Discussions of Fluorescence in Selenium Chemistry: Recently Reported Probes, Particles, and a Clearer Biological Knowledge †
Abstract
1. Introduction
1.1. Se in Biochemistry—New Findings Regarding Selenium in Biology and the Environment
1.2. Biology and Disease
1.3. Environment
2. Selenium as Analyte
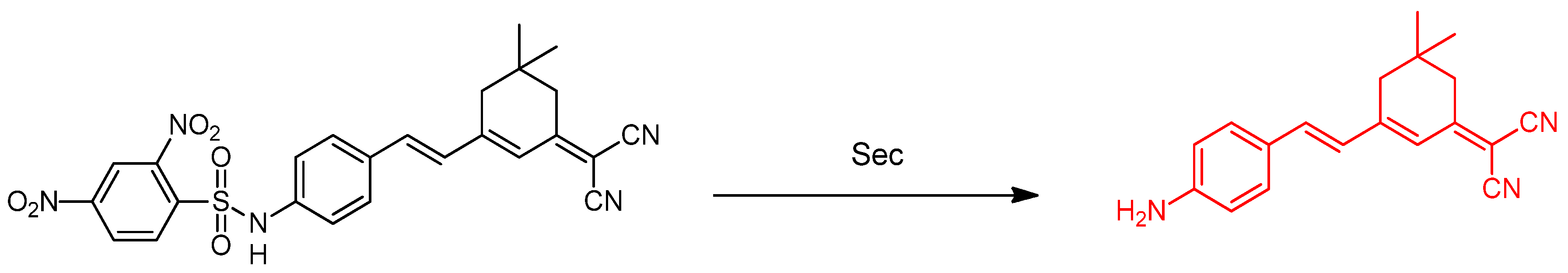
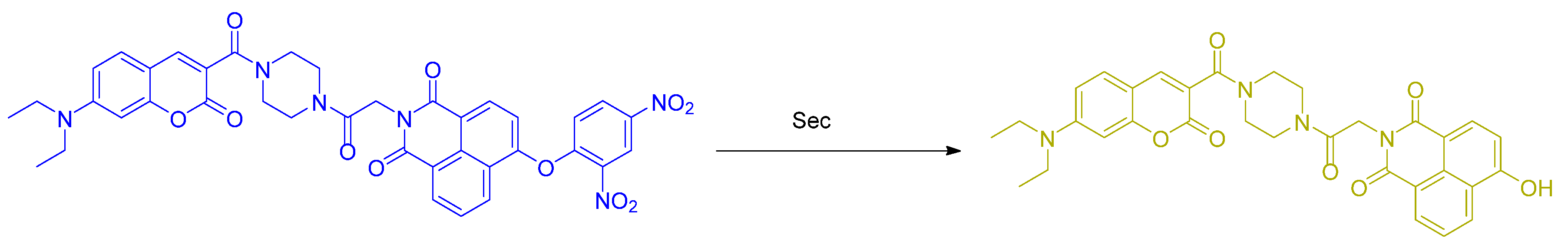
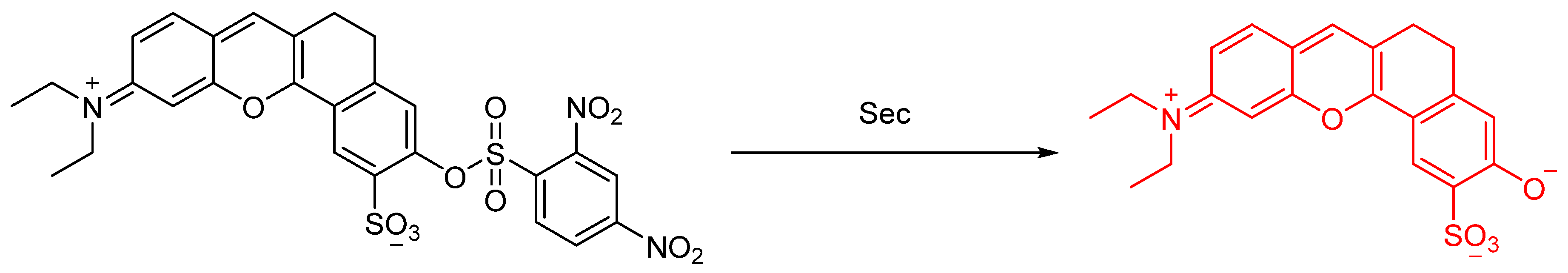
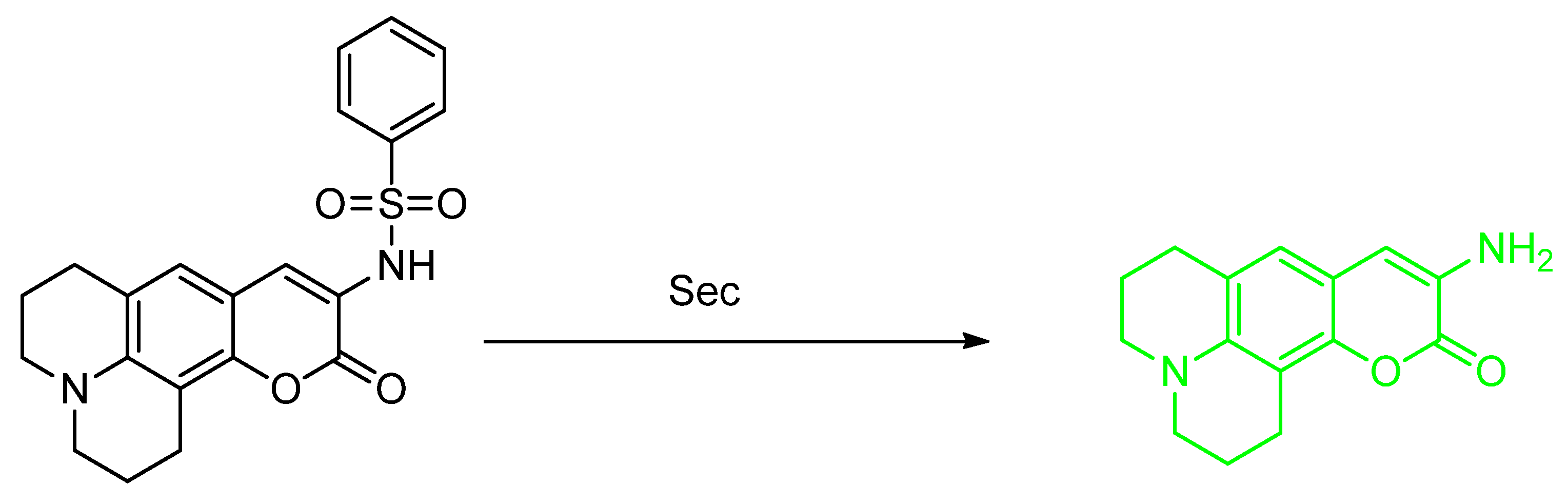
2.1. Selenocysteine (Sec)
2.2. Selenium in Therapeutics, Medicinal Chemistry and Skeleton Cores
3. Selenium as an Active Site Constituent of Small Molecule Chemosensors
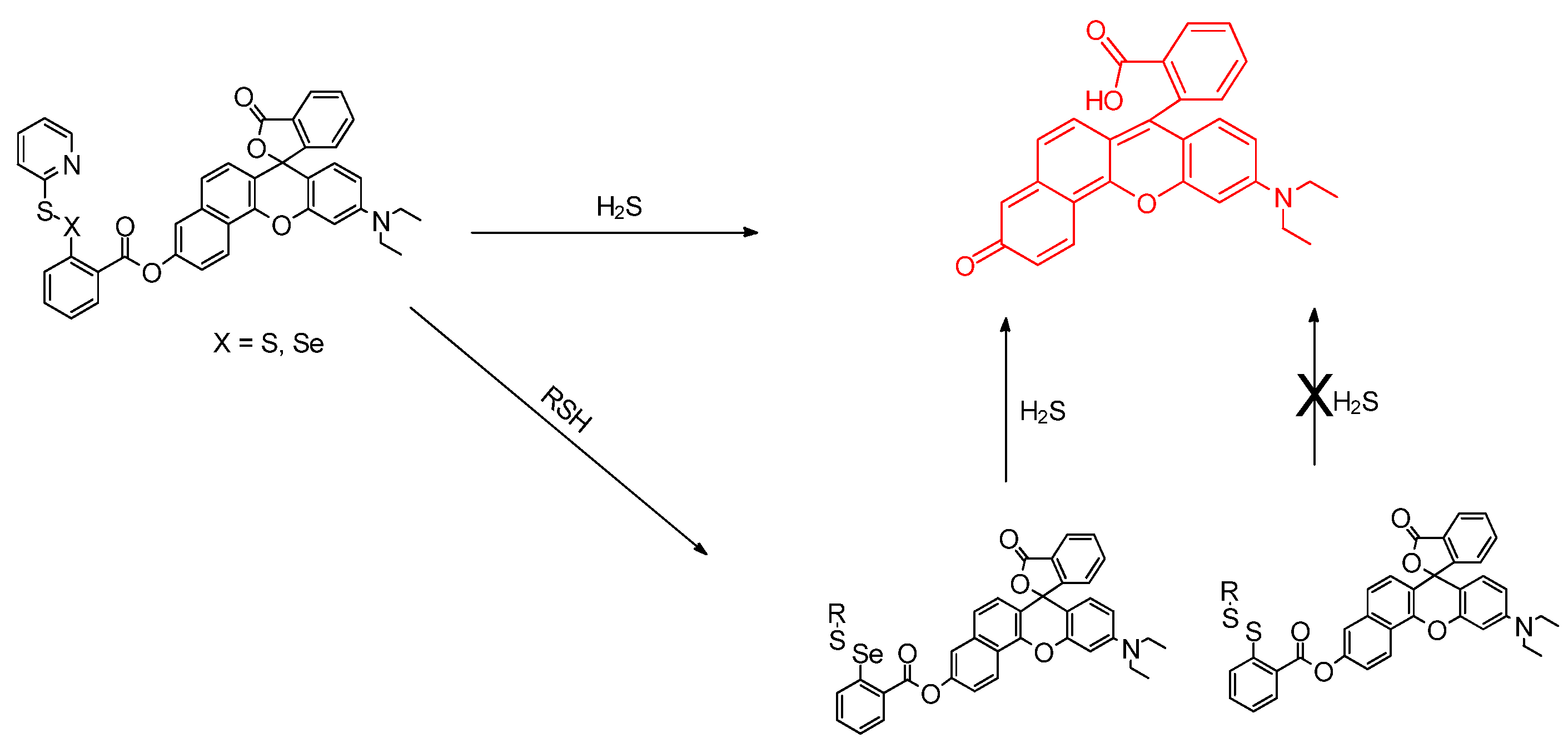
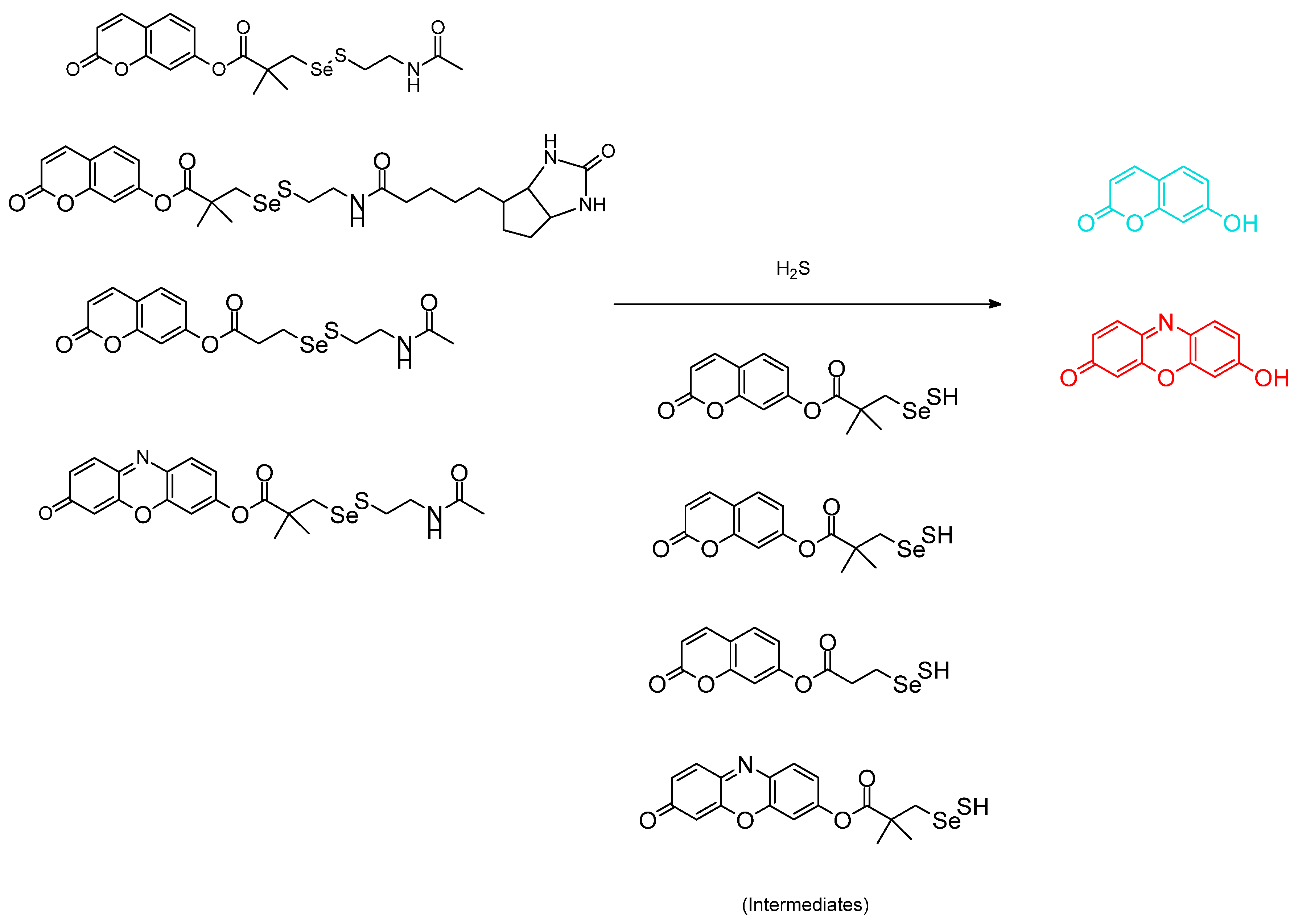
3.1. H2S, Sulfane, Biothiols, Glutathione, and Anions as Analytes
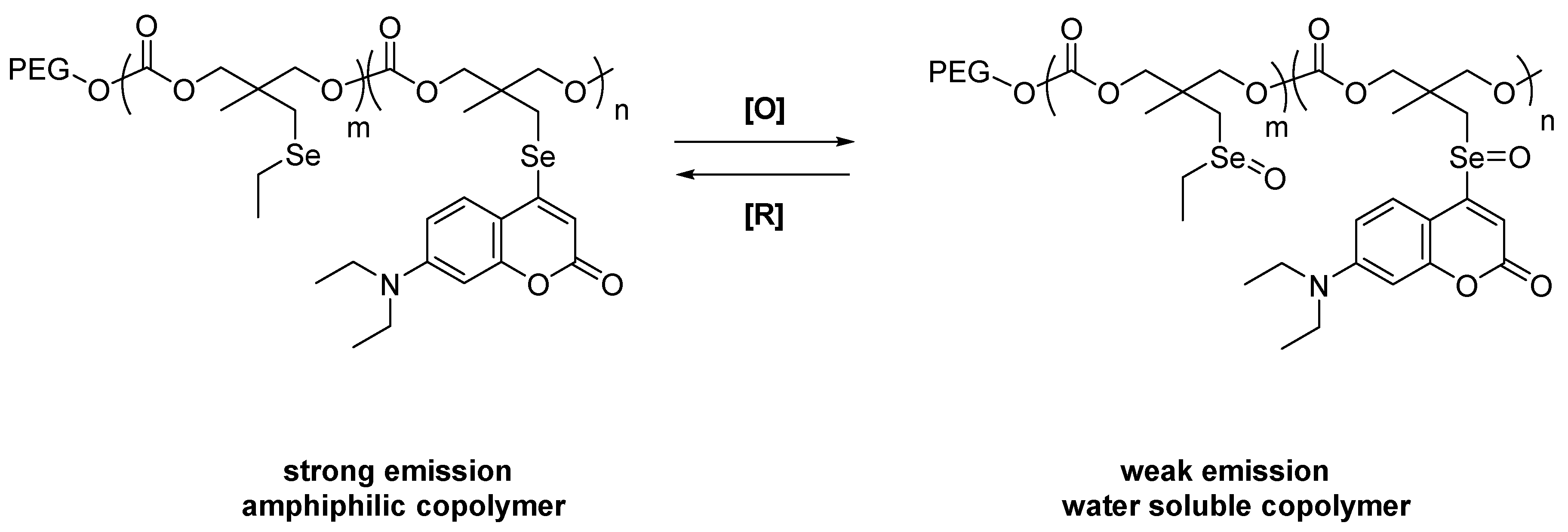
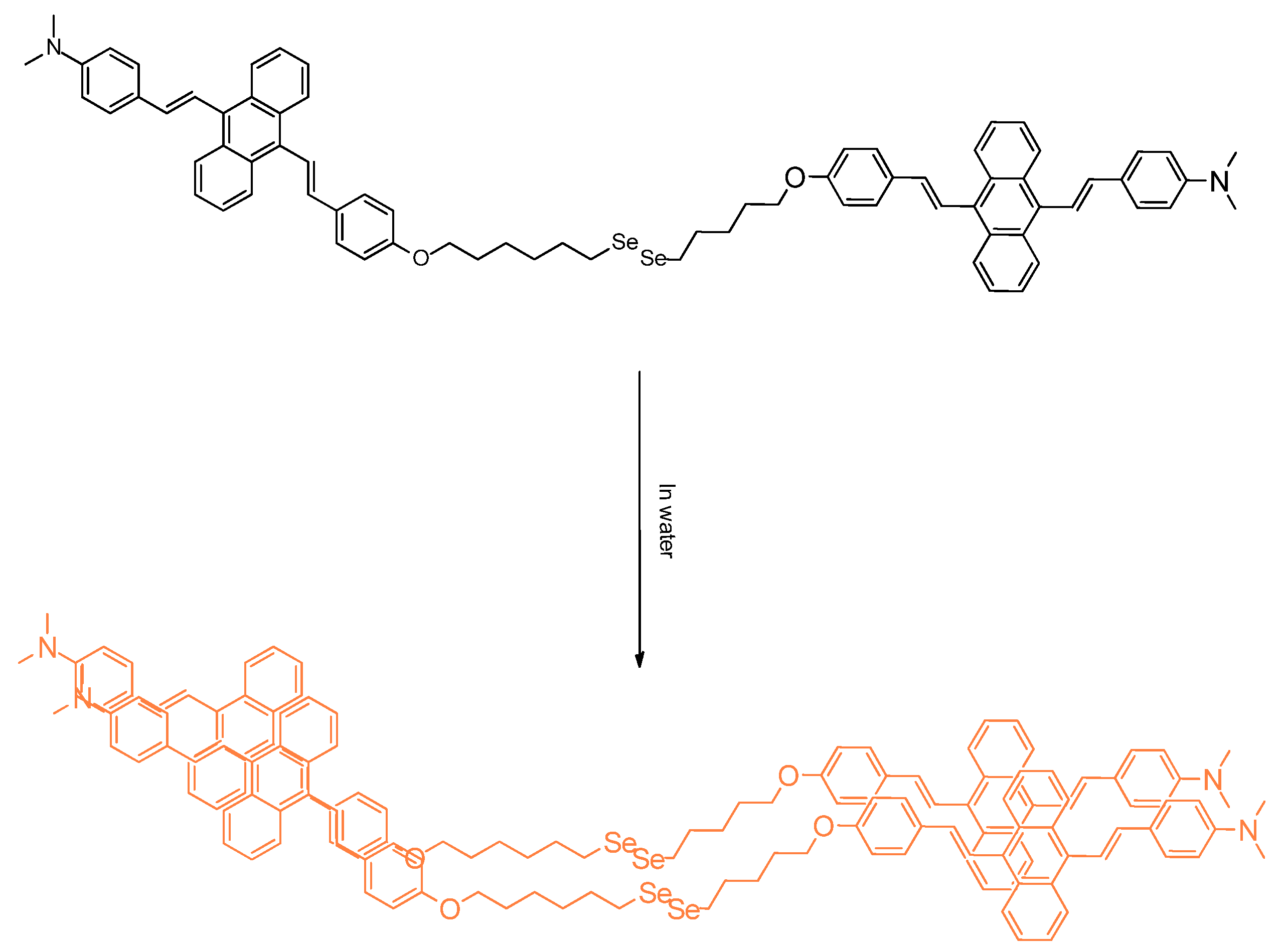
3.2. Free Radicals and ROS
4. Analytes Containing Selenium
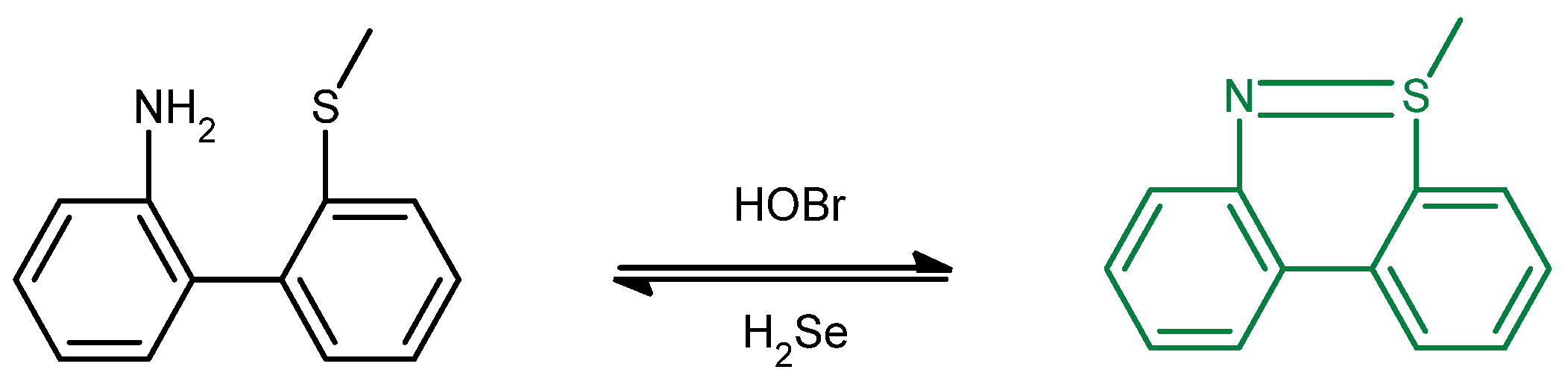
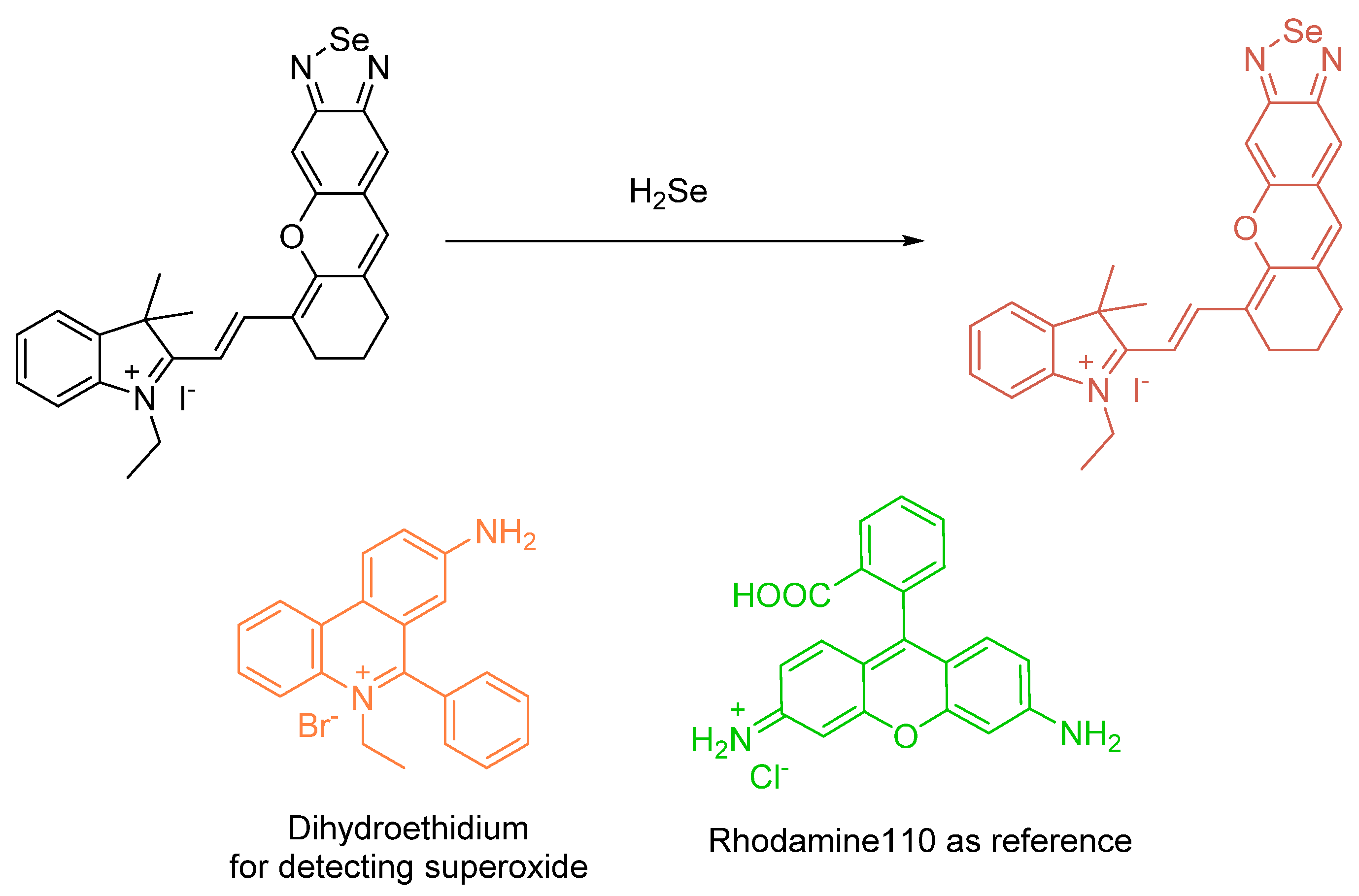
Reduced Organic Selenium (Selenocysteine) (Biological)) e.g., H2Se
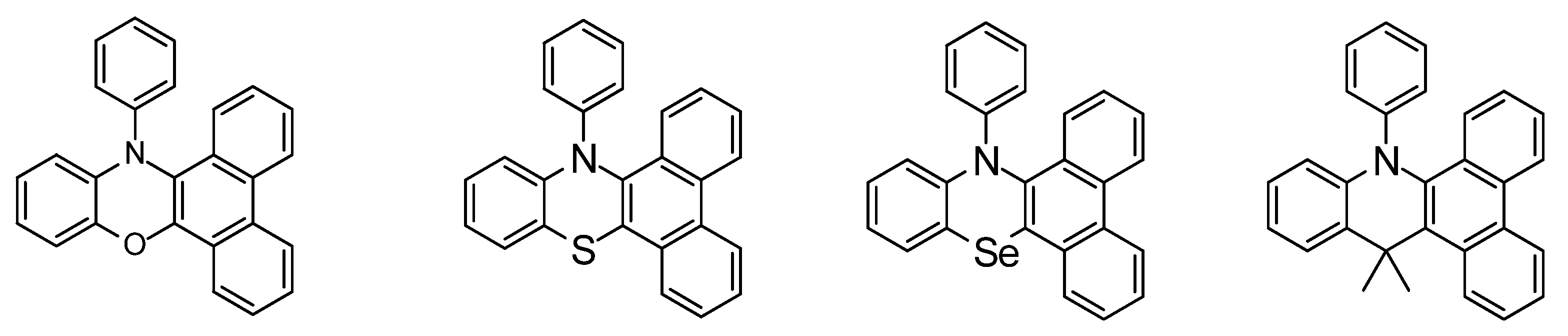
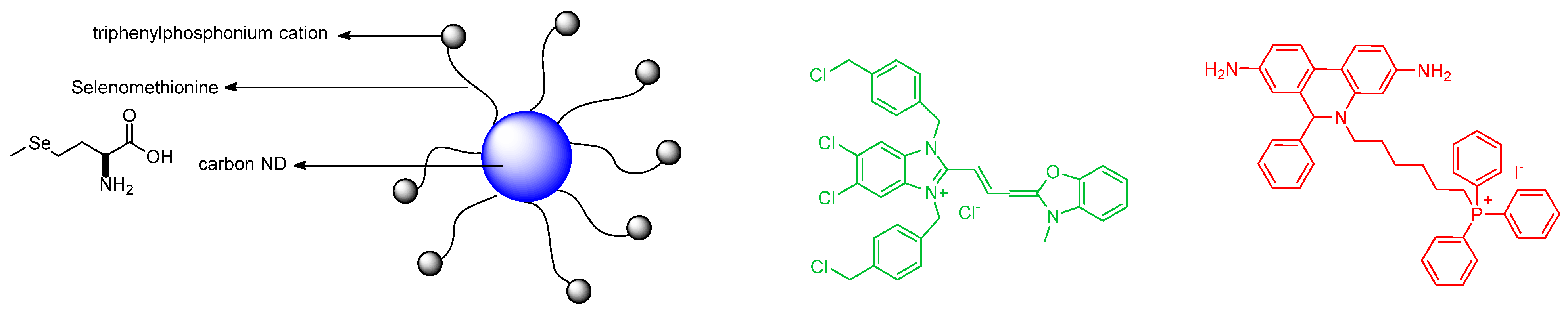
5. Selenium in Physics, Surfaces, and Nanoscience with Regard to Sensing and Fluorescence
6. Catalyst Poisons
7. Solar Conversions Related to Probing
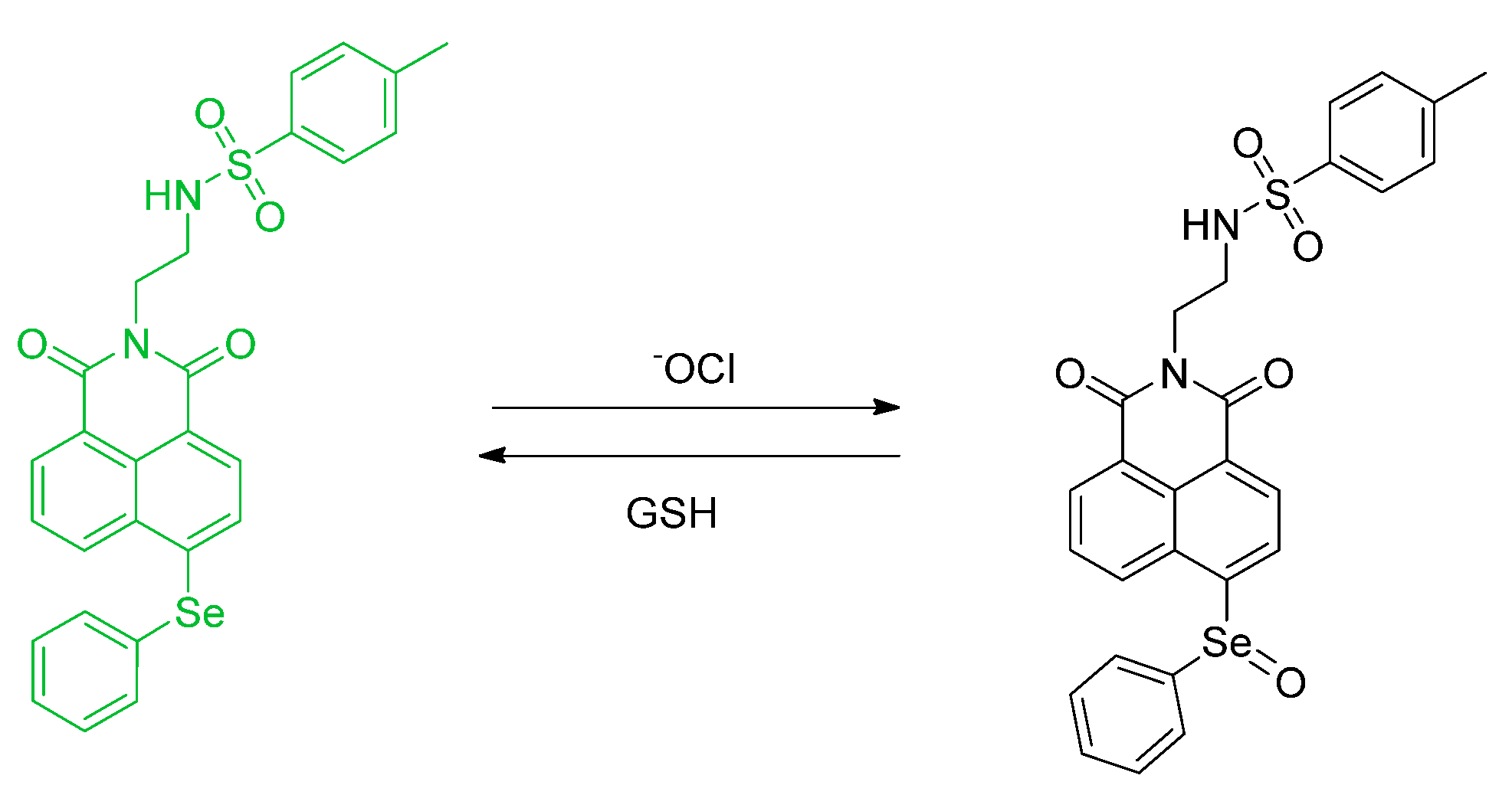
8. A Recent Report about Reductants and ROS in the Context of Probing and Fluorescence
9. Future Outlook and Suggestions
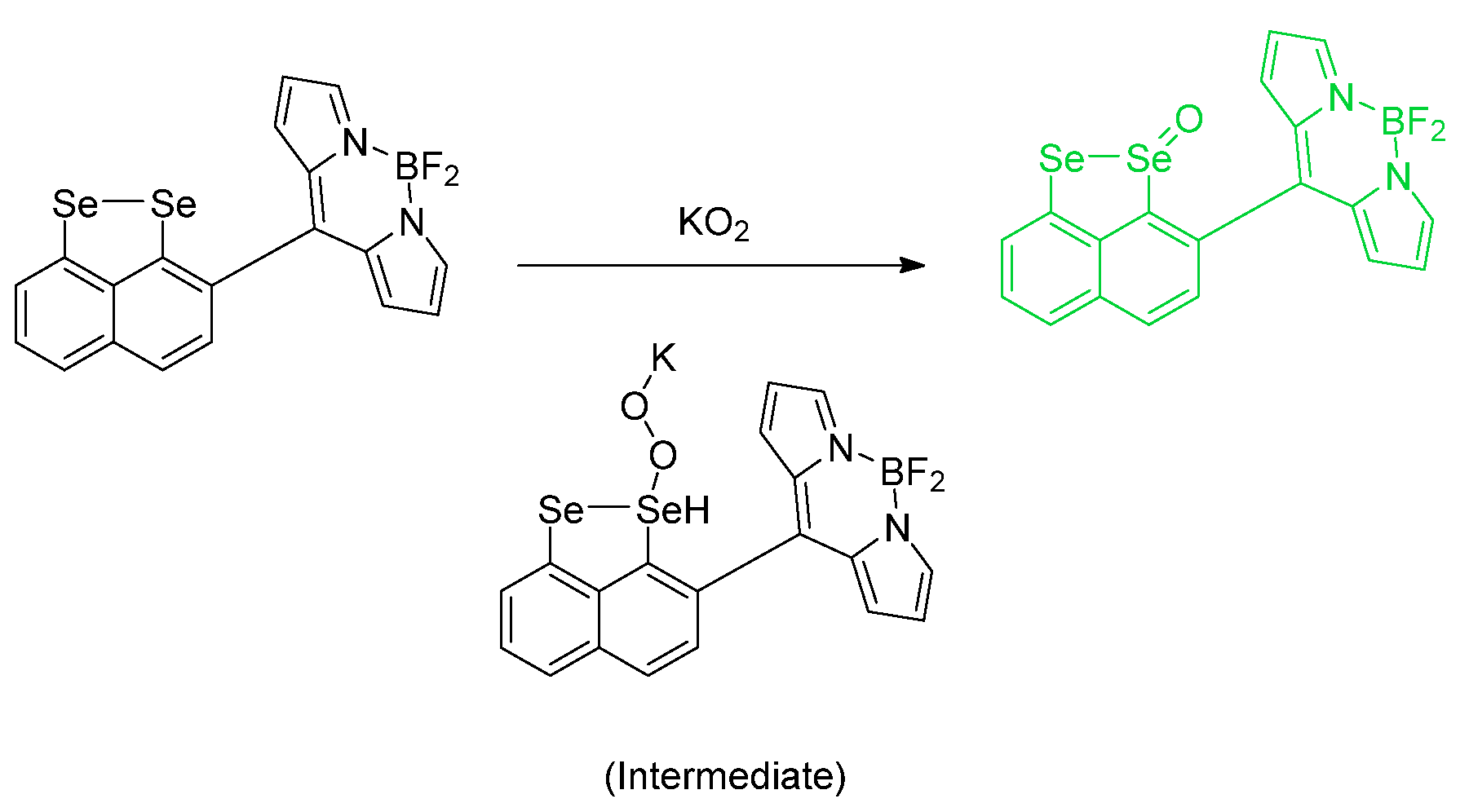
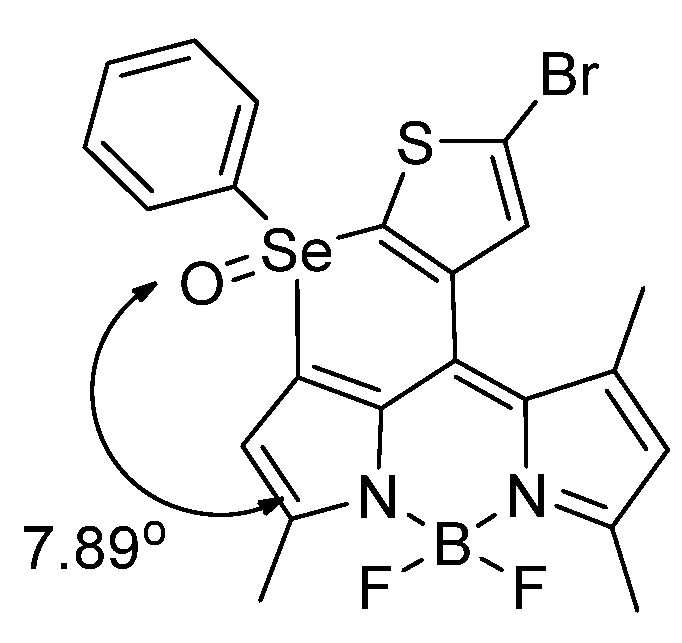
- Relevant to reference [50], it would be important to consider mixed systems in which we have the presence of TeSe or SeTe bond.
- Pertaining to reference [50], the sterics of the fluorophore near the active site can be tuned, to allow for one of the two atoms to undergo reaction first, and to explore bot the kinetics and steric considerations.
- Relevant to [62], we seek to determine whether the structure is the same in solution. Might it have a chance to dissociate and reassociate? If so, how would that help extend the concept of such probes?
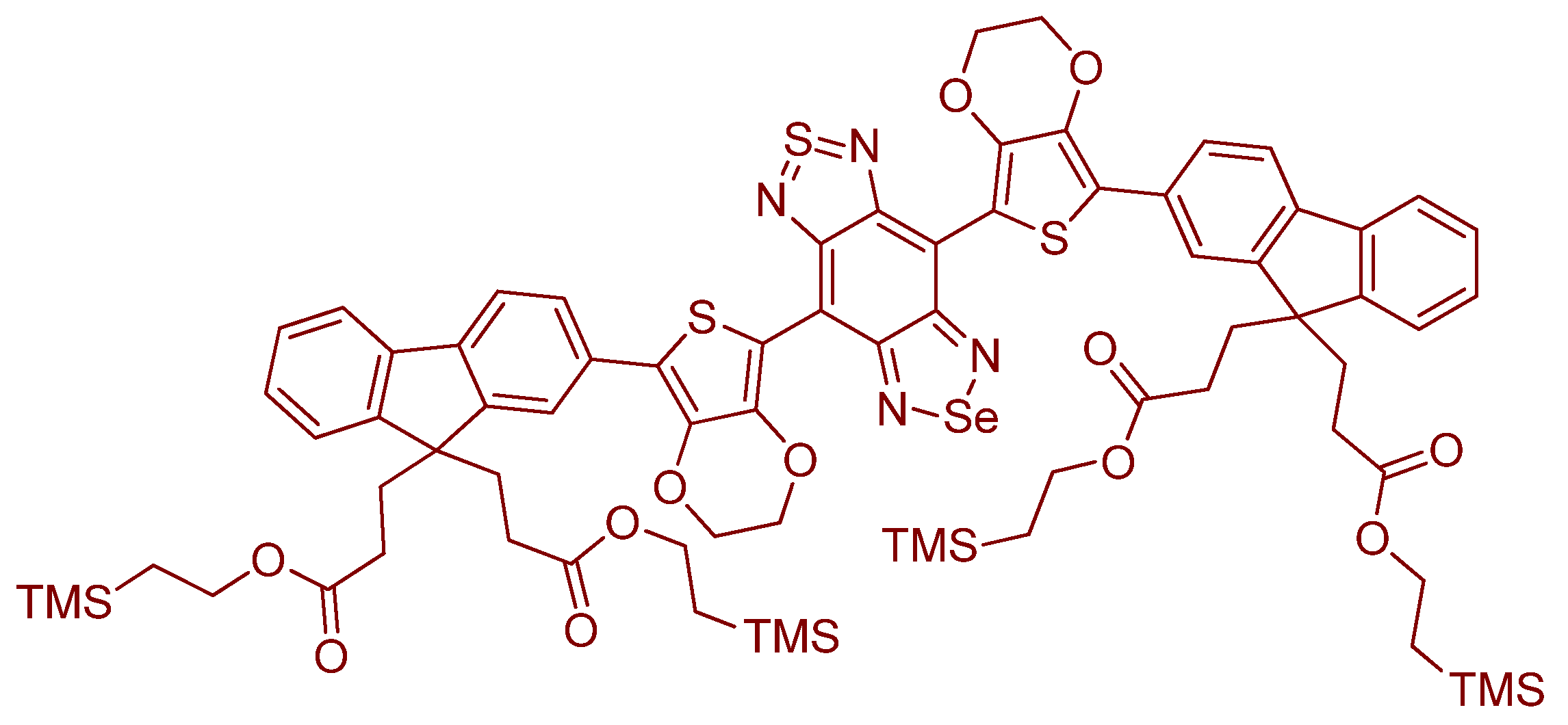
- Pertaining to reference [173], it would be important to consider the synthesis and study of a more expanded π–system version of this probe. In this way, we could have e.g., a NIR version of this (exact) system for use (comparison) with the same analytes and under the same conditions [141]. In terms of particles and their surfaces, there is a little information as to the inertness of SeO32− and SeO42− for example in not interfering with the so-called nanozymes.
- There may be much more interplay between small molecules and particles than we are currently aware of in current probing and therefore many more investigations are necessary [174].
- Further understanding of the fundamental chemistry/biochemistry of RSeSRs and RSeSH systems are required [18]. This will help us understand what all of their possible intracellular targets are, and to what extent they impact signaling. Besides antioxidant regeneration and peroxide radical reduction, the roles of RSeSR and RSeSHs in other systems need to be further explored.
- We sometimes simplistically say we cannot have ROS without the involvement of metals or irradiation. What about this state of the art holds true for RSSs? Also, that we produce ROS when we biology with light energy is important to added.
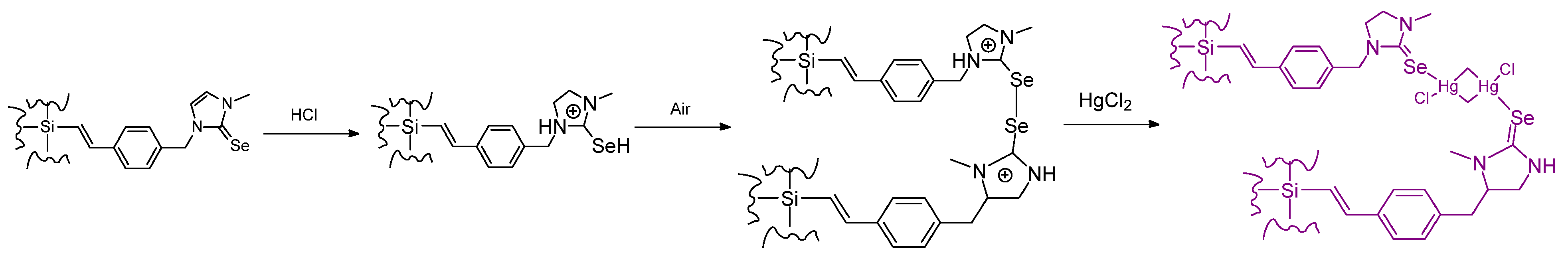
- There are naturally more open questions to explore [146] with the dynamics of the “belt” positions; but whether it is possible to ever go from a “belt” position to a “cubane” frame position remains to be seen. It is interesting that KSeCN can supply the Se to the cofactor; with this simple reagent, more can be discovered quickly.
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hoover, G.C.; Seferos, D.S. Photoactivity and optical applications of organic materials containing selenium and tellurium. Chem. Sci. 2019, 10, 9182–9188. [Google Scholar] [CrossRef] [PubMed]
- Seçkin, H.Y.; Kalkan, G.; Bütün, I.; Akbaş, A.; Baş, Y.; Karakuş, N.; Benli, I. Analysis of Manganese Superoxide Dismutase and Glutathione Peroxidase 1 Gene Polymorphisms in Vitiligo. Biochem. Genet. 2016, 54, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Chen, L.; Kwon, N.; Yoon, J. Fluorescent Probes Containing Selenium as a Guest or Host. Chem 2016, 1, 674–698. [Google Scholar] [CrossRef]
- Xiao, Y.; Xu, S.; Zhao, S.; Liu, K.; Lu, Z.; Hou, Z. Protective effects of selenium against zearalenone-induced apoptosis in chicken spleen lymphocyte via an endoplasmic reticulum stress signaling pathway. Cell Stress Chaperon. 2019, 24, 77–89. [Google Scholar] [CrossRef]
- Hussain, R.; Luo, K. Geochemical valuation and intake of F, As, and Se in coal wastes contaminated areas and their potential impacts on local inhabitants, Shaanxi China. Environ. Geochem. Health 2018, 40, 2667–2683. [Google Scholar] [CrossRef]
- Kharma, A.; Grman, M.; Misak, A.; Domínguez-Álvarez, E.; Nasim, M.J.; Ondrias, K.; Chovanec, M.; Jacob, C. Inorganic Polysulfides and Related Reactive Sulfur–Selenium Species from the Perspective of Chemistry. Molecules 2019, 24, 1359. [Google Scholar] [CrossRef]
- Thomas, R.K.; Sukumaran, S.; Sudarsanakumar, C. An insight into the comparative binding affinities of chlorogenic acid functionalized gold and silver nanoparticles with ctDNA along with its cytotoxicity analysis. J. Mol. Liq. 2019, 287, 110911. [Google Scholar] [CrossRef]
- Mitra, M.; Ahamed, S.T.; Ghosh, A.; Mondal, A.; Kargupta, K.; Ganguly, S.; Banerjee, D. Polyaniline/Reduced Graphene Oxide Composite-Enhanced Visible-Light-Driven Photocatalytic Activity for the Degradation of Organic Dyes. ACS Omega 2019, 4, 1623–1635. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Q.; Liu, X.; Zhang, J.; Yang, X.-F.; Li, Z.; Li, H. Sensitive and Selective Fluorescent Probe for Selenol in Living Cells Designed via a pKa Shift Strategy. Anal. Chem. 2018, 90, 4119–4125. [Google Scholar] [CrossRef]
- Xu, C.; Qian, Y.; Qi, Z.; Lu, C.-G.; Cui, Y. A conjugated BODIPY–triphenylamine multi-aldoxime: Sonogashira coupling, ratiometric chemodosimeter and rapid detection of hypochlorite with two-photon excited fluorescence. New J. Chem. 2018, 42, 6910–6917. [Google Scholar] [CrossRef]
- Xiao, Z.; Li, S.; Guo, J.; Rao, Z.; Liu, C.; Jia, Z.; Suo, D.; Wang, S.; Li, Y.; Fan, X. Application of enzymatic probe sonication for selenium speciation in animal feeds. J. Chromatogr. A 2017, 1530, 51–58. [Google Scholar] [CrossRef] [PubMed]
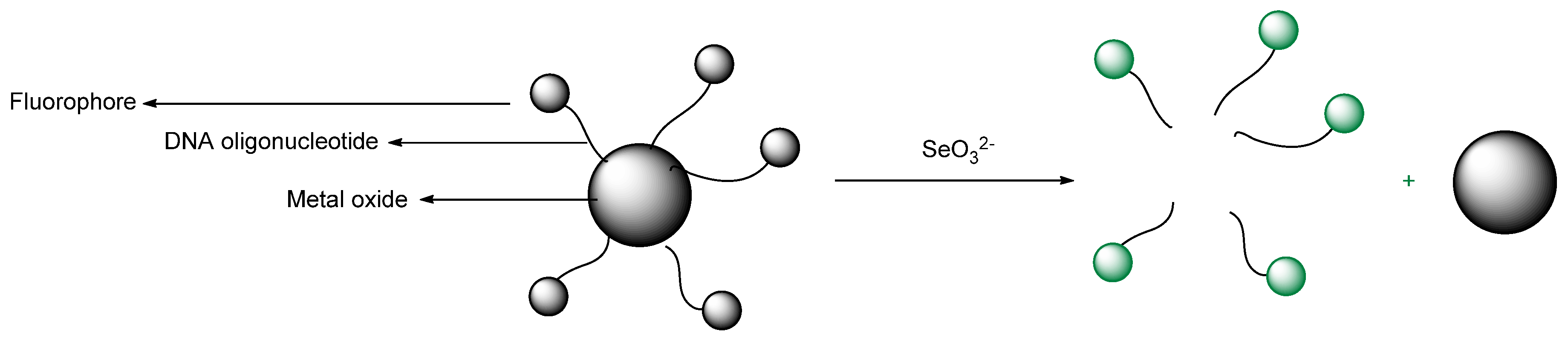
- Yu, T.; Liu, B. Adsorption of Selenite and Selenate by Metal Oxides Studied with Fluorescent DNA Probes for Analytical Application. J. Anal. Test. 2017, 1, 1–7. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, P.; Dyba, M.; Sousa, R.; Stagno, J.R.; Wang, Y.-X. Applications of PLOR in labeling large RNAs at specific sites. Methods 2016, 103, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Förster, H.-J.; Bindi, L.; Stanley, C.J. Grundmannite, CuBiSe2, the Se-analogue of emplectite, a new mineral from the El Dragón mine, Potosí, Bolivia. Eur. J. Miner. 2016, 28, 467–477. [Google Scholar] [CrossRef]
- Alker, W.; Schwerdtle, T.; Schomburg, L.; Haase, H. A Zinpyr-1-based Fluorimetric Microassay for Free Zinc in Human Serum. Int. J. Mol. Sci. 2019, 20, 4006. [Google Scholar] [CrossRef]
- Chen, P.; Wang, L.-X.; Sui, X.-J.; Li, S.-M.; Wang, Y.; Liu, Q.; Ni, J.-Z. Comparative Serum Proteomic Analysis of the Effects of Sodium Selenate on a Mouse Model of Alzheimer’s Disease. Biol. Trace Elem. Res. 2019, 192, 263–276. [Google Scholar] [CrossRef]
- Piagentini, M.; Silva, D.; Dell’Aqua, C.; Moya-Araujo, C.; Codognoto, V.; Ramos, A.; Oba, E. Effect of selenium supplementation on semen characteristics of Brazil’s ram. Reprod. Domest. Anim. 2017, 52, 355–358. [Google Scholar] [CrossRef]
- Hamsath, A.; Xian, M. Chemistry and Chemical Biology of Selenenyl Sulfides and Thioseleninic Acids. Antioxid. Redox Signal. 2020, 33, 1143–1157. [Google Scholar] [CrossRef]
- Engilberge, S.; Riobé, F.; Di Pietro, S.; Lassalle, L.; Coquelle, N.; Arnaud, C.-A.; Pitrat, D.; Mulatier, J.-C.; Madern, D.; Breyton, C.; et al. Crystallophore: A versatile lanthanide complex for protein crystallography combining nucleating effects, phasing properties, and luminescence. Chem. Sci. 2017, 8, 5909–5917. [Google Scholar] [CrossRef]
- Nuthanakanti, A.; Boerneke, M.A.; Hermann, T.; Srivatsan, S.G. Structure of the Ribosomal RNA Decoding Site Containing a Selenium-Modified Responsive Fluorescent Ribonucleoside Probe. Angew. Chem. Int. Ed. 2017, 56, 2640–2644. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, X.; Yu, Y.; Zhao, Q.; Tang, C.; Zhang, J. A review of bioselenol-specific fluorescent probes: Synthesis, properties, and imaging applications. Anal. Chim. Acta 2020, 1110, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Devi, P.; Jain, R.; Thakur, A.; Kumar, M.; Labhsetwar, N.K.; Nayak, M.; Kumar, P. A systematic review and meta-analysis of voltammetric and optical techniques for inorganic selenium determination in water. TrAC Trends Anal. Chem. 2017, 95, 69–85. [Google Scholar] [CrossRef]
- Dolgova, N.V.; Nehzati, S.; Choudhury, S.; Macdonald, T.C.; Regnier, N.R.; Crawford, A.M.; Ponomarenko, O.; George, G.N.; Pickering, I.J. X-ray spectroscopy and imaging of selenium in living systems. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2383–2392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Kai, X.; Zhang, Y.; Zheng, Y.; Xue, Y.; Yin, X.; Zhao, J. A reaction-based near-infrared fluorescent probe that can visualize endogenous selenocysteine in vivo in tumor-bearing mice. Analyst 2018, 143, 4860–4869. [Google Scholar] [CrossRef]
- Han, X.; Wang, R.; Song, X.; Yu, F.; Chen, L. Evaluation Selenocysteine Protective Effect in Carbon Disulfide Induced Hepatitis with a Mitochondrial Targeting Ratiometric Near-Infrared Fluorescent Probe. Anal. Chem. 2018, 90, 8108–8115. [Google Scholar] [CrossRef]
- Pan, Z.; Zhang, J.; Ji, K.; Chittavong, V.; Ji, X.; Wang, B. Organic CO Prodrugs Activated by Endogenous ROS. Org. Lett. 2018, 20, 8–11. [Google Scholar] [CrossRef]
- Meng, F.; Chen, Y.-A.; Chen, C.-L.; Chou, P. Syntheses and Excited-State Intramolecular Proton Transfer of 3-Hydroxythioflavone and Its Sulfone Analogue. ChemPhotoChem 2018, 2, 475–480. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Deng, J.; Huang, Y.; Pan, Z.; Yu, Y.; Cao, H. Electrochemical diselenylation of indolizines via intermolecular C–Se formation with 2-methylpyridines, α-bromoketones and diselenides. Chem. Commun. 2020, 56, 735–738. [Google Scholar] [CrossRef]
- Chen, Y.-A.; Chen, D.; Wu, C.; Chang, K.; Meng, F.; Chen, M.; Lin, J.; Huang, C.; Su, J.; Tian, H.; et al. Mono-Heteroatom Substitution for Harnessing Excited-State Structural Planarization of Dihydrodibenzo[a,c]phenazines. Chem. A Eur. J. 2019, 25, 16755–16764. [Google Scholar] [CrossRef]
- Da Silva, R.B.; Coelho, F.L.; Rodembusch, F.S.; Schwab, R.S.; Schneider, J.M.F.M.; Rampon, D.S.; Schneider, P.H. Straightforward synthesis of photoactive chalcogen functionalized benzimidazo[1,2-a]quinolines. New J. Chem. 2019, 43, 11596–11603. [Google Scholar] [CrossRef]
- Yu, L.; Ke, H.-L.; Du, F.-S.; Li, Z.-C. Redox-Responsive Fluorescent Polycarbonates Based on Selenide for Chemotherapy of Triple-Negative Breast Cancer. Biomacromolecules 2019, 20, 2809–2820. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Du, Y.; Liang, Q.; Cheng, Z.; Tian, J. A selenium-containing selective histone deacetylase 6 inhibitor for targeted in vivo breast tumor imaging and therapy. J. Mater. Chem. B 2019, 7, 3528–3536. [Google Scholar] [CrossRef]
- Soares, A.T.G.; Rodrigues, L.B.L.; Salgueiro, W.; Dal Forno, A.H.D.C.; Rodrigues, C.F.; Sacramento, M.; Franco, J.L.; Alves, D.; Oliveira, R.D.P.; Pinton, S.; et al. Organoselenotriazoles attenuate oxidative damage induced by mitochondrial dysfunction in mev-1 Caenorhabditis elegans mutants. J. Trace Elements Med. Biol. 2019, 53, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Ferrell, A.J.; Chen, W.; Wang, D.; Xian, M. Cyclic Acyl Disulfides and Acyl Selenylsulfides as the Precursors for Persulfides (RSSH), Selenylsulfides (RSeSH), and Hydrogen Sulfide (H2S). Org. Lett. 2018, 20, 852–855. [Google Scholar] [CrossRef]
- Ecker, A.; Ledur, P.C.; Da Silva, R.S.; Leal, D.B.R.; Rodrigues, O.E.D.; Ardisson-Araújo, D.; Waczuk, E.P.; Da Rocha, J.B.T.; Barbosa, N.B.D.V. Chalcogenozidovudine Derivatives with Antitumor Activity: Comparative Toxicities in Cultured Human Mononuclear Cells. Toxicol. Sci. 2017, 160, 30–46. [Google Scholar] [CrossRef]
- Domracheva, I.; Kanepe-Lapsa, I.; Jackevica, L.; Vasiljeva, J.; Arsenyan, P. Selenopheno quinolinones and coumarins promote cancer cell apoptosis by ROS depletion and caspase-7 activation. Life Sci. 2017, 186, 92–101. [Google Scholar] [CrossRef]
- Cheignon, C.; Cordeau, E.; Prache, N.; Cantel, S.; Martinez, J.; Subra, G.; Arnaudguilhem, C.; Bouyssiere, B.; Enjalbal, C. Receptor–Ligand Interaction Measured by Inductively Coupled Plasma Mass Spectrometry and Selenium Labeling. J. Med. Chem. 2018, 61, 10173–10184. [Google Scholar] [CrossRef]
- Wu, W.; Yang, Y.; Yang, Y.; Yang, Y.; Zhang, K.; Guo, L.; Ge, H.; Chen, X.; Liu, J.; Feng, H. Molecular Engineering of an Organic NIR-II Fluorophore with Aggregation-Induced Emission Characteristics for In Vivo Imaging. Small 2019, 15, e1805549. [Google Scholar] [CrossRef]
- Zhan, R.; Li, X.; Zang, L.; Xu, K. An Au–Se nanoprobe for the evaluation of the invasive potential of breast cancer cells via imaging the sequential activation of uPA and MMP-2. Analyst 2020, 145, 1008–1013. [Google Scholar] [CrossRef]
- Guan, H.; Zhang, A.; Li, P.; Xia, L.; Guo, F. ESIPT Fluorescence Probe Based on Double-Switch Recognition Mechanism for Selective and Rapid Detection of Hydrogen Sulfide in Living Cells. ACS Omega 2019, 4, 9113–9119. [Google Scholar] [CrossRef]
- Huang, L.; Zhu, Q.; Zhu, J.; Luo, L.; Pu, S.; Zhang, W.; Zhu, W.; Sun, J.; Wang, J. Portable Colorimetric Detection of Mercury(II) Based on a Non-Noble Metal Nanozyme with Tunable Activity. Inorg. Chem. 2019, 58, 1638–1646. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.C.; Su, S.-B.; Fernández-Lodeiro, J.; Dos Santos, A.A. A selective emissive chromogenic and fluorogenic seleno-coumarin probe for Cu2+detection in aprotic media. Photochem. Photobiol. Sci. 2017, 16, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Gil Choi, M.; Ryu, H.; Cho, M.J.; Lee, S.K.; Chang, S.-K. Dual signaling of hypochlorite in tap water by selective oxidation of phenylselenylated dichlorofluorescein. Sens. Actuators B Chem. 2017, 244, 307–313. [Google Scholar] [CrossRef]
- Zhang, D.; Hu, M.; Yuan, X.; Wu, Y.-X.; Hu, X.; Xu, S.; Liu, H.-W.; Zhang, X.-B.; Liu, Y.; Tan, W. Engineering Self-Calibrating Nanoprobes with Two-Photon-Activated Fluorescence Resonance Energy Transfer for Ratiometric Imaging of Biological Selenocysteine. ACS Appl. Mater. Interfaces 2019, 11, 17722–17729. [Google Scholar] [CrossRef] [PubMed]
- Mulay, S.V.; Yudhistira, T.; Choi, M.; Kim, Y.; Kim, J.; Jang, Y.J.; Jon, S.; Churchill, D.G. Substituent Effects in BODIPY in Live Cell Imaging. Chem. Asian J. 2016, 11, 3598–3605. [Google Scholar] [CrossRef]
- Rafique, J.; Canto, R.F.S.; Saba, S.; Barbosa, F.A.R.; Braga, A.L. Recent Advances in the Synthesis of Biologically Relevant Selenium-containing 5-Membered Heterocycles. Curr. Org. Chem. 2015, 20, 166–188. [Google Scholar] [CrossRef]
- Vahter, J.; Viht, K.; Uri, A.; Manoharan, G.B.; Enkvist, E. Thiazole- and selenazole-comprising high-affinity inhibitors possess bright microsecond-scale photoluminescence in complex with protein kinase CK2. Bioorgan. Med. Chem. 2018, 26, 5062–5068. [Google Scholar] [CrossRef]
- Soares-Paulino, A.A.; Giroldo, L.; Pradie, N.A.; Reis, J.S.; Back, D.F.; Braga, A.A.; Stefani, H.A.; Fernández-Lodeiro, J.; Dos Santos, A.A. Nanomolar Detection of Palladium (II) through a Novel Seleno-Rhodamine-based fluorescent and colorimetric chemosensor. Dye. Pigment. 2020, 179, 108355. [Google Scholar] [CrossRef]
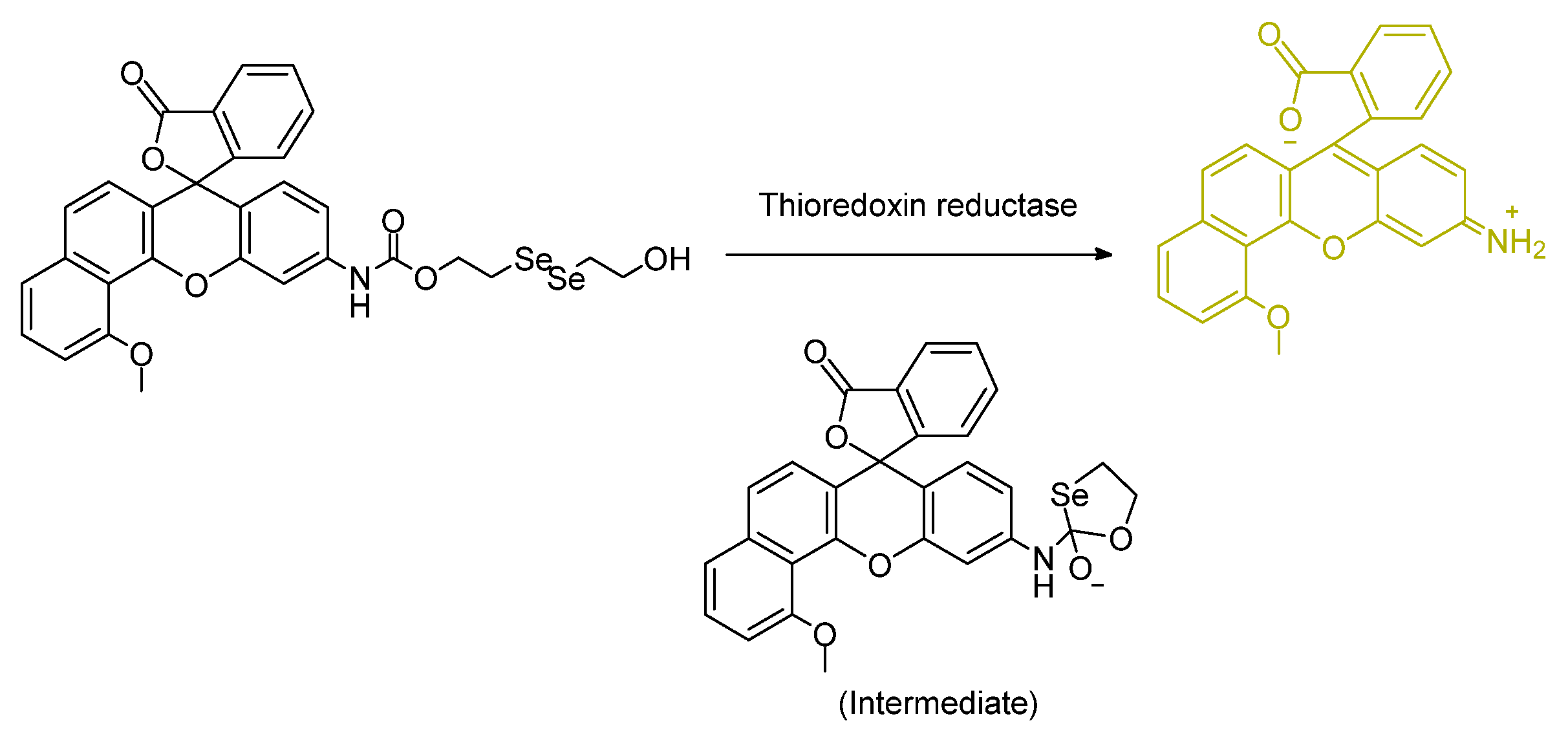
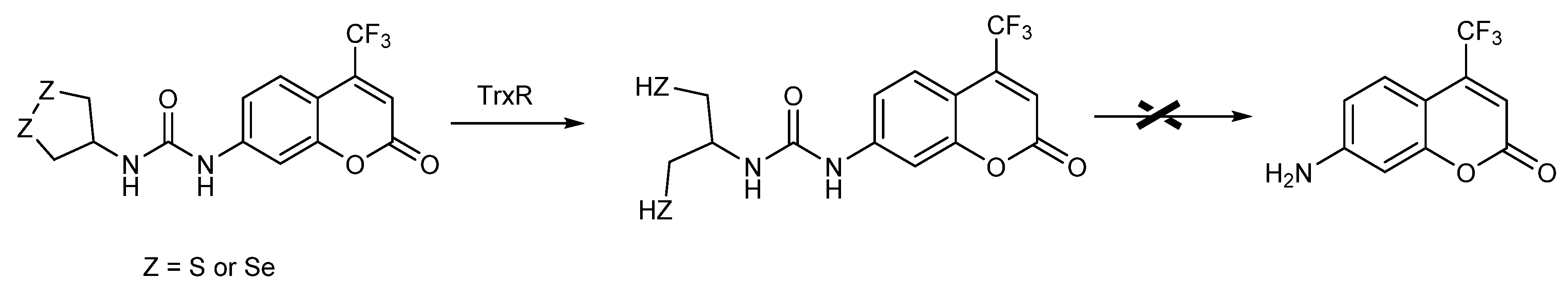
- Mafireyi, T.J.; Laws, M.; Bassett, J.W.; Cassidy, P.B.; Escobedo, J.O.; Strongin, R.M. A Diselenide Turn-On Fluorescent Probe for the Detection of Thioredoxin Reductase. Angew. Chem. Int. Ed. 2020, 59, 15147–15151. [Google Scholar] [CrossRef]
- Madibone, K.S.; Deshmukh, P.P.; Navalkar, A.; Maji, S.K.; Badani, P.M.; Manjare, S.T. Cyclic Organoselenide BODIPY-Based Probe: Targeting Superoxide in MCF-7 Cancer Cells. ACS Omega 2020, 5, 14186–14193. [Google Scholar] [CrossRef]
- Zhang, X.; Qu, W.; Liu, H.; Ma, Y.; Wang, L.; Sun, Q.; Yu, F. Visualizing hydrogen sulfide in living cells and zebrafish using a red-emitting fluorescent probe via selenium-sulfur exchange reaction. Anal. Chim. Acta 2020, 1109, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Shen, Z.; Chen, B.; Wang, X.; Xia, Q.; Ge, Z.; Xu, A.-W.; Li, X. Selenium-doped two-photon fluorescent carbon nanodots for in-situ free radical scavenging in mitochondria. J. Colloid Interface Sci. 2020, 567, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Zang, S.; Kong, X.; Cui, J.; Su, S.; Shu, W.; Zhang, X.; Zhang, X. Revealing the redox status in endoplasmic reticulum by a selenium fluorescence probe. J. Mater. Chem. B 2020, 8, 2660–2665. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.C.; Capelo, J.L.; Fernández-Lodeiro, J.; Dos Santos, A.A. A seleno-pyrene selective probe for Hg2+ detection in either aqueous or aprotic systems. Sens. Actuators B Chem. 2017, 239, 311–318. [Google Scholar] [CrossRef]
- Casula, A.; Llopis-Lorente, A.; Garau, A.; Isaia, F.; Kubicki, M.; Lippolis, V.; Sancenón, F.; Martínez-Máñez, R.; Owczarzak, A.; Santi, C.; et al. A new class of silica-supported chromo-fluorogenic chemosensors for anion recognition based on a selenourea scaffold. Chem. Commun. 2017, 53, 3729–3732. [Google Scholar] [CrossRef]
- Chen, L.; Wang, R.; Yu, F.; Li, B.; Chen, L. Imaging of intracellular sulfane sulfur expression changes under hypoxic stress via a selenium-containing near-infrared fluorescent probe. J. Mater. Chem. B 2018, 6, 6637–6645. [Google Scholar] [CrossRef]
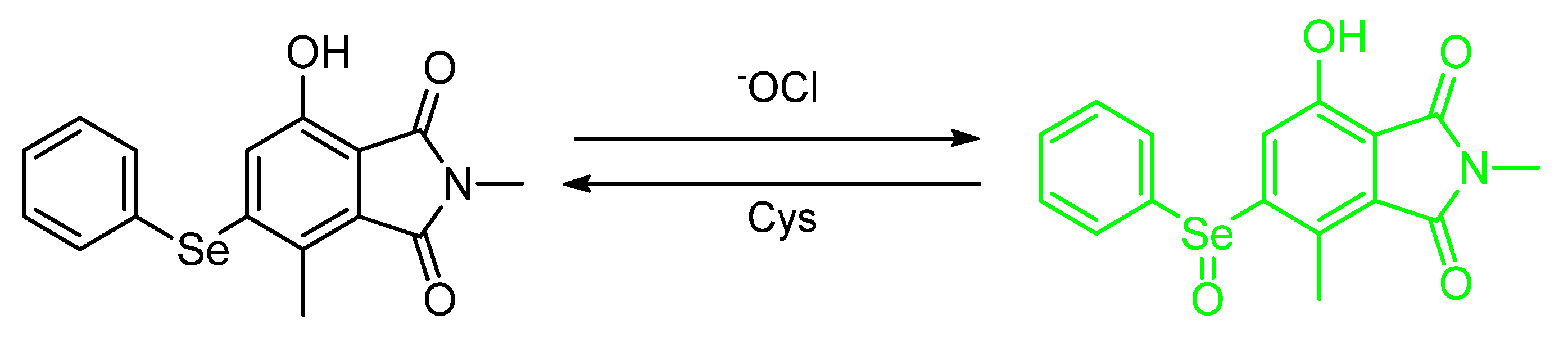
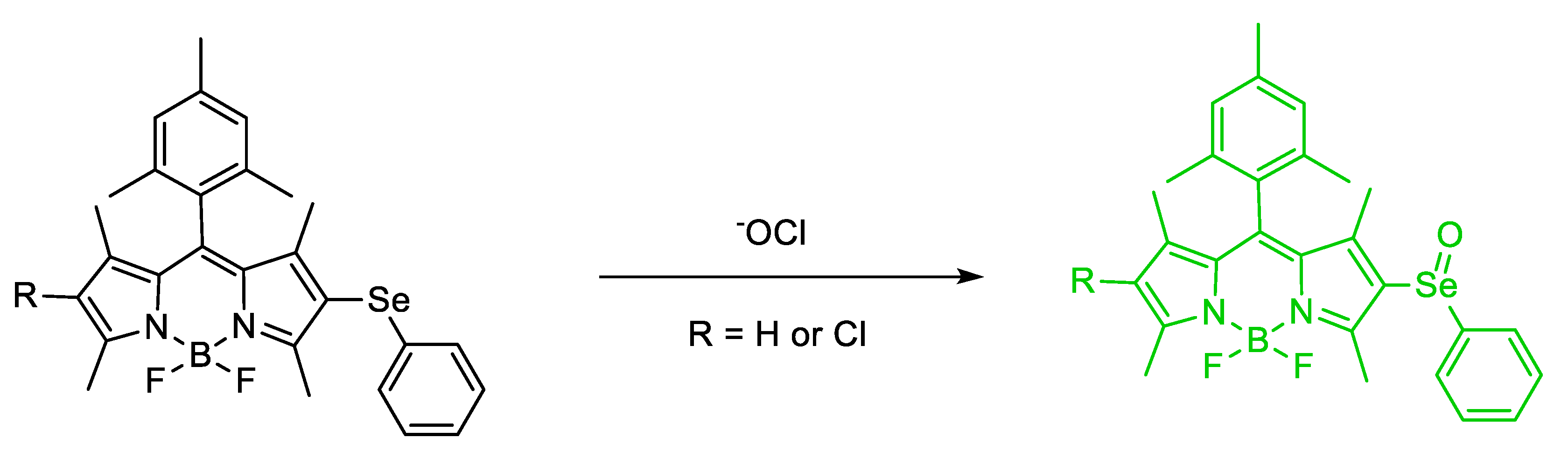
- Xie, X.; Wu, T.; Wang, X.; Li, Y.; Wang, K.; Zhao, Z.; Jiao, X.; Tang, B. A two-photon fluorescent probe for ratiometric visualization of hypochlorous acid in live cells and animals based on a selenide oxidation/elimination tandem reaction. Chem. Commun. 2018, 54, 11965–11968. [Google Scholar] [CrossRef]
- Honson, N.S.; Jensen, J.R.; Darby, M.V.; Kuret, J. Potent inhibition of tau fibrillization with a multivalent ligand. Biochem. Biophys. Res. Commun. 2007, 363, 229–234. [Google Scholar] [CrossRef][Green Version]
- Kim, Y.; Jun, T.; Mulay, S.V.; Manjare, S.T.; Kwak, J.; Lee, Y.; Churchill, D.G. Novel intramolecular π–π-interaction in a BODIPY system by oxidation of a single selenium center: Geometrical stamping and spectroscopic and spectrometric distinctions. Dalton Trans. 2017, 46, 4111–4117. [Google Scholar] [CrossRef]
- Zhao, X.; Yuan, G.; Ding, H.; Zhou, L.; Lin, Q. A TP-FRET-based fluorescent sensor for ratiometric visualization of selenocysteine derivatives in living cells, tissues and zebrafish. J. Hazard. Mater. 2020, 381, 120918. [Google Scholar] [CrossRef]
- Suarez, S.I.; Ambrose, R.; Kalk, M.A.; Lukesh, J.C. Selenosulfides Tethered to gem -Dimethyl Esters: A Robust and Highly Versatile Framework for H 2 S Probe Development. Chem. A Eur. J. 2019, 25, 15736–15740. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, Z.; Feng, S.; Wang, D.; Liu, H. A Selenone-Functionalized Polyhedral Oligomeric Silsesquioxane for Selective Detection and Adsorption of Hg2+ ions in Aqueous Solutions. Polymers 2019, 11, 2084. [Google Scholar] [CrossRef] [PubMed]
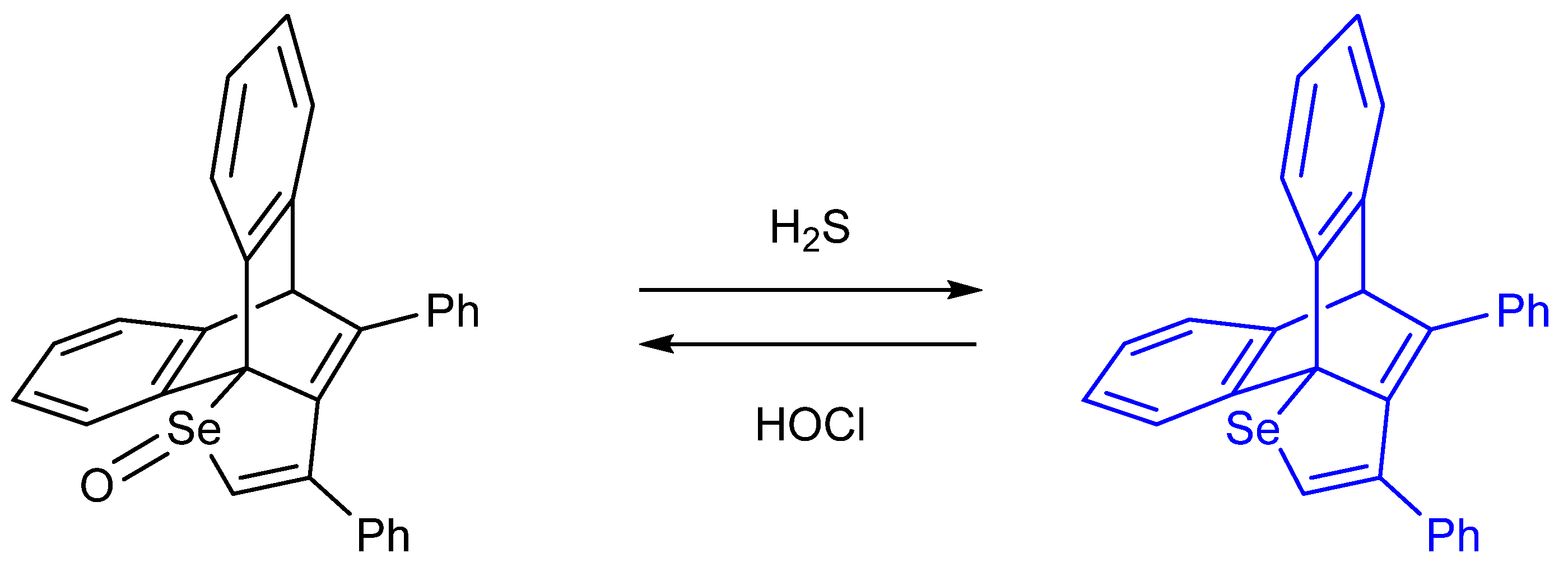
- Ishii, A.; Shibata, M.; Nakata, N. 1,4-Diaryl-1-oxy-1,3-butadiene Conjugated System Incorporated in a Dibenzobarrelene Skeleton: Synthesis, Photophysical Properties, and Comparison with the Heavier Group 16 Congeners. Bull. Chem. Soc. Jpn. 2016, 89, 1470–1479. [Google Scholar] [CrossRef]
- Annaka, T.; Nakata, N.; Ishii, A. A reversible and turn-on type fluorescence behaviour of hydrogen sulfide via a redox cycle between selenoxide and selenide. New J. Chem. 2019, 43, 11643–11652. [Google Scholar] [CrossRef]
- Li, X.; Zhang, B.; Yan, C.; Li, J.; Wang, S.; Wei, X.; Jiang, X.; Zhou, P.; Fang, J. A fast and specific fluorescent probe for thioredoxin reductase that works via disulphide bond cleavage. Nat. Commun. 2019, 10, 2745. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, P.P.; Navalkar, A.; Maji, S.K.; Manjare, S.T. Phenylselenyl containing turn-on dibodipy probe for selective detection of superoxide in mammalian breast cancer cell line. Sens. Actuators B Chem. 2019, 281, 8–13. [Google Scholar] [CrossRef]
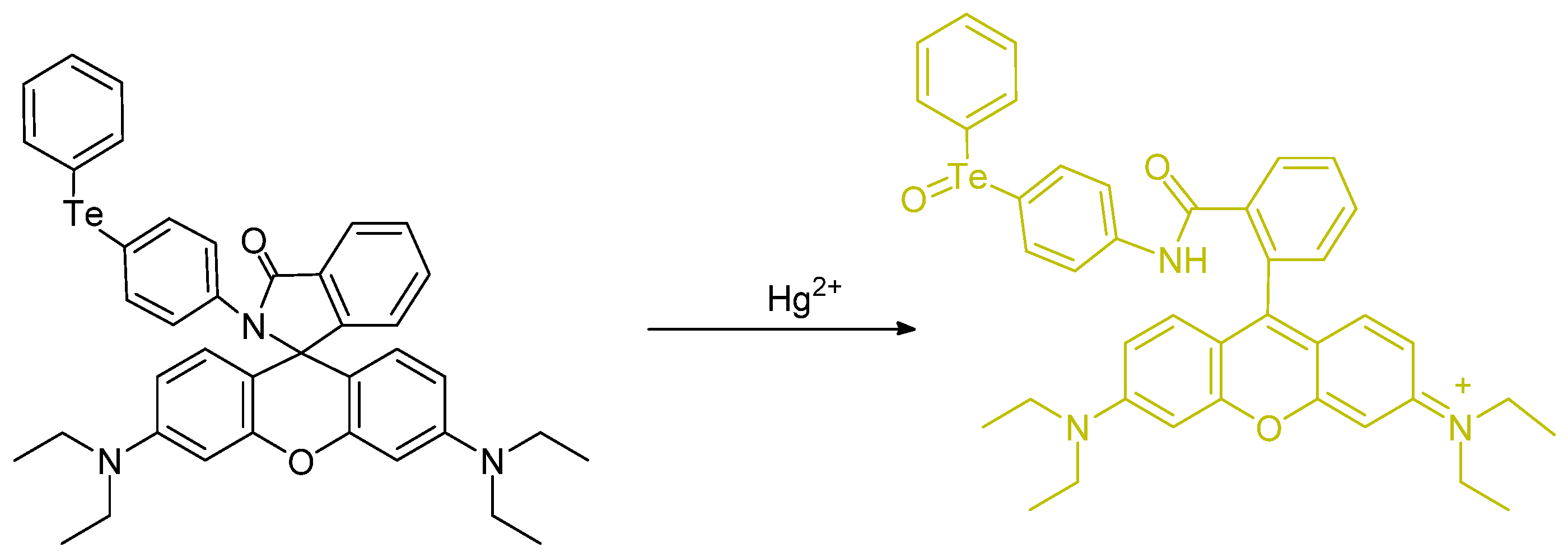
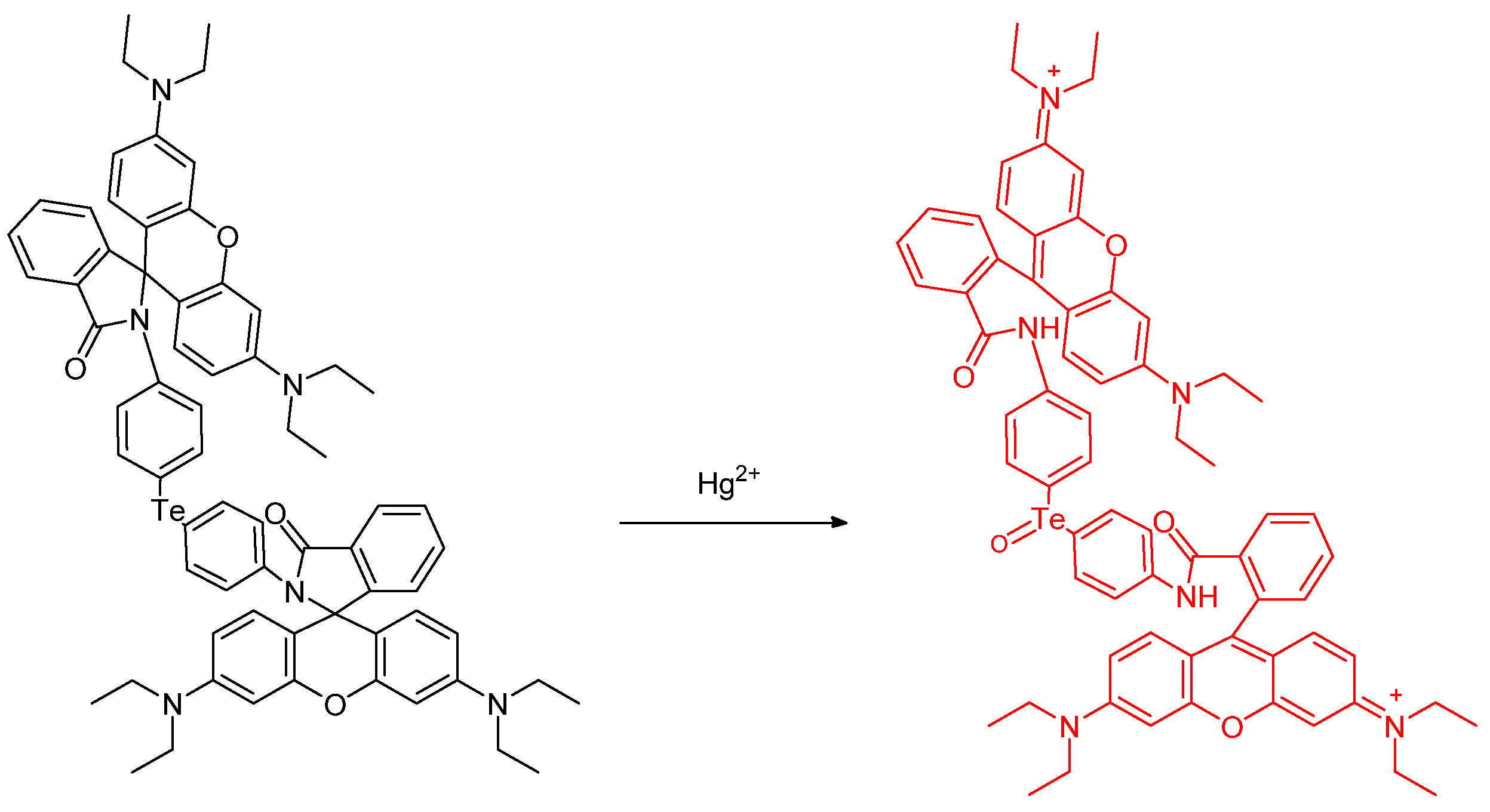
- Paulino, A.A.; Giroldo, L.; Celante, G.; Oliveira, E.; Santos, S.M.; Mendes, R.F.; Paz, F.A.A.; Fioroto, A.M.; Oliveira, P.V.; Serrano, S.H.P.; et al. Oxidation of tellurium dyes induced by mercury: More insights on the naked-eye and fluorescent Hg2+ detection. Dye. Pigment. 2019, 160, 208–216. [Google Scholar] [CrossRef]
- Soares-Paulino, A.A.; Giroldo, L.; Celante, G.; Lodeiro, C.; Dos Santos, A.A. Formation of an emissive telluroxide promoted by Hg2+ in aqueous environment: A new naked-eye and ratiometric rhodamine dimer fluorescent mercury(II) probe. Dye. Pigment. 2018, 159, 121–127. [Google Scholar] [CrossRef]
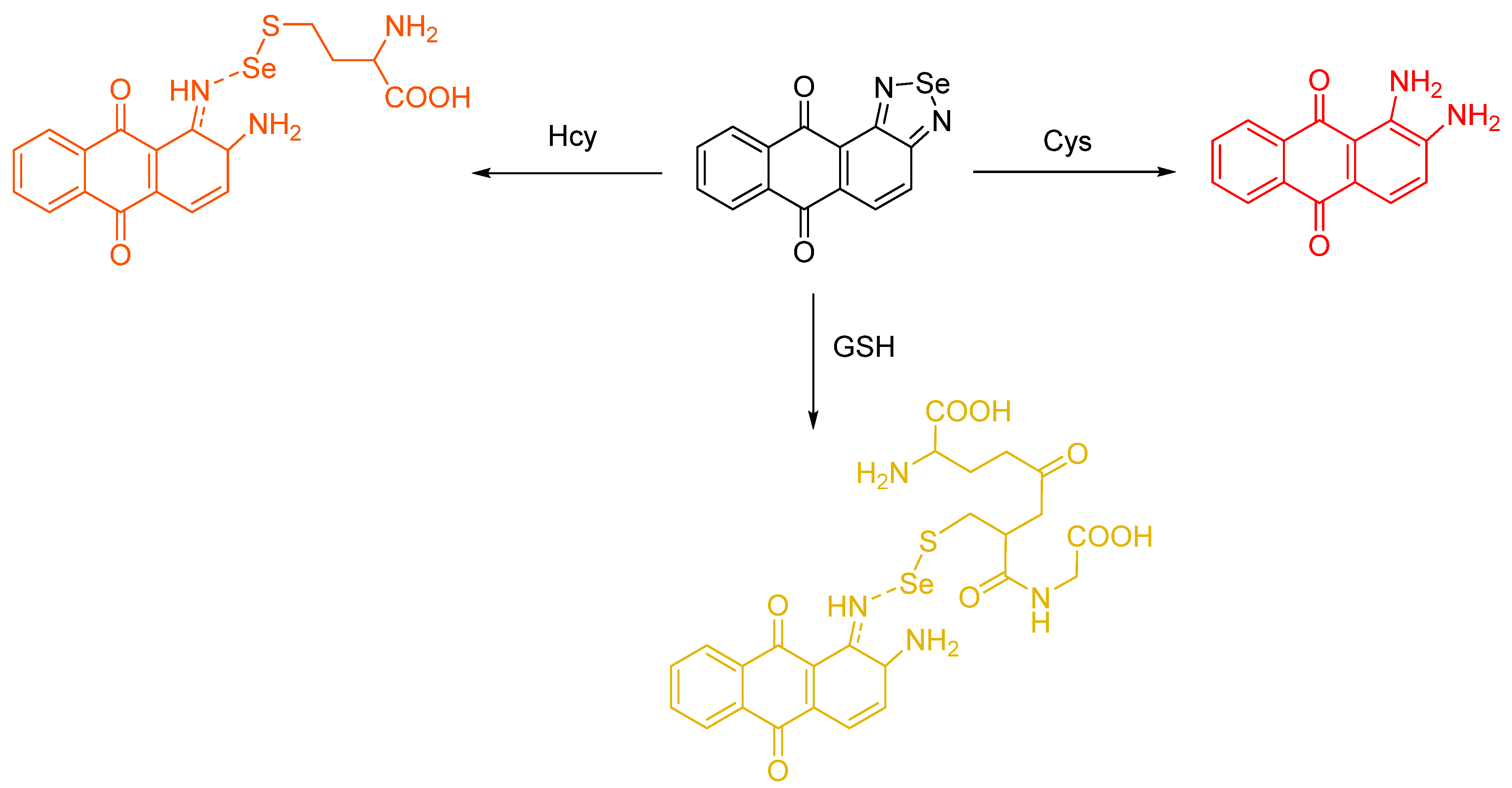
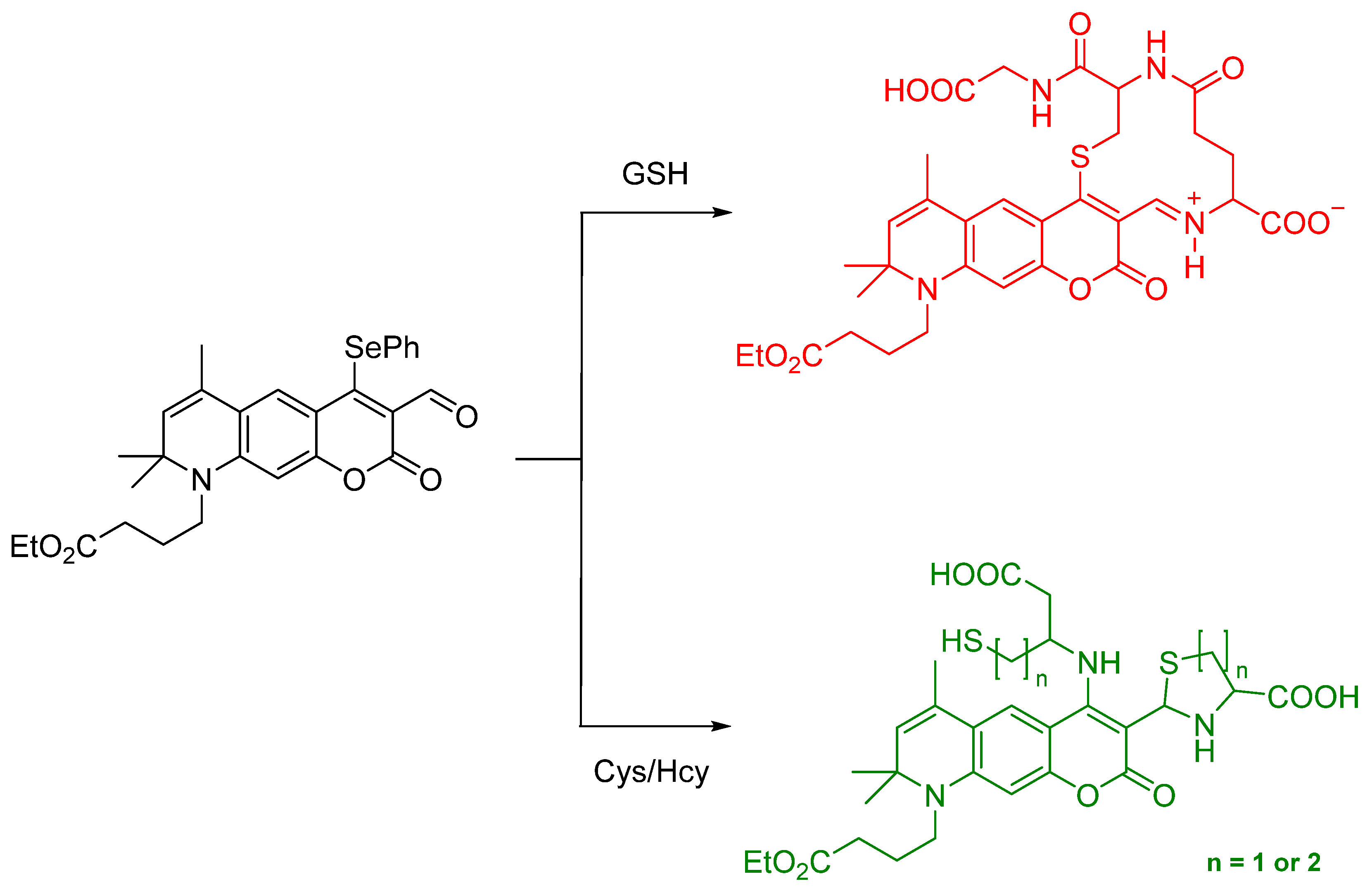
- Tian, Y.; Zhu, B.; Yang, W.; Jing, J.; Zhang, X. A fluorescent probe for differentiating Cys, Hcy and GSH via a stepwise interaction. Sens. Actuators B Chem. 2018, 262, 345–349. [Google Scholar] [CrossRef]
- Mulay, S.V.; Kim, Y.; Choi, M.; Lee, D.Y.; Choi, J.; Lee, Y.; Jon, S.; Churchill, D.G. Enhanced Doubly Activated Dual Emission Fluorescent Probes for Selective Imaging of Glutathione or Cysteine in Living Systems. Anal. Chem. 2018, 90, 2648–2654. [Google Scholar] [CrossRef]
- Mulay, S.V.; Choi, M.; Jang, Y.J.; Kim, Y.; Jon, S.; Churchill, D.G. Enhanced Fluorescence Turn-on Imaging of Hypochlorous Acid in Living Immune and Cancer Cells. Chem. A Eur. J. 2016, 22, 9642–9648. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Choi, M.; Manjare, S.T.; Jon, S.; Churchill, D.G. Diselenide-based probe for the selective imaging of hypochlorite in living cancer cells. RSC Adv. 2016, 6, 32013–32017. [Google Scholar] [CrossRef]
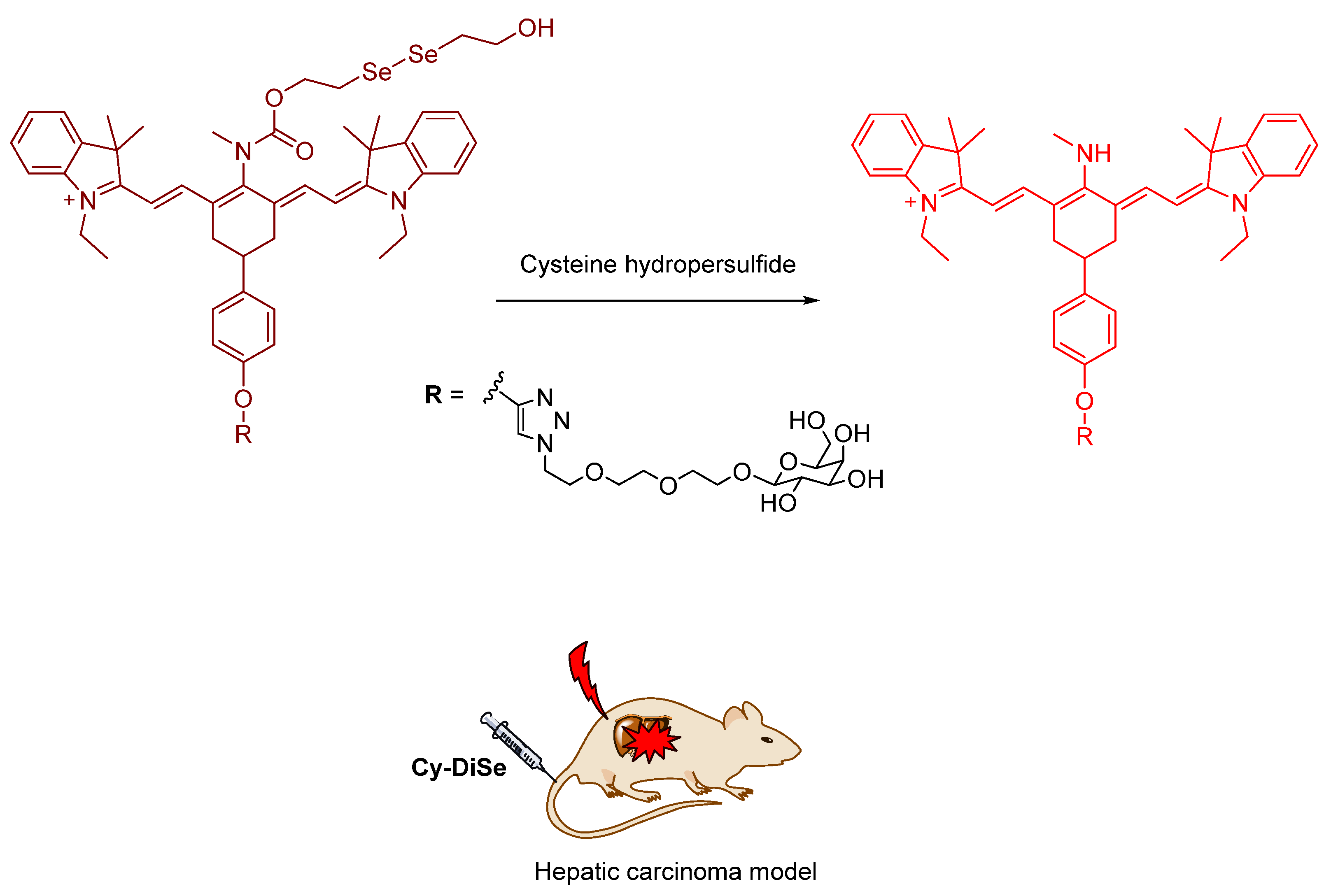
- Han, X.; Yu, F.; Song, X.; Chen, L. Quantification of cysteine hydropersulfide with a ratiometric near-infrared fluorescent probe based on selenium–sulfur exchange reaction. Chem. Sci. 2016, 7, 5098–5107. [Google Scholar] [CrossRef] [PubMed]
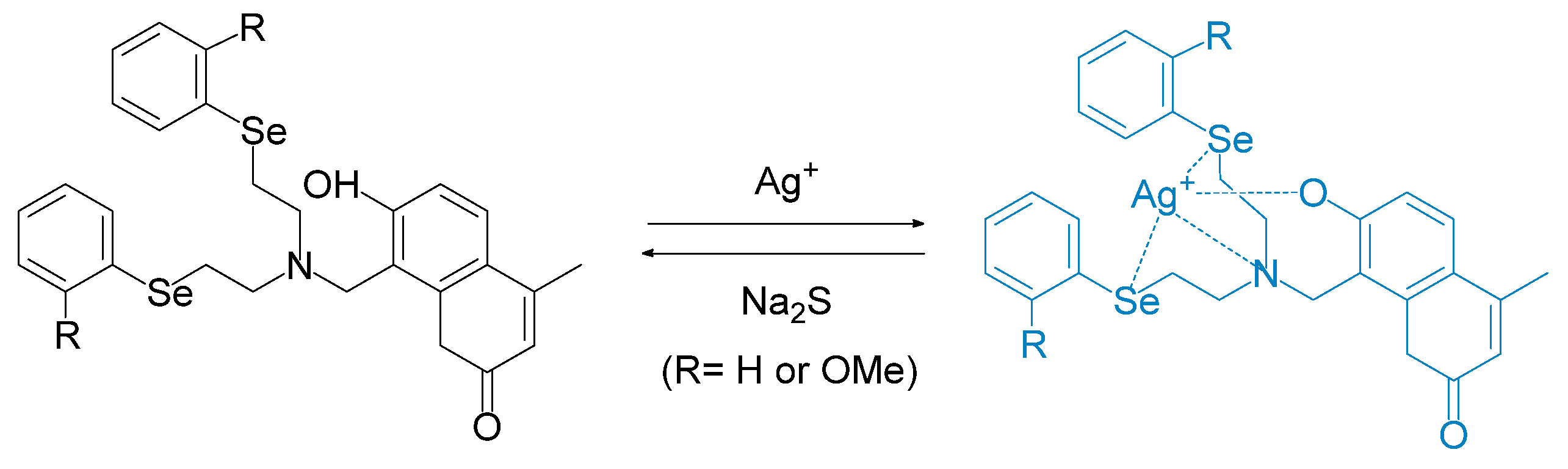
- Su, J.; Huang, S.; He, S.; Zeng, X. Sensitive and selective fluorescent chemosensors combining multiple PET processes for Ag+ sensing. Chem. Res. Chin. Univ. 2016, 32, 20–27. [Google Scholar] [CrossRef]
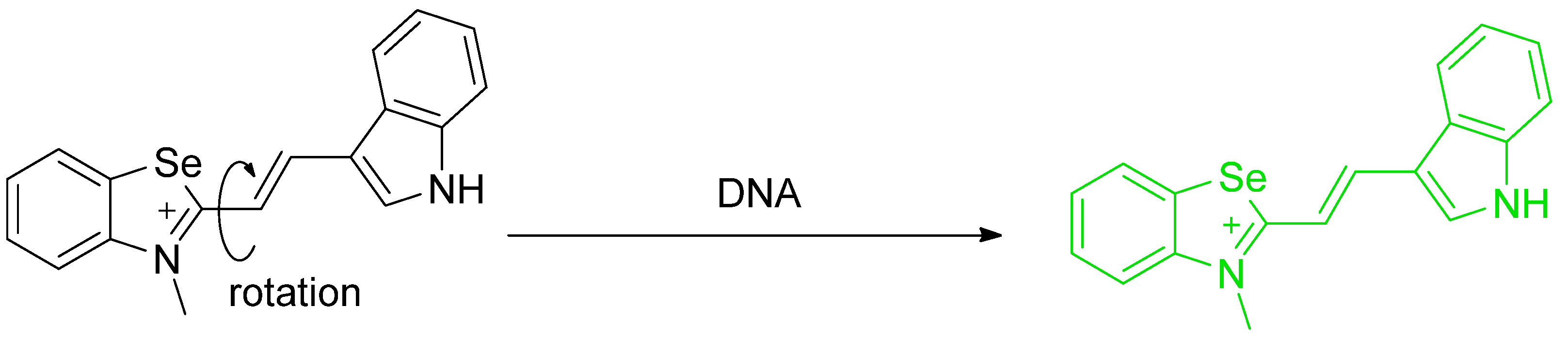
- Gaur, P.; Kumar, A.; Dey, G.; Kumar, R.; Bhattacharyya, S.; Ghosh, S. Selenium Incorporated Cationic Organochalcogen: Live Cell Compatible and Highly Photostable Molecular Stain for Imaging and Localization of Intracellular DNA. ACS Appl. Mater. Interfaces 2016, 8, 10690–10699. [Google Scholar] [CrossRef]
- Martins, T.D.; Pacheco, M.L.; Boto, R.E.; Almeida, P.; Farinha, J.P.S.; Reis, L.V. Synthesis, characterization and protein-association of dicyanomethylene squaraine dyes. Dye. Pigment. 2017, 147, 120–129. [Google Scholar] [CrossRef]
- Han, X.; Song, X.; Xiaoyue, H.; Chen, L. A ratiometric fluorescent probe for imaging and quantifying anti-apoptotic effects of GSH under temperature stress. Chem. Sci. 2017, 8, 6991–7002. [Google Scholar] [CrossRef]
- Gao, M.; Wang, R.; Yu, F.; Chen, L. Evaluation of sulfane sulfur bioeffects via a mitochondria-targeting selenium-containing near-infrared fluorescent probe. Biomaterials 2018, 160, 1–14. [Google Scholar] [CrossRef]
- Xu, C.; Qian, Y. A selenamorpholine-based redox-responsive fluorescent probe for targeting lysosome and visualizing exogenous/endogenous hydrogen peroxide in living cells and zebrafish. J. Mater. Chem. B 2019, 7, 2714–2721. [Google Scholar] [CrossRef]
- Tian, Y.; Xin, F.; Jing, J.; Zhang, X. Ratiometric fluorescence imaging for sodium selenite in living cells. Dye. Pigment. 2019, 164, 133–138. [Google Scholar] [CrossRef]
- Guan, M.; Mi, H.; Fei, Q.; Shan, H.; Huan, Y.; Lv, S.; Feng, G.; Xu, H. Study of Fluorescent Imaging of Se (IV) in Living Cells Using a Turn-on Fluorescent Probe Based on a Rhodamine Spirolactame Derivative. J. Fluoresc. 2016, 27, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Song, X.; Yu, F.; Chen, L. A Ratiometric Near-Infrared Fluorescent Probe for Quantification and Evaluation of Selenocysteine-Protective Effects in Acute Inflammation. Adv. Funct. Mater. 2017, 27, 1–9. [Google Scholar] [CrossRef]
- Pooja, D.; Kaur, G.; Thakur, A.; Kaur, N.; Grewal, A.; Kumar, P. Waste derivitized blue luminescent carbon quantum dots for selenite sensing in water. Talanta 2017, 170, 49–55. [Google Scholar] [CrossRef]
- Xing, Z.; Yang, M.; Guo, W.; Jin, L.; Liu, Z.; Hu, S. Elemental imaging method based on a dielectric barrier discharge probe coupled with inductively coupled plasma mass spectrometry. Spectrochim. Acta Part B At. Spectrosc. 2018, 147, 141–148. [Google Scholar] [CrossRef]
- Pan, X.; Song, X.; Wang, C.; Cheng, T.; Luan, D.; Xu, K.; Tang, B. H2Se Induces Reductive Stress in HepG2 Cells and Activates Cell Autophagy by Regulating the Redox of HMGB1 Protein under Hypoxia. Theranostics 2019, 9, 1794–1808. [Google Scholar] [CrossRef]
- Nehzati, S.; Dolgova, N.V.; Sokaras, D.; Kroll, T.; Cotelesage, J.J.H.; Pickering, I.J.; George, G.N. A Photochemically Generated Selenyl Free Radical Observed by High Energy Resolution Fluorescence Detected X-ray Absorption Spectroscopy. Inorg. Chem. 2018, 57, 10867–10872. [Google Scholar] [CrossRef]
- Tong, X.; Li, X.; Channa, A.I.; Liu, R.; Xu, J.-Y.; Yu, P.; Chang, L.; Ji, H.; Wang, Q.; Wang, Z.M. Engineering the Optoelectronic Properties of Colloidal Alloyed Copper Chalcogenide Quantum Dots for High-Efficiency Solar Energy Conversion. Sol. RRL 2019, 3, 1–8. [Google Scholar] [CrossRef]
- Yuan, A.; Wu, X.; Li, X.; Hao, C.; Xu, C.; Kuang, H. Au@gap@AuAg Nanorod Side-by-Side Assemblies for Ultrasensitive SERS Detection of Mercury and its Transformation. Small 2019, 15, e1901958. [Google Scholar] [CrossRef]
- Devi, P.; Thakur, A.; Chopra, S.; Kaur, N.; Kumar, P.; Singh, N.; Kumar, M.; Shivaprasad, S.M.; Nayak, M.K. Ultrasensitive and Selective Sensing of Selenium Using Nitrogen-Rich Ligand Interfaced Carbon Quantum Dots. ACS Appl. Mater. Interfaces 2017, 9, 13448–13456. [Google Scholar] [CrossRef]
- Guo, Y.; Luo, Y.; Wang, N.; Tang, M.; Xiao, J.; Chen, S.-W.; Wang, J. Au nanoparticle-based probe for selenol in living cells and selenium-rich tea and rice. Talanta 2020, 212, 120583. [Google Scholar] [CrossRef]
- Hu, B.; Cheng, R.; Liu, X.; Pan, X.; Kong, F.; Gao, W.; Xu, K.; Tang, B. A nanosensor for in vivo selenol imaging based on the formation of Au Se bonds. Biomaterials 2016, 92, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Areti, S.; Verma, S.K.; Bellare, J.; Rao, C.P. Selenocysteine vs Cysteine: Tuning the Derivatization on Benzenesulfonyl Moiety of a Triazole Linked Dansyl Connected Glycoconjugate for Selective Recognition of Selenocysteine and the Applicability of the Conjugate in Buffer, in Serum, on Silica Gel, and in HepG2 Cells. Anal. Chem. 2016, 88, 7259–7267. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Feng, W.; Zhai, Q.; Feng, G. Selenocysteine detection and bioimaging in living cells by a colorimetric and near-infrared fluorescent turn-on probe with a large stokes shift. Biosens. Bioelectron. 2017, 87, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shi, Y.; Sheng, Z.; Zhang, Y.; Kai, X.; Li, M.; Yin, X. Bioluminescence Imaging of Selenocysteine in Vivo with a Highly Sensitive Probe. ACS Sens. 2019, 4, 3147–3155. [Google Scholar] [CrossRef]
- Ungati, H.; Govindaraj, V.; Narayanan, M.; Mugesh, G. Probing the Formation of a Seleninic Acid in Living Cells by the Fluorescence Switching of a Glutathione Peroxidase Mimetic. Angew. Chem. 2019, 131, 8240–8244. [Google Scholar] [CrossRef]
- Tian, Y.; Xin, F.; Zhang, X.; Zhang, X. Fluorescence imaging of lysosomal hydrogen selenide under oxygen-controlled conditions. J. Mater. Chem. B 2019, 7, 2829–2834. [Google Scholar] [CrossRef]
- Dai, C.-G.; Wang, J.-L.; Song, Q.-H. Red fluorescent probes based on a Bodipy analogue for selective and sensitive detection of selenols in solutions and in living systems. J. Mater. Chem. B 2016, 4, 6726–6733. [Google Scholar] [CrossRef]
- Kong, F.; Ge, L.; Pan, X.; Xu, K.; Liu, X.; Tang, B. A highly selective near-infrared fluorescent probe for imaging H2Se in living cells and in vivo. Chem. Sci. 2015, 7, 1051–1056. [Google Scholar] [CrossRef]
- Liu, Q.; Huan, Y.; Zheng, Q.; Fei, Q.; Fei, Y.; Fan, Q.; Feng, G.; Shan, H.-Y. A selective and sensitive fluorescence probe for Se(IV) based on fluorescence quenching of gatifloxacin. Chem. Res. Chin. Univ. 2016, 32, 736–741. [Google Scholar] [CrossRef]
- Zhang, P.; Ding, Y.; Liu, W.; Niu, G.; Zhang, H.; Ge, J.; Wu, J.; Li, Y.; Wang, P. Red emissive fluorescent probe for the rapid detection of selenocysteine. Sens. Actuators B Chem. 2018, 264, 234–239. [Google Scholar] [CrossRef]
- Luan, D.; Gao, X.; Kong, F.; Song, X.; Zheng, A.; Liu, X.; Xu, K.; Tang, B. Cyclic Regulation of the Sulfilimine Bond in Peptides and NC1 Hexamers via the HOBr/H2Se Conjugated System. Anal. Chem. 2018, 90, 9523–9528. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Guo, W.; Ge, L.; Kong, F.; Xu, K.; Tang, B. Double-ratiometric fluorescence imaging of H2Se and O2˙− under hypoxia for exploring Na2SeO3-induced HepG2 cells’ apoptosis. RSC Adv. 2018, 8, 40984–40988. [Google Scholar] [CrossRef]
- Zwolak, I. The effect of selenium, as selenite, on vanadate-induced ROS generation in CHO-K1 cells measured using dichloro-dihydro-fluorescein diacetate (DCFH-DA) assay. Trace Elem. Electrolytes 2018, 35, 136–141. [Google Scholar] [CrossRef]
- Arnab, S.M.; Kabir, M.Z. Impact of Lubberts Effect on Amorphous Selenium Indirect Conversion Avalanche Detector for Medical X-ray Imaging. IEEE Trans. Radiat. Plasma Med. Sci. 2017, 1, 1. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Veluthandath, A.V.; Murugan, G.S.; Bisht, P.B. Temperature dependence of whispering gallery modes of quantum dot-doped microbottle resonators. J. Lumin. 2020, 221, 117050. [Google Scholar] [CrossRef]
- Lang, C.; Zhang, X.; Dong, Z.; Luo, Q.; Qiao, S.; Huang, Z.; Fan, X.; Xu, J.; Liu, J. Selenium-containing organic nanoparticles as silent precursors for ultra-sensitive thiol-responsive transmembrane anion transport. Nanoscale 2016, 8, 2960–2966. [Google Scholar] [CrossRef]
- Lewis, C.S.; Liu, H.; Han, J.; Wang, L.; Yue, S.; Brennan, N.A.; Wong, S.S. Probing charge transfer in a novel class of luminescent perovskite-based heterostructures composed of quantum dots bound to RE-activated CaTiO3 phosphors. Nanoscale 2016, 8, 2129–2142. [Google Scholar] [CrossRef]
- Ye, J.-H.; Jung, E.-Y.; Choi, S.-H. Synthesis and Characterization of TGA-Capped CdSe QDs for Electrochemiluminescence (ECL) Biosensor. Adv. Eng. Res. 2016, 153–155. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, P.; Jiang, Q.; Wang, F.; Pang, D.-W.; Liu, X. Assembly-enhanced fluorescence from metal nanoclusters and quantum dots for highly sensitive biosensing. Sens. Actuators B Chem. 2019, 279, 334–341. [Google Scholar] [CrossRef]
- Singh, S.; Kaur, V.; Kumar, N.; Garg, M.; Pandey, S.K.; Meena, V.K. Cadmium chalcogenide derived fluorescent quanta-sensor for melamine detection. Sens. Actuators B Chem. 2018, 273, 505–510. [Google Scholar] [CrossRef]
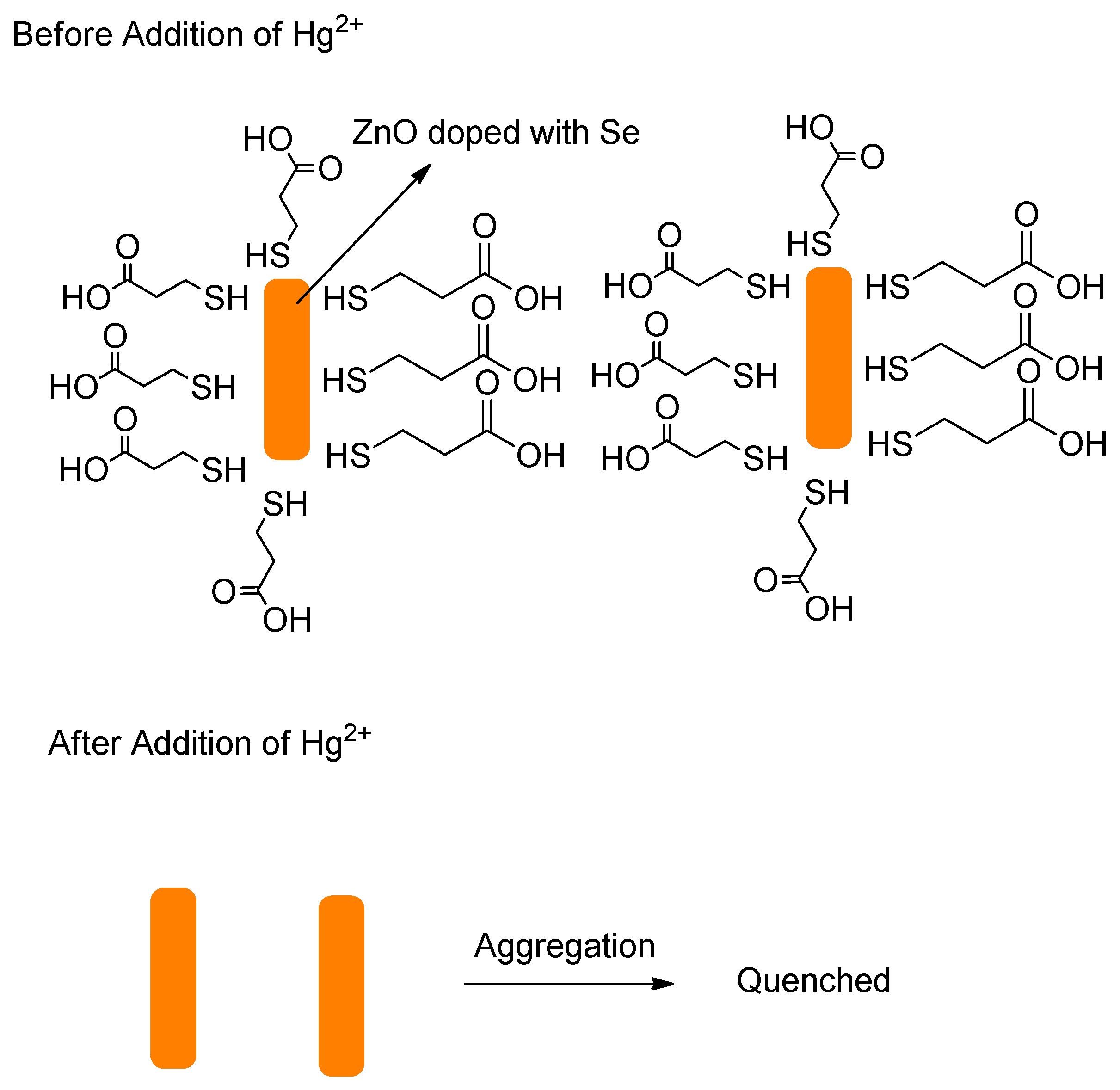
- Rao, A.V.R.K.; Reddy, R.B.; Sengupta, S.; Chelvam, V. Efficient “turn-on” nanosensor by dual emission-quenching mechanism of functionalized Se doped ZnO nanorods for mercury (II) detection. Appl. Nanosci. 2018, 8, 1973–1987. [Google Scholar] [CrossRef]
- Simlandy, A.K.; Bhattacharyya, B.; Pandey, A.; Mukherjee, S. Picosecond Electron Transfer from Quantum Dots Enables a General and Efficient Aerobic Oxidation of Boronic Acids. ACS Catal. 2018, 8, 5206–5211. [Google Scholar] [CrossRef]
- Jang, Y.J.; Lee, J.; Jeong, J.-H.; Lee, K.-B.; Kim, D.; Lee, Y. Complementary Characterization of Cu(In,Ga)Se2 Thin-Film Photovoltaic Cells Using Secondary Ion Mass Spectrometry, Auger Electron Spectroscopy, and Atom Probe Tomography. J. Nanosci. Nanotechnol. 2018, 18, 3548–3556. [Google Scholar] [CrossRef]
- Ding, Y.; Yin, H.; Hao, X.; Kyratzis, I.L.; Shirley, S.; Liu, F.; Musameh, M.M.; Sun, K. Novel composite films of polysaccharides and glutathione capped zinc selenide (GSH@ZnSe) quantum dots for detection of Cd2+ and Cu2+. New J. Chem. 2018, 42, 4871–4880. [Google Scholar] [CrossRef]
- Kaur, G.; Bharti, S.; Tripathi, S. Interactions between thioglycolic acid capped CdSe/ZnS nanoparticles and papain. J. Lumin. 2018, 195, 375–384. [Google Scholar] [CrossRef]
- Zhang, J.; Teng, Z.; Yuan, Y.; Zeng, Q.-Z.; Lou, Z.; Lee, S.-H.; Wang, Q. Development, physicochemical characterization and cytotoxicity of selenium nanoparticles stabilized by beta-lactoglobulin. Int. J. Biol. Macromol. 2018, 107, 1406–1413. [Google Scholar] [CrossRef]
- Pansare, A.V.; Shedge, A.A.; Patil, V.R. Discrete SeNPs-Macromolecule Binding Manipulated by Hydrophilic Interaction. Int. J. Biol. Macromol. 2018, 107, 1982–1987. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, R.; Liu, W.; Wang, X.; Li, P.; Zhang, W.; Wang, H.; Tang, B. Te-containing carbon dots for fluorescence imaging of superoxide anion in mice during acute strenuous exercise or emotional changes. Chem. Sci. 2018, 9, 721–727. [Google Scholar] [CrossRef]
- Zhao, Q.; Tao, G.; Ge, C.; Cai, Y.; Qiao, Q.; Jia, X. Ultrasensitive fluorescent probe for copper ion based on cadmium selenide/cadmium sulfide quantum dots capped with dimercaprol. Spectrosc. Lett. 2018, 51, 216–222. [Google Scholar] [CrossRef]
- Huang, N.; Chen, X.; Zhu, X.; Xu, M.; Liu, J. Ruthenium complexes/polypeptide self-assembled nanoparticles for identification of bacterial infection and targeted antibacterial research. Biomaterials 2017, 141, 296–313. [Google Scholar] [CrossRef]
- Prasanth, S.; Sudarsanakumar, C. Elucidating the interaction of l-cysteine-capped selenium nanoparticles and human serum albumin: Spectroscopic and thermodynamic analysis. New J. Chem. 2017, 41, 9521–9530. [Google Scholar] [CrossRef]
- Liu, N.; Kumbham, M.; Pita, I.; Guo, Y.; Bianchini, P.; Diaspro, A.; Tofail, S.A.M.; Peremans, A.; Silien, C. Far-Field Subdiffraction Imaging of Semiconductors Using Nonlinear Transient Absorption Differential Microscopy. ACS Photon. 2016, 3, 478–485. [Google Scholar] [CrossRef]
- Karimi, B.; Arabi, A.M.; Afarani, M.S. Synthesis of carbon nanotube-cadmium selenide luminescent nanocomposites using electrophoretic deposition technique. J. Mater. Sci. Mater. Electron. 2016, 28, 1247–1252. [Google Scholar] [CrossRef]
- Chinnathambi, S.; Abu, N.; Hanagata, N. Biocompatible CdSe/ZnS quantum dot micelles for long-term cell imaging without alteration to the native structure of the blood plasma protein human serum albumin. RSC Adv. 2017, 7, 2392–2402. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, H.; Tang, J.; Deng, S.; Yan, F.; Li, W.; Qu, M. Carbon dots doped with heteroatoms for fluorescent bioimaging: A review. Microchim. Acta 2017, 184, 343–368. [Google Scholar] [CrossRef]
- Sajwan, R.K.; Bagbi, Y.; Sharma, P.; Solanki, P.R. L-cysteine and 3-mercaptopropionic acid capped cadmium selenide quantum dots based metal ion probes. J. Lumin. 2017, 187, 126–132. [Google Scholar] [CrossRef]
- Bergeron, A.; Ibrahim, J.; Leonelli, R.; Francoeur, S. Oxidation dynamics of ultrathin GaSe probed through Raman spectroscopy. Appl. Phys. Lett. 2017, 110, 241901. [Google Scholar] [CrossRef]
- Li, F.; Li, T.; Sun, C.; Xia, J.; Jiao, Y.; Xu, H. Selenium-Doped Carbon Quantum Dots for Free-Radical Scavenging. Angew. Chem. Int. Ed. 2017, 56, 9910–9914. [Google Scholar] [CrossRef]
- Laatar, F.; Harizi, A.; Zarroug, A.; Ghrib, M.; Hassen, M.; Gaidi, M.; Ezzaouia, H. Novel CdSe nanorods/porous anodic alumina nanocomposite-based ethanol sensor: Sensitivity enhancement by visible light illumination. J. Mater. Sci. Mater. Electron. 2017, 28, 12259–12267. [Google Scholar] [CrossRef]
- Chen, T.; Zeng, L.; Mai, X.; Shi, C.; Luo, L.; Chen, T. Nucleolin-targeted selenium nanocomposites with enhanced theranostic efficacy to antagonize glioblastoma. J. Mater. Chem. B 2017, 5, 3024–3034. [Google Scholar] [CrossRef]
- Waldron, D.L.; Burke, R.; Preske, A.; Krauss, T.D.; Zawodny, J.M.; Gupta, M.C. Temperature-dependent optical properties of lead selenide quantum dot polymer nanocomposites. Appl. Opt. 2017, 56, 1982–1989. [Google Scholar] [CrossRef] [PubMed]
- Vibin, M.; Vinayakan, R.; Fernandez, F.B.; John, A.; Abraham, A. A Novel Fluorescent Quantum Dot Probe for the Rapid Diagnostic High Contrast Imaging of Tumor in Mice. J. Fluoresc. 2017, 27, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Karatay, A.; Yuksek, M.; Ertap, H.; Mak, A.K.; Karabulut, M.; Elmali, A. Influence of boron concentration on nonlinear absorption and ultrafast dynamics in GaSe crystals. Opt. Mater. 2016, 60, 74–80. [Google Scholar] [CrossRef]
- Huang, Z.; Zeng, Q.; Bai, Z.; Qin, S. Regulating the Fluorescence Emission of CdSe Quantum Dots Based on the Surface Ligand Exchange with MAA. Polym. Adv. Technol. 2020, 31, 2667–2675. [Google Scholar] [CrossRef]
- Oleshko, V.; Tarasenko, V.F.; Erofeev, M.V.; Vil’chinskaya, S.S. Pulsed X-Ray and Cathodoluminescence of Pure and Alloyed Zinc Selenide Single Crystals. Russ. Phys. J. 2020, 63, 311–316. [Google Scholar] [CrossRef]
- Singh, M.R.; Persaud, P.D.; Yastrebov, S. A study of two-photon florescence in metallic nanoshells. Nanotechnology 2020, 31, 265203. [Google Scholar] [CrossRef]
- Shehata, N.; Samir, E.; Kandas, I. Gold/QDs-Embedded-Ceria Nanoparticles: Optical Fluorescence Enhancement as a Quenching Sensor. Appl. Sci. 2020, 10, 1236. [Google Scholar] [CrossRef]
- Chowdhury, A.D.; Takemura, K.; Khorish, I.M.; Nasrin, F.; Tun, M.M.N.; Morita, K.; Park, E.Y. The detection and identification of dengue virus serotypes with quantum dot and AuNP regulated localized surface plasmon resonance. Nanoscale Adv. 2020, 2, 699–709. [Google Scholar] [CrossRef]
- Singh, P.; Singh, S.; Singh, P.; Prakash, R.; Rai, S. Generation of red-NIR bi-modal fluorescence in hybrid nanostructure. Mater. Res. Bull. 2020, 122. [Google Scholar] [CrossRef]
- Sadeghi, S.; Davami, A. CdSe quantum dots capped with a deep eutectic solvent as a fluorescent probe for copper(II) determination in various drinks. Microchim. Acta 2020, 187, 1–9. [Google Scholar] [CrossRef]
- Chang, Y.; Liu, M.; Liu, J. Highly Selective Fluorescent Sensing of Phosphite through Recovery of Poisoned Nickel Oxide Nanozyme. Anal. Chem. 2020, 92, 3118–3124. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Seelen, E.A.; Mazrui, N.M.; Kerns, P.; Suib, S.L.; Zhao, J.; Mason, R.P. The interaction of mercury and methylmercury with chalcogenide nanoparticles. Environ. Pollut. 2019, 255, 113346. [Google Scholar] [CrossRef] [PubMed]
- Horstmann, C.; Kim, D.S.; Campbell, C.; Kim, K. Transcriptome Profile Alteration with Cadmium Selenide/Zinc Sulfide Quantum Dots in Saccharomyces cerevisiae. Biomolecules 2019, 9, 653. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, Z.; Xu, L.; Xu, Y.; Lin, Y.; Zhang, Y.; Sun, Y.; Yang, G. Rational Design of a Multifunctional Molecular Dye with Single Dose and Laser for Efficiency NIR-II Fluorescence/Photoacoustic Imaging Guided Photothermal Therapy. Anal. Chem. 2019, 91, 12476–12483. [Google Scholar] [CrossRef] [PubMed]
- Deka, A.; Saha, A.; Mohanta, D. Consequence of surfactant coating on the Raman active modes and highly symmetric blue-emission decay dynamics of cubic phase MnSe quantum dots. Phys. E Low Dimens. Syst. Nanostruct. 2019, 113, 226–232. [Google Scholar] [CrossRef]
- Henthorn, J.T.; Arias, R.J.; Koroidov, S.; Kroll, T.; Sokaras, D.; Bergmann, U.; Rees, D.C.; Debeer, S. Localized Electronic Structure of Nitrogenase FeMoco Revealed by Selenium K-Edge High Resolution X-ray Absorption Spectroscopy. J. Am. Chem. Soc. 2019, 141, 13676–13688. [Google Scholar] [CrossRef]
- Subramaniyan, S.B.; Anbazhagan, V. Water soluble cadmium selenide quantum dots for ultrasensitive detection of organic, inorganic and elemental mercury in biological fluids and live cells. RSC Adv. 2019, 9, 22274–22281. [Google Scholar] [CrossRef]
- Han, Y.; Wang, T.; Liu, H.; Zhang, S.; Zhang, H.; Li, M.; Sun, Q.; Li, Z. The release and detection of copper ions from ultrasmall theranostic Cu2−xSe nanoparticles. Nanoscale 2019, 11, 11819–11829. [Google Scholar] [CrossRef]
- Sun, Q.; Yang, S.-H.; Wu, L.; Dong, Q.-J.; Yang, W.-C.; Yang, G.-F. Detection of Intracellular Selenol-Containing Molecules Using a Fluorescent Probe with Near-Zero Background Signal. Anal. Chem. 2016, 88, 6084–6091. [Google Scholar] [CrossRef]
- Rupp, E.C.; Granite, E.J.; Stanko, D.C. Laboratory scale studies of Pd/γ-Al2O3 sorbents for the removal of trace contaminants from coal-derived fuel gas at elevated temperatures. Fuel 2013, 108, 131–136. [Google Scholar] [CrossRef]
- Gao, F.; Yang, L.; Tang, J.; Du, Z.; Wang, Y.; Hu, Z.; Han, D.; Huang, L.; Belfiore, L.A. Fin-like CdSeS Nanoplatelets for Pesticide Sensing. ACS Appl. Nano Mater. 2019, 2, 3459–3466. [Google Scholar] [CrossRef]
- Jang, M.-H.; Yoo, S.-S.; Kramer, M.T.; Dhar, N.K.; Gupta, M.C. Electrical transport properties of sensitized PbSe thin films for IR imaging sensors. Semicond. Sci. Technol. 2019, 34, 065009. [Google Scholar] [CrossRef]
- Sivasankaran, U.; Jesny, S.; Jose, A.R.; Kumar, K.G. Fluorescence Determination of Glutathione Using Tissue Paper-derived Carbon Dots as Fluorophores. Anal. Sci. 2017, 33, 281–285. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Jiang, X.; Kou, Y.; Lu, J.; Tan, L. Investigation of photo-induced electron transfer between amino-functionalized graphene quantum dots and selenium nanoparticle and it’s application for sensitive fluorescent detection of copper ions. Talanta 2019, 197, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Alifu, N.; Cai, Y.; Lam, J.W.Y.; He, X.; Su, H.; Zhang, P.; Qian, J.; Tang, B.Z. Synthesis of an efficient far-red/near-infrared luminogen with AIE characteristics for in vivo bioimaging applications. Chem. Commun. 2019, 55, 5615–5618. [Google Scholar] [CrossRef]
- Liu, X.; Song, X.; Luan, D.; Hu, B.; Xu, K.; Tang, B. Real-Time in Situ Visualizing of the Sequential Activation of Caspase Cascade Using a Multicolor Gold–Selenium Bonding Fluorescent Nanoprobe. Anal. Chem. 2019, 91, 5994–6002. [Google Scholar] [CrossRef]
- Walekar, L.S.; Zheng, M.; Zheng, L.; Long, M. Selenium and nitrogen co-doped carbon quantum dots as a fluorescent probe for perfluorooctanoic acid. Microchim. Acta 2019, 186, 278. [Google Scholar] [CrossRef]
- Dias, E.H.; Pereira, D.F.; De Sousa, B.B.; Matias, M.S.; De Queiroz, M.R.; Santiago, F.M.; Silva, A.C.; Dantas, N.O.; Santos-Filho, N.A.; De Oliveira, C.J. In vitro tracking of phospholipase A2 from snake venom conjugated with magic-sized quantum dots. Int. J. Biol. Macromol. 2019, 122, 461–468. [Google Scholar] [CrossRef]
- Ramaraj, S.; Sakthivel, M.; Chen, S.-M.; Ho, K.-C. Ultrasound-assisted synthesis of two-dimensional layered ytterbium substituted molybdenum diselenide nanosheets with excellent electrocatalytic activity for the electrochemical detection of diphenylamine anti-scald agent in fruit extract. Ultrason. Sonochem. 2019, 50, 265–277. [Google Scholar] [CrossRef]
- Huang, K.; Xu, K.; Zhu, W.; Yang, L.; Hou, X.; Zheng, C. Hydride Generation for Headspace Solid-Phase Extraction with CdTe Quantum Dots Immobilized on Paper for Sensitive Visual Detection of Selenium. Anal. Chem. 2015, 88, 789–795. [Google Scholar] [CrossRef]
- Killingsworth, M.C.; Bobryshev, Y.V. Correlative Light- and Electron Microscopy Using Quantum Dot Nanoparticles. J. Vis. Exp. 2016, e54307. [Google Scholar] [CrossRef] [PubMed]
- Mir, I.A.; Rawat, K.; Bohidar, H.B. CuInGaSe nanocrystals for detection of trace amount of water in D2O (at ppm level). Cryst. Res. Technol. 2016, 51, 561–568. [Google Scholar] [CrossRef]
- Rao, Y.N.; Datta, A.; Das, S.K.; Saha, A. Irradiation route to aqueous synthesis of highly luminescent ZnSe quantum dots and its function as a copper ion fluorescence sensor. Mater. Res. Bull. 2016, 80, 280–287. [Google Scholar] [CrossRef]
- Chopra, S.S.; Theis, T.L. Comparative cradle-to-gate energy assessment of indium phosphide and cadmium selenide quantum dot displays. Environ. Sci. Nano 2016, 4, 244–254. [Google Scholar] [CrossRef]
- Perez, R.V.; Machado, C.G.; Santos-Bezerra, D.P.; Admoni, S.N.; Patente, T.A.; Monteiro, M.B.; Cavaleiro, A.M.; Queiroz, M.S.; Nery, M.; Correa-Giannella, M.L. Allelic variations in genes belonging to glutathione system increase proliferative retinopathy risk in type 1 diabetes individuals. Gene 2019, 703, 120–124. [Google Scholar] [CrossRef]
- Gong, D.; Han, S.-C.; Iqbal, A.; Qian, J.; Cao, T.; Liu, W.; Liu, W.; Qin, W.; Guo, H. Fast and Selective Two-Stage Ratiometric Fluorescent Probes for Imaging of Glutathione in Living Cells. Anal. Chem. 2017, 89, 13112–13119. [Google Scholar] [CrossRef]
- Gong, D.; Ru, J.; Cao, T.; Qian, J.; Liu, W.; Iqbal, A.; Liu, W.; Qin, W.; Guo, H. Two-stage ratiometric fluorescent responsive probe for rapid glutathione detection based on BODIPY thiol-halogen nucleophilic mono- or disubstitution. Sens. Actuators B Chem. 2018, 258, 72–79. [Google Scholar] [CrossRef]
- Chen, L.; Park, J.-S.; Wu, D.; Kim, C.-H.; Yoon, J. A colorimetric and fluorescent probe for rapid detection of glutathione and its application to tissue specific bio-imaging in living cells and zebrafish. Sens. Actuators B Chem. 2018, 262, 306–312. [Google Scholar] [CrossRef]
- Eteshola, E.O.U.; Haupt, D.A.; Koos, S.I.; Siemer, L.A.; Morris, D.L. The role of metal ion binding in the antioxidant mechanisms of reduced and oxidized glutathione in metal-mediated oxidative DNA damage. Metallomics 2020, 12, 79–91. [Google Scholar] [CrossRef]
- Wang, K.; Nie, G.; Ran, S.; Wang, H.; Liu, X.; Zheng, Z.; Zhang, Y. A “turn-on” near-infrared fluorescent probe with high sensitivity for detecting reduced glutathione based on red shift in vitro and in vivo. Dye. Pigment. 2020, 172, 107837. [Google Scholar] [CrossRef]
- Li, Y.; Fan, X.; Chen, L.; Zeng, H.; Chen, X.; Lun, W.; Fan, X.; Wong, W.-Y. A time-resolved near-infrared phosphorescent iridium(iii) complex for fast and highly specific peroxynitrite detection and bioimaging applications. J. Mater. Chem. B 2019, 7, 7612–7618. [Google Scholar] [CrossRef]
- Kim, S.; Ko, C.W.; Lim, T.; Yoo, S.; Ham, H.J.; Kang, S.-Y.; Kang, S.; Cho, S.K.; Han, M.S. A hydrazone-based turn-on fluorescent probe for peroxynitrite detection and live-cell imaging. Dye. Pigment. 2019, 171, 107762. [Google Scholar] [CrossRef]
- Xin, F.; Tian, Y.; Zhang, X. Ratiometric fluorescent probe for highly selective detection of gaseous H2Se. Dye. Pigment. 2020, 177, 108274. [Google Scholar] [CrossRef]
- Zare, B.; Nami, M.; Shahverdi, A.-R. Tracing Tellurium and Its Nanostructures in Biology. Biol. Trace Element Res. 2017, 180, 171–181. [Google Scholar] [CrossRef]




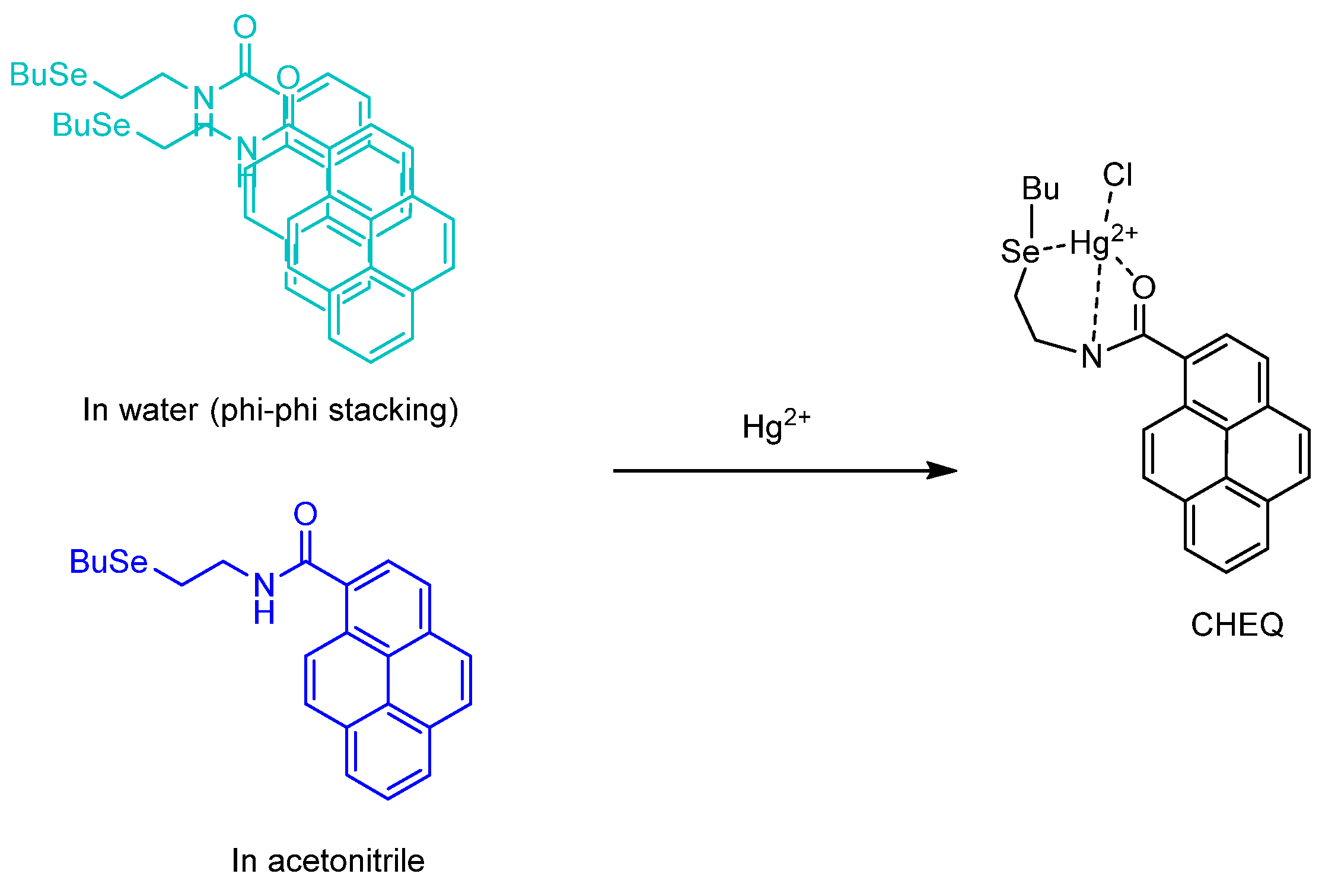
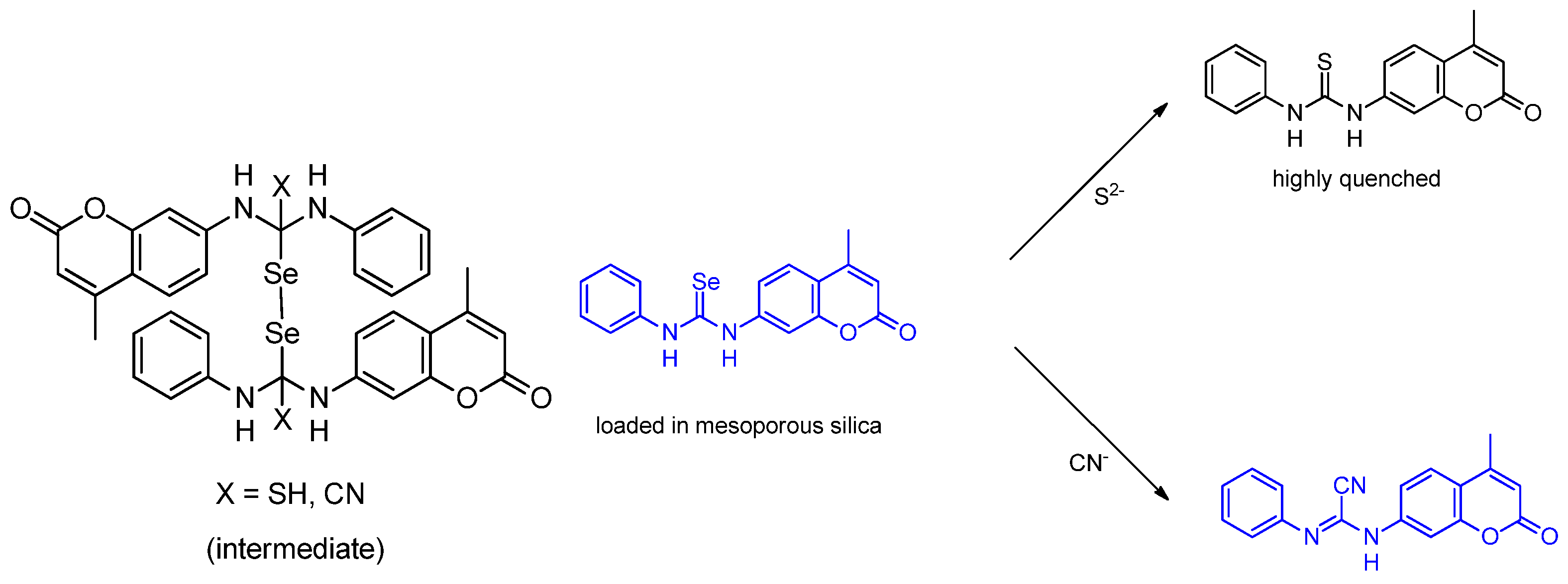
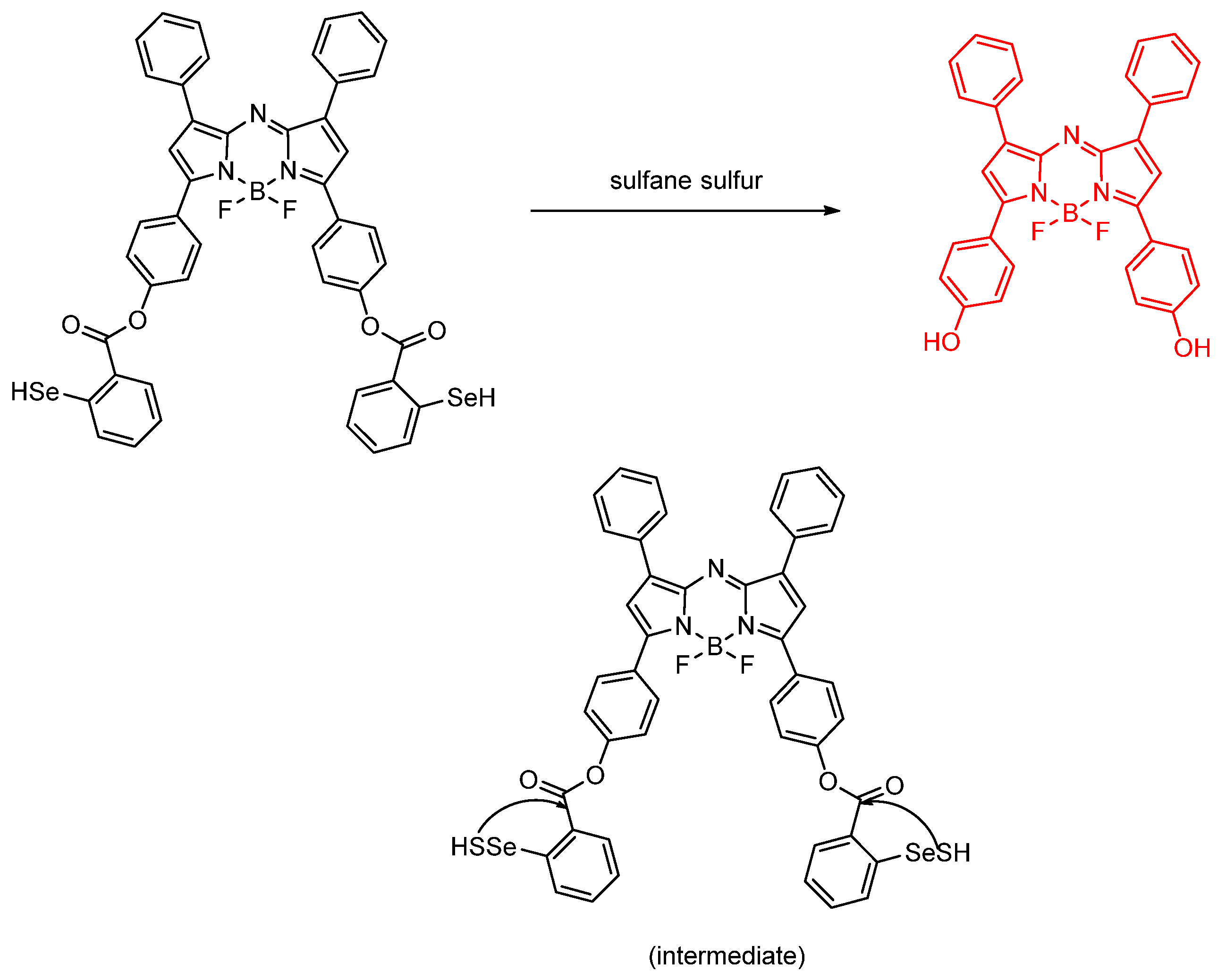
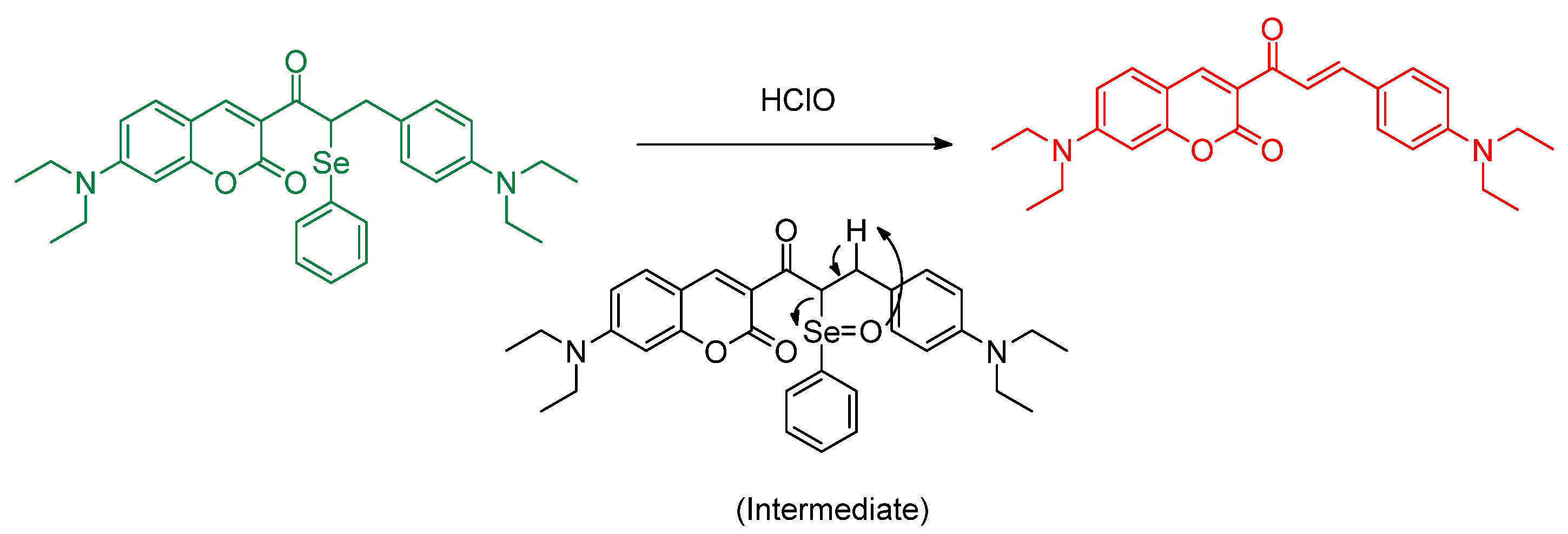
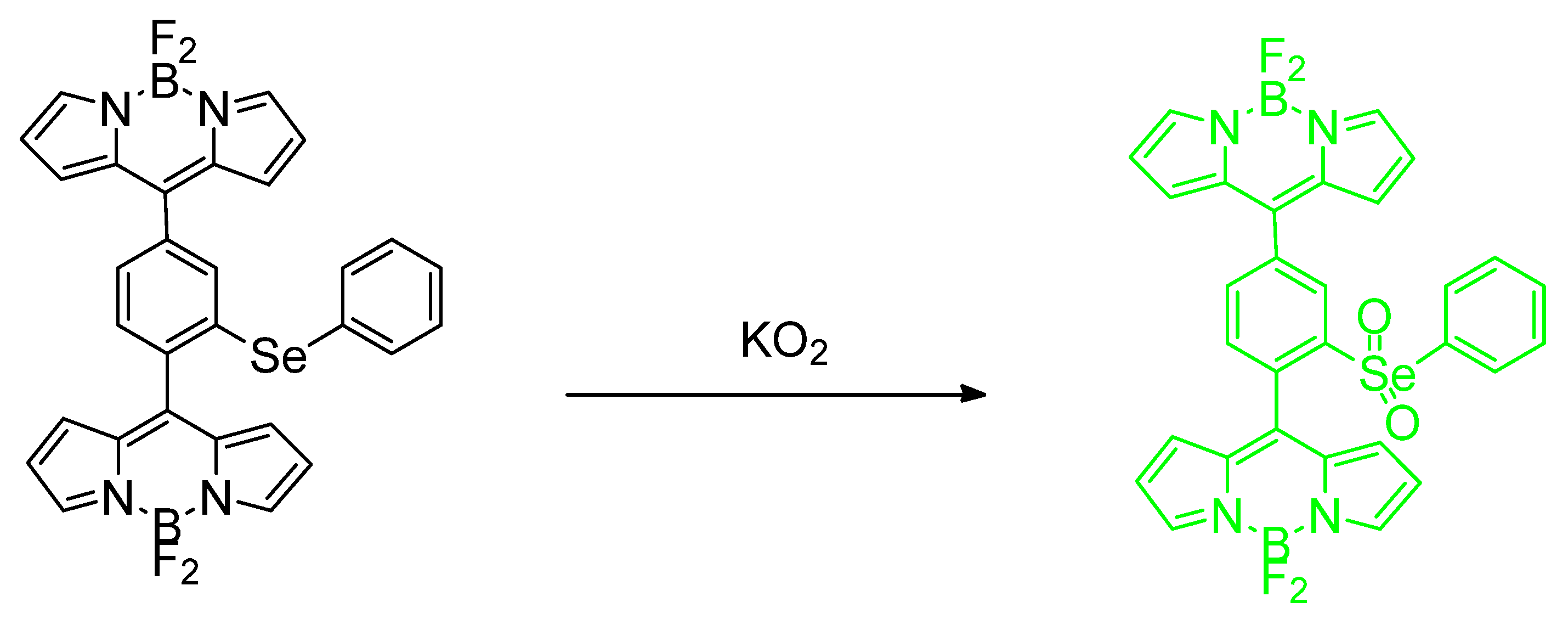


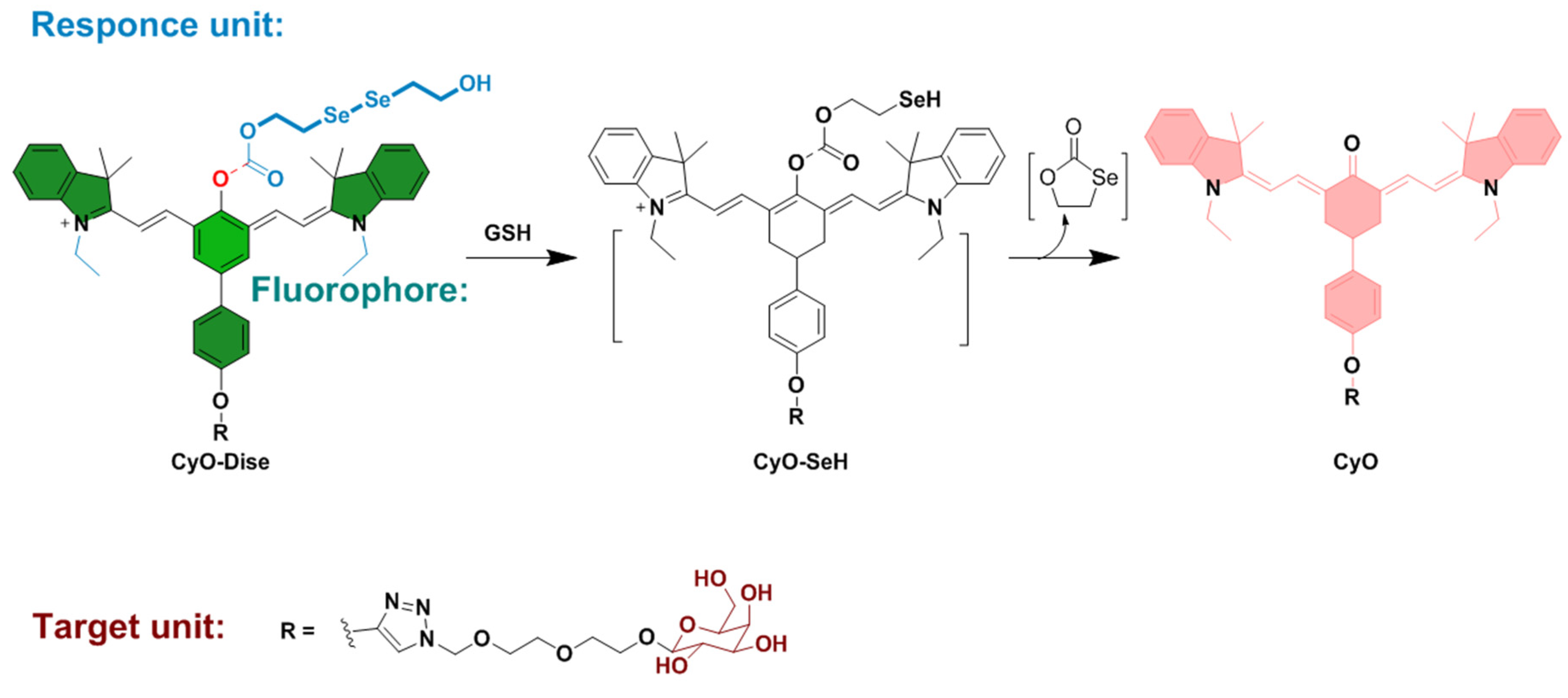
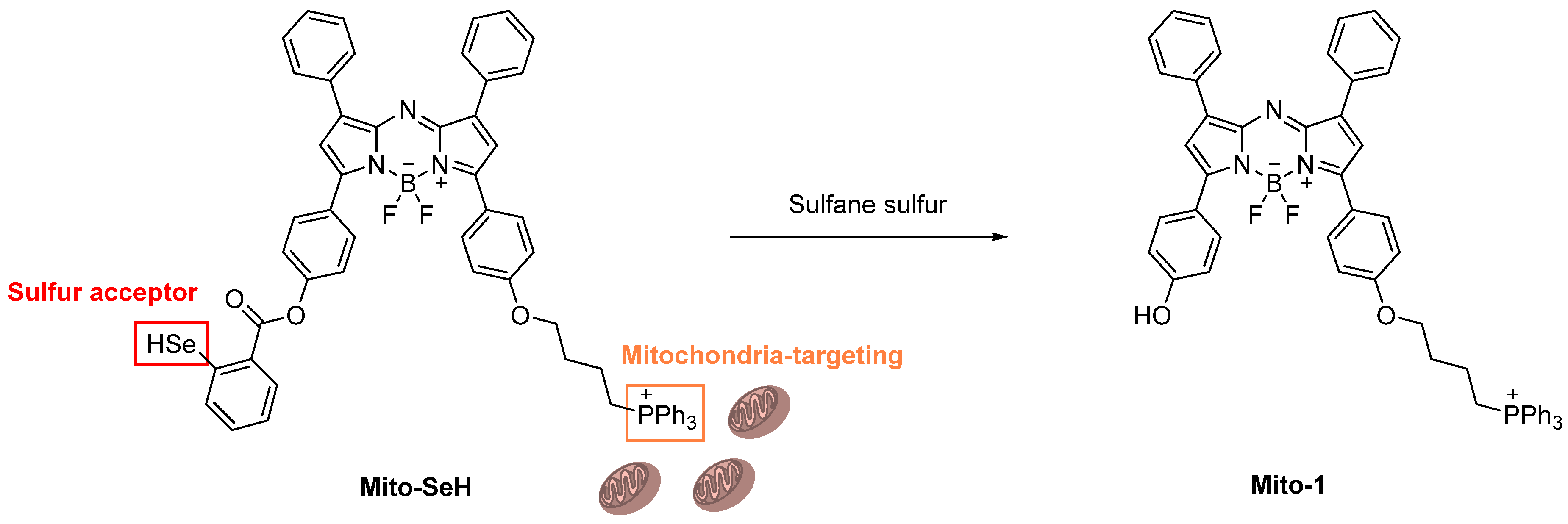
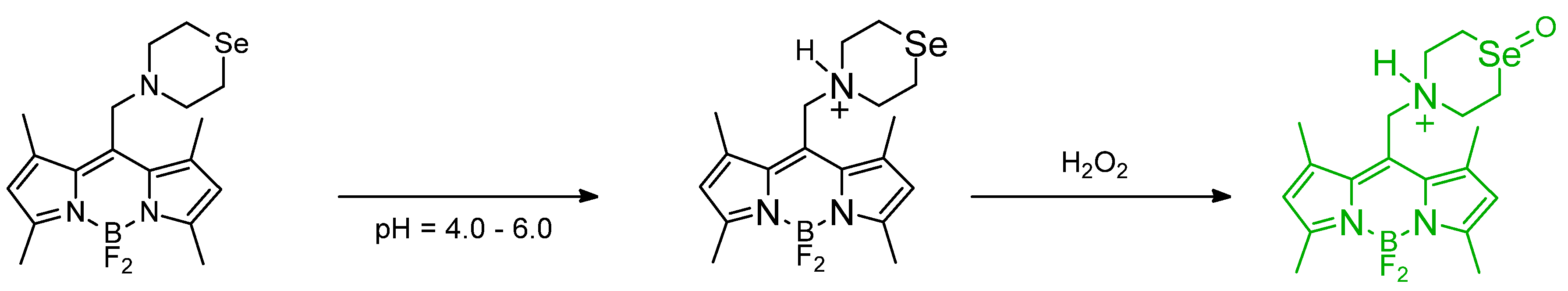
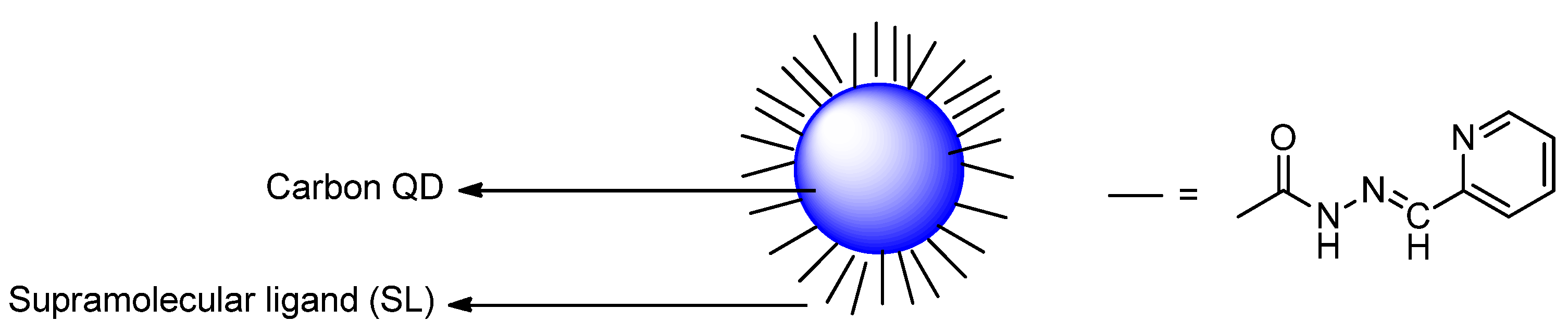

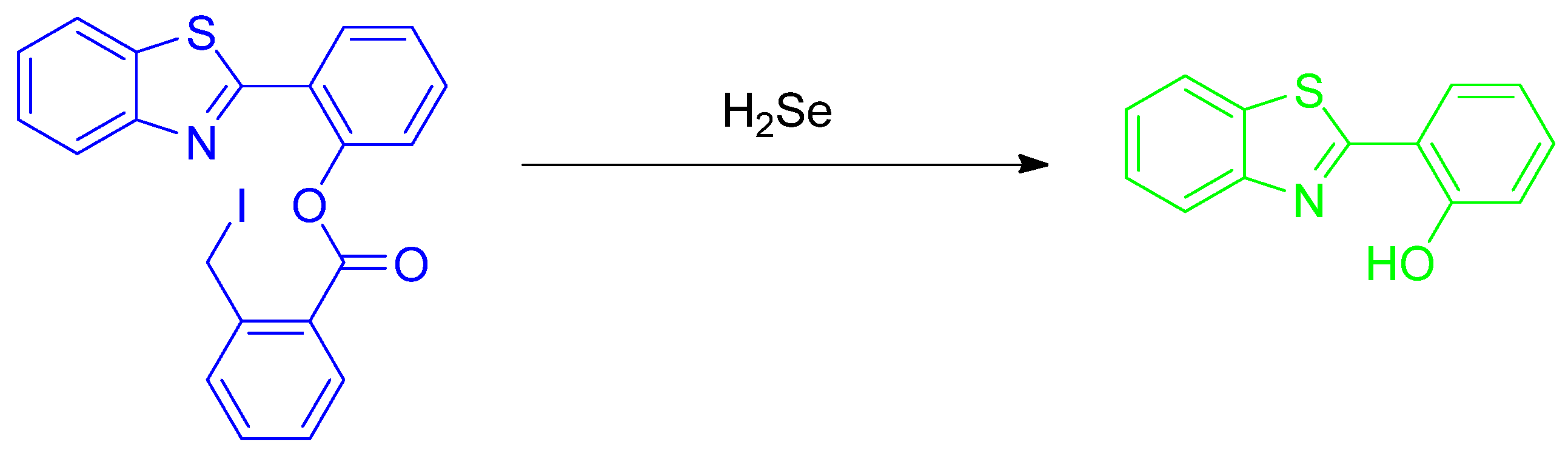
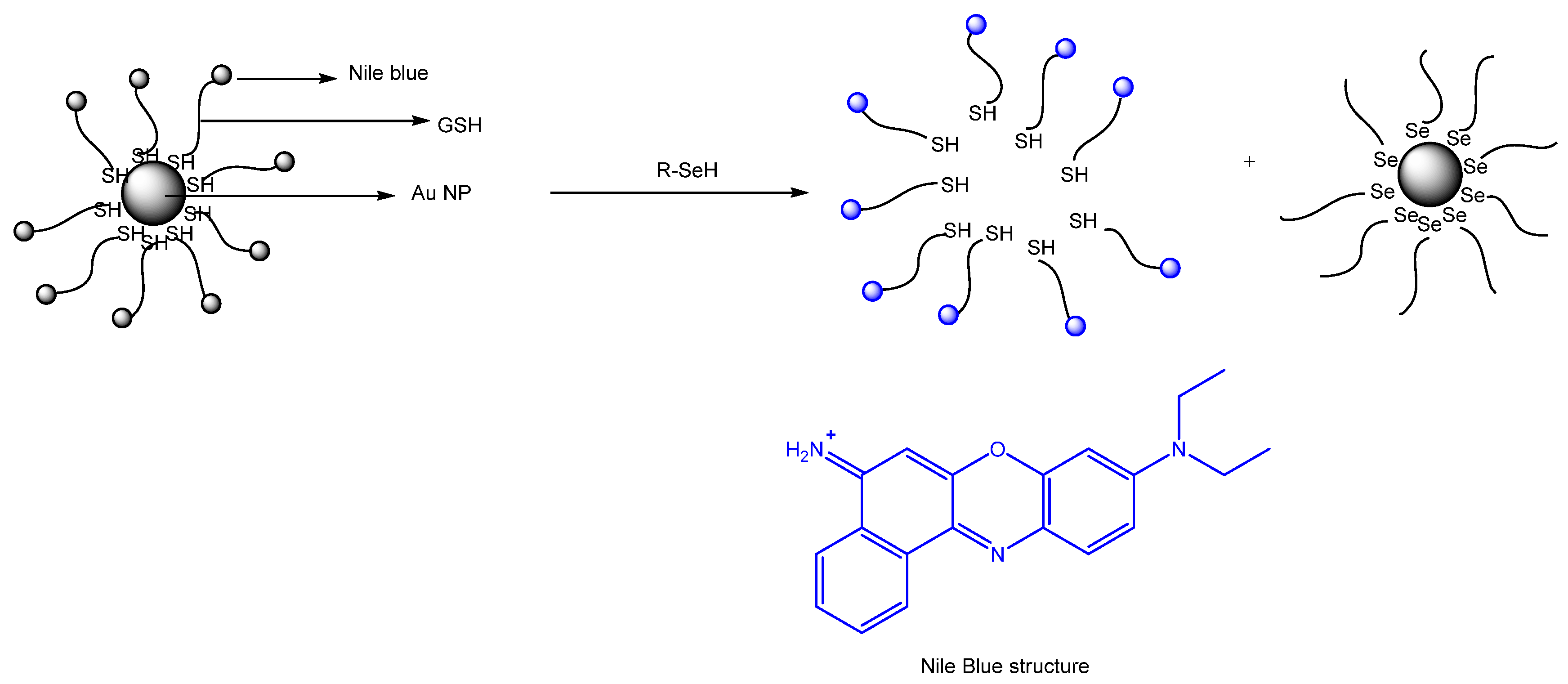
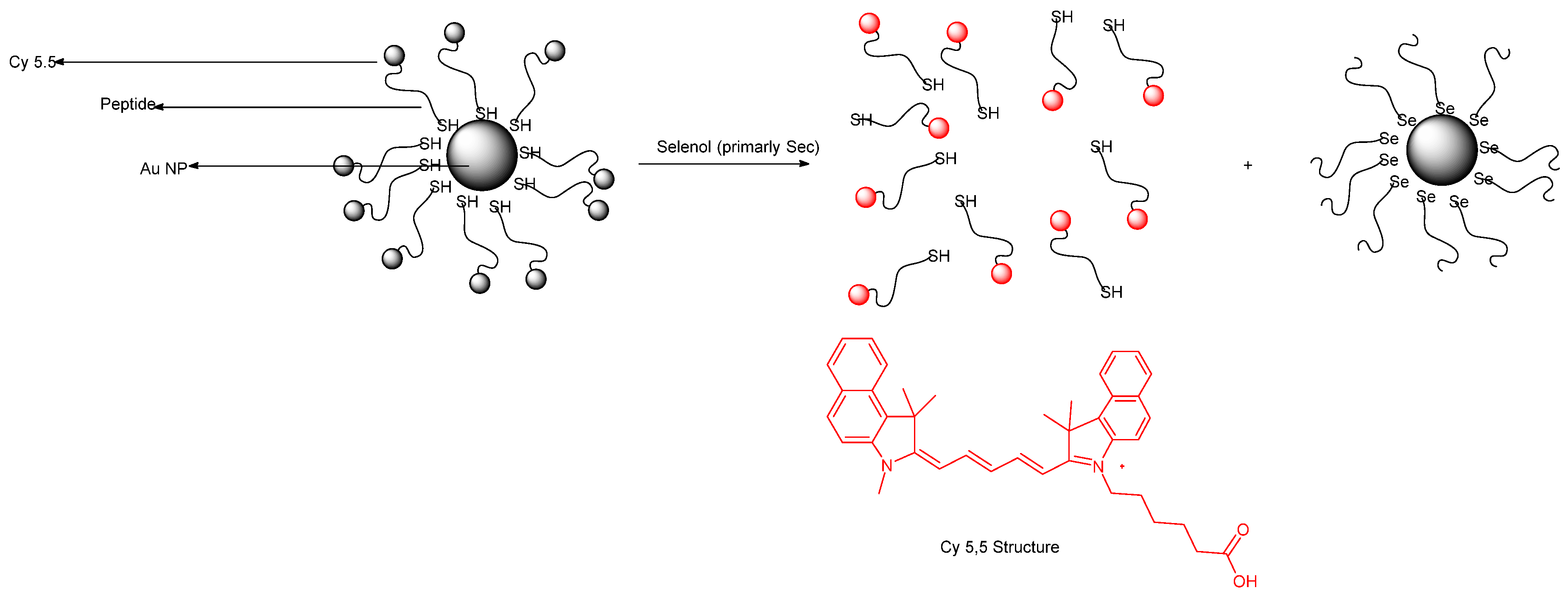
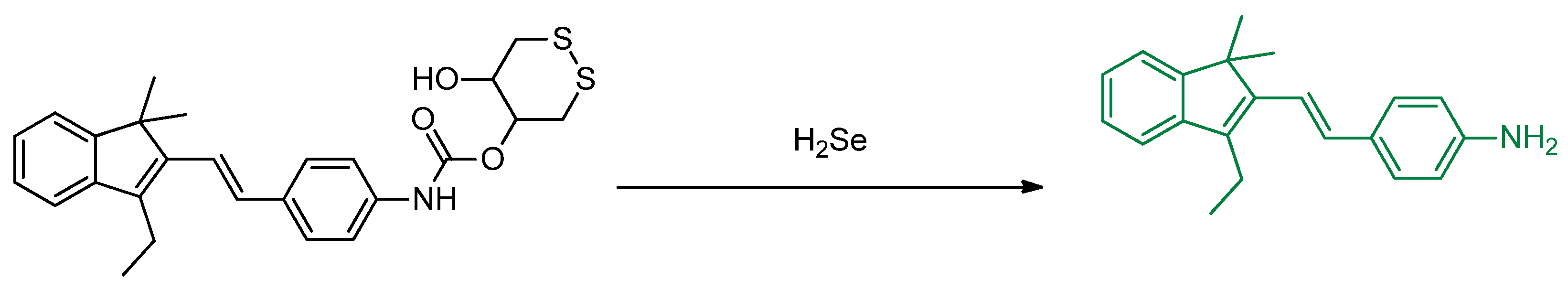
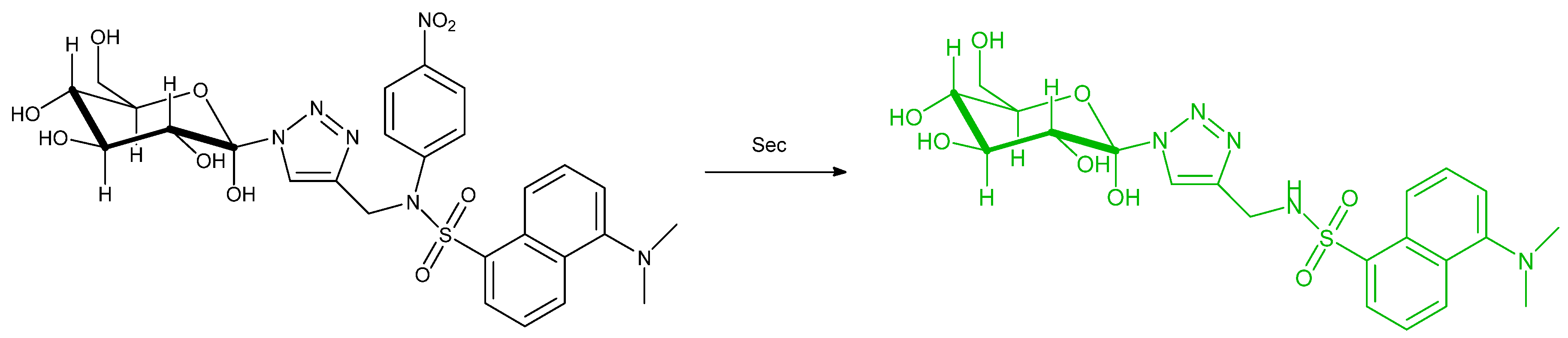
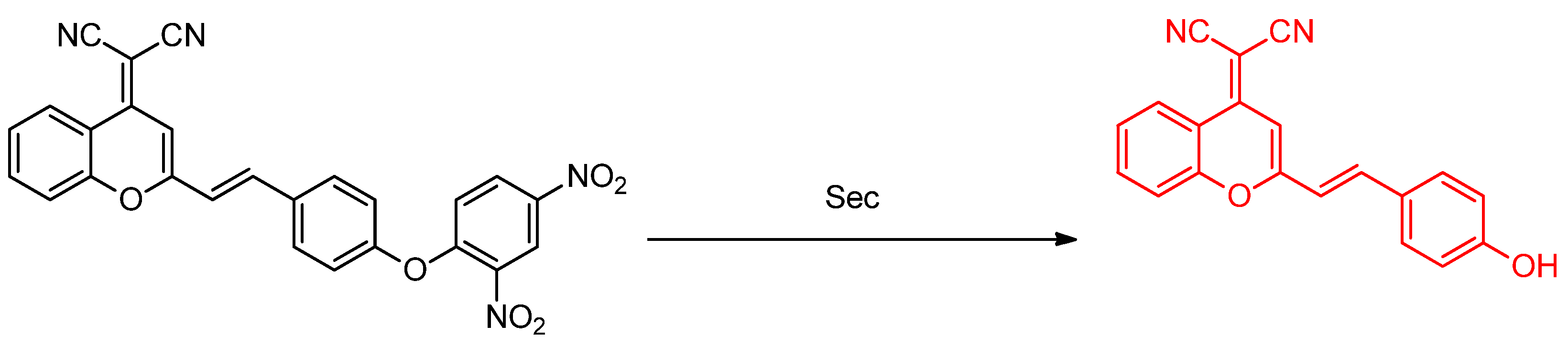
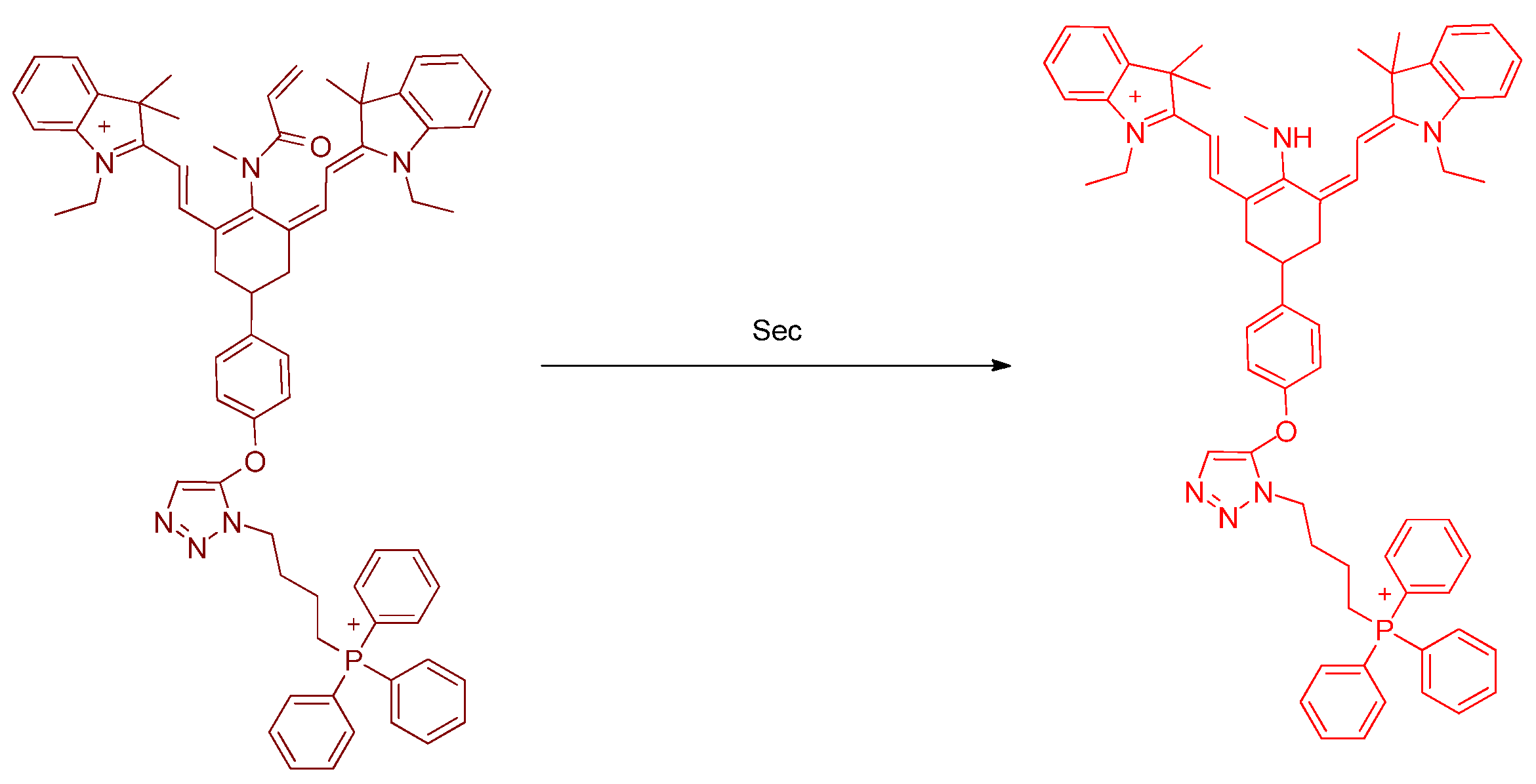

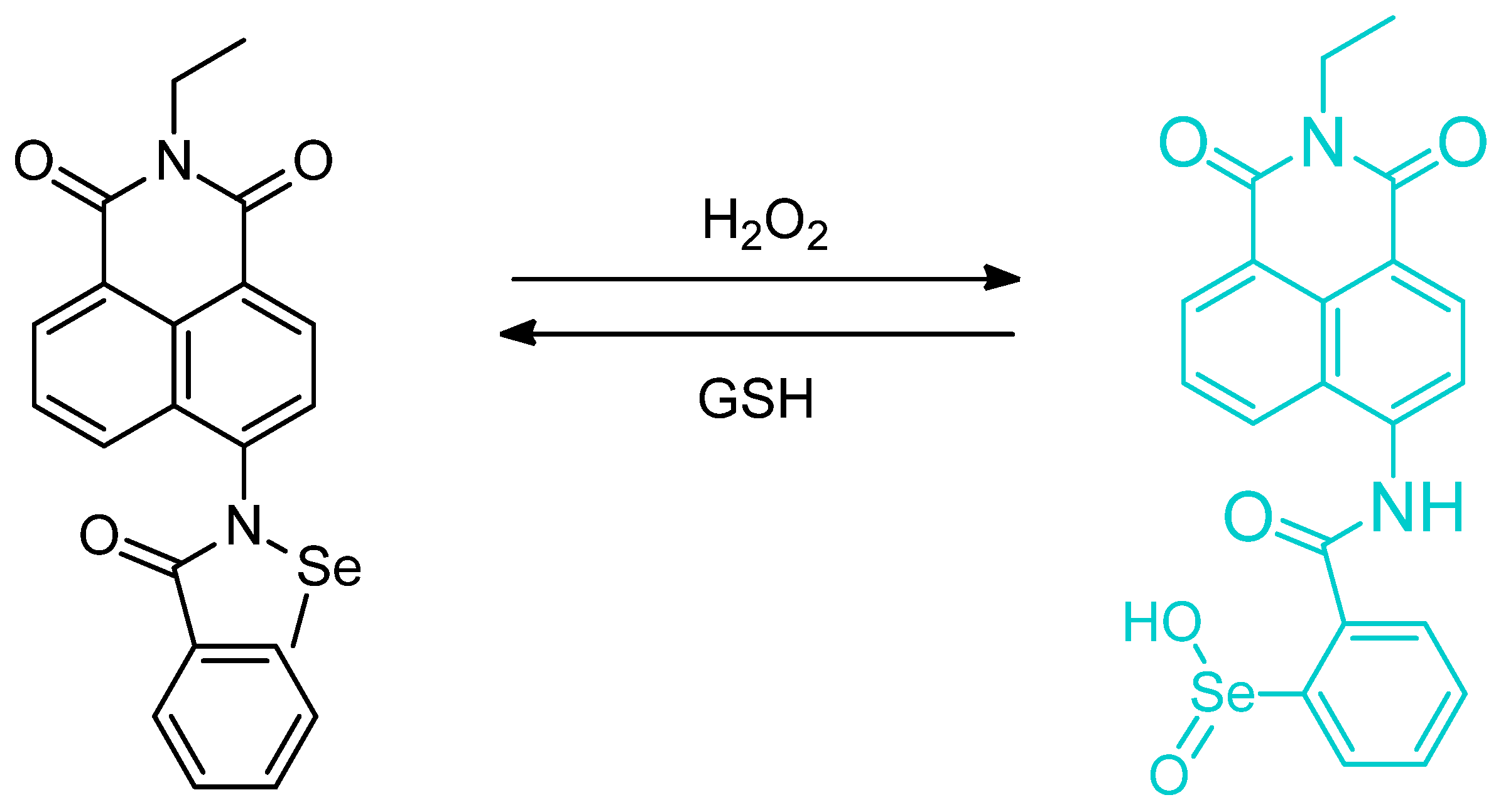
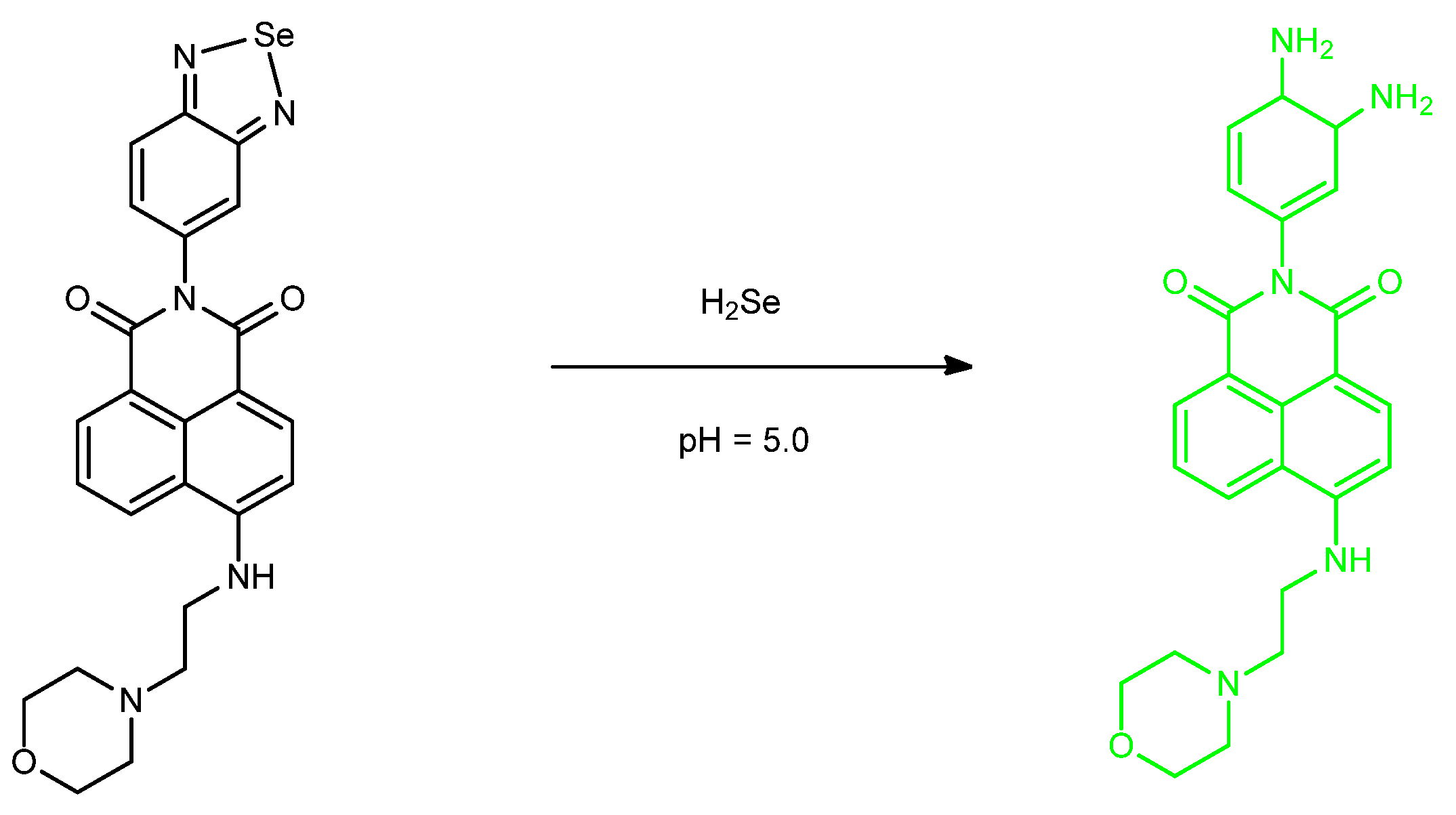
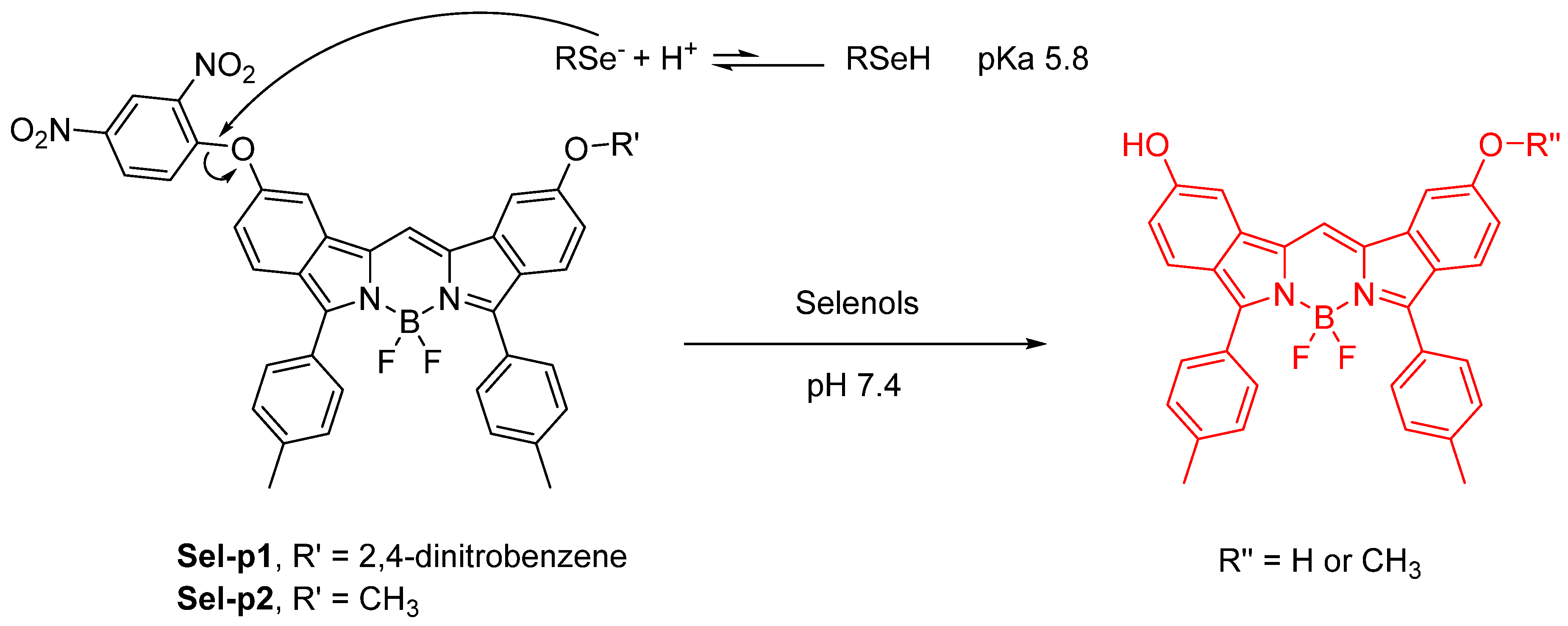
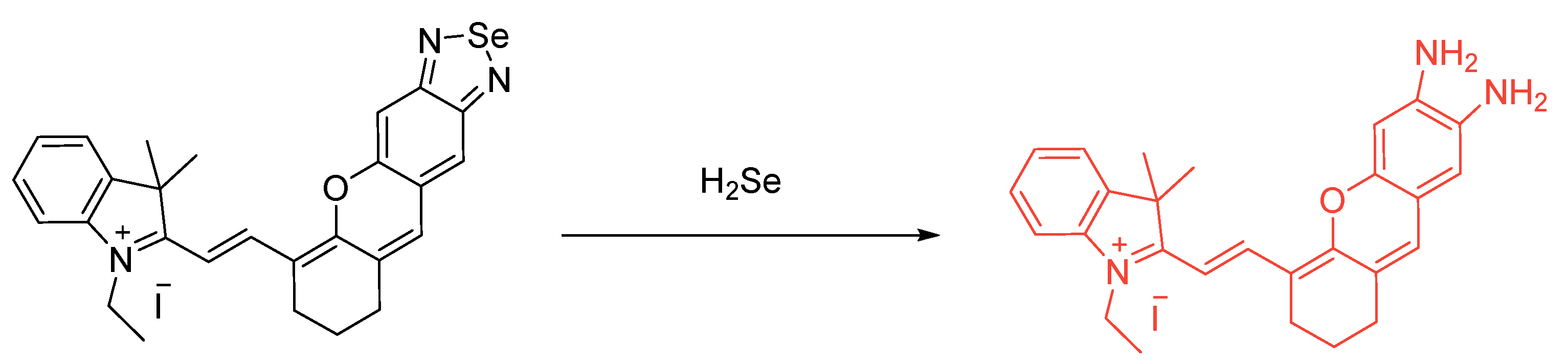
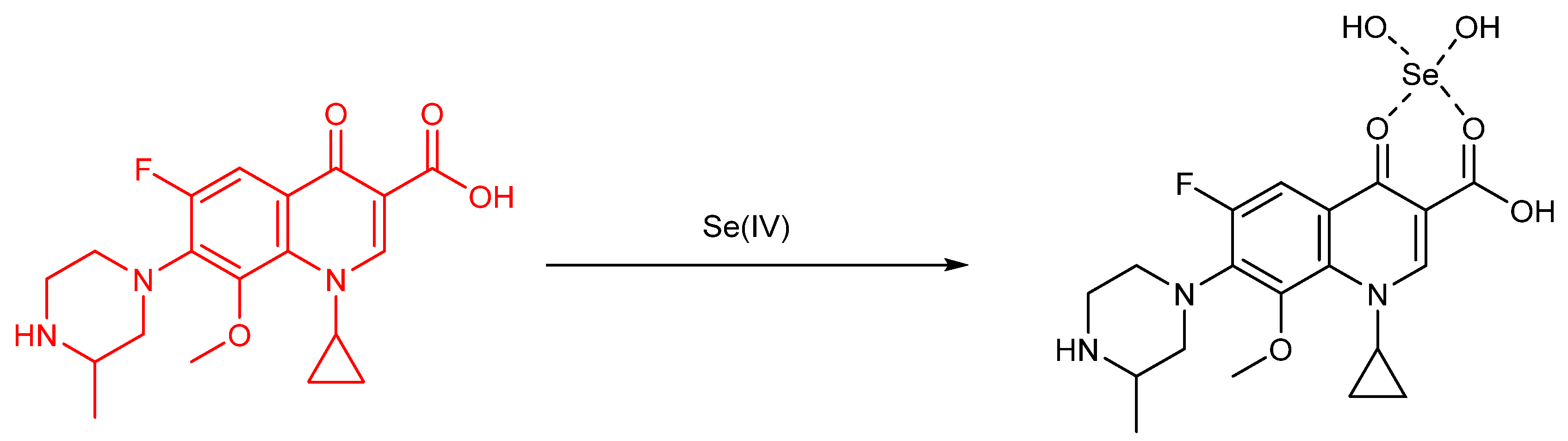
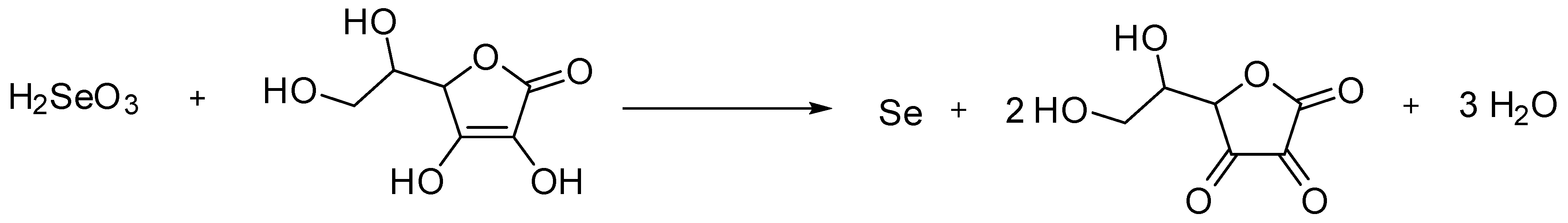
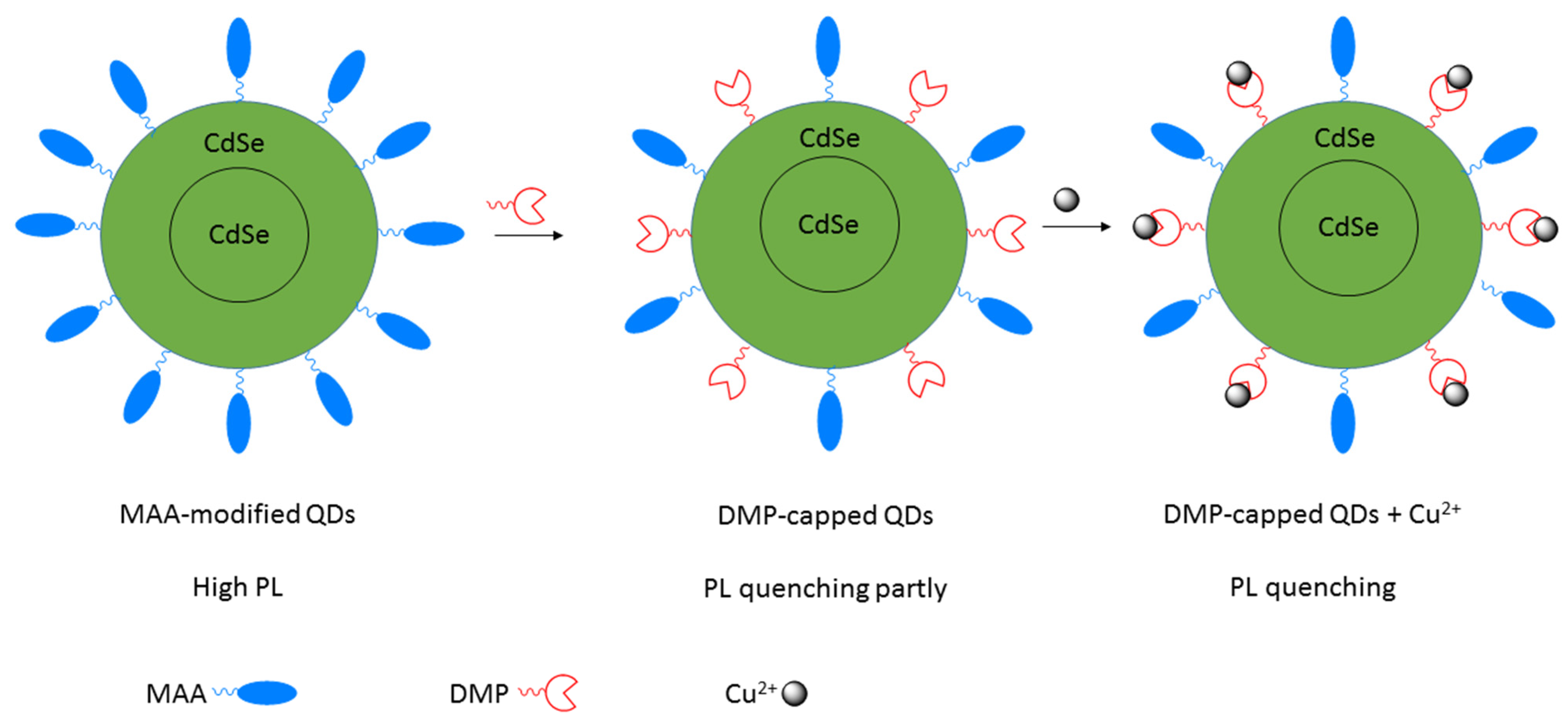

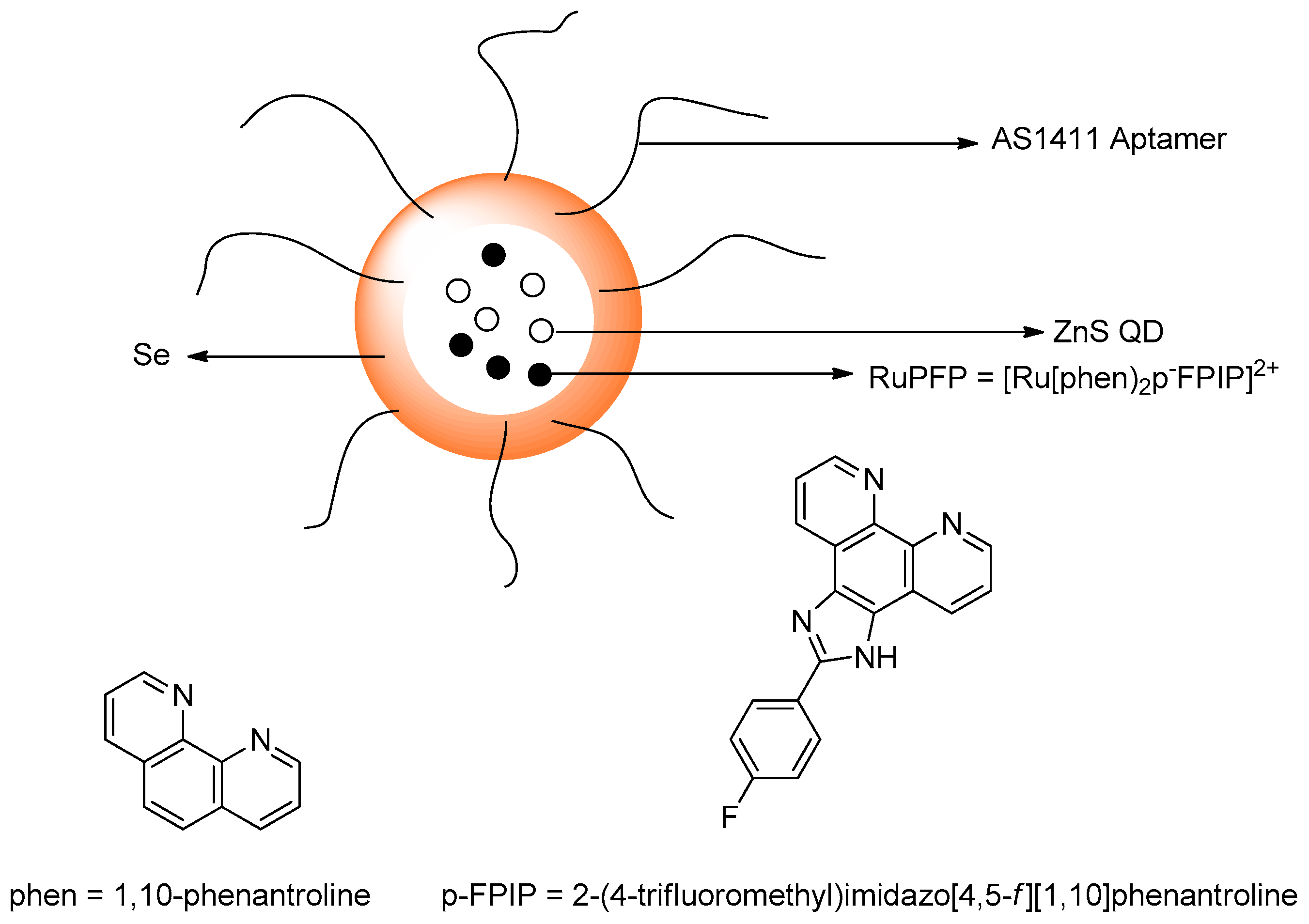

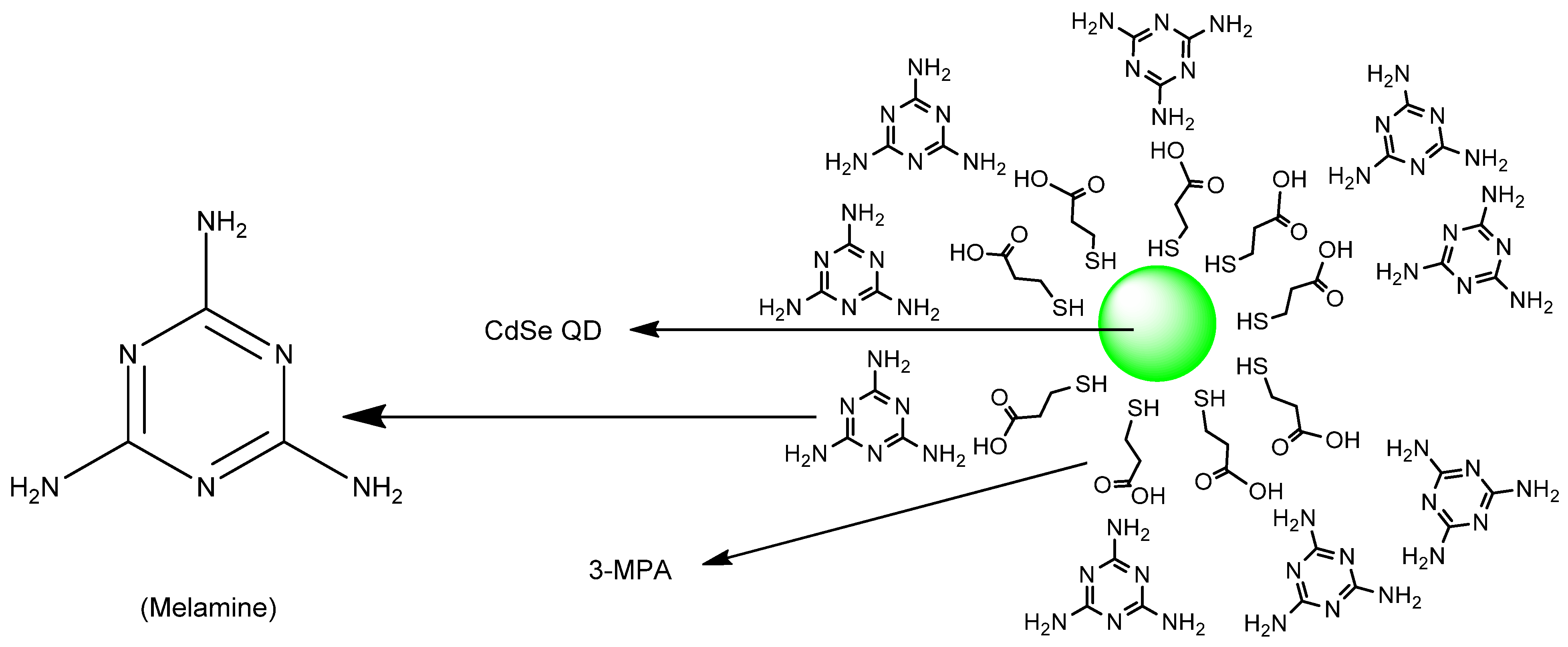

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdillah, A.; Sonawane, P.M.; Kim, D.; Mametov, D.; Shimodaira, S.; Park, Y.; Churchill, D.G. Discussions of Fluorescence in Selenium Chemistry: Recently Reported Probes, Particles, and a Clearer Biological Knowledge. Molecules 2021, 26, 692. https://doi.org/10.3390/molecules26030692
Abdillah A, Sonawane PM, Kim D, Mametov D, Shimodaira S, Park Y, Churchill DG. Discussions of Fluorescence in Selenium Chemistry: Recently Reported Probes, Particles, and a Clearer Biological Knowledge. Molecules. 2021; 26(3):692. https://doi.org/10.3390/molecules26030692
Chicago/Turabian StyleAbdillah, Ariq, Prasad M. Sonawane, Donghyeon Kim, Dooronbek Mametov, Shingo Shimodaira, Yunseon Park, and David G. Churchill. 2021. "Discussions of Fluorescence in Selenium Chemistry: Recently Reported Probes, Particles, and a Clearer Biological Knowledge" Molecules 26, no. 3: 692. https://doi.org/10.3390/molecules26030692
APA StyleAbdillah, A., Sonawane, P. M., Kim, D., Mametov, D., Shimodaira, S., Park, Y., & Churchill, D. G. (2021). Discussions of Fluorescence in Selenium Chemistry: Recently Reported Probes, Particles, and a Clearer Biological Knowledge. Molecules, 26(3), 692. https://doi.org/10.3390/molecules26030692






