Self-Assembled Nanomaterials Based on Complementary Sn(IV) and Zn(II)-Porphyrins, and Their Photocatalytic Degradation for Rhodamine B Dye
Abstract
1. Introduction
2. Results and Discussion
2.1. Syntheses
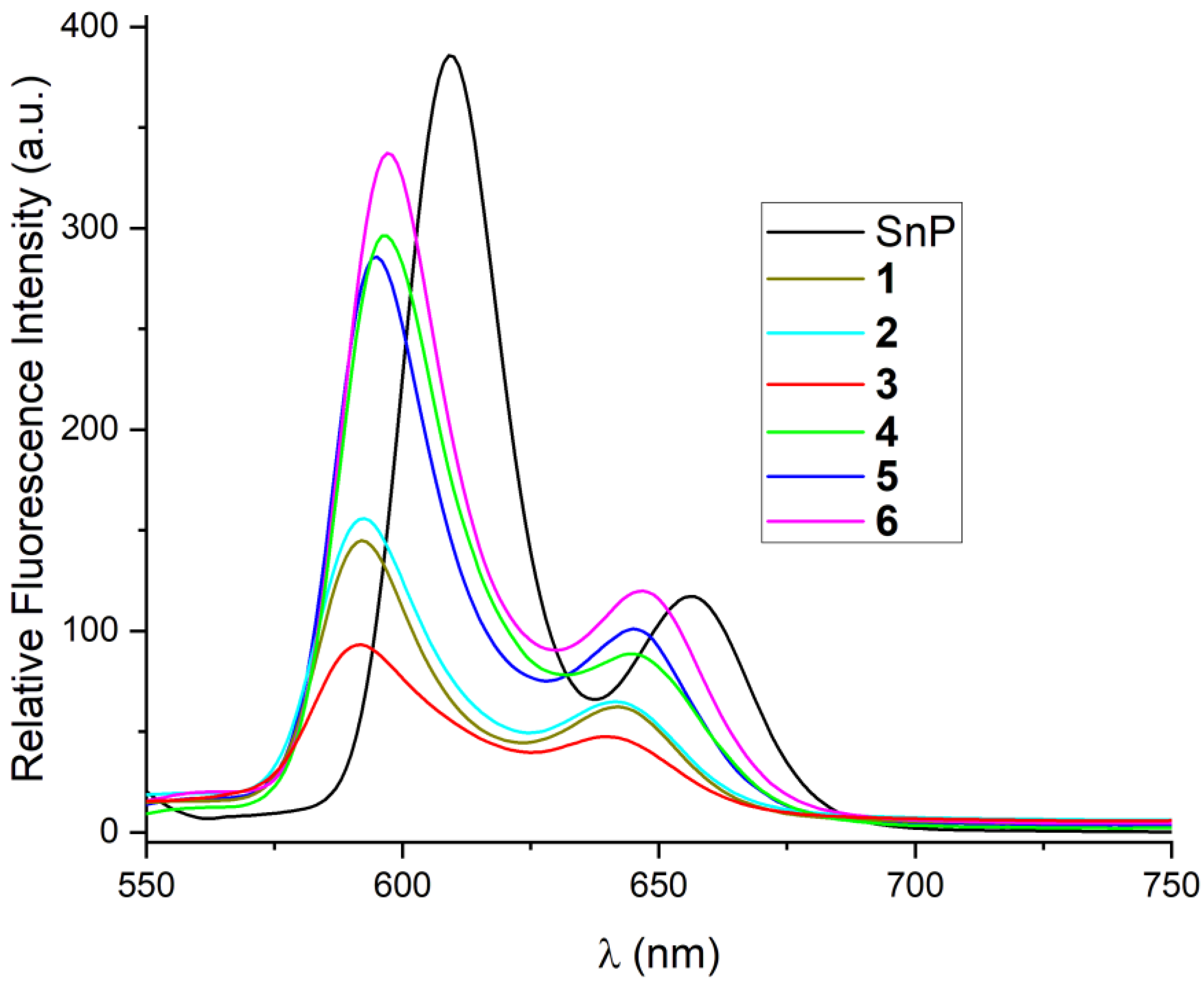
2.2. Spectroscopic Characterization
2.3. Supramolecular Self-Assembly to Nanostructures
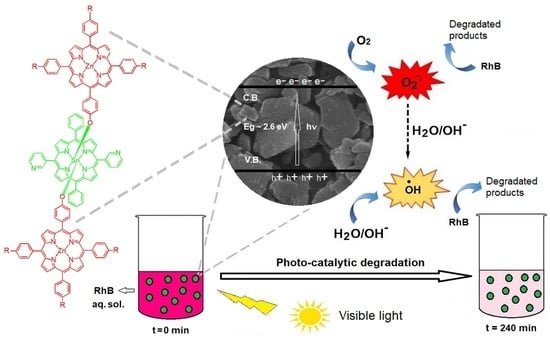
2.4. Photocatalysis for the Degradation of Rhodamine B (RhB) Dye
3. Conclusions
4. Materials and Methods
4.1. General Procedure for the Synthesis of Free Porphyrin H2Ln
4.1.1. Synthesis of Meso-5-(4-hydroxyphenyl)-10,15,20-tris(phenyl)porphyrin H2L1
4.1.2. Synthesis of Meso-5-(4-hydroxyphenyl)-10,15,20-tris(4-methylphenyl)porphyrin H2L2
4.1.3. Synthesis of Meso-5-(4-hydroxyphenyl)-10,15,20-tris(4-tert-butylphenyl)porphyrin H2L3
4.1.4. Synthesis of Meso-5-(4-hydroxyphenyl)-10,15,20-tris(4-methoxyphenyl)porphyrin H2L4
4.1.5. Synthesis of Meso-5-(4-hydroxyphenyl)-10,15,20-tris(4-chlorophenyl)porphyrin H2L5
4.1.6. Synthesis of Meso-5-(4-hydroxyphenyl)-10,15,20-tris(4-nitrophenyl)porphyrin H2L6
4.2. General Procedure for the Synthesis of Zn(II)-Porphyrin ZnLn
4.2.1. Synthesis of Meso-5-(4-hydroxyphenyl)-10,15,20-tris(phenyl)porphyrinato)zinc(II) ZnL1
4.2.2. Synthesis of Meso-5-(4-hydroxyphenyl)-10,15,20-tris(4-methylphenyl)porphyrinato)zinc(II) ZnL2
4.2.3. Synthesis of Meso-5-(4-hydroxyphenyl)-10,15,20-tris(4-tert-butylphenyl)porphyrinato)zinc(II) ZnL3
4.2.4. Synthesis of Meso-5-(4-hydroxyphenyl)-10,15,20-tris(4-methoxyphenyl)porphyrinato)zinc(II) ZnL4
4.2.5. Synthesis of Meso-5-(4-hydroxyphenyl)-10,15,20-tris(4-chlorophenyl)porphyrinato)zinc(II) ZnL5
4.2.6. Synthesis of Meso-5-(4-hydroxyphenyl)-10,15,20-tris(4-nitrophenyl)porphyrinato)zinc(II) ZnL6
4.3. General Procedure for the Synthesis of the Triad
4.3.1. Synthesis of Triad 1
4.3.2. Synthesis of Triad 2
4.3.3. Synthesis of Triad 3
4.3.4. Synthesis of Triad 4
4.3.5. Synthesis of Triad 5
4.3.6. Synthesis of Triad 6
4.4. Photocatalytic Degradation of Rhodamine B by Nanoaggregates of Triad 1–6
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Astruc, D. Introduction: Nanoparticles in Catalysis. Chem. Rev. 2020, 120, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Uchaker, E.; Candelaria, S.L.; Cao, G. Nanomaterials for energy conversion and storage. Chem. Soc. Rev. 2013, 42, 3127–3171. [Google Scholar] [CrossRef]
- Sun, H.; Ren, J.; Qu, X. Carbon Nanomaterials and DNA: From Molecular Recognition to Applications. Acc. Chem. Res. 2016, 49, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Munonde, T.S.; Nomngongo, P.N. Nanocomposites for Electrochemical Sensors and Their Applications on the Detection of Trace Metals in Environmental Water Samples. Sensors 2020, 21, 131. [Google Scholar] [CrossRef]
- Montaseri, H.; Kruger, C.A.; Abrahamse, H. recent advances in porphyrin-based inorganic nanoparticles for cancer treatment. Int. J. Mol. Sci. 2020, 21, 3358. [Google Scholar] [CrossRef]
- Minea, A.A. A Review on Electrical Conductivity of Nanoparticle-Enhanced Fluids. Nanomaterials 2019, 9, 1592. [Google Scholar] [CrossRef]
- Wang, J.; Mu, X.; Sun, M.; Mu, T. Optoelectronic properties and applications of graphene-based hybrid nanomaterials and van der Waals hetero structures. Appl. Mater. Today 2019, 16, 1–20. [Google Scholar] [CrossRef]
- Saji, V.S.; Cook, R. Corrosion Protection and Control Using Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- O’Regan, B.; Grätzel, M. A Low-Cost, High-Efficiency Solar Cell Based on Dye-Sensitized Colloidal TiO2 Films. Renew. Energy 2018, 335, 208–213. [Google Scholar] [CrossRef]
- Wang, S.-P.; Lin, W.; Wang, X.; Cen, T.-Y.; Xie, H.; Huang, J.; Zhu, B.-Y.; Zhang, Z.; Song, A.; Hao, J.; et al. Controllable hierarchical self-assembly of porphyrin-derived supra-amphiphiles. Nat. Commun. 2019, 10, 1399. [Google Scholar] [CrossRef]
- Whitesides, G.M.; Mathius, J.P. Molecular self-assembly and nanochemistry: A chemical strategy for the synthesis of nanostructures. Science 1991, 254, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Elemans, J.A.A.W.; Van Hameren, R.; Nolte, R.J.M.; Rowan, A.E. Molecular Materials by Self-Assembly of Porphyrins, Phthalocyanines, and Perylenes. Adv. Mater. 2006, 18, 1251–1266. [Google Scholar] [CrossRef]
- Liu, H.; Xu, J.; Li, Y.; Li, Y. Aggregate Nanostructures of Organic Molecular Materials. Acc. Chem. Res. 2010, 43, 1496–1508. [Google Scholar] [CrossRef]
- Gong, X.; Milic, T.; Xu, C.; Batteas, J.D.; Drain, C.M. Preparation and Characterization of Porphyrin Nanoparticles. J. Am. Chem. Soc. 2002, 124, 14290–14291. [Google Scholar] [CrossRef] [PubMed]
- Medforth, C.J.; Wang, Z.; Martin, K.E.; Song, Y.; Jacobsen, J.L.; Shelnutt, J.A. Self-assembled porphyrin nanostructures. Chem. Commun. 2009, 7261–7277. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Beavers, C.M.; Busani, T.; Martin, K.E.; Jacobsen, J.L.; Mercado, B.Q.; Swartzentruber, B.S.; Van Swol, F.; Medforth, C.J.; Shelnutt, J.A. Binary ionic porphyrin nanosheets: Electronic and light-harvesting properties regulated by crystal structure. Nanoscale 2012, 4, 1695–1700. [Google Scholar] [CrossRef]
- Hasobe, T. Photo- and electro-functional self-assembled architectures of porphyrins. Phys. Chem. Chem. Phys. 2012, 14, 15975–15987. [Google Scholar] [CrossRef] [PubMed]
- Lehn, J.-M. Perspectives in Supramolecular Chemistry—From Molecular Recognition towards Molecular Information Pro-cessing and Self-Organization. Angew. Chem. Int. Ed. Engl. 1990, 29, 1304–1319. [Google Scholar] [CrossRef]
- Lee, S.J.; Malliakas, C.D.; Kanatzidis, M.G.; Hupp, J.T.; Nguyen, S.T. Amphiphilic Porphyrin Nanocrystals: Morphology Tuning and Hierarchical Assembly. Adv. Mater. 2008, 20, 3543–3549. [Google Scholar] [CrossRef]
- Drain, C.M.; Varotto, A.; Radivojevic, I. ChemInform Abstract: Self-Organized Porphyrinic Materials. ChemInform 2009, 40, 1630–1658. [Google Scholar] [CrossRef]
- Beletskaya, I.; Tyurin, V.S.; Tsivadze, A.Y.; Guilard, R.; Stern, C. Supramolecular Chemistry of Metalloporphyrins. Chem. Rev. 2009, 109, 1659–1713. [Google Scholar] [CrossRef]
- Shao, S.; Rajendiran, V.; Lovell, J.F. Metalloporphyrin nanoparticles: Coordinating diverse theranostic functions. Co-ord. Chem. Rev. 2019, 379, 99–120. [Google Scholar] [CrossRef]
- Hu, J.-S.; Guo, Y.-G.; Liang, H.-P.; Wan, L.-J.; Jiang, L. Three-Dimensional Self-Organization of Supramolecular Self-Assembled Porphyrin Hollow Hexagonal Nanoprisms. J. Am. Chem. Soc. 2005, 127, 17090–17095. [Google Scholar] [CrossRef]
- Martin, K.E.; Tian, Y.; Busani, T.; Medforth, C.J.; Franco, R.; Van Swol, F.; Shelnutt, J.A. Charge Effects on the Structure and Composition of Porphyrin Binary Ionic Solids: ZnTPPS/SnTMePyP Nanomaterials. Chem. Mater. 2013, 25, 441–447. [Google Scholar] [CrossRef]
- Maeda, H.; Hasegawa, M.; Hashimoto, T.; Kakimoto, T.; Nishio, S.; Nakanishi, T. Nanoscale Spherical Architectures Fabri-cated by Metal Coordination of Multiple Dipyrrin Moieties. J. Am. Chem. Soc. 2006, 128, 10024–10025. [Google Scholar] [CrossRef] [PubMed]
- Hasobe, T.; Oki, H.; Sandanayakaa, A.S.D.; Murata, H. Sonication-assisted supramolecular nanorods of meso-diaryl-substituted porphy-rins. Chem. Commun. 2008, 724–726. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, P.; Dong, H.; Zhen, Y.; Liu, M.; Hu, W. Porphyrin Supramolecular 1D Structures via Surfactant-Assisted Self-Assembly. Adv. Mater. 2015, 27, 5379–5387. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Medforth, A.C.J.; Shelnutt, J.A. Porphyrin Nanotubes by Ionic Self-Assembly. J. Am. Chem. Soc. 2004, 126, 15954–15955. [Google Scholar] [CrossRef]
- Jang, J.H.; Jeon, K.-S.; Oh, S.; Kim, H.-J.; Asahi, T.; Masuhara, H.; Yoon, M. Synthesis of Sn-Porphyrin-Intercalated Trititanate Nanofibers: Optoelectronic Properties and Photocatalytic Activities. Chem. Mater. 2007, 19, 1984–1991. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Z.; Medforth, C.J.; Shelnutt, J.A. Self-Assembly and Self-Metallization of Porphyrin Nanosheets. J. Am. Chem. Soc. 2007, 129, 2440–2441. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Busani, T.; Uyeda, G.H.; Martin, K.E.; van Swol, F.; Medforth, C.J.; Montan, G.A.; Shelnutt, J.A. Hierarchical coop-erative binary ionic porphyrin nanocomposites. Chem. Commun. 2012, 48, 4863–4865. [Google Scholar] [CrossRef]
- Jacobsen, J.L.; Berget, P.E.; Varela, M.C.; Vu, T.; Schore, N.E.; Martin, K.E.; Shelnutt, J.A.; Santos, L.M.; Medforth, C.J. Synthesis and nanostructures of 5,10,15,20-tetrakis(4-piperidyl)porphyrin. Tetrahedron 2013, 69, 10507–10515. [Google Scholar] [CrossRef]
- Chen, Y.; Li, A.; Huang, Z.-H.; Wang, L.-N.; Kang, F. Porphyrin-Based Nanostructures for Photocatalytic Applications. Nanomaterials 2016, 6, 51. [Google Scholar] [CrossRef]
- Li, C.; Park, K.-M.; Kim, H.-J. Ionic assembled hybrid nanoparticle consisting of tin(IV) porphyrin cations and polyoxomo-lybdate anions, and photocatalytic hydrogen production by its visible light sensitization. Inorg. Chem. Commun. 2015, 60, 8–11. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, K.-M.; Ahn, T.K.; Kim, S.K.; Kim, K.S.; Kim, D.; Kim, H.-J. Novel fullerene–porphyrin–fullerene triad linked by metal axial coordination: Synthesis, X-ray crystal structure, and spectroscopic characterizations of trans-bis([60]fullerenoacetato)tin(iv) porphyrin. Chem. Commun. 2004, 2594–2595. [Google Scholar] [CrossRef]
- Kim, H.-J.; Jo, H.J.; Kim, J.; Kim, S.-Y.; Kim, N.; Kim, K. Supramolecular self-assembly of tin(iv) porphyrin channels stabilizing single-file chains of water molecules. CrystEngComm 2005, 7, 417–420. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, M.K.; Kim, H.-J. Fluorescent chemosensing for aromatic compounds by a supramolecular complex composed of tin(iv) porphyrin, viologen, and cucurbit[8]uril. Chem. Commun. 2019, 55, 10575–10578. [Google Scholar] [CrossRef]
- Kim, M.K.; Shee, N.K.; Lee, J.; Yoon, M.; Kim, H.-J. Photoinduced electron transfer upon supramolecular complexation of (porphyrinato)Sn-viologen with cucurbit[7]uril. Photochem. Photobiol. Sci. 2019, 18, 1996–2002. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Shee, N.K.; Park, K.M.; Kim, H.-J. Assembly and X-ray crystal structures of heterometallic multiporphyrins with complementary coordination between ruthenium (II) and tin (IV) porphyrins. Inorg. Chim. Acta 2019, 488, 1–7. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, M.K.; Kim, H.-J. Supramolecular Porphyrin Nanostructures Based on Coordination-Driven Self-Assembly and Their Visible Light Catalytic Degradation of Methylene Blue Dye. Nanomaterials 2020, 10, 2314. [Google Scholar] [CrossRef]
- Giribabu, L.; Rao, T.A.; Maiya, B.G. “Axial-Bonding”-Type Hybrid Porphyrin Arrays: Synthesis, Spectroscopy, Electrochemistry, and Singlet State Properties. Inorg. Chem. 1999, 38, 4971–4980. [Google Scholar] [CrossRef]
- Shetti, V.S.; Ravikanth, M. Sn(IV) Porphyrin Based Axial-Bonding Type Porphyrin Triads Containing Heteroporphyrins as Axial Ligands. Inorg. Chem. 2010, 49, 2692–2700. [Google Scholar] [CrossRef] [PubMed]
- Amati, A.; Cavigli, P.; Demitri, N.; Natali, M.; Indelli, M.T.; Iengo, E. Sn(IV) Multiporphyrin Arrays as Tunable Photoactive Systems. Inorg. Chem. 2019, 58, 4399–4411. [Google Scholar] [CrossRef]
- Rani, J.; Grover, V.; Dhamija, S.; Titi, H.M.; Patra, R. Computational insight into the halogen bonded self-assembly of hexa-coordinated metalloporphyrins. Phys. Chem. Chem. Phys. 2020, 22, 11558–11566. [Google Scholar] [CrossRef]
- Larowska, D.; O’Brien, J.M.; Senge, M.O.; Burdzinski, G.; Marciniak, B.; Lewandowska-Andralojc, A. Graphene Oxide Func-tionalized with Cationic Porphyrins as Materials for the Photodegradation of Rhodamine B. J. Phys. Chem. C 2020, 124, 15769–15780. [Google Scholar] [CrossRef] [PubMed]
- Gligorovski, S.; Strekowski, R.; Barbati, S.; Vione, D. Environmental Implications of Hydroxyl Radicals (•OH). Chem. Rev. 2015, 115, 13051–13092. [Google Scholar] [CrossRef] [PubMed]
- Hubenko, K.; Yefimova, S.; Tkacheva, T.; Maksimchuk, P.; Borovoy, I.; Klochkov, V.; Kavok, N.; Opolonin, O.; Malyukin, Y. Reactive oxygen species generation in aqueous solutions containing GdVO4:Eu3+ nanoparticles and their complexes with methylene blue. Nanoscale Res. Lett. 2018, 13, 100. [Google Scholar] [CrossRef]
- Zhang, Z.; Fan, J.; Du, J.; Peng, X. Two-channel responsive luminescent chemosensors for dioxygen species: Molecular oxygen, singlet oxygen and superoxide anion. Coord. Chem. Rev. 2021, 427, 213575. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shee, N.K.; Kim, H.-J. Self-Assembled Nanomaterials Based on Complementary Sn(IV) and Zn(II)-Porphyrins, and Their Photocatalytic Degradation for Rhodamine B Dye. Molecules 2021, 26, 3598. https://doi.org/10.3390/molecules26123598
Shee NK, Kim H-J. Self-Assembled Nanomaterials Based on Complementary Sn(IV) and Zn(II)-Porphyrins, and Their Photocatalytic Degradation for Rhodamine B Dye. Molecules. 2021; 26(12):3598. https://doi.org/10.3390/molecules26123598
Chicago/Turabian StyleShee, Nirmal K., and Hee-Joon Kim. 2021. "Self-Assembled Nanomaterials Based on Complementary Sn(IV) and Zn(II)-Porphyrins, and Their Photocatalytic Degradation for Rhodamine B Dye" Molecules 26, no. 12: 3598. https://doi.org/10.3390/molecules26123598
APA StyleShee, N. K., & Kim, H.-J. (2021). Self-Assembled Nanomaterials Based on Complementary Sn(IV) and Zn(II)-Porphyrins, and Their Photocatalytic Degradation for Rhodamine B Dye. Molecules, 26(12), 3598. https://doi.org/10.3390/molecules26123598