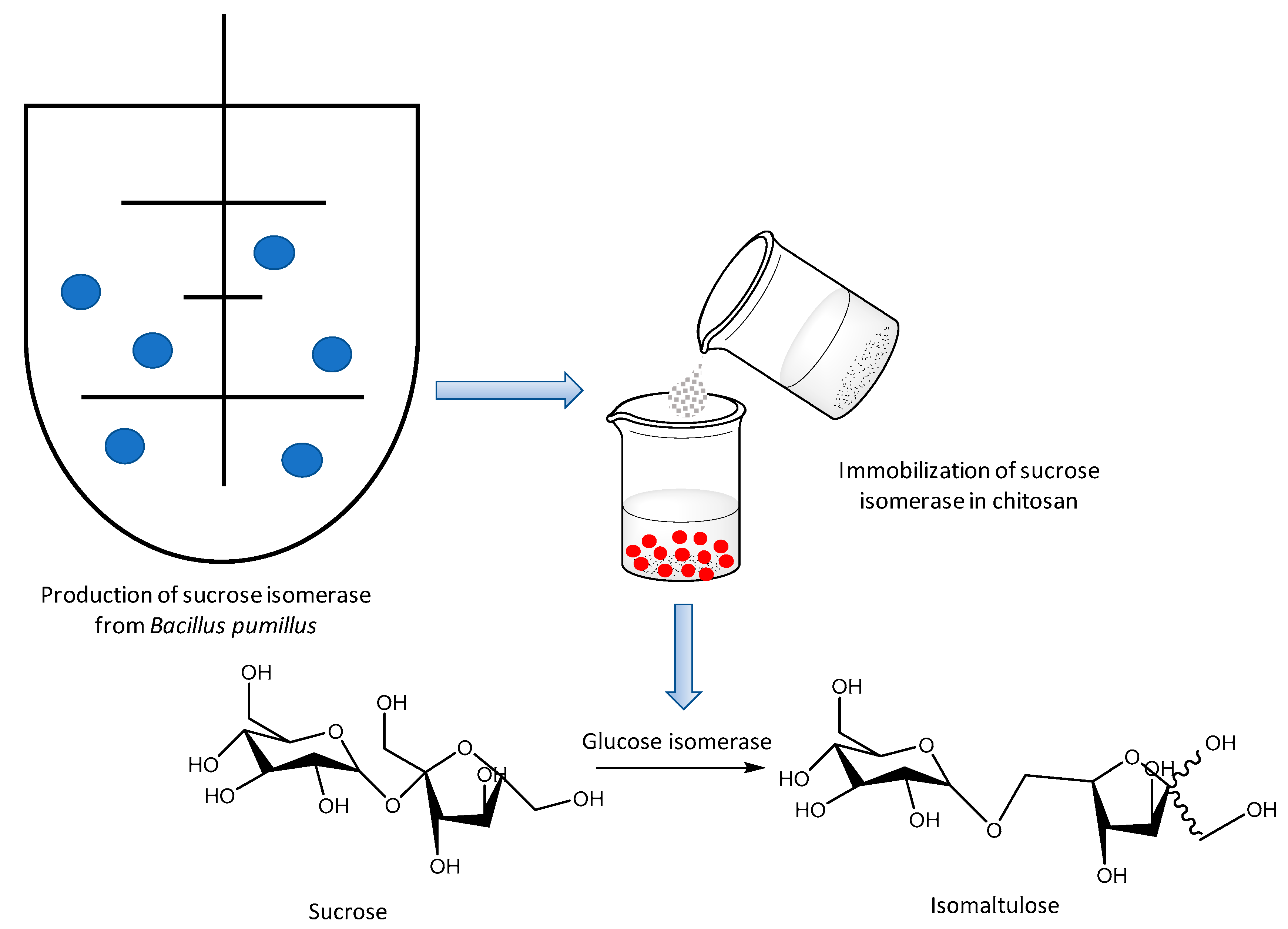
- Jing, W.; Sheng, C.; Xuguo, D.; Menghua, G. Immobilization Method of Sucrose Isomerase. CN Patent 106367377A, 25 October 2019. [Google Scholar]
- Jianyu, G.; Xiong, Z.; Xuehui, L.; Jinxing, L.; Zhixiang, X.; Weijie, Z.; Changhua, S. A Kind of Method that Alkali Ionic Liquid Catalysis Glucose Isomerase Prepares Fructose. CN Patent 106632522B, 12 July 2019. [Google Scholar]
- Mallinath, L.A.; Anil, O.A.; Joanna, V.J.; Gunvant, C.V.; Chandrashekar, W.P.; Laxmiputra, P.M.; Shivajirao, P.P.; Ravindra, A.B.; Ramray, M.C.; Indra, P.; et al. Process for Production of Pure Glucose from Cellulose. U.S. Patent 10465257B2, 5 November 2019. [Google Scholar]
- Khan, N.R.; Rathod, V.K. Enzyme catalyzed synthesis of cosmetic esters and its intensification: A review. Process. Biochem. 2015, 50, 1793–1806. [Google Scholar] [CrossRef]
- Chia-Hung, K.; Chwen-Jen, S.; Jen-Min, K. One Lipase Packed Bed Bioreactor for the Continuous Synthesis of Docosahexaenoic Acid and Eicosapentaenoic Acid Ester-Based. TW Patent I646195B, 1 January 2019. [Google Scholar]
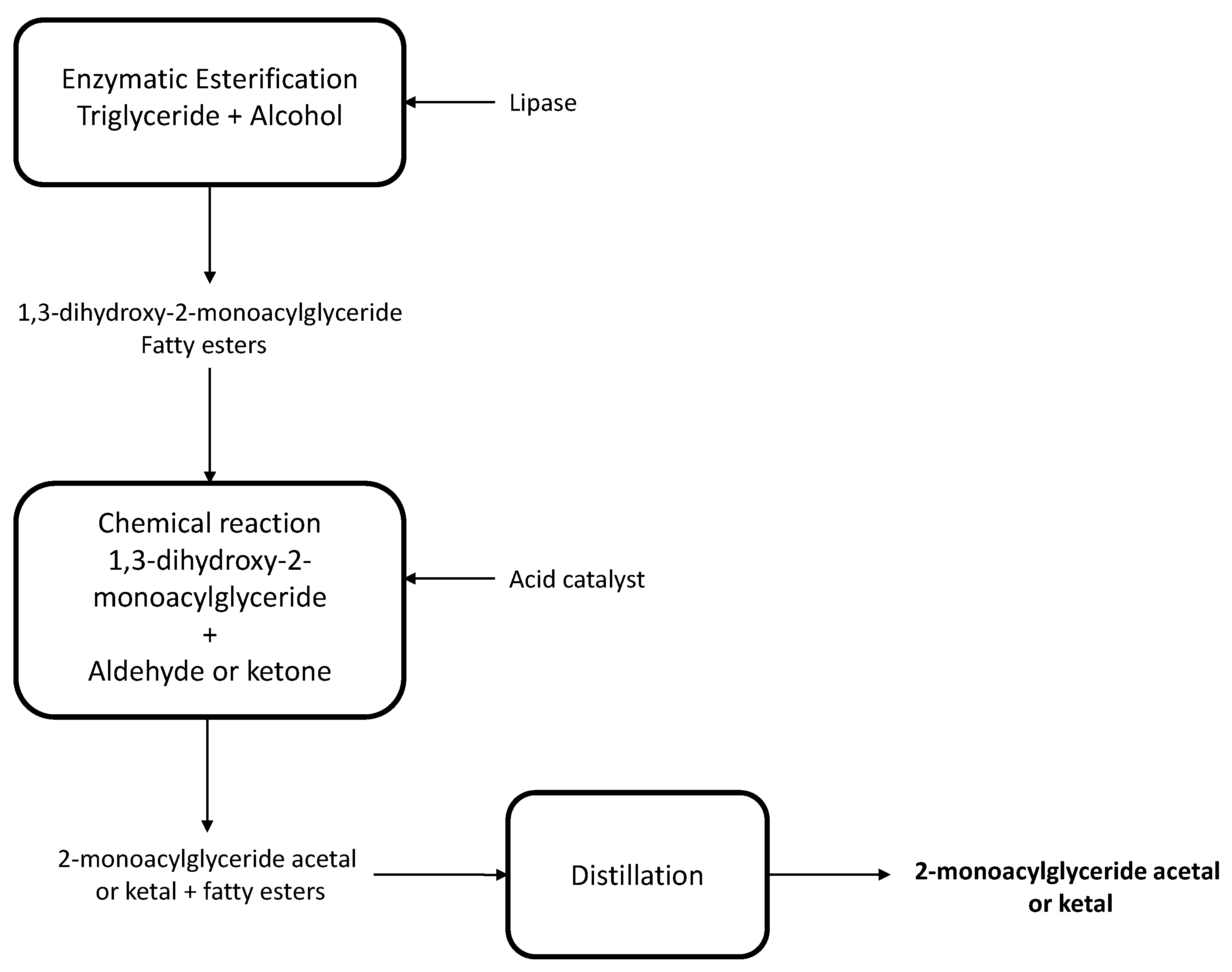
- Jin Sup, S.; Young, K.M.; Chang, K.Y.; Chan, K.B. Improved Method for Preparing Monoacylglycerol by Using Enzymatic Alcoholysis. KR Patent 101969467B1, 17 April 2019. [Google Scholar]
- Rongione, J.C. Method for Preparing 2-Monoacylgycerides. EP Patent 3317385B1, 3 April 2019. [Google Scholar]
- Wei, D.; Lingmei, D.; Dehua, L. The Coupling Technique of Production by Enzymes Biodiesel and Enrichment of Polybasic Unsaturated Fatty Acid Ester. CN Patent 105925628B, 14 May 2019. [Google Scholar]
- Xiaosan, W.; Wenjia, T.; Jianhua, H.; Qingzhe, J.; Xingguo, W. A Kind of Enzymatic Synthesis Method of Symmetrical Structure Triglycerides. CN Patent 105063115B, 7 August 2018. [Google Scholar]
- Pandal, N. Global Markets for Flavors and Fragrances. BCC Res. MA: Wellesley. 2016. Available online: https://www.bccresearch.com/report/download/report/chm034d (accessed on 30 January 2021).
- Mordor Intelligence. Global Cosmetics Products Market–Segmented by Product Type, Distribution Channel (Direct Selling, Supermarkets, Specialty Stores), and Region–Growth, Trends and Forecasts (2018–2023). Hyderabad, India. 2018. Available online: https://www.researchandmarkets.com/reports/4472918/global-cosmetics-products-market-segmented-by (accessed on 30 January 2021).
- Van Wyk, N.; Kroukamp, H.; Pretorius, I.S. The Smell of Synthetic Biology: Engineering Strategies for Aroma Compound Production in Yeast. Fermentation 2018, 4, 54. [Google Scholar] [CrossRef]
- Zijun, X.; Xiaoyuan, H.; Jingyi, Z.; Lijun, X.; Jing, L.; Jianren, L. A Kind of Method of Lipase-Catalyzed Synthesis 3-Hydroxy-2-Butanone Fatty Acid Ester. CN Patent 104498543B, 31 October 2017. [Google Scholar]
- Caimei, Z.; Xiaofeng, W.; Shengmei, L. A Kind of Alpha-Linolenic Acid Monoglyceride and Preparation Method Thereof. CN Patent 105331648B, 18 December 2018. [Google Scholar]
- Guajardo, N.; Domínguez de María, P. Biocatalytic valorization of furans: Opportunities for inherently unstable substrates. ChemSusChem 2017, 10, 4123–4134. [Google Scholar]
- Chen, Y.Z.; Yang, Y.F.; Ji, F.; Liao, R.F.; Zhou, J.H. Method for Preparing 5-Hydroxymethyl Furfural from Raw Materials Containing Glucose. Patent TWI664291B, 1 July 2019. [Google Scholar]
- Cao, F.; Huang, T.; Wu, H.; Zhou, H.; Wei, P. A Kind of Method of Coproduction Gluconic Acid and Hydroxymethyl Furfural. CN Patent 105837433B, 10 April 2018. [Google Scholar]
- Li, N.; Zhang, X.; Zong, M. Comamonas testosteroni and Application Thereof in Synthesis of 5-Hydroxymethyl Furoic Acid. CN Patent 107365724B, 28 April 2020. [Google Scholar]
- Ruijssenaars, H.J. Dehydrogenase-Catalysed Production of FDCA. EP Patent 3259349B1, 17 June 2020. [Google Scholar]
- Badoux, F. Process for the Preparation of 2,5-FDCA. DE Patent 102016118154B3, 23 November 2017. [Google Scholar]
- Law, P.; Mines, P.; Carnell, A.; McKenna, S. Processes for the Formation of Furandicarboxylic Acid (FDCA) via a Multistep Biocatalytic Oxidation Reaction of 5-Hydroxymethylfurfural (HMF). U.S. Patent 10344307B2, 9 July 2019. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guajardo, N.; Domínguez de María, P. Production of Bulk Chemicals with Biocatalysis: Drivers and Challenges Reflected in Recent Industrial Granted Patents (2015–2020). Molecules 2021, 26, 736. https://doi.org/10.3390/molecules26030736
Guajardo N, Domínguez de María P. Production of Bulk Chemicals with Biocatalysis: Drivers and Challenges Reflected in Recent Industrial Granted Patents (2015–2020). Molecules. 2021; 26(3):736. https://doi.org/10.3390/molecules26030736
Chicago/Turabian StyleGuajardo, Nadia, and Pablo Domínguez de María. 2021. "Production of Bulk Chemicals with Biocatalysis: Drivers and Challenges Reflected in Recent Industrial Granted Patents (2015–2020)" Molecules 26, no. 3: 736. https://doi.org/10.3390/molecules26030736
APA StyleGuajardo, N., & Domínguez de María, P. (2021). Production of Bulk Chemicals with Biocatalysis: Drivers and Challenges Reflected in Recent Industrial Granted Patents (2015–2020). Molecules, 26(3), 736. https://doi.org/10.3390/molecules26030736