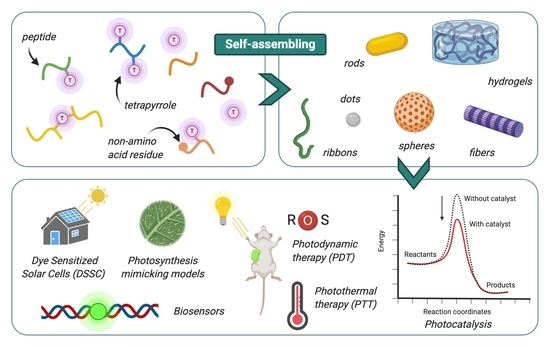
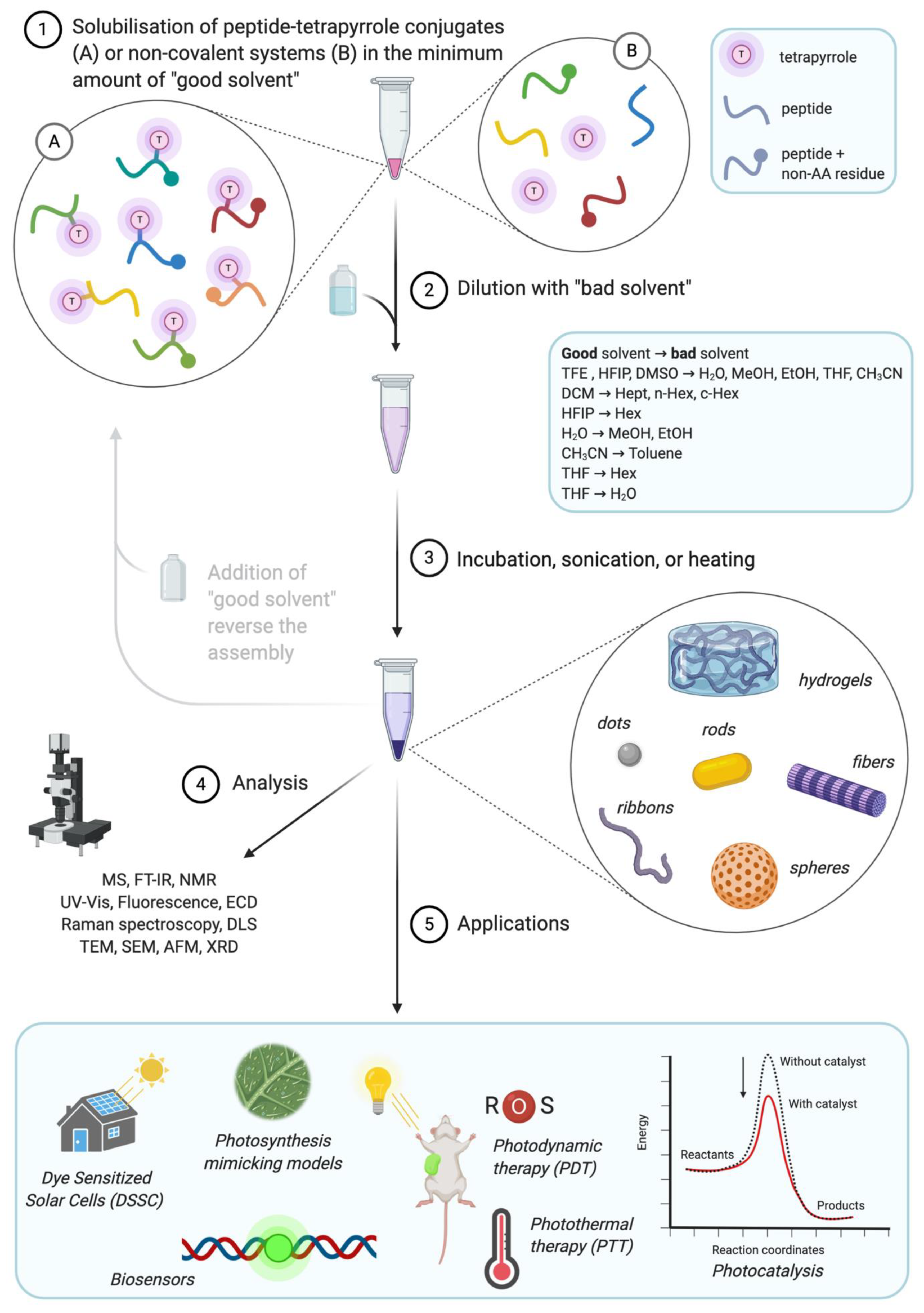
Peptide-Tetrapyrrole Supramolecular Self-Assemblies: State of the Art
Abstract
1. Introduction
1.1. Assembly of Peptides and Tetrapyrroles
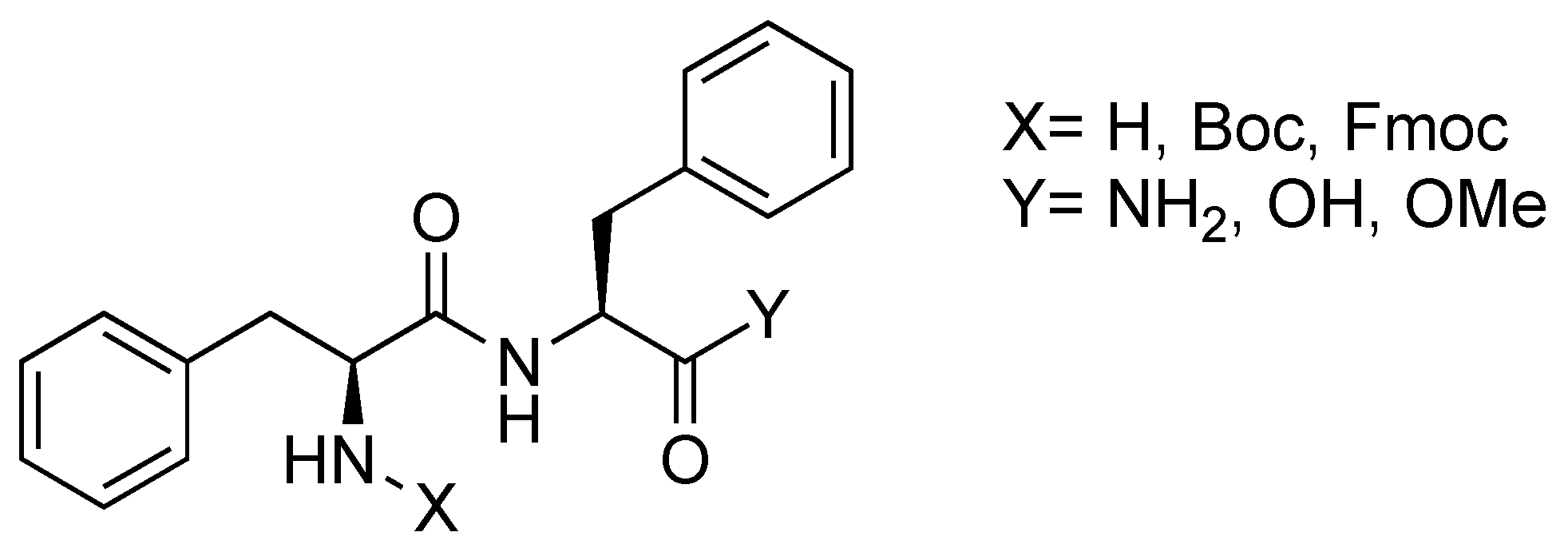
2. Diphenylalanine
| Entry | Peptide | Tetrapyrrole | Interaction (Linker/Bond) | Ref. |
|---|---|---|---|---|
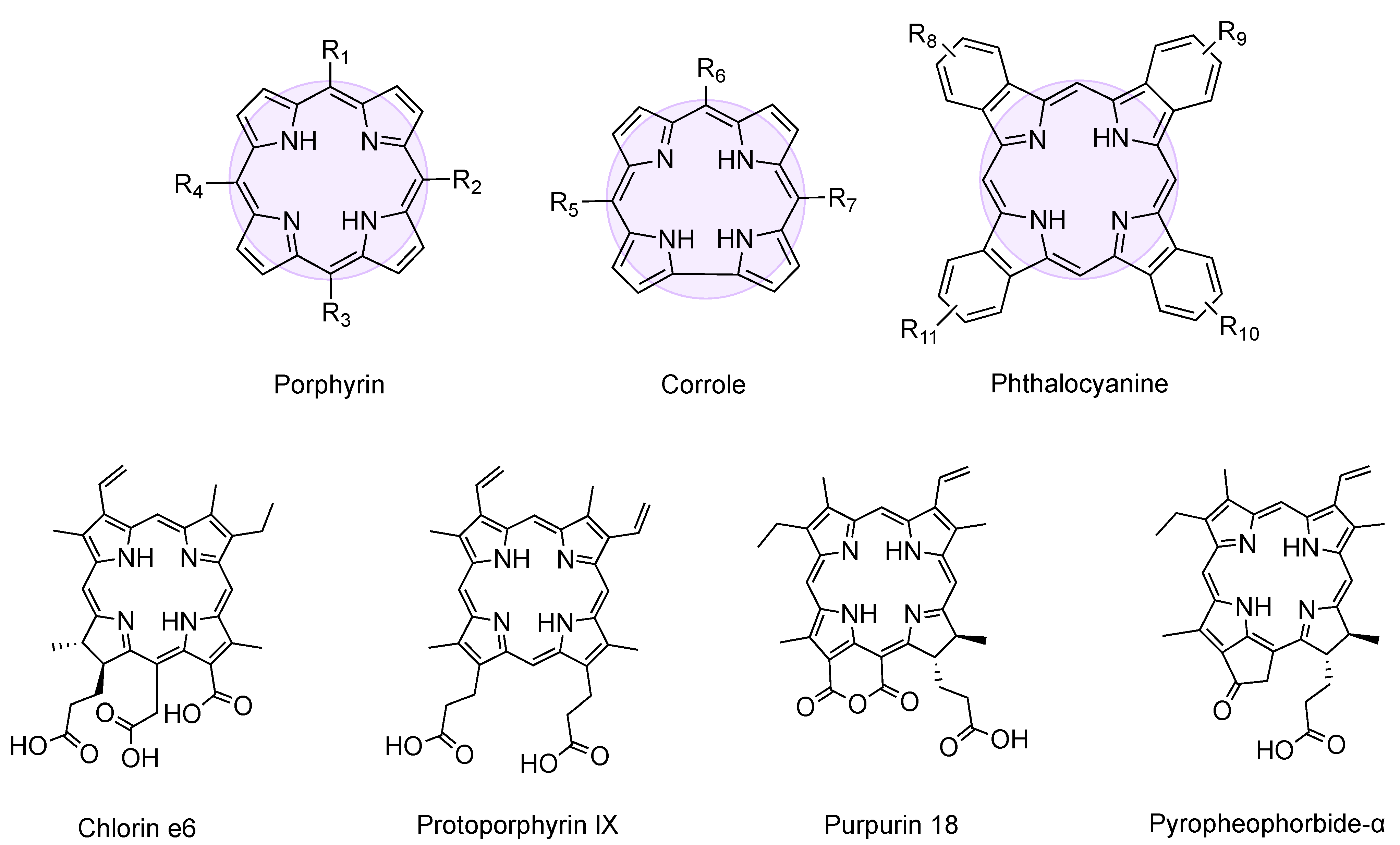
| 1 | (a) FF (b) Boc-FF (c) Fmoc-FF | Porphyrin R1 = 4-aminophenyl R2, R3, R4 = phenyl | Covalent (amide) | [52,53] |
| 2 | FF-OMe | Porphyrin R1 = 4-aminophenyl R2, R3, R4 = phenyl | Covalent (triazine) | [54] |
| 3 | Boc-FF | Porphyrin R1 = 3,4-diaminophenyl R2, R3, R4 = phenyl | Covalent (amide) | [54] |
| 4 | Boc-FF | Porphyrin [SnOH2] R1, R2, R3, R4 = phenyl | Metal coordination | [54] |
| 5 | (a) Fmoc-FF (b) FF-OMe | Corrole R5, R7 = pentafluorophenyl R6 = 4-aminophenyl | Covalent (a) amide (b) triazine | [54] |
| 6 | Boc-FF | Porphyrin R1 = 4-carboxyphenyl R2, R4 = mesityl R3 = 4-aminophenyl | Covalent (amide) | [55] |
| 7 | (a) FF (b) Boc-FF (c) Fmoc-FF | Porphyrin R1, R2, R3, R4 = 4-aminophenyl | Covalent (amide) | [56] |
| 8 | FF-BODIPY | Porphyrin R1 = 4-carboxyphenyl R2, R3, R4 = phenyl | Covalent (amide) | [56] |
| 9 | FF | Porphyrin R1 = 4-carboxyphenyl R2, R3, R4 = phenyl | Covalent (amide) | [57] |
| 10 | FF | Porphyrin R1 = 4-aminophenyl R2, R3, R4 = phenyl | Covalent (triazine) | [58] |
| 11 | FF | Porphyrin R1 = 4-aminophenyl R2, R3, R4 = phenyl | Covalent (glutaric acid) | [59] |
| 12 | FF | Phthalocyanine [Zn] R8, R9, R10 = H, R11 = OH | Covalent (butanoic acid) | [60,61] |
| 13 | FF-NH2·HCl | Chlorin e6 | Noncovalent | [62] |
| 14 | FF | Porphyrin R1, R2, R3, R4 = hydroxyphenyl | Noncovalent | [63] |
| 15 | Fmoc-FF | Porphyrin [Zn]; [Sn] R1, R2, R3, R4 = 4-pyridyl | Noncovalent | [64] |
| 16 | Fmoc-FF | Porphyrin (a) R1, R2, R3, R4 = 4-carboxyphenyl (b) R1, R2, R3, R4 = 4-aminophenyl | Noncovalent | [65] |
| 17 | FF | Porphyrin R1, R2, R3, R4 = 4-sulfonatophenyl | Noncovalent | [66] |
| 18 | D-F-D-F-NH2 | Porphyrin [Sn(OH)2] R1, R2, R3, R4 = 4-tolyl | Noncovalent | [67] |
| 19 | FF | Phthalocyanine [Zn] (a) R8, R9, R10, R11 = solketal (b) R8, R9, R10, R11 = glyceryl (c) R8, R9, R10 = solketal; R11= methyl (d) R8, R9, R10 = glyceryl; R11= methyl | Noncovalent | [68] |
3. Other Dipeptides
| Entry | Peptide | Tetrapyrrole | Interaction (Linker/Bond) | Ref. |
|---|---|---|---|---|
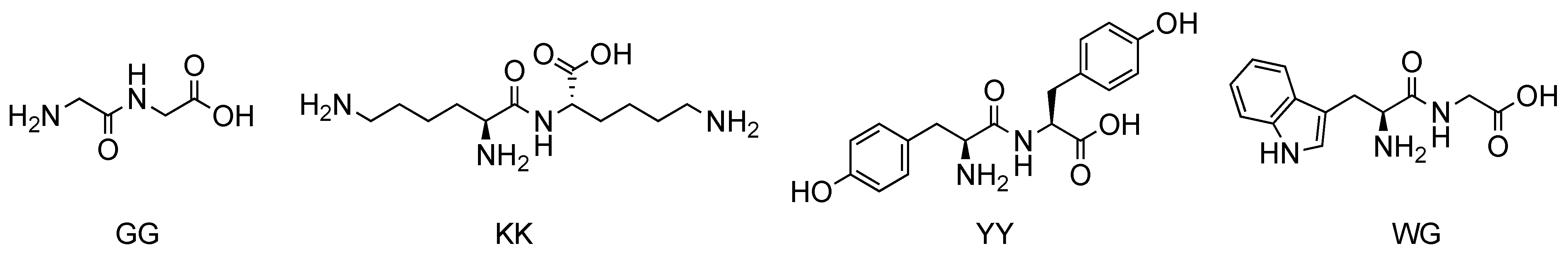
| 20 | GG | Porphyrin R1 = 4-carboxyphenyl R2, R3, R4 = 3-methoxyphenyl | Covalent (amide) | [69] |
| 21 | KK | Porphyrin R1, R2, R3, R4 = 4-sulfonatophenyl | Noncovalent | [70,71] |
| 22 | YY | PorphyrinR1 = 1,3-bis(octyloxy)phenyl R3 = 4-(bromomethyl)phenyl R2, R4 = H | Covalent (ether) | [72] |
| 23 | WG | Porphyrin R1, R3 = 4-carboxyphenyl R2, R4 = phenyl | Covalent (amide) | [73] |
4. Medium and Long Peptides
| Entry | Peptide | Tetrapyrrole | Interaction (linker/bond) | Ref. |
|---|---|---|---|---|
| 24 | Ac-CKVKV-NH2 | Porphyrin (a) R1 = 4-bromoacetamidophenyl (b) R1 = 2-bromoacetamidophenyl R2, R3, R4 = 4-tolyl | Covalent (thioether) | [74,75] |
| 25 | Ac-CKVSVKV-NH2 | Porphyrin R1 = 4-bromoacetamidophenyl R2, R3, R4 = 4-tolyl | Covalent (thioether) | [74,75] |
| 26 | Ac-NAEASAESAY-NH2 | Porphyrin R1 = 4-aminophenyl R2, R3, R4 = phenyl | Covalent (amide) | [76] |
| 27 | (a) Ac-IQQLKNQIKQLLKQ-NH2 (b) Ac-IQQLKNQIKQLLKQAAIQ QLQNQIQQLLQQ-NH2 | Porphyrin R1, R2, R3, R4 = 4-sulfonatophenyl | Noncovalent | [77,78,79] |
| 28 | (a) Ac-K(IEALEGK)2(IEALEHK) (IEALEGK)G-NH2 (b) Ac-Q(IAALEQK)(IAALE-4-Pal-K) (IAALEQK)2G-NH2 | Protoporphyrin IX [Co] | Metal coordination | [80,81] |
| 29 | KKKKK | Phthalocyanine [Zn] R8, R9, R10 = H, R11 = COOH | Covalent (amide) | [82] |
| 30 | GAGAG-NH2 | Porphyrin [Zn] R1 = 4-carboxyphenyl R2, R3, R4 = 4-tert-butylphenyl | Covalent (amide) | [83] |
| 31 | (a) GIGKFLHSAKKFGKAFVGEILNS(b) GIGKALHSAKKFGKAFVGEILNS | Porphyrin R1 = 4-carboxyphenyl R2, R3, R4 = phenyl | Covalent (amide) | [84] |
| 32 | Ac-IIIIKK-NH2 | Porphyrin R1, R2, R3, R4 = 4-sulfonatophenyl | Noncovalent | [85,86,87] |
| 33 | PLG | Pyropheophorbide-α | Covalent (amide) | [88] |
| 34 | YVHD | Purpurin-18 | Covalent (amide) | [89] |
| 35 | (a) QRLGVGFPK (b) QKVPHVGQK | Purpurin-18 | Covalent (amide) | [90] |
| 36 | GTFG | Purpurin-18 | Covalent (amide) | [91] |
| 37 | AKC | Purpurin-18 | Covalent (amide) | [92] |
| 38 | (a) RRR (b) RRRRRRR | Purpurin-18 | Covalent (amide) | [93] |
| 39 | KLVFF | Porphyrin R1 = 4-carboxyphenyl R2, R3, R4 = phenyl | Covalent (amide) | [94] |
| 40 | FFYSV | Protoporphyrin IX | Covalent (amide) | [95] |
5. Peptides Containing Non-Amino Acid Components
| Entry | Peptide | Tetrapyrrole | Interaction (Linker/Bond) | Ref. |
|---|---|---|---|---|
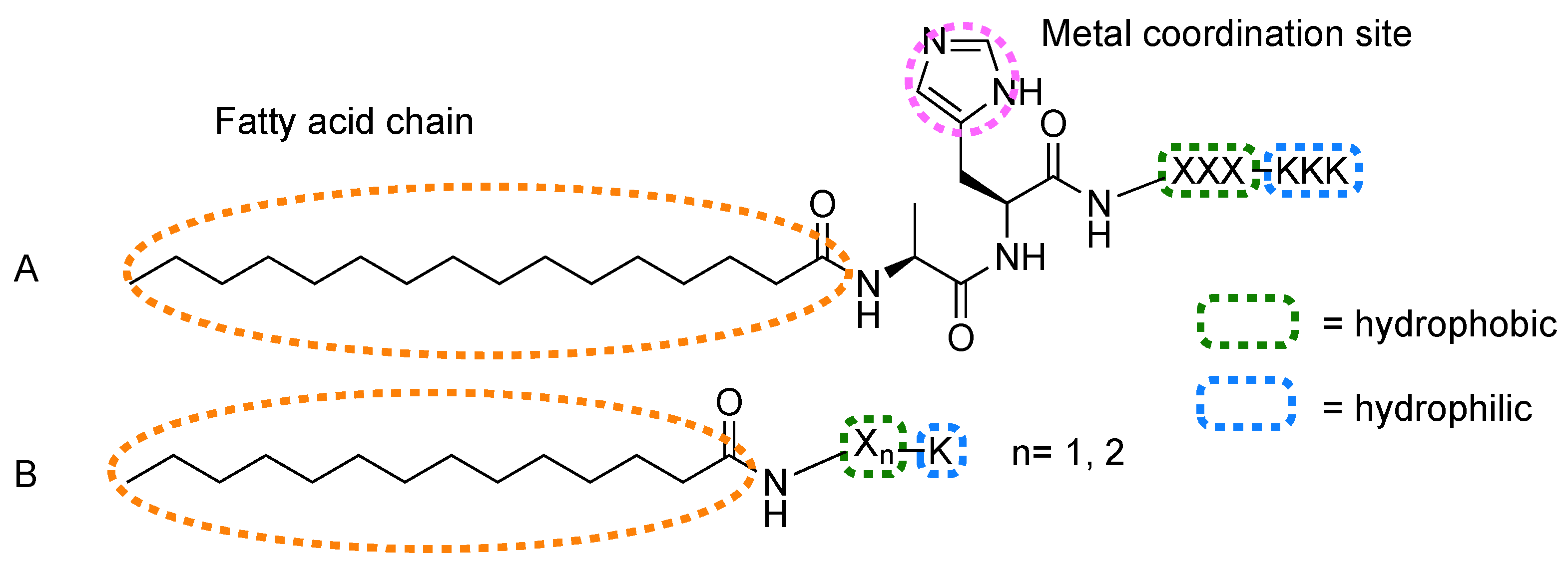
| 41 | (a) c16-AHLLLKKK (b) c16-AHALLLKKK (c) c16-AHWWWKKK (d) c16-AHFFFKKK (e) c16-AHIIIKKK (f) c16-AHVVVKKK (g) c16-AHAAAKKK | Protoporphyrin IX [Zn] | Metal coordination | [97,98,99,100] |
| 42 | (a) c16-AALLLKKK (b) c16-AHLLLKKK (c) c16-HHLLLKKK (d) c16-MHLLLKKK | Protoporphyrin IX [Fe] | Metal coordination | [101] |
| 43 | (a) c16-AHLLLKKK (b) c16-AHLLLKKKKKKKKK | Porphyrin [Zn] R1, R3 = phenyl R2, R4 = H | Metal coordination | [102] |
| 44 | (a) c14-FFK (b) c14-FK (c) c14-YYK (d) c14-YK | Porphyrin [Zn] R1, R2, R3, R4 = 4-pyridyl | Noncovalent | [103] |
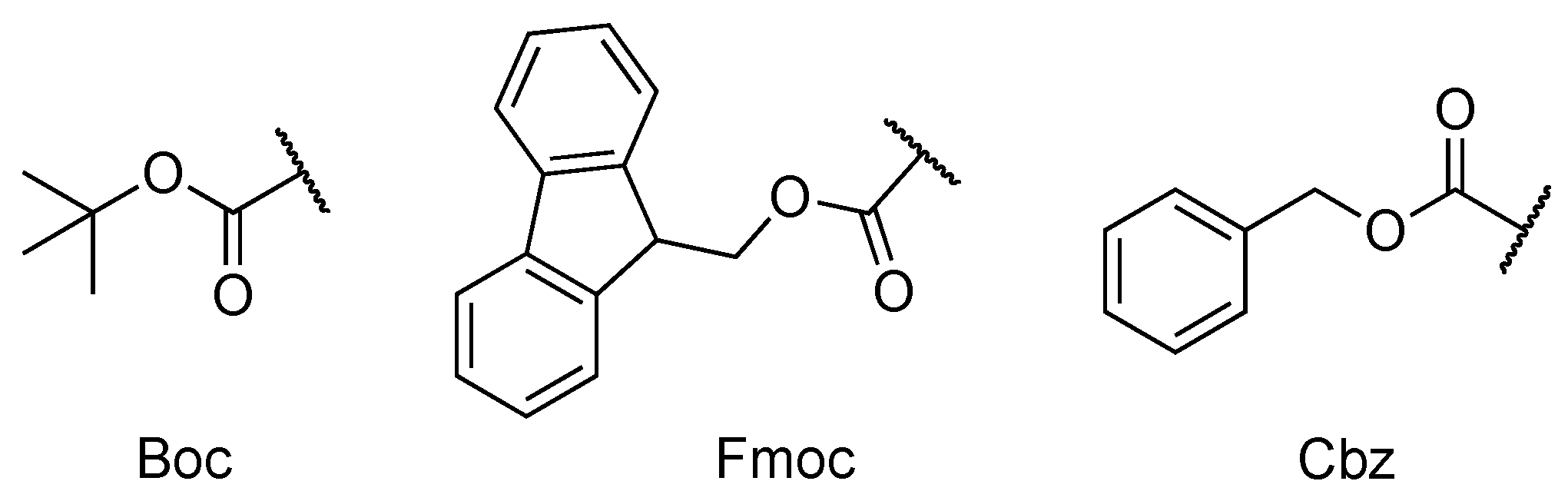
| 45 | (a) Boc-II (b) Fmoc-II (c) Cbz-II (d) II-OMe | Porphyrin (a, b, c) R1 = 4-aminophenyl (d) R1 = 4-carboxyphenyl R2, R3, R4 = phenyl | Covalent (amide) | [104] |
| 46 | (a) Boc-AI (b) Fmoc-AI (c) Cbz-AI (d) AI-OMe | Porphyrin (a, b, c) R1 = 4-aminophenyl (d) R1 = 4-carboxyphenyl R2, R3, R4 = phenyl | Covalent (amide) | [104] |
| 47 | Fmoc-TL-NH2 | Porphyrin R1, R2, R3, R4 = 4-carboxyphenyl | Noncovalent | [105] |
| 48 | Fmoc-LLL-OMe | Porphyrin R1, R2, R3, R4 = phenyl | Noncovalent | [106] |
| 49 | Fmoc-LLL-OMe | Porphyrin R1, R2, R3, R4 = 4-hydroxyphenyl | Noncovalent | [107,108] |
| 50 | Cbz-HF | Chlorin e6 [Zn] | Metal coordination | [109] |
| 51 | (a) Fmoc-ChaChaGK-NH2 (b) Fmoc-FFGK-NH2 (c) Ac-ChaChaGK-NH2 (d) Ac-FFGK-NH2 | Porphyrin R1, R2, R3, R4 = 4-sulfonatophenyl | Noncovalent | [110,111] |
| 52 | (a) GGK(Biotin)-COOH (b) GGK(Biotin)-CONH2 | Phthalocyanine [Zn] R8, R9, R10 = H R11 = 4-(3-propanoyl)phenoxy | Covalent | [112] |
| 53 | Thy-AAibAAibAAibAAib-Ade | Porphyrin R1 = 4-aminophenyl R2, R3, R4 = phenyl | Noncovalent | [113] |
| 54 | Ac-VE(NDI)VKVE(NDI)V-NH2 | PorphyrinR1 = 4-carboxyphenyl R2, R3, R4 = phenyl | Covalent (amide) | [114] |
| 55 | (a) KK (b) KKKK (c) KKKKKKKK (d) KKKKKKKKKKKKKKKK | Porphyrin R1 = 4-[(4-butanoyl)oxy]phenyl R2, R3, R4 = 1,3-di-tert-butylphenyl | Covalent (amide) | [115,116] |
5.1. Fatty Acids
5.2. Protecting Groups
5.3. Other Biomolecules
5.4. Electron Acceptor Units
6. Cyclic Peptides
| Entry | Peptide | Tetrapyrrole | Interaction (Linker/Bond) | Ref. |
|---|---|---|---|---|
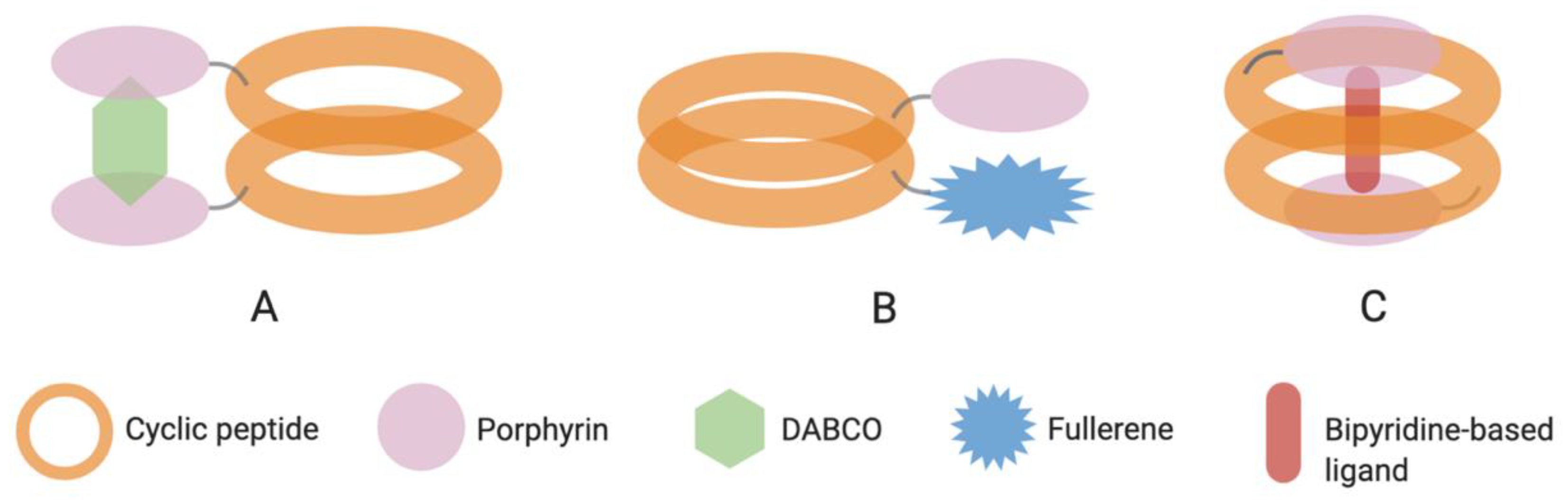
| 56 | c[(S-d-MeN-γ-Ach-F-d-MeN-γ-Ach)2] | Porphyrin [Zn] R1 = 4-carboxyphenyl R2, R3, R4 = 4-pentylphenyl | Covalent (ester) | [119] |
| 57 | c[S-d-MeN-γ-Acp-(F-d-MeN-γ-Acp)2] | Porphyrin [Zn] R1 = 4-carboxyphenyl R2, R3, R4 = 4-pentylphenyl | Covalent (ester) | [120] |
| 58 | c[(I-MeN-γ-Acp-I-NHNHAcN-γ-Acp-)2] | Porphyrin [Zn] R1, R3 = 3-formylphenyl R2, R4 = mesityl | Covalent (hydrazone) | [121] |
7. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Croce, R.; Van Amerongen, H. Natural strategies for photosynthetic light harvesting. Nat. Chem. Biol. 2014, 10, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yan, H.; Wang, K.; Kuang, T.; Zhang, J.; Gui, L.; An, X.; Chang, W. Crystal structure of spinach major light-harvesting complex at 2.72 Å resolution. Nature 2004, 428, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Battersby, A.R. Tetrapyrroles: The pigments of life. Nat. Prod. Rep. 2000, 17, 507–526. [Google Scholar] [CrossRef]
- Lombardi, A.; Nastri, F.; Pavone, V. Peptide-based heme-protein models. Chem. Rev. 2001, 101, 3165–3189. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Liu, K.; Abbas, M.; Yan, X. Peptide-Modulated Self-Assembly of Chromophores toward Biomimetic Light-Harvesting Nanoarchitectonics. Adv. Mater. 2016, 28, 1031–1043. [Google Scholar] [CrossRef]
- Jones, R.G.; Ober, C.K.; Hodge, P.; Kratochvíl, P.; Moad, G.; Vert, M. Terminology for aggregation and self-assembly in polymer science (IUPAC recommendations 2013). Pure Appl. Chem. 2013, 85, 463–492. [Google Scholar] [CrossRef]
- Fairman, R.; Åkerfeldt, K.S. Peptides as novel smart materials. Curr. Opin. Struct. Biol. 2005, 15, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Zelzer, M.; Ulijn, R.V. Next-generation peptide nanomaterials: Molecular networks, interfaces and supramolecular functionality. Chem. Soc. Rev. 2010, 39, 3351–3357. [Google Scholar] [CrossRef]
- Gupta, S.; Singh, I.; Sharma, A.K.; Kumar, P. Ultrashort Peptide Self-Assembly: Front-Runners to Transport Drug and Gene Cargos. Front. Bioeng. Biotechnol. 2020, 8, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Dehsorkhi, A.; Castelletto, V.; Hamley, I.W. Self-assembling amphiphilic peptides. J. Pept. Sci. 2014, 20, 453–467. [Google Scholar] [CrossRef]
- Claussen, R.C.; Rabatic, B.M.; Stupp, S.I. Aqueous Self-Assembly of Unsymmetric Peptide Bolaamphiphiles into Nanofibers with Hydrophilic Cores and Surfaces. J. Am. Chem. Soc. 2003, 125, 12680–12681. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Chen, Y.; Tang, C.; Zhou, Q.; Wang, C.; Shi, Y.K.; Zhao, X. De Novo design of a bolaamphiphilic peptide with only natural amino acids. Macromol. Biosci. 2008, 8, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Hamley, I.W. Peptide nanotubes. Angew. Chem. Int. Ed. 2014, 53, 6866–6881. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, D.; Morin, S.A.; Jin, S.; Raines, R.T. Self-assembled collagen-like peptide fibers as templates for metallic nanowires. J. Mater. Chem. 2008, 18, 3865–3870. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xing, R.; Bai, S.; Yan, X. Recent advances of self-assembling peptide-based hydrogels for biomedical applications. Soft Matter 2019, 15, 1704–1715. [Google Scholar] [CrossRef] [PubMed]
- Lian, M.; Chen, X.; Lu, Y.; Yang, W. Self-Assembled Peptide Hydrogel as a Smart Biointerface for Enzyme-Based Electrochemical Biosensing and Cell Monitoring. ACS Appl. Mater. Interfaces 2016, 8, 25036–25042. [Google Scholar] [CrossRef]
- Fan, T.; Yu, X.; Shen, B.; Sun, L. Peptide Self-Assembled Nanostructures for Drug Delivery Applications. J. Nanomater. 2017, 2017. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, H.; Zhao, X. Designer Self-Assembling Peptide Hydrogels to Engineer 3D Cell Microenvironments for Cell Constructs Formation and Precise Oncology Remodeling in Ovarian Cancer. Adv. Sci. 2020, 7. [Google Scholar] [CrossRef]
- Reches, M.; Gazit, E. Casting metal nanowires within discrete self-assembled peptide nanotubes. Science 2003, 300, 625–627. [Google Scholar] [CrossRef]
- Ravichandran, R.; Griffith, M.; Phopase, J. Applications of self-assembling peptide scaffolds in regenerative medicine: The way to the clinic. J. Mater. Chem. B 2014, 2, 8466–8478. [Google Scholar] [CrossRef]
- Chen, J.; Zou, X. Self-assemble peptide biomaterials and their biomedical applications. Bioact. Mater. 2019, 4, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Zhang, S. Designer nanomaterials using chiral self-assembling peptide systems and their emerging benefit for society. Chem. Soc. Rev. 2012, 41, 4736–4754. [Google Scholar] [CrossRef] [PubMed]
- Rad-Malekshahi, M.; Lempsink, L.; Amidi, M.; Hennink, W.E.; Mastrobattista, E. Biomedical Applications of Self-Assembling Peptides. Bioconjug. Chem. 2016, 27, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Gerbelli, B.B.; Vassiliades, S.V.; Rojas, J.E.U.; Pelin, J.N.B.D.; Mancini, R.S.N.; Pereira, W.S.G.; Aguilar, A.M.; Venanzi, M.; Cavalieri, F.; Giuntini, F.; et al. Hierarchical Self-Assembly of Peptides and its Applications in Bionanotechnology. Macromol. Chem. Phys. 2019, 220, 1–22. [Google Scholar] [CrossRef]
- Cook, L.; Brewer, G.; Wong-Ng, W. Structural aspects of porphyrins for functional materials applications. Crystals 2017, 7, 223. [Google Scholar] [CrossRef]
- Mandal, A.K.; Taniguchi, M.; Diers, J.R.; Niedzwiedzki, D.M.; Kirmaier, C.; Lindsey, J.S.; Bocian, D.F.; Holten, D. Photophysical properties and electronic structure of porphyrins bearing zero to four meso-Phenyl substituents: New insights into seemingly well understood tetrapyrroles. J. Phys. Chem. A 2016, 120, 9719–9731. [Google Scholar] [CrossRef]
- MacDonald, I.J.; Dougherty, T.J. Basic principles of photodynamic therapy. J. Porphyr. Phthalocyanines 2001, 5, 105–129. [Google Scholar] [CrossRef]
- Giuntini, F.; Alonso, C.M.A.; Boyle, R.W. Synthetic approaches for the conjugation of porphyrins and related macrocycles to peptides and proteins. Photochem. Photobiol. Sci. 2011, 10, 759–791. [Google Scholar] [CrossRef]
- Giuntini, F.; Chauhan, V.M.; Aylott, J.W.; Rosser, G.A.; Athanasiadis, A.; Beeby, A.; MacRobert, A.J.; Brown, R.A.; Boyle, R.W. Conjugatable water-soluble Pt(ii) and Pd(ii) porphyrin complexes: Novel nano- and molecular probes for optical oxygen tension measurement in tissue engineering. Photochem. Photobiol. Sci. 2014, 13, 1039–1051. [Google Scholar] [CrossRef]
- Barona-Castaño, J.C.; Carmona-Vargas, C.C.; Brocksom, T.J.; De Oliveira, K.T.; Graça, M.; Neves, P.M.S.; Amparo, M.; Faustino, F. Porphyrins as catalysts in scalable organic reactions. Molecules 2016, 21, 310. [Google Scholar] [CrossRef]
- Wang, H.; Wang, B.W.; Bian, Y.; Gao, S.; Jiang, J. Single-molecule magnetism of tetrapyrrole lanthanide compounds with sandwich multiple-decker structures. Coord. Chem. Rev. 2016, 306, 195–216. [Google Scholar] [CrossRef]
- Wang, X.F.; Tamiaki, H. Cyclic tetrapyrrole based molecules for dye-sensitized solar cells. Energy Environ. Sci. 2010, 3, 94–106. [Google Scholar] [CrossRef]
- Ohno, O.; Kaizu, Y.; Kobayashi, H. J-aggregate formation of a water-soluble porphyrin in acidic aqueous media. J. Chem. Phys. 1993, 99, 4128–4139. [Google Scholar] [CrossRef]
- Maiti, N.C.; Mazumdar, S.; Periasamy, N. J- and H-aggregates of porphyrin—Surfactant complexes: Time-resolved fluorescence and other spectroscopic studies. J. Phys. Chem. B 1998, 102, 1528–1538. [Google Scholar] [CrossRef]
- Serra, V.V.; Andrade, S.M.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Costa, S.M.B. J-aggregate formation in bis-(4-carboxyphenyl)porphyrins in water:pH and counterion dependence. New J. Chem. 2010, 34, 2757–2765. [Google Scholar] [CrossRef]
- Isago, H. Spectral properties of a novel antimony(III)-phthalocyanine complex that behaves like J-aggregates in non-aqueous media. Chem. Commun. 2003, 3, 1864–1865. [Google Scholar] [CrossRef]
- Bayda, M.; Dumoulin, F.; Hug, G.L.; Koput, J.; Gorniak, R.; Wojcik, A. Fluorescent H-aggregates of an asymmetrically substituted mono-amino Zn(II) phthalocyanine. Dalton Trans. 2017, 46, 1914–1926. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Andeme Edzang, J.; Diallo, A.K.; Dutronc, T.; Balaban, T.S.; Videlot-Ackermann, C.; Terazzi, E.; Canard, G. Light absorption and hole-transport properties of copper corroles: From aggregates to a liquid crystal mesophase. New J. Chem. 2015, 39, 7140–7146. [Google Scholar] [CrossRef]
- Bobe, M.S.R.; Al Kobaisi, M.; Bhosale, S.V.; Bhosale, S.V. Solvent-Tuned Self-Assembled Nanostructures of Chiral l/d-Phenylalanine Derivatives of Protoporphyrin IX. ChemistryOpen 2015, 4, 516–522. [Google Scholar] [CrossRef]
- Jintoku, H.; Sagawa, T.; Sawada, T.; Takafuji, M.; Ihara, H. Versatile chiroptics of peptide-induced assemblies of metalloporphyrins. Org. Biomol. Chem. 2010, 8, 1344–1350. [Google Scholar] [CrossRef] [PubMed]
- Koti, A.S.R.; Periasamy, N. Self-assembly of template-directed J-aggregates of porphyrin. Chem. Mater. 2003, 15, 369–371. [Google Scholar] [CrossRef]
- Lauceri, R.; Raudino, A.; Scolaro, L.M.; Micali, N.; Purrello, R. From achiral porphyrins to template-imprinted chiral aggregates and further. Self-replication of chiral memory from scratch. J. Am. Chem. Soc. 2002, 124, 894–895. [Google Scholar] [CrossRef] [PubMed]
- Purrello, R.; Bellacchio, E.; Gurrieri, S.; Lauceri, R.; Raudino, A.; Scolaro, L.M.; Santoro, A.M. pH modulation of porphyrins self-assembly onto polylysine. J. Phys. Chem. B 1998, 102, 8852–8857. [Google Scholar] [CrossRef]
- Li, F.; Wang, T.; Wang, L.; Zhao, C.; Yang, F.; Sun, H.; Liu, D.; Hu, W.; Li, W.; Zhou, X. Using glycine-induced nanoparticle to enhance photo-induced electron transfer efficiency in donor-acceptor system. Dye. Pigment. 2017, 140, 116–121. [Google Scholar] [CrossRef]
- Azriel, R.; Gazit, E. Analysis of the minimal amyloid-forming fragment of the islet amyloid polypeptide. An experimental support for the key role of the phenylalanine residue in amyloid formation. J. Biol. Chem. 2001, 276, 34156–34161. [Google Scholar] [CrossRef] [PubMed]
- Gazit, E. A possible role for π-stacking in the self-assembly of amyloid fibrils. FASEB J. 2002, 16, 77–83. [Google Scholar] [CrossRef]
- Caughey, B.; Lansbury, P.T. Protofibrils, pores, fibrils, and neurodegeneration: Separating the responsible protein aggregates from the innocent bystanders. Annu. Rev. Neurosci. 2003, 26, 267–298. [Google Scholar] [CrossRef]
- Nelson, R.; Sawaya, M.R.; Balbirnie, M.; Madsen, A.; Riekel, C.; Grothe, R.; Eisenberg, D. Structure of the cross-β spine of amyloid-like fibrils. Nature 2005, 435, 773–778. [Google Scholar] [CrossRef]
- Adler-Abramovich, L.; Reches, M.; Sedman, V.L.; Allen, S.; Tendler, S.J.B.; Gazit, E. Thermal and chemical stability of diphenylalanine peptide nanotubes: Implications for nanotechnological applications. Langmuir 2006, 22, 1313–1320. [Google Scholar] [CrossRef]
- Gazit, E. Self-assembled peptide nanostructures: The design of molecular building blocks and their technological utilization. Chem. Soc. Rev. 2007, 36, 1263–1269. [Google Scholar] [CrossRef]
- Yan, X.; Zhu, P.; Li, J. Self-assembly and application of diphenylalanine-based nanostructures. Chem. Soc. Rev. 2010, 39, 1877–1890. [Google Scholar] [CrossRef] [PubMed]
- Charalambidis, G.; Kasotakis, E.; Lazarides, T.; Mitraki, A.; Coutsolelos, A.G. Self-assembly into spheres of a hybrid diphenylalanine-porphyrin: Increased fluorescence lifetime and conserved electronic properties. Chem. A Eur. J. 2011, 17, 7213–7219. [Google Scholar] [CrossRef] [PubMed]
- Charalambidis, G.; Georgilis, E.; Panda, M.K.; Anson, C.E.; Powell, A.K.; Doyle, S.; Moss, D.; Jochum, T.; Horton, P.N.; Coles, S.J.; et al. A switchable self-assembling and disassembling chiral system based on a porphyrin-substituted phenylalanine-phenylalanine motif. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Karikis, K.; Georgilis, E.; Charalambidis, G.; Petrou, A.; Vakuliuk, O.; Chatziioannou, T.; Raptaki, I.; Tsovola, S.; Papakyriacou, I.; Mitraki, A.; et al. Corrole and Porphyrin Amino Acid Conjugates: Synthesis and Physicochemical Properties. Chem. A Eur. J. 2016, 22, 11245–11252. [Google Scholar] [CrossRef] [PubMed]
- Charalambidis, G.; Karikis, K.; Georgilis, E.; M’Sabah, B.L.; Pellegrin, Y.; Planchat, A.; Lucas, B.; Mitraki, A.; Bouclé, J.; Odobel, F.; et al. Supramolecular architectures featuring the antenna effect in solid state DSSCs. Sustain. Energy Fuels 2017, 1, 387–395. [Google Scholar] [CrossRef]
- Nikoloudakis, E.; Orphanos, E.; Agapaki, E.; Nikolaou, V.; Charisiadis, A.; Charalambidis, G.; Mitraki, A.; Coutsolelos, A.G. Molecular self-assembly of porphyrin and BODIPY chromophores connected with diphenylalanine moieties. J. Porphyr. Phthalocyanines 2020, 24, 775–785. [Google Scholar] [CrossRef]
- Tao, K.; Xue, B.; Frere, S.; Slutsky, I.; Cao, Y.; Wang, W.; Gazit, E. Multiporous Supramolecular Microspheres for Artificial Photosynthesis. Chem. Mater. 2017, 29, 4454–4460. [Google Scholar] [CrossRef]
- Li, F.; Liu, D.; Wang, T.; Hu, J.; Meng, F.; Sun, H.; Shang, Z.; Li, P.; Feng, W.; Li, W.; et al. J-aggregation in porphyrin nanoparticles induced by diphenylalanine. J. Solid State Chem. 2017, 252, 86–92. [Google Scholar] [CrossRef]
- Zou, Q.; Abbas, M.; Zhao, L.; Li, S.; Shen, G.; Yan, X. Biological photothermal nanodots based on self-assembly of peptide-porphyrin conjugates for antitumor therapy. J. Am. Chem. Soc. 2017, 139, 1921–1927. [Google Scholar] [CrossRef]
- Zhao, L.; Li, S.; Liu, Y.; Xing, R.; Yan, X. Kinetically Controlled Self-Assembly of Phthalocyanine–Peptide Conjugate Nanofibrils Enabling Superlarge Redshifted Absorption. CCS Chem. 2019, 1, 173–180. [Google Scholar] [CrossRef]
- Li, S.; Zhao, L.; Chang, R.; Xing, R.; Yan, X. Spatiotemporally Coupled Photoactivity of Phthalocyanine–Peptide Conjugate Self-Assemblies for Adaptive Tumor Theranostics. Chem. A Eur. J. 2019, 25, 13429–13435. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Xing, R.; Zou, Q.; Ma, G.; Möhwald, H.; Yan, X. Simple Peptide-Tuned Self-Assembly of Photosensitizers towards Anticancer Photodynamic Therapy. Angew. Chem. Int. Ed. 2016, 55, 3036–3039. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, M.; Lee, J.S.; Park, C.B. Self-assembled light-harvesting peptide nanotubes for mimicking natural photosynthesis. Angew. Chem. Int. Ed. 2012, 124, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Nam, D.H.; Lee, Y.W.; Nam, Y.S.; Park, C.B. Self-assembly of metalloporphyrins into light-harvesting peptide nanofiber hydrogels for solar water oxidation. Small 2014, 10, 1272–1277. [Google Scholar] [CrossRef]
- Li, Y.; Männel, M.J.; Hauck, N.; Patel, H.P.; Auernhammer, G.K.; Chae, S.; Fery, A.; Li, J.; Thiele, J. Embedment of quantum dots and biomolecules in an in-situ formed dipeptide hydrogel using microfluidics. Angew. Chem. Int. Ed. 2020, anie.202015340. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Zhang, L.; Yan, X.; Wang, A.; Ma, G.; Li, J.; Möhwald, H.; Mann, S. Multifunctional porous microspheres based on peptide-porphyrin hierarchical co-assembly. Angew. Chem. Int. Ed. 2014, 53, 2366–2370. [Google Scholar] [CrossRef] [PubMed]
- Parayil, S.K.; Lee, J.; Yoon, M. Highly fluorescent peptide nanoribbon impregnated with Sn-porphyrin as a potent DNA sensor. Photochem. Photobiol. Sci. 2013, 12, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.I.; Prieto, T.; Rodrigues, T.; Ferreira, F.F.; Nascimento, F.B.; Ribeiro, A.O.; Silva, E.R.; Giuntini, F.; Alves, W.A. Conjugation with L,L-diphenylalanine Self-Assemblies Enhances in Vitro Antitumor Activity of Phthalocyanine Photosensitizer. Sci. Rep. 2017, 7, 13166. [Google Scholar] [CrossRef]
- Teixeira, R.; Andrade, S.M.; Vaz Serra, V.; Paulo, P.M.R.; Sánchez-Coronilla, A.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Costa, S.M.B. Reorganization of self-assembled dipeptide porphyrin J-aggregates in water-ethanol mixtures. J. Phys. Chem. B 2012, 116, 2396–2404. [Google Scholar] [CrossRef]
- Liu, K.; Xing, R.; Chen, C.; Shen, G.; Yan, L.; Zou, Q.; Ma, G.; Möhwald, H.; Yan, X. Peptide-induced hierarchical long-range order and photocatalytic activity of porphyrin assemblies. Angew. Chem. Int. Ed. 2015, 54, 500–505. [Google Scholar] [CrossRef]
- Liu, K.; Xing, R.; Li, Y.; Zou, Q.; Möhwald, H.; Yan, X. Mimicking Primitive Photobacteria: Sustainable Hydrogen Evolution Based on Peptide–Porphyrin Co-Assemblies with a Self-Mineralized Reaction Center. Angew. Chem. Int. Ed. 2016, 55, 12503–12507. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Heo, J.; Ham, S.; Yoo, H.; Lee, C.H.; Yoon, H.; Ryu, D.; Kim, D.; Jang, W.D. Cyclodipeptide-bridged porphyrin dimer supramolecular assemblies. Chem. Commun. 2011, 47, 2405–2407. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sun, B.; Chang, R.; Cao, S.; Yuan, C.; Zhao, L.; Yang, H.; Li, J.; Yan, X.; van Hest, J.C.M. Acid-Activatable Transmorphic Peptide-Based Nanomaterials for Photodynamic Therapy. Angew. Chem. Int. Ed. 2020, 59, 20582–20588. [Google Scholar] [CrossRef] [PubMed]
- Arai, T.; Inudo, M.; Ishimatsu, T.; Sasaki, T.; Kato, T.; Nishino, N. CD investigation of porphyrin-porphyrin interaction with links to acyclic β-sheet peptide self-assembled in an aqueous media. Chem. Lett. 2001, 1240–1241. [Google Scholar] [CrossRef]
- Arai, T.; Inudo, M.; Ishimatsu, T.; Akamatsu, C.; Tokusaki, Y.; Sasaki, T.; Nishino, N. Self-assembling of the porphyrin-linked acyclic penta- and heptapeptides in aqueous trifluoroethanol. J. Org. Chem. 2003, 68, 5540–5549. [Google Scholar] [CrossRef] [PubMed]
- Dunetz, J.R.; Sandstrom, C.; Young, E.R.; Baker, P.; Van Name, S.A.; Cathopolous, T.; Fairman, R.; De Paula, J.C.; Åkerfeldt, K.S. Self-assembling porphyrin-modified peptides. Org. Lett. 2005, 7, 2559–2561. [Google Scholar] [CrossRef] [PubMed]
- Kovaric, B.C.; Kokona, B.; Schwab, A.D.; Twomey, M.A.; De Paula, J.C.; Fairman, R. Self-assembly of peptide porphyrin complexes: Toward the development of smart biomaterials. J. Am. Chem. Soc. 2006, 128, 4166–4167. [Google Scholar] [CrossRef]
- Kokona, B.; Kim, A.M.; Roden, R.C.; Daniels, J.P.; Pepe-Mooney, B.J.; Kovaric, B.C.; De Paula, J.C.; Johnson, K.A.; Fairman, R. Self assembly of coiled-coil peptide-porphyrin complexes. Biomacromolecules 2009, 10, 1454–1459. [Google Scholar] [CrossRef]
- Pepe-Mooney, B.J.; Kokona, B.; Fairman, R. Characterization of mesoscale coiled-coil peptide-porphyrin complexes. Biomacromolecules 2011, 12, 4196–4203. [Google Scholar] [CrossRef]
- Carvalho, I.M.M.; Ogawa, M.Y. Self-organization of porphyrin-peptide units by metal-mediated peptide assembly. J. Braz. Chem. Soc. 2010, 21, 1390–1394. [Google Scholar] [CrossRef]
- Zaytsev, D.V.; Xie, F.; Mukherjee, M.; Bludin, A.; Demeler, B.; Breece, R.M.; Tierney, D.L.; Ogawa, M.Y. Nanometer to millimeter scale peptide-porphyrin materials. Biomacromolecules 2010, 11, 2602–2609. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Li, J.; Chen, J.; Hu, P.; Jiang, L.; Chen, X.; Huang, M.; Chen, Z.; Xu, P. Smart Photosensitizer: Tumor-Triggered Oncotherapy by Self-Assembly Photodynamic Nanodots. ACS Appl. Mater. Interfaces 2018, 10, 15369–15380. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, Y.; Ma, P.; Lu, J.; Zhang, X.; Jiang, J. Morphology and chirality controlled self-assembled nanostructures of porphyrin-pentapeptide conjugate: Effect of the peptide secondary conformation. J. Mater. Chem. 2011, 21, 8057–8065. [Google Scholar] [CrossRef]
- Cimino, R.; Grelloni, E.; Magna, G.; Monti, D.; Stefanelli, M.; Gatto, E.; Placidi, E.; Biscaglia, F.; Gobbo, M.; Venanzi, M. Tuning the morphology of mesoscopic structures of porphyrin macrocycles functionalized by an antimicrobial peptide. J. Porphyr. Phthalocyanines 2020, 24, 920–928. [Google Scholar] [CrossRef]
- Wang, S.; Li, M.; Patil, A.J.; Sun, S.; Tian, L.; Zhang, D.; Cao, M.; Mann, S. Design and construction of artificial photoresponsive protocells capable of converting day light to chemical energy. J. Mater. Chem. A 2017, 5, 24612–24616. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, D.; Zhang, X.; Yu, D.; Jiang, X.; Wang, Z.; Cao, M.; Xia, Y.; Liu, H. Short peptide-regulated aggregation of porphyrins for photoelectric conversion. Sustain. Energy Fuels 2019, 3, 529–538. [Google Scholar] [CrossRef]
- Xiu, Y.; Zhang, D.; Xu, L.; Li, J.; Chen, Y.; Xia, Y.; Cao, M.; Wang, S. Bioinspired construction of light-harvesting antenna via hierarchically co-assembling approach. J. Colloid Interface Sci. 2020. [Google Scholar] [CrossRef]
- Li, L.L.; Ma, H.L.; Qi, G.B.; Zhang, D.; Yu, F.; Hu, Z.; Wang, H. Pathological-Condition-Driven Construction of Supramolecular Nanoassemblies for Bacterial Infection Detection. Adv. Mater. 2016, 28, 254–262. [Google Scholar] [CrossRef]
- Li, L.L.; Zeng, Q.; Liu, W.J.; Hu, X.F.; Li, Y.; Pan, J.; Wan, D.; Wang, H. Quantitative Analysis of Caspase-1 Activity in Living Cells Through Dynamic Equilibrium of Chlorophyll-Based Nano-assembly Modulated Photoacoustic Signals. ACS Appl. Mater. Interfaces 2016, 8, 17936–17943. [Google Scholar] [CrossRef]
- Li, L.L.; Qiao, S.L.; Liu, W.J.; Ma, Y.; Wan, D.; Pan, J.; Wang, H. Intracellular construction of topology-controlled polypeptide nanostructures with diverse biological functions. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef]
- Lin, Y.X.; Wang, Y.; Qiao, S.L.; An, H.W.; Wang, J.; Ma, Y.; Wang, L.; Wang, H. “In vivo self-assembled” nanoprobes for optimizing autophagy-mediated chemotherapy. Biomaterials 2017, 141, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Fei, Y.; Hu, L.; Huang, Z.; Li, L.L.; Wang, H. Chemotaxis-Instructed Intracellular Staphylococcus aureus Infection Detection by a Targeting and Self-Assembly Signal-Enhanced Photoacoustic Probe. Nano Lett. 2018, 18, 6229–6236. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Gao, Z.J.; Cai, J.Q.; Li, L.L.; Wang, H. Synthesis and Self-Assembly Behavior of Chlorophyll Derivatives for Ratiometric Photoacoustic Signal Optimization. Langmuir 2020. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ma, M.; Yu, D.; Ren, J.; Qu, X. Target-driven supramolecular self-assembly for selective amyloid-β photooxygenation against Alzheimer’s disease. Chem. Sci. 2020, 11, 11003–11008. [Google Scholar] [CrossRef]
- Ren, C.; Wang, Z.; Zhang, X.; Gao, J.; Gao, Y.; Zhang, Y.; Liu, J.; Yang, C.; Liu, J. Construction of all-in-one peptide nanomedicine with photoacoustic imaging guided mild hyperthermia for enhanced cancer chemotherapy. Chem. Eng. J. 2021, 405. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, S.; Chen, J.; Deng, Y.; Luo, Z.; Chen, H.; Hamblin, M.R.; Huang, M. Pentalysine β-carbonylphthalocyanine zinc: An effective tumor-targeting photosensitizer for photodynamic therapy. ChemMedChem 2010, 5, 890–898. [Google Scholar] [CrossRef]
- Fry, H.C.; Garcia, J.M.; Medina, M.J.; Ricoy, U.M.; Gosztola, D.J.; Nikiforov, M.P.; Palmer, L.C.; Stupp, S.I. Self-assembly of highly ordered peptide amphiphile metalloporphyrin arrays. J. Am. Chem. Soc. 2012, 134, 14646–14649. [Google Scholar] [CrossRef]
- Fry, H.C.; Solomon, L.A.; Diroll, B.T.; Liu, Y.; Gosztola, D.J.; Cohn, H.M. Morphological Control of Chromophore Spin State in Zinc Porphyrin-Peptide Assemblies. J. Am. Chem. Soc. 2020, 142, 233–241. [Google Scholar] [CrossRef]
- Solomon, L.A.; Sykes, M.E.; Wu, Y.A.; Schaller, R.D.; Wiederrecht, G.P.; Fry, H.C. Tailorable Exciton Transport in Doped Peptide-Amphiphile Assemblies. ACS Nano 2017, 11, 9112–9118. [Google Scholar] [CrossRef]
- Solomon, L.A.; Wood, A.R.; Sykes, M.E.; Diroll, B.T.; Wiederrecht, G.P.; Schaller, R.D.; Fry, H.C. Microenvironment control of porphyrin binding, organization, and function in peptide nanofiber assemblies. Nanoscale 2019, 11, 5412–5421. [Google Scholar] [CrossRef]
- Solomon, L.A.; Kronenberg, J.B.; Fry, H.C. Control of Heme Coordination and Catalytic Activity by Conformational Changes in Peptide-Amphiphile Assemblies. J. Am. Chem. Soc. 2017, 139, 8497–8507. [Google Scholar] [CrossRef] [PubMed]
- Fry, H.C.; Liu, Y.; Dimitrijevic, N.M.; Rajh, T. Photoinitated charge separation in a hybrid titanium dioxide metalloporphyrin peptide material. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Tao, K.; Jacoby, G.; Burlaka, L.; Beck, R.; Gazit, E. Design of Controllable Bio-Inspired Chiroptic Self-Assemblies. Biomacromolecules 2016, 17, 2937–2945. [Google Scholar] [CrossRef] [PubMed]
- Nikoloudakis, E.; Mitropoulou, K.; Landrou, G.; Charalambidis, G.; Nikolaou, V.; Mitraki, A.; Coutsolelos, A.G. Self-assembly of aliphatic dipeptides coupled with porphyrin and BODIPY chromophores. Chem. Commun. 2019, 55, 14103–14106. [Google Scholar] [CrossRef] [PubMed]
- Wijerathne, N.K.; Kumar, M.; Ulijn, R.V. Fmoc-Dipeptide/Porphyrin Molar Ratio Dictates Energy Transfer Efficiency in Nanostructures Produced by Biocatalytic Co-Assembly. Chem. A Eur. J. 2019, 25, 11847–11851. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Wang, A.; Ren, P.; Wang, M.; Dong, Q.; Li, J.; Bai, S. Self-Assembled Peptide Hydrogel With Porphyrin as a Dopant for Enhanced Photocurrent Generation. Colloids Interface Sci. Commun. 2018, 23, 29–33. [Google Scholar] [CrossRef]
- Li, J.; Wang, A.; Zhao, L.; Dong, Q.; Wang, M.; Xu, H.; Yan, X.; Bai, S. Self-Assembly of Monomeric Hydrophobic Photosensitizers with Short Peptides Forming Photodynamic Nanoparticles with Real-Time Tracking Property and without the Need of Release in Vivo. ACS Appl. Mater. Interfaces 2018, 10, 28420–28427. [Google Scholar] [CrossRef]
- Li, J.; Wang, A.; Ren, P.; Yan, X.; Bai, S. One-step co-assembly method to fabricate photosensitive peptide nanoparticles for two-photon photodynamic therapy. Chem. Commun. 2019, 55, 3191–3194. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zou, Q.; Li, Y.; Yuan, C.; Xing, R.; Yan, X. Smart Peptide-Based Supramolecular Photodynamic Metallo-Nanodrugs Designed by Multicomponent Coordination Self-Assembly. J. Am. Chem. Soc. 2018, 140, 10794–10802. [Google Scholar] [CrossRef] [PubMed]
- Xiu, Y.; Zhang, X.; Feng, Y.; Wei, R.; Wang, S.; Xia, Y.; Cao, M.; Wang, S. Peptide-mediated porphyrin based hierarchical complexes for light-to-chemical conversion. Nanoscale 2020, 12, 15201–15208. [Google Scholar] [CrossRef] [PubMed]
- Xiu, Y.; Xu, L.; Zhang, X.; Wang, X.; Liu, F.; Xia, Y.; Cao, M.; Wang, S. Mechanistic Process Understanding of the Biomimetic Construction of Porphyrin-Based Light-Capturing Antennas from Self-Assembled Fmoc-Blocked Peptide Templates. ACS Sustain. Chem. Eng. 2020, 8, 15761–15771. [Google Scholar] [CrossRef]
- Li, Y.; Wong, R.C.H.; Yan, X.; Ng, D.K.P.; Lo, P.C. Self-Assembled Nanophotosensitizing Systems with Zinc(II) Phthalocyanine-Peptide Conjugates as Building Blocks for Targeted Chemo-Photodynamic Therapy. ACS Appl. Bio Mater. 2020, 3, 5463–5473. [Google Scholar] [CrossRef]
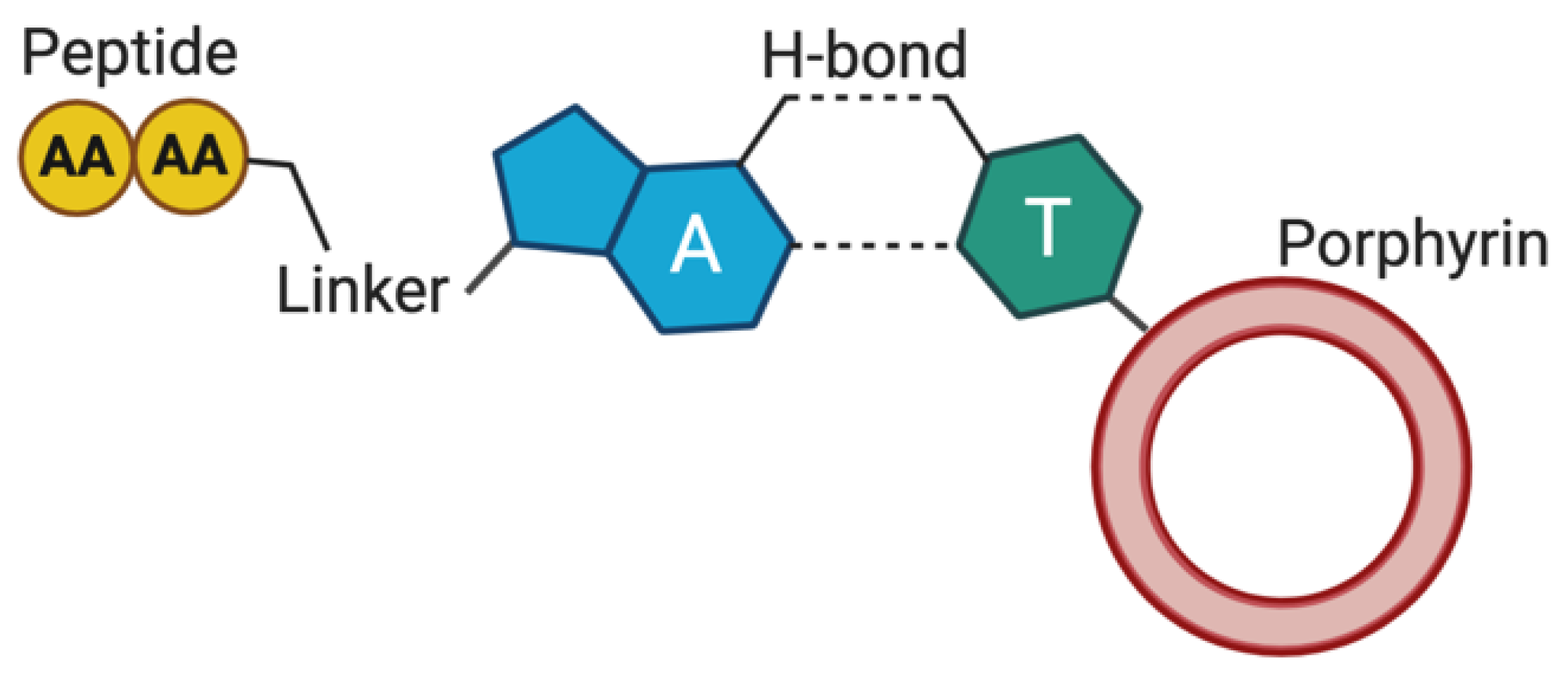
- Marafon, G.; Menegazzo, I.; De Zotti, M.; Crisma, M.; Toniolo, C.; Moretto, A. Tuning morphological architectures generated through living supramolecular assembly of a helical foldamer end-capped with two complementary nucleobases. Soft Matter 2017, 13, 4231–4240. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Tashiro, K.; Takei, T.; Yamamoto, Y. Controlled self-assembly of oligopeptides bearing electron donor and acceptor units on the side chains to form β-sheets with selective π-stacking configuration. Chem. Lett. 2017, 46, 423–425. [Google Scholar] [CrossRef]
- Hasobe, T.; Kamat, P.V.; Troiani, V.; Solladié, N.; Ahn, T.K.; Kim, S.K.; Kim, D.; Kongkanand, A.; Kuwabata, S.; Fukuzumi, S. Enhancement of light-energy conversion efficiency by multi-porphyrin arrays of porphyrin-peptide oligomers with fullerene clusters. J. Phys. Chem. B 2005, 109, 19–23. [Google Scholar] [CrossRef]
- Hasobe, T.; Saito, K.; Kamat, P.V.; Troiani, V.; Qiu, H.; Solladié, N.; Kim, K.S.; Park, J.K.; Kim, D.; D’Souza, F.; et al. Organic solar cells. Supramolecular composites of porphyrins and fullerenes organized by polypeptide structures as light harvesters. J. Mater. Chem. 2007, 17, 4160–4170. [Google Scholar] [CrossRef]
- Nikoloudakis, E.; Karikis, K.; Han, J.; Kokotidou, C.; Charisiadis, A.; Folias, F.; Douvas, A.M.; Mitraki, A.; Charalambidis, G.; Yan, X.; et al. A self-assembly study of PNA-porphyrin and PNA-BODIPY hybrids in mixed solvent systems. Nanoscale 2019, 11, 3557–3566. [Google Scholar] [CrossRef]
- Solladie, N.; Hamel, A.; Gross, M. Synthesis of multiporphyrinic α-polypeptides: Towards the study of the migration of an excited state for the mimicking of the natural light harvesting device. Tetrahedron Lett. 2000, 41, 6075–6078. [Google Scholar] [CrossRef]
- Hernández-Eguía, L.P.; Brea, R.J.; Castedo, L.; Ballester, P.; Granja, J.R. Regioisomeric control induced by DABCO coordination to rotatable self-assembled bis-and tetraporphyrin α,γ-Cyclic octapeptide dimers. Chem. A Eur. J. 2011, 17, 1220–1229. [Google Scholar] [CrossRef]
- Aragay, G.; Ventura, B.; Guerra, A.; Pintre, I.; Chiorboli, C.; García-Fandiño, R.; Flamigni, L.; Granja, J.R.; Ballester, P. Self-sorting of cyclic peptide homodimers into a heterodimeric assembly featuring an efficient photoinduced intramolecular electron-transfer process. Chem. A Eur. J. 2014, 20, 3427–3438. [Google Scholar] [CrossRef]
- Ozores, H.L.; Amorín, M.; Granja, J.R. Self-assembling molecular capsules based on α,γ-cyclic peptides. J. Am. Chem. Soc. 2017, 139, 776–784. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dognini, P.; Coxon, C.R.; Alves, W.A.; Giuntini, F. Peptide-Tetrapyrrole Supramolecular Self-Assemblies: State of the Art. Molecules 2021, 26, 693. https://doi.org/10.3390/molecules26030693
Dognini P, Coxon CR, Alves WA, Giuntini F. Peptide-Tetrapyrrole Supramolecular Self-Assemblies: State of the Art. Molecules. 2021; 26(3):693. https://doi.org/10.3390/molecules26030693
Chicago/Turabian StyleDognini, Paolo, Christopher R. Coxon, Wendel A. Alves, and Francesca Giuntini. 2021. "Peptide-Tetrapyrrole Supramolecular Self-Assemblies: State of the Art" Molecules 26, no. 3: 693. https://doi.org/10.3390/molecules26030693
APA StyleDognini, P., Coxon, C. R., Alves, W. A., & Giuntini, F. (2021). Peptide-Tetrapyrrole Supramolecular Self-Assemblies: State of the Art. Molecules, 26(3), 693. https://doi.org/10.3390/molecules26030693