Evaluation of [64Cu]Cu-NOTA-PEG7-H-Tz for Pretargeted Imaging in LS174T Xenografts—Comparison to [111In]In-DOTA-PEG11-BisPy-Tz
Abstract
1. Introduction
2. Results and Discussion
2.1. Comparison of Physicochemical Properties and In Vitro Stability
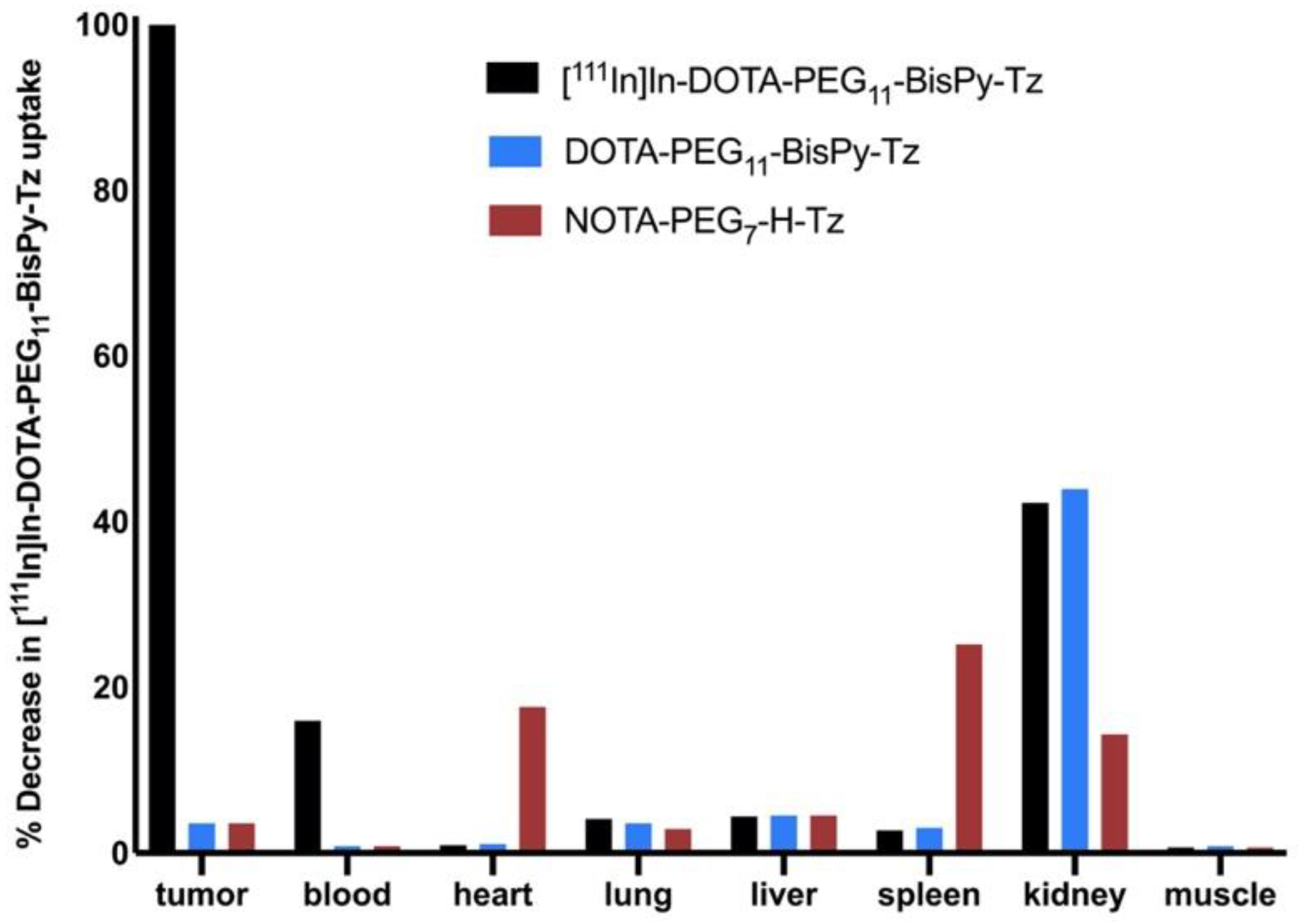
2.2. Ex Vivo Blocking Study
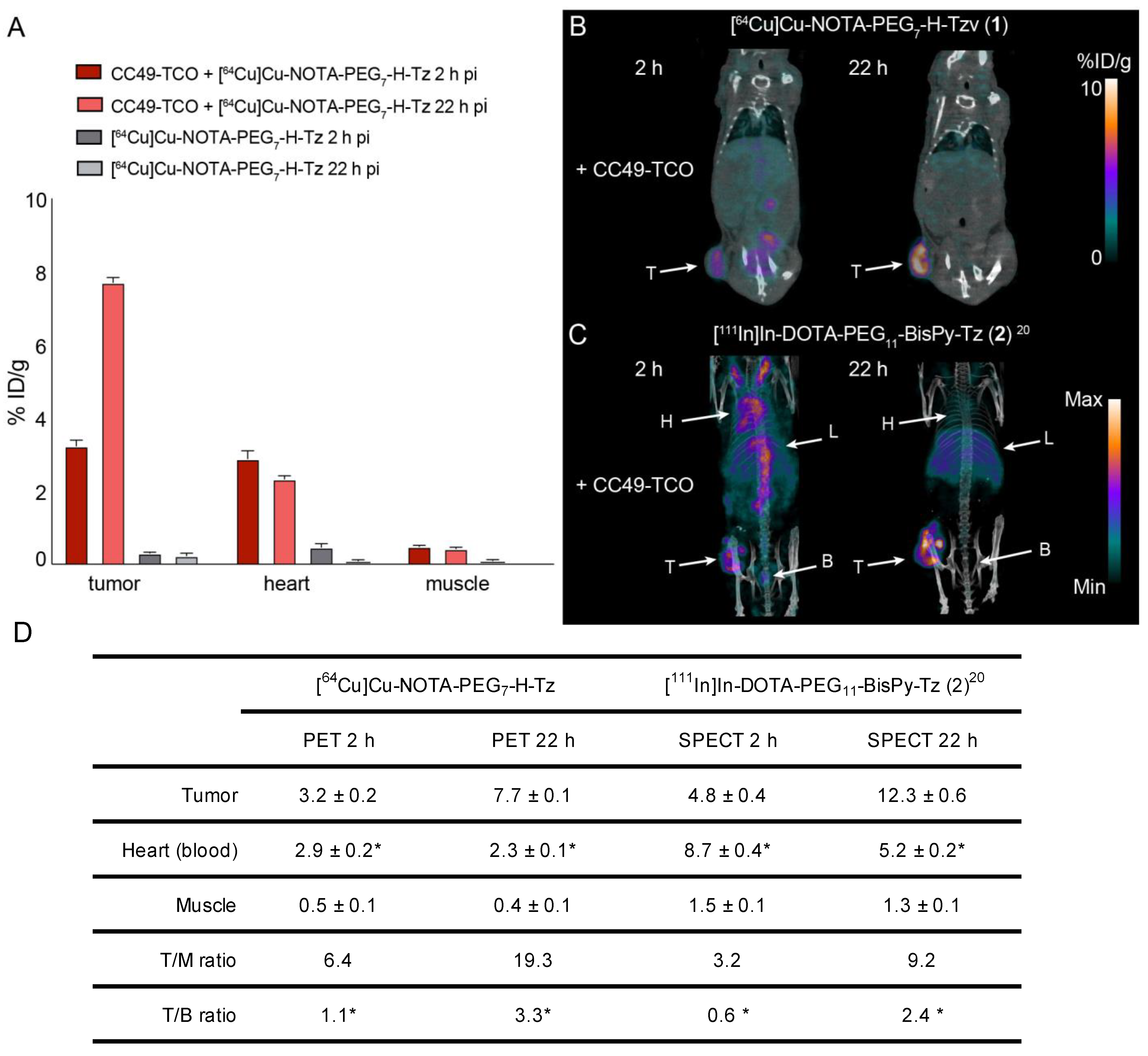
2.3. Pretargeted Imaging
3. Experimental Section
3.1. Organic Chemistry
3.2. Radiochemistry
3.3. Animal Model
3.4. Ex Vivo Blocking Assay
3.5. Pretargeted Imaging
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Stéen, E.J.L.; Edem, P.E.; Nørregaard, K.; Jørgensen, J.T.; Shalgunov, V.; Kjaer, A.; Herth, M.M. Pretargeting in Nuclear Imaging and Radionuclide Therapy: Improving Efficacy of Theranostics and Nanomedicines. Biomaterials 2018, 179, 209–245. [Google Scholar] [CrossRef] [PubMed]
- Altai, M.; Membreno, R.; Cook, B.; Tolmachev, V.; Zeglis, B.M. Pretargeted Imaging and Therapy. J. Nucl. Med. 2017, 58, 1553–1559. [Google Scholar] [CrossRef] [PubMed]
- Zeglis, B.M.; Brand, C.; Abdel-Atti, D.; Carnazza, K.E.; Cook, B.E.; Carlin, S.; Reiner, T.; Lewis, J.S. Optimization of a Pretargeted Strategy for the PET Imaging of Colorectal Carcinoma via the Modulation of Radioligand Pharmacokinetics. Mol. Pharm. 2015, 12, 3575–3587. [Google Scholar] [CrossRef] [PubMed]
- Rossin, R.; Verkerk, P.R.; Van Den Bosch, S.M.; Vulders, R.C.M.; Verel, I.; Lub, J.; Robillard, M.S. In Vivo Chemistry for Pretargeted Tumor Imaging in Live Mice. Angew. Chem. Int. Ed. 2010, 49, 3375–3378. [Google Scholar] [CrossRef] [PubMed]
- Stéen, E.J.L.; Jørgensen, J.T.; Johann, K.; Nørregaard, K.; Sohr, B.; Svatunek, D.; Birke, A.; Shalgunov, V.; Edem, P.E.; Rossin, R.; et al. Trans-Cyclooctene-Functionalized PeptoBrushes with Improved Reaction Kinetics of the Tetrazine Ligation for Pretargeted Nuclear Imaging. ACS Nano 2020, 14, 568–584. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, B.L.; Guo, Z.; Bernardes, G.J.L. Inverse Electron Demand Diels–Alder Reactions in Chemical Biology. Chem. Soc. Rev. 2017, 46, 4895–4950. [Google Scholar] [CrossRef] [PubMed]
- Blackman, M.L.; Royzen, M.; Fox, J.M. Tetrazine Ligation: Fast Bioconjugation Based on Inverse-Electron-Demand Diels−Alder Reactivity. J. Am. Chem. Soc. 2008, 130, 13518–13519. [Google Scholar] [CrossRef]
- Wei, W.; Rosenkrans, Z.T.; Liu, J.; Huang, G.; Luo, Q.-Y.; Cai, W. ImmunoPET: Concept, Design, and Applications. Chem. Rev. 2020, 120, 3787–3851. [Google Scholar] [CrossRef]
- Rossin, R.; Lappchen, T.; van den Bosch, S.M.; Laforest, R.; Robillard, M.S. Diels-Alder Reaction for Tumor Pretargeting: In Vivo Chemistry Can Boost Tumor Radiation Dose Compared with Directly Labeled Antibody. J. Nucl. Med. 2013, 54, 1989–1995. [Google Scholar] [CrossRef]
- Minnix, M.; Li, L.; Yazaki, P.; Chea, J.; Poku, E.; Colcher, D.; Shively, J.E. Improved Targeting of an Anti-TAG-72 Antibody Drug Conjugate for the Treatment of Ovarian Cancer. Cancer Med. 2020, 9, 4756–4767. [Google Scholar] [CrossRef]
- Edem, P.E.; Jørgensen, J.T.; Nørregaard, K.; Rossin, R.; Yazdani, A.; Valliant, J.F.; Robillard, M.; Herth, M.M.; Kjaer, A. Evaluation of a 68Ga-Labeled DOTA-Tetrazine as a PET Alternative to 111In-SPECT Pretargeted Imaging. Molecules 2020, 25, 463. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, A.; Bilton, H.; Vito, A.; Genady, A.R.; Rathmann, S.M.; Ahmad, Z.; Janzen, N.; Czorny, S.; Zeglis, B.M.; Francesconi, L.C.; et al. A Bone-Seeking Trans-Cyclooctene for Pretargeting and Bioorthogonal Chemistry: A Proof of Concept Study Using 99mTc- and 177Lu-Labeled Tetrazines. J. Med. Chem. 2016, 59, 9381–9389. [Google Scholar] [CrossRef] [PubMed]
- Keinänen, O.; Fung, K.; Pourat, J.; Jallinoja, V.; Vivier, D.; Pillarsetty, N.K.; Airaksinen, A.J.; Lewis, J.S.; Zeglis, B.M.; Sarparanta, M. Pretargeting of Internalizing Trastuzumab and Cetuximab with a 18F-Tetrazine Tracer in Xenograft Models. EJNMMI Res. 2017, 7, 95. [Google Scholar] [CrossRef] [PubMed]
- Denk, C.; Svatunek, D.; Mairinger, S.; Stanek, J.; Filip, T.; Matscheko, D.; Kuntner, C.; Wanek, T.; Mikula, H. Design, Synthesis, and Evaluation of a Low-Molecular-Weight 11C-Labeled Tetrazine for Pretargeted PET Imaging Applying Bioorthogonal in Vivo Click Chemistry. Bioconjug. Chem. 2016, 27, 1707–1712. [Google Scholar] [CrossRef] [PubMed]
- Herth, M.M.; Andersen, V.L.; Lehel, S.; Madsen, J.; Knudsen, G.M.; Kristensen, J.L. Development of a 11C-Labeled Tetrazine for Rapid Tetrazine–Trans-Cyclooctene Ligation. Chem. Commun. 2013, 49, 3805. [Google Scholar] [CrossRef] [PubMed]
- Denk, C.; Svatunek, D.; Filip, T.; Wanek, T.; Lumpi, D.; Fröhlich, J.; Kuntner, C.; Mikula, H. Development of a 18F-Labeled Tetrazine with Favorable Pharmacokinetics for Bioorthogonal PET Imaging. Angew. Chem. Int. Ed. 2014, 53, 9655–9659. [Google Scholar] [CrossRef]
- Edem, P.E.; Sinnes, J.-P.; Pektor, S.; Bausbacher, N.; Rossin, R.; Yazdani, A.; Miederer, M.; Kjær, A.; Valliant, J.F.; Robillard, M.S.; et al. Evaluation of the Inverse Electron Demand Diels-Alder Reaction in Rats Using a Scandium-44-Labelled Tetrazine for Pretargeted PET Imaging. EJNMMI Res. 2019, 9, 49. [Google Scholar] [CrossRef]
- Shah, M.A.; Zhang, X.; Rossin, R.; Robillard, M.S.; Fisher, D.R.; Bueltmann, T.; Hoeben, F.J.M.; Quinn, T.P. Metal-Free Cycloaddition Chemistry Driven Pretargeted Radioimmunotherapy Using α-Particle Radiation. Bioconjug. Chem. 2017, 28, 3007–3015. [Google Scholar] [CrossRef]
- Meyer, J.-P.; Houghton, J.L.; Kozlowski, P.; Abdel-Atti, D.; Reiner, T.; Pillarsetty, N.V.K.; Scholz, W.W.; Zeglis, B.M.; Lewis, J.S. 18F-Based Pretargeted PET Imaging Based on Bioorthogonal Diels–Alder Click Chemistry. Bioconjug. Chem. 2016, 27, 298–301. [Google Scholar] [CrossRef]
- García, M.F.; Zhang, X.; Shah, M.; Newton-Northup, J.; Cabral, P.; Cerecetto, H.; Quinn, T. 99mTc-Bioorthogonal Click Chemistry Reagent for in Vivo Pretargeted Imaging. Bioorg. Med. Chem. 2016, 24, 1209–1215. [Google Scholar] [CrossRef]
- Rahmim, A.; Zaidib, H. PET versus SPECT: Strengths, limitations and challenges. Nuclear Med. Commun. 2008, 25, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Frey, E.C.; Humm, J.L.; Ljungberg, M. Accuracy and Precision of Radioactivity Quantification in Nuclear Medicine Images. Semin. Nucl. Med. 2012, 42, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Läppchen, T.; Rossin, R.; van Mourik, T.R.; Gruntz, G.; Hoeben, F.J.M.; Versteegen, R.M.; Janssen, H.M.; Lub, J.; Robillard, M.S. DOTA-Tetrazine Probes with Modified Linkers for Tumor Pretargeting. Nucl. Med. Biol. 2017, 55, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Stéen, J.; Jørgensen, J.T.; Denk, C.; Battisti, U.M.; Nørregaard, K.; Edem, P. Lipophilicity and Click Reactivity Determine the Performance of Bioorthogonal Tetrazine Tools in Pretargeted in Vivo Chemistry. ChemRxiv Prepr. 2020. [Google Scholar] [CrossRef]
- Nichols, B.; Qin, Z.; Yang, J.; Vera, D.R.; Devaraj, N.K. 68Ga Chelating Bioorthogonal Tetrazine Polymers for the Multistep Labeling of Cancer Biomarkers. Chem. Commun. 2014, 50, 5215–5217. [Google Scholar] [CrossRef]

| [64Cu]Cu-NOTA-PEG7-H-Tz (1) | [111In]In-DOTA-PEG11-BisPy-Tz (2) | |
|---|---|---|
| MW (g/mol) | 1163.18 | 1386.31 |
| Metal Complex Net Charge | −1 | 0 |
| logD7.4 | −2.44 ± 0.08c | −4.51 ± 0.07 b |
| Rate constants (M−1 s−1) (in dioxane @ 25 °C) | 216 d | 230 d |
| Stability (%) -PBS (after 2h @ 37 °C) | 87.0 ± 0.6 c | 96.7 ± 0.3 e |
| Stability (%) -Serum (after 2h @ 37 °C) | 84.7 ± 4.8 c | 86.8 ± 1.1 e |
| Serum protein binding (%) | N.D. | 5.6 ± 1.2 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poulie, C.B.M.; Jørgensen, J.T.; Shalgunov, V.; Kougioumtzoglou, G.; Jeppesen, T.E.; Kjaer, A.; Herth, M.M. Evaluation of [64Cu]Cu-NOTA-PEG7-H-Tz for Pretargeted Imaging in LS174T Xenografts—Comparison to [111In]In-DOTA-PEG11-BisPy-Tz. Molecules 2021, 26, 544. https://doi.org/10.3390/molecules26030544
Poulie CBM, Jørgensen JT, Shalgunov V, Kougioumtzoglou G, Jeppesen TE, Kjaer A, Herth MM. Evaluation of [64Cu]Cu-NOTA-PEG7-H-Tz for Pretargeted Imaging in LS174T Xenografts—Comparison to [111In]In-DOTA-PEG11-BisPy-Tz. Molecules. 2021; 26(3):544. https://doi.org/10.3390/molecules26030544
Chicago/Turabian StylePoulie, Christian B. M., Jesper T. Jørgensen, Vladimir Shalgunov, Georgios Kougioumtzoglou, Troels Elmer Jeppesen, Andreas Kjaer, and Matthias M. Herth. 2021. "Evaluation of [64Cu]Cu-NOTA-PEG7-H-Tz for Pretargeted Imaging in LS174T Xenografts—Comparison to [111In]In-DOTA-PEG11-BisPy-Tz" Molecules 26, no. 3: 544. https://doi.org/10.3390/molecules26030544
APA StylePoulie, C. B. M., Jørgensen, J. T., Shalgunov, V., Kougioumtzoglou, G., Jeppesen, T. E., Kjaer, A., & Herth, M. M. (2021). Evaluation of [64Cu]Cu-NOTA-PEG7-H-Tz for Pretargeted Imaging in LS174T Xenografts—Comparison to [111In]In-DOTA-PEG11-BisPy-Tz. Molecules, 26(3), 544. https://doi.org/10.3390/molecules26030544








