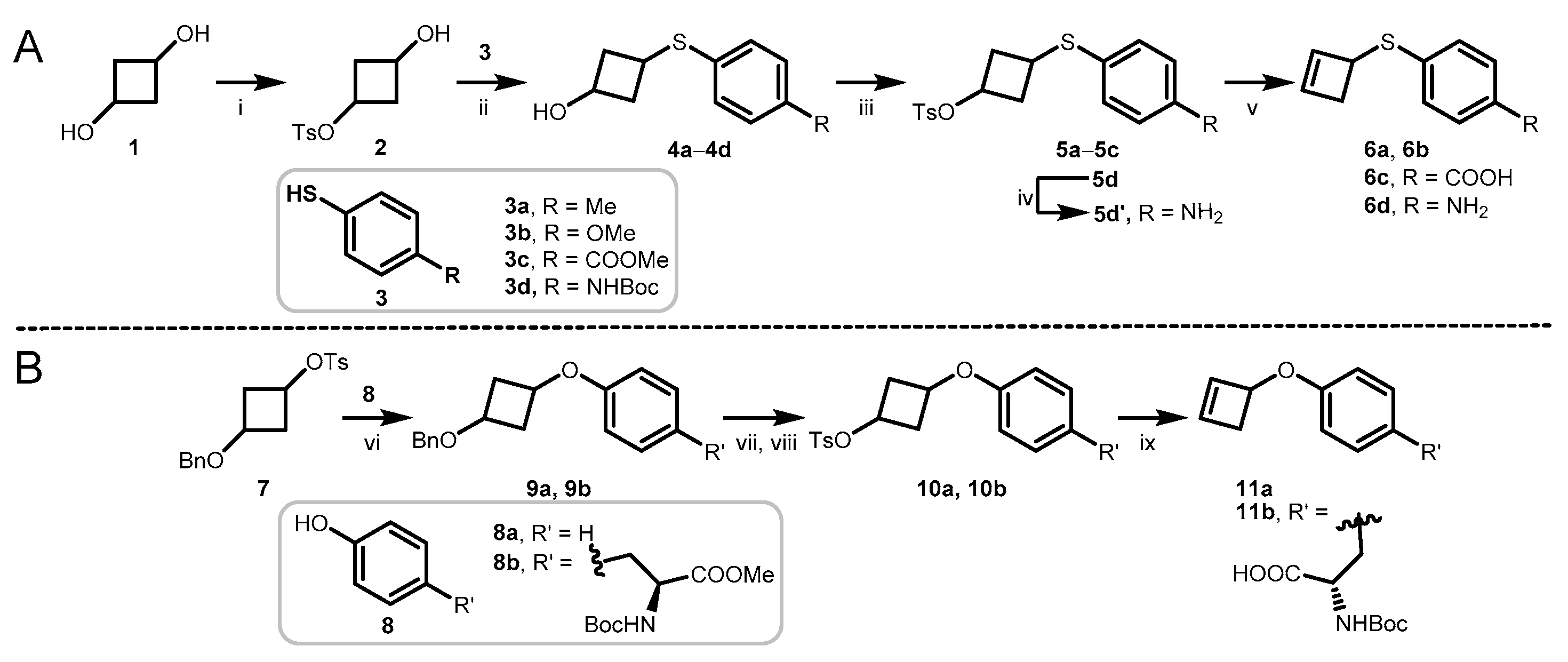
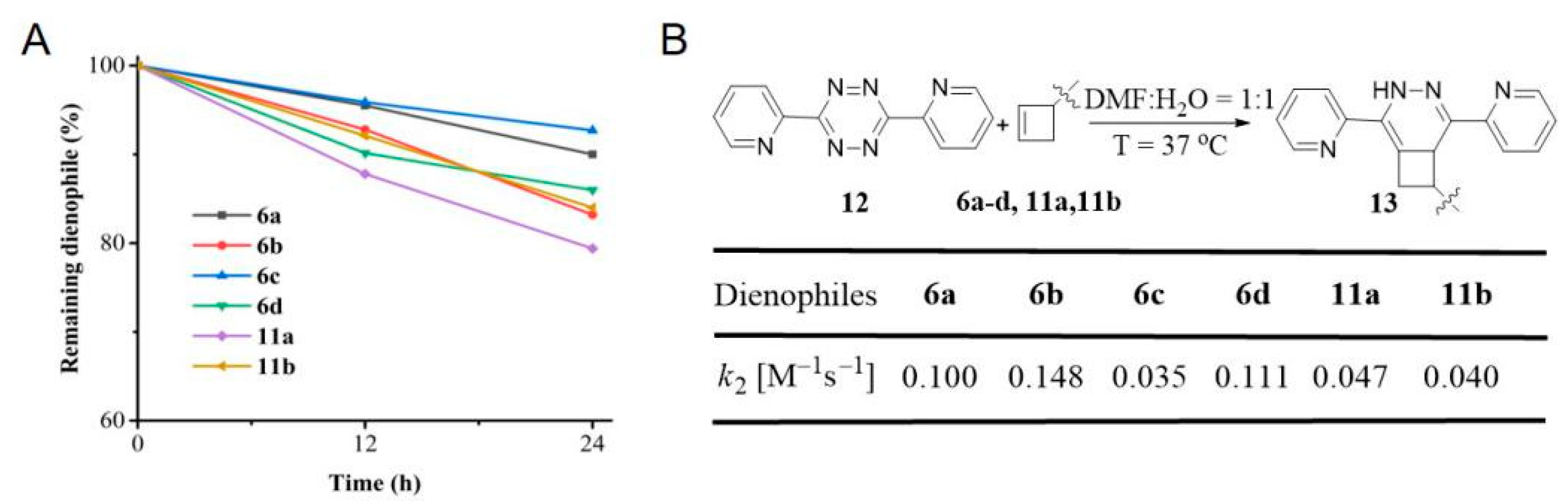
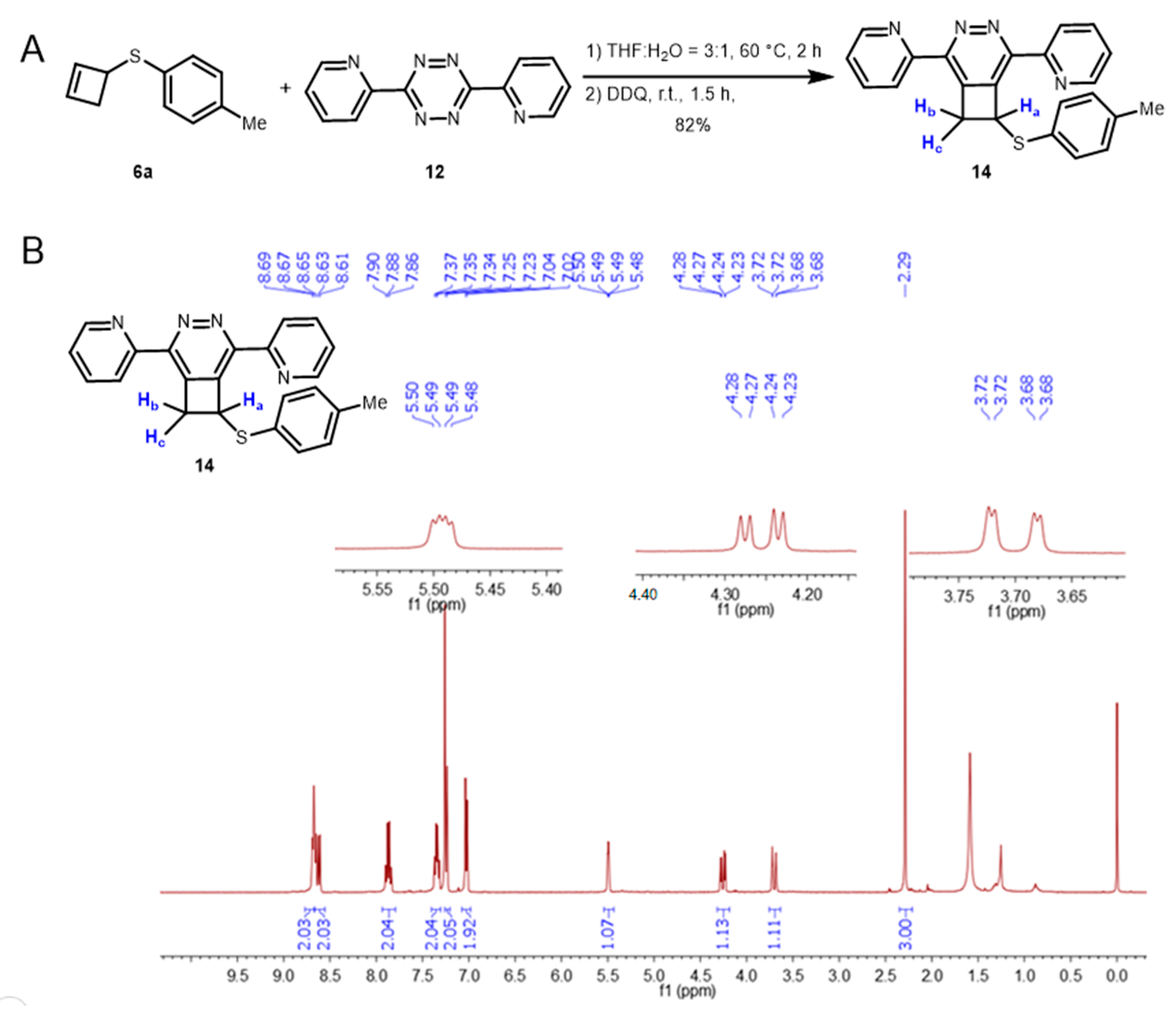
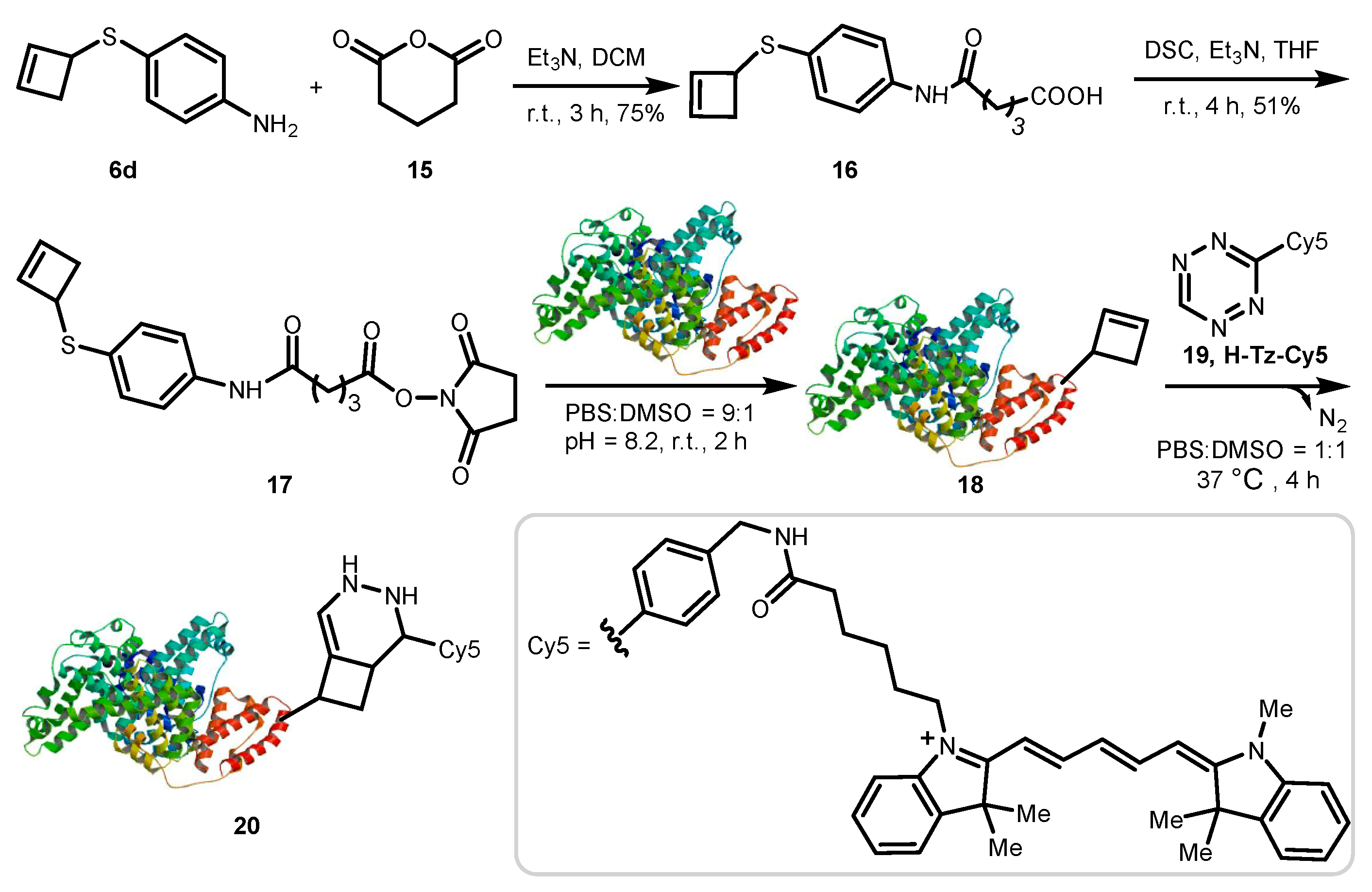
3.2. Synthesis and Characterization
3.2.1. Synthesis of 3-Hydroxycyclobutyl 4-Methylbenzenesulfonate (2)
A solution of tosyl chloride (TsCl, 22 mg, 0.12 mmol, 0.7 equiv) in dichloromethane (DCM, 0.5 mL) was added dropwise with stirring to a mixture of 1,3-cyclobutanediol (15 mg, 0.17 mmol, 1.0 equiv) and TEA (47 μL, 0.34 mmol, 2.0 equiv) in DCM (0.3 mL) at 0 °C under Ar. The reaction mixture was stirred at room temperature for 9 h, followed by evaporation in vacuo. The obtained residue was purified by silica gel column chromatography (PE/EA = 2:1) to afford the desired analogue 2 in 38% yield as a light yellow oil.
3.2.2. Synthesis of Compounds 4a–4d
To synthesize 3-(p-tolylthio)cyclobutanol (4a), potassium tert-butoxide (265 mg, 2.35 mmol, 1.3 equiv) was added under intense stirring to a solution of 3-hydroxycyclobutyl-4-methylbenzenesulfonate (439 mg, 1.81 mmol, 1.0 equiv) and 4-methylbenzenethiol (270 mg, 2.18 mmol, 1.2 equiv) in tert-butanol (8 mL) at room temperature under Ar. The mixture was stirred at 80 °C for 7 h. After completion of the reaction monitored by TLC, the crude mixture was quenched with water and extracted with ethyl acetate. The combined organic phases were washed with brine, dried over anhydrous Na2SO4, and evaporated in vacuo. The residue was purified by silica gel column chromatography (PE/EA = 3:1) to afford 4a (186 mg, 86%) as a yellow solid. 1H–NMR (400 MHz, CDCl3) δ 7.16 (d, J = 8.1 Hz, 2H), 7.10 (d, J = 8.0 Hz, 2H), 4.63–4.56 (m, 1H), 3.85–3.79 (m, 1H), 2.46–2.34 (m, 4H), 2.32 (s, 3H), 1.99 (s, 1H). 13C–NMR (101 MHz, CDCl3) δ 136.2, 132.9, 129.8, 129.7, 66.3, 39.6, 33.9, 21.1. HRMS [M+H]+ m/z calcd. for [C11H15OS]+ 195.0838, found 195.0837.
To synthesize 3-((4-methoxyphenyl)thio)cyclobutanol (4b), potassium tert-butoxide (105 mg, 0.94 mmol, 1.3 equiv) was added under intense stirring to a solution of 3-hydroxycyclobutyl-4-methylbenzenesulfonate (170.3 mg, 0.72 mmol, 1.0 equiv) and 4-methoxybenzenethiol (121 mg, 0.86 mmol, 1.2 equiv) in tert-butanol (4 mL) at room temperature under Ar. The mixture was stirred at 80 °C for 7 h. After completion of the reaction monitored by TLC, the crude mixture was quenched with water and extracted with ethyl acetate. The combined organic phases were washed with brine, dried over anhydrous Na2SO4, and evaporated in vacuo. The residue was purified by silica gel column chromatography (PE/EA = 20:1) to afford 4b (81 mg, 77%) as a white solid. 1H–NMR (400 MHz, CDCl3) δ 7.31–7.26 (m, 2H), 6.87–6.81 (m, 2H), 4.58–4.52 (m, 1H), 3.79 (s, 3H), 3.77–3.69 (m, 1H), 2.34 (t, J = 6.4 Hz, 4H), 1.90 (s, 1H). 13C–NMR (101 MHz, CDCl3) δ 159.1, 133.2, 126.4, 114.7, 66.2, 55.4, 39.5, 35.2.
To synthesize methyl 4-((3-hydroxycyclobutyl)thio)benzoate (4c), potassium tert-butoxide (304 mg, 2.70 mmol, 1.3 equiv) was added under intense stirring to a solution of 3-hydroxycyclobutyl-4-methylbenzenesulfonate (504 mg, 2.08 mmol, 1.0 equiv) and methyl 4-mercaptobenzoate (420 mg, 2.50 mmol, 1.2 equiv) in tert-butanol (8 mL) at room temperature under Ar. The mixture was stirred at 80 °C for 7 h. After completion of the reaction monitored by TLC, the crude mixture was quenched with water and extracted with ethyl acetate. The combined organic phases were washed with brine, dried over anhydrous Na2SO4, and evaporated in vacuo. The residue was purified by silica gel column chromatography (PE/EA = 2:1) to afford 4c (200 mg, 48%) as a white solid. 1H–NMR (400 MHz, CDCl3) δ 7.91 (d, J = 8.3 Hz, 2H), 7.15 (d, J = 8.3 Hz, 2H), 4.75–4.57 (m, 1H), 3.98–3.92 (m, 1H), 3.89 (s, 3H), 2.60–2.48 (m, 2H), 2.43–2.37 (m, 2H), 2.13 (s, 1H). 13C–NMR (101 MHz, CDCl3) δ 167.0, 144.5, 130.1, 126.7, 126.0, 66.1, 52.2, 52.1, 39.4, 31.9. HRMS [M+Na]+ m/z calcd. for [C12H14NaO3S]+ 261.0556, found 261.0563.
To synthesize tert-butyl (4-((3-hydroxycyclobutyl)thio)phenyl)carbamate (4d), potassium tert-butoxide (200 mg, 1.78 mmol, 1.3 equiv) was added under intense stirring to a solution of 3-hydroxycyclobutyl-4-methylbenzenesulfonate (331 mg, 1.36 mmol, 1.0 equiv) and methyl tert-butyl (4-mercaptophenyl)carbamate (370 mg, 1.64 mmol, 1.2 equiv) in tert-butanol (8 mL) at room temperature under Ar. The mixture was stirred at 80 °C for 7 h. After completion of the reaction monitored by TLC, the crude mixture was quenched with water and extracted with ethyl acetate. The combined organic phases were washed with brine, dried over anhydrous Na2SO4, and evaporated in vacuo. The residue was purified by silica gel column chromatography (PE/EA = 2:1) to afford 4d (146 mg, 32%) as a brown solid. 1H–NMR (400 MHz, CDCl3) δ 7.29 (d, J = 8.4 Hz, 2H), 7.22 (d, J = 8.6 Hz, 2H), 6.48 (s, 1H), 4.63–4.49 (m, 1H), 3.82–3.72 (m, 1H), 2.36 (dd, J = 12.8, 6.4 Hz, 4H), 1.86 (s, 1H), 1.51 (s, 9H). 13C–NMR (101 MHz, CDCl3) δ 152.7, 137.2, 131.4, 129.7, 119.2, 80.8, 66.2, 39.5, 34.5, 28.4. HRMS [M-H]− m/z calcd. for [C15H20NO3S]− 294.1169, found 294.1167.
3.2.3. Synthesis of Compounds 5a–5d
To synthesize 3-(p-Tolylthio)cyclobutyl-4-methylbenzenesulfonate (5a), a solution of TsCl (365 mg, 1.91 mmol, 2.0 equiv) in DCM (5 mL) was added dropwise under stirring to a mixture of 3-(p-tolylthio)cyclobutanol (186 mg, 0.96 mmol, 1.0 equiv), TEA (400 μL, 2.87 mmol, 3 equiv), and 4-dimethylaminopyridine (DMAP) (23 mg, 0.19 mmol, 0.2 equiv) in DCM (5 mL) at 0 °C under Ar. The resulting mixture was stirred at room temperature overnight. After completion, the reaction was quenched with water and extracted with ethyl acetate. The combined organic phases were washed with brine, dried over anhydrous Na2SO4, and evaporated in vacuo. The residue was purified by silica gel column chromatography (PE/EA = 2:1) to afford 5a (261 mg, 78%) as a light yellow oil. 1H–NMR (400 MHz, CDCl3) δ 7.76 (d, J = 8.3 Hz, 2H), 7.33 (d, J = 8.0 Hz, 2H), 7.11 (q, J = 8.3 Hz, 4H), 5.03–4.96 (m, 1H), 3.84–3.73 (m, 1H), 2.70–2.56 (m, 2H), 2.45 (s, 3H), 2.36–2.26 (m, 5H). 13C–NMR (101 MHz, CDCl3) δ 145.0, 136.9, 133.8, 131.7, 130.4, 130.0, 129.9, 128.0, 73.9, 37.2, 34.6, 21.8, 21.2. HRMS [M+Na]+ m/z calcd. for [C18H20NaO3S2]+ 371.0746, found 371.0749.
To synthesize 3-((4-methoxyphenyl)thio)cyclobutyl 4-methylbenzenesulfonate (5b), a solution of TsCl (90.7 mg, 0.48 mmol, 2.0 equiv) in DCM (2 mL) was added dropwise under stirring to a mixture of 3-((4-methoxyphenyl)thio)cyclobutanol (50 mg, 0.24 mmol, 1.0 equiv), triethylamine (TEA, 100 μL, 0.71 mmol, 3 equiv), and 4-dimethylaminopyridine (DMAP) (5.8 mg, 0.05 mmol, 0.2 equiv) in DCM (2 mL) at 0 °C under Ar. The resulting mixture was stirred at room temperature overnight. After completion, the reaction was quenched with water and extracted with ethyl acetate. The combined organic phases were washed with brine, dried over anhydrous Na2SO4, and evaporated in vacuo. The residue was purified by silica gel column chromatography (PE/EA = 5:1) to afford 5b (47.6 mg, 55%) as a white solid. 1H–NMR (400 MHz, CDCl3) δ 7.75 (d, J = 8.3 Hz, 2H), 7.33 (d, J = 8.1 Hz, 2H), 7.25 (d, J = 8.9 Hz, 2H), 6.83 (d, J = 8.8 Hz, 2H), 4.97–4.90 (m, 1H), 3.79 (s, 3H), 3.74–3.67 (m, 1H), 2.63–2.51 (m, 2H), 2.44 (s, 3H), 2.30–2.25 (m, 2H). 13C–NMR (101 MHz, CDCl3) δ 159.5, 145.0, 133.9, 133.7, 130.0, 127.9, 125.2, 114.8, 73.9, 55.4, 37.0, 35.8, 21.7. HRMS [M+Na]+ m/z calcd. for [C18H20NaO4S2]+ 387.0695, found 387.0700.
To synthesize methyl 4-((3-(tosyloxy)cyclobutyl)thio)benzoate (5c), a solution of TsCl (318.8 mg, 1.67 mmol, 2.0 equiv) in DCM (3 mL) was added dropwise under stirring to a mixture of methyl 4-((3-hydroxycyclobutyl)thio)benzoate (199 mg, 0.84 mmol, 1.0 equiv), TEA (350 μL, 2.5 mmol, 3 equiv), and 4-dimethylaminopyridine (DMAP) (20.4 mg, 0.16 mmol, 0.2 equiv) in DCM (4 mL) at 0 °C under Ar. The resulting mixture was stirred at room temperature overnight. After completion, the reaction was quenched with water and extracted with ethyl acetate. The combined organic phases were washed with brine, dried over anhydrous Na2SO4, and evaporated in vacuo. The residue was purified by silica gel column chromatography (PE/EA = 5:1) to afford 5c (260 mg, 79%) as a white solid. 1H–NMR (400 MHz, CDCl3) δ 7.91 (d, J = 8.6 Hz, 2H), 7.77 (d, J = 8.3 Hz, 2H), 7.34 (d, J = 8.0 Hz, 2H), 7.11 (d, J = 8.5 Hz, 2H), 5.09–5.02 (m, 1H), 3.98–3.93 (m, 1H), 3.89 (s, 3H), 2.85–2.69 (m, 2H), 2.45 (s, 3H), 2.40–2.33 (m, 2H). 13C–NMR (101 MHz, CDCl3) δ 166.7, 145.1, 143.2, 133.6, 130.2, 130.0, 127.9, 127.2, 126.4, 73.4, 52.2, 37.0, 32.5, 21.8. HRMS [M+Na]+ m/z calcd. for [C19H20NaO5S2]+ 415.0644, found 415.0652.
To synthesize 3-((4-((tert-butoxycarbonyl)amino)phenyl)thio)cyclobutyl 4-methylbenzene- sulfonate (5d), a solution of TsCl (155 mg, 0.81 mmol, 2.0 equiv) in DCM (2 mL) was added dropwise under stirring to a mixture of tert-butyl (4-((3-hydroxycyclobutyl)thio)phenyl)carbamate (120 mg, 0.41 mmol, 1.0 equiv), TEA (170 μL, 1.22 mmol, 3 equiv), and 4-dimethylaminopyridine (DMAP) (10 mg, 0.08 mmol, 0.2 equiv) in DCM (2 mL) at 0 °C under Ar. The resulting mixture was stirred at room temperature overnight. After completion, the reaction was quenched with water and extracted with ethyl acetate. The combined organic phases were washed with brine, dried over anhydrous Na2SO4, and evaporated in vacuo. The residue was purified by silica gel column chromatography (PE/EA = 5:1) to afford 5d (136 mg, 74%) as a light yellow oil. 1H–NMR (400 MHz, CDCl3) δ 7.75 (d, J = 8.3 Hz, 2H), 7.31 (dd, J = 15.9, 8.4 Hz, 4H), 7.19 (d, J = 8.6 Hz, 2H), 6.51 (s, 1H), 5.04–4.89 (m, 1H), 3.77–3.71 (m, 1H), 2.64–2.52 (m, 2H), 2.44 (s, 3H), 2.31–2.25 (m, 2H), 1.51 (s, 9H). 13C–NMR (101 MHz, CDCl3) δ 152.7, 145.0, 137.8, 133.7, 132.2, 130.0, 128.3, 127.9, 119.2, 80.9, 73.9, 37.0, 35.1, 28.4, 21.8. HRMS [M-H]− m/z calcd. for [C22H26NO5S2]− 448.1258, found 448.1256.
3.2.4. Synthesis of Compounds 6a–6d
To synthesize cyclobut-2-en-1-yl(p-tolyl)sulfane (6a), potassium tert-butoxide (56 mg, 0.5 mmol, 2.0 equiv) was dissolved in dry DMSO (1 mL) under Ar, forming a colorless solution. Then, a solution of 3-(p-tolylthio)cyclobutyl-4-methylbenzenesulfonate (87 mg, 0.25 mmol, 1.0 equiv) in dry DMSO (l mL) was added slowly to the colorless solution, and the resulting mixture was stirred at room temperature for 1 h. Water was then added slowly, followed by ethyl acetate. The separated water phase was extracted with ethyl acetate three times, and the combined organic phases were washed once with water. The final organic phase was dried over anhydrous Na2SO4 and evaporated in vacuo. The residue was purified by silica gel column chromatography (PE) to afford compound 6a (38 mg, 86%) as a light yellow oil. 1H–NMR (400 MHz, CDCl3): δ 7.25 (d, J = 8.0 Hz, 2H), 7.09 (d, J = 7.9 Hz, 2H), 6.10 (dd, J = 17.4, 2.5 Hz, 2H), 4.28 (d, J = 3.7 Hz, 1H), 3.03 (dd, J = 13.8, 3.9 Hz, 1H), 2.49 (d, J = 13.8 Hz, 1H), 2.31 (s, 3H). 13C–NMR (101 MHz, CDCl3): δ 138.1, 137.5, 136.5, 132.4, 130.7, 129.7, 46.9, 40.1, 21.2. HRMS [M+H]+ m/z calcd. for [C11H13S]+ 177.0732, found 177.0740.
To synthesize cyclobut-2-en-1-yl(4-methoxyphenyl)sulfane (6b), potassium tert-butoxide (29.3 mg, 0.26 mmol, 2.0 equiv) was dissolved in dry DMSO (1 mL) under Ar, forming a colorless solution. Then, a solution of 3-((4-methoxyphenyl)thio)cyclobutyl 4-methylbenzenesulfonate (47.6 mg, 0.13 mmol, 1.0 equiv) in dry DMSO (l mL) was added slowly to the colorless solution, and the resulting mixture was stirred at room temperature for 1 h. Water was then added slowly, followed by ethyl acetate. The separated water phase was extracted with ethyl acetate three times, and the combined organic phases were washed once with water. The final organic phase was dried over anhydrous Na2SO4 and evaporated in vacuo. The residue was purified by silica gel column chromatography (PE) to afford compound 6b (24.5 mg, 89%) as a light yellow oil. 1H–NMR (400 MHz, CDCl3) δ 7.35 (d, J = 8.7 Hz, 2H), 6.84 (d, J = 8.7 Hz, 2H), 6.07 (dd, J = 14.4, 2.6 Hz, 2H), 4.19 (d, J = 3.9 Hz, 1H), 3.80 (s, 3H), 2.97 (dd, J = 13.8, 4.0 Hz, 1H), 2.46 (d, J = 13.8 Hz, 1H). 13C–NMR (101 MHz, CDCl3) δ 159.2, 138.3, 137.2, 134.1, 125.6, 114.4, 55.4, 47.9, 39.8. HRMS [M+H]+ m/z calcd. for [C11H13OS]+ 193.0682, found 193.0686.
To synthesize 4-(cyclobut-2-en-1-ylthio)benzoic acid (6c), potassium tert-butoxide (11.45 mg, 0.10 mmol, 2.0 equiv) was dissolved in dry DMSO (0.5 mL) under Ar, forming a colorless solution. Then, a solution of methyl 4-((3-(tosyloxy)cyclobutyl)thio)benzoate (20 mg, 0.05 mmol, 1.0 equiv) in dry DMSO (0.5 mL) was added slowly to the colorless solution, and the resulting mixture was stirred at room temperature for 1 h. Water was then added slowly, followed by ethyl acetate. The separated water phase was extracted with ethyl acetate three times, and the combined organic phases were washed once with water. The final organic phase was dried over anhydrous Na2SO4 and evaporated in vacuo. The residue was purified by silica gel column chromatography (PE/EA = 1:1) to afford compound 6c (5.4 mg, 47%) as a white solid. 1H–NMR (400 MHz, DMSO) δ 7.85 (d, J = 8.4 Hz, 2H), 7.34 (d, J = 8.4 Hz, 2H), 6.25 (d, J = 24.8 Hz, 2H), 4.57 (s, 1H), 3.19 (dd, J = 13.9, 3.8 Hz, 1H), 2.43 (d, J = 14.0 Hz, 1H). 13C–NMR (101 MHz, DMSO) δ 167.0, 143.3, 138.3, 137.2, 129.8, 129.1, 126.2, 43.8, 39.6. HRMS [M-H]− m/z calcd. for [C11H9O2S]− 205.0329, found 205.0330.
To synthesize 4-(cyclobut-2-en-1-ylthio)aniline (6d), 3-((4-((tert-butoxycarbonyl)amino)phenyl) thio)cyclobutyl 4-methylbenzenesulfonate (22 mg, 0.049 mmol, 1.0 equiv) was dissolved in DCM (0.7 mL) and trifluoroacetic acid was added (0.3 mL). The resulting mixture was stirred at room temperature for 1 h. Upon completion monitored by TLC, the reaction mixture was evaporated under reduced pressure. The residue 5d’ was used for the next step without further purification. Potassium tert-butoxide (11 mg, 0.097 mmol, 2.0 equiv) was dissolved in dry DMSO (0.4 mL) under Ar, forming a colorless solution. Then, a solution of methyl 3-((4-aminophenyl)thio)cyclobutyl 4-methylbenzenesulfonate (17.1 mg, 0.049 mmol, 1.0 equiv) in dry DMSO (0.4 mL) was added slowly to the colorless solution, and the resulting mixture was stirred at room temperature for 1 h. Water was then added slowly, followed by ethyl acetate. The separated water phase was extracted with ethyl acetate three times, and the combined organic phases were washed once with water. The final organic phase was dried over anhydrous Na2SO4 and evaporated in vacuo. The residue was purified by silica gel column chromatography (PE/EA = 5:1) to afford compound 6d (4 mg, 46%) as a light yellow solid. 1H–NMR (400 MHz, CDCl3) δ 7.25 (d, J = 9.3 Hz, 3H), 6.61 (d, J = 8.5 Hz, 2H), 6.05 (dd, J = 10.9, 2.6 Hz, 2H), 4.13 (d, J = 3.9 Hz, 1H), 3.73 (s, 2H), 2.93 (dd, J = 13.8, 4.0 Hz, 1H), 2.44 (d, J = 13.7 Hz, 1H). 13C–NMR (101 MHz, CDCl3) δ 146.2, 138.5, 137.0, 134.8, 122.2, 115.4, 48.3, 39.6. HRMS [M+H]+ m/z calcd. for [C10H12NS]+ 178.0685, found 178.0687.
3.2.5. Synthesis of Compounds 9a and 9b
A solution of 3-(benzyloxy)cyclobutyl-4-methylbenzenesulfonate (1.0 equiv), compound 8a or 8b (2.0 equiv), and Cs2CO3 (2.0 equiv) was prepared in DMF, and the resulting mixture was stirred at 80 °C for 8 h. Then the reaction was quenched with water and extracted with ethyl acetate. The combined organic phases were washed with brine, dried over anhydrous Na2SO4, and evaporated in vacuo. The residue was purified by silica gel column chromatography (PE/EA = 40:1) to afford the desired product 9a or 9b.
For the synthesis of (3-(benzyloxy)cyclobutoxy)benzene (9a), 1.32 g of 8a was used, giving 484 mg of compound 9a (48% yield) as a light yellow oil.
For the synthesis of methyl 3-(4-(3-(benzyloxy)cyclobutoxy)phenyl) -2-((tert-butoxycarbonyl)amino)propanoate (9b), 261 mg of 8b was used, giving 250 mg of compound 9b (70% yield) as a white solid.
3.2.6. Synthesis of Compounds 10a and 10b
To prepare 3-phenoxycyclobutyl-4-methylbenzenesulfonate (10a), compound 9a (469 mg, 1.84 mmol, 1.0 equiv) was dissolved in ethanol (10 mL), followed by the addition of Pd/C (94 mg). The resulting solution was purged with hydrogen five times, and the mixture was then hydrogenated with a H2 balloon overnight at room temperature. After completion of the reaction monitored by TLC, the catalyst was removed by filtration over celite, and the filter cake was washed three times with ethyl acetate. The combined organic layers were evaporated in vacuo, affording a crude mixture that was used in the next step without purification. TEA (772 μL, 5.53 mmol, 3.0 equiv) and DMAP (45 mg, 0.37 mmol, 0.2 equiv) were dissolved in DCM (4 mL) under Ar and added to the crude mixture. The resulting mixture was cooled to 0 °C, and a solution of TsCl (1.05 g, 5.53 mmol, 3.0 equiv) in DCM (6 mL) was added. The reaction mixture was stirred at room temperature for 20 h. Then the reaction was quenched with water and extracted with ethyl acetate. The combined organic phases were washed with brine, dried over anhydrous Na2SO4, and evaporated in vacuo. The residue was purified by silica gel column chromatography (PE/EA = 5:1) to afford 10a in 86% yield (508 mg) as a light yellow oil. 1H–NMR (400 MHz, CDCl3) δ 7.79 (d, J = 8.2 Hz, 2H), 7.35 (d, J = 8.3 Hz, 2H), 7.25 (t, J = 8.0 Hz, 2H), 6.94 (t, J = 7.4 Hz, 1H), 6.71 (d, J = 7.9 Hz, 2H), 5.11–5.00 (m, 1H), 4.84–4.79 (m, 1H), 2.67–2.58 (m, 2H), 2.54–2.48 (m, 2H), 2.45 (s, 3H). 13C–NMR (101 MHz, CDCl3) δ 157.2, 145.1, 133.7, 130.1, 129.7, 128.0, 121.3, 114.9, 73.2, 68.2, 37.8, 21.8. HRMS [M+Na]+ m/z calcd. for[C17H18NaO4S]+ 341.0818, found 341.0826.
Methyl 2-((tert-butoxycarbonyl)amino)-3-(4-(3-(tosyloxy)cyclobutoxy)phenyl)propanoate (10b) was isolated in 70% yield from compound 9b and TsCl as a light red solid by a method analogous to that described for the synthesis of 10a above.
3.2.7. Synthesis of Compounds 11a and 11b
To prepare cyclobut-2-en-1-yloxy)benzene (11a), potassium tert-butoxide (198 mg, 1.77 mmol, 2.0 equiv) was dissolved in dry DMSO (2 mL) under Ar, forming a colorless solution. A solution of compound 10a (281 mg, 0.88 mmol, 1.0 equiv) in dry DMSO (2 mL) was then added slowly to the colorless solution, and the reaction mixture was left stirring at room temperature for 1 h. After completion of the reaction monitored by TLC, water was added slowly, followed by ethyl acetate. The separated water phase was extracted with ethyl acetate three times, and the combined organic layers were washed once with water. The final organic phase was dried over anhydrous Na2SO4 and evaporated in vacuo. The residue was purified by silica gel column chromatography (PE) to afford 11a (73.6 mg, 57%) as a colorless oil. 1H–NMR (400 MHz, CDCl3) δ 7.28 (t, J = 9.8 Hz, 2H), 6.95 (t, J = 7.4 Hz, 1H), 6.89 (d, J = 7.8 Hz, 2H), 6.31 (d, J = 33.7 Hz, 2H), 5.10 (d, J = 3.2 Hz, 1H), 3.00 (dd, J = 13.4, 3.5 Hz, 1H), 2.63 (dd, J = 13.4, 1.0 Hz, 1H). 13C–NMR (101 MHz, CDCl3) δ 158.2, 139.3, 137.0, 129.7, 121.0, 115.0, 74.5, 39.7.
The compound 2-((tert-butoxycarbonyl)amino)-3-(4-(cyclobut-2-en-1-yloxy)phenyl)propanoic acid (11b) was obtained in 45% yield as a white solid from compound 10b and potassium tert-butoxide by a method analogous to that described for the synthesis of 11a above. 1H–NMR (400 MHz, DMSO-d6) δ 12.49 (s, 1H), 7.11 (d, J = 8.4 Hz, 2H), 6.98 (d, J = 8.3 Hz, 1H), 6.77 (d, J = 8.5 Hz, 2H), 6.30 (d, J = 32.6 Hz, 2H), 5.02 (d, J = 2.8 Hz, 1H), 4.02–3.96 (m, 1H), 2.98–2.84 (m, 2H), 2.71 (dd, J = 13.6, 10.3 Hz, 1H), 2.48–2.35 (m, 2H), 1.28 (s, 8H). 13C–NMR (101 MHz, DMSO-d6) δ 173.7, 156.2, 155.5, 138.9, 137.2, 130.2, 130.1, 114.4, 78.0, 73.8, 55.4, 40.4, 35.7, 28.2, 27.9. HRMS [M-H]− m/z calcd. for [C18H22NO5]− 332.1503, found 332.1493.
3.2.8. Synthesis of 5-((4-(Cyclobut-2-en-1-Ylthio)Phenyl)Amino)-5-Oxopentanoic Acid (16)
A mixture of compound 6d (5 mg, 0.028 mmol, 1.0 equiv), glutaric anhydride (3.5 mg, 0.031 mmol, 1.1 equiv), and TEA (10 μL, 0.07 mmol, 2.5 equiv) was dissolved in DCM (0.5 mL) under Ar. The resulting mixture was stirred at room temperature for 3 h, and then quenched with water and extracted with ethyl acetate. The combined organic phases were washed with brine, dried over anhydrous Na2SO4, and evaporated in vacuo. Due to the small scale of the reaction, the residue was purified by thin-layer chromatography to afford 6.2 mg (75%) of 16 as a white solid. 1H–NMR (400 MHz, DMSO-d6) δ 9.96 (s, 1H), 7.56 (d, J = 8.6 Hz, 2H), 7.28 (d, J = 8.6 Hz, 2H), 6.15 (dd, J = 15.0, 2.6 Hz, 2H), 4.30 (d, J = 3.6 Hz, 1H), 3.00 (dd, J = 13.8, 3.9 Hz, 1H), 2.35 (dd, J = 13.7, 6.2 Hz, 3H), 2.26 (t, J = 7.3 Hz, 2H), 1.80 (p, J = 7.3 Hz, 2H). 13C–NMR (101 MHz, DMSO-d6): δ 174.2, 170.8, 138.1, 137.4, 131.0, 128.3, 119.5, 46.1, 39.4, 35.4, 33.1, 20.5. HRMS [M+Na]+ m/z calcd. for [C15H17NNaO3S]+ 314.0821, found 314.0821.
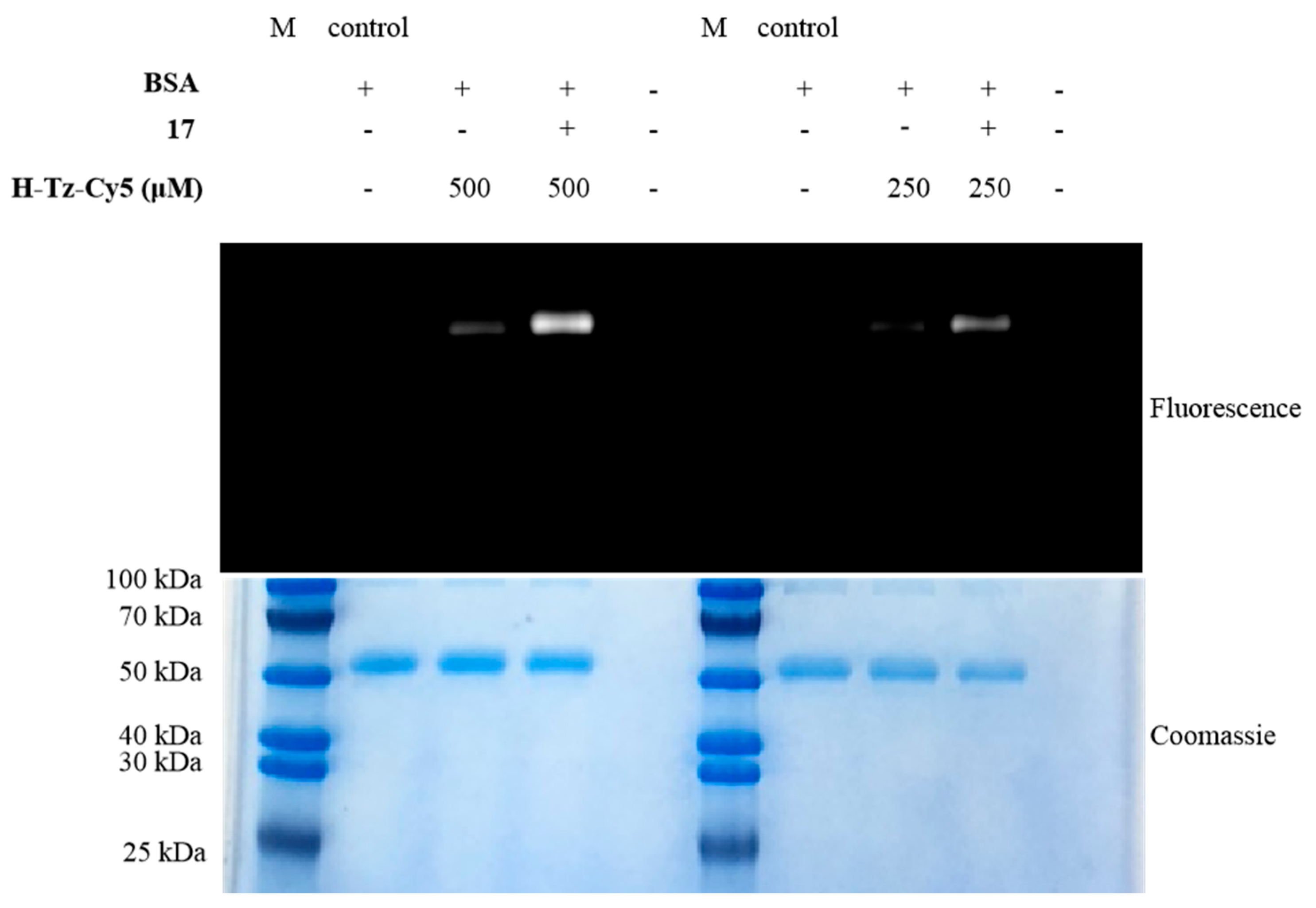
3.2.9. Synthesis of 2,5-Dioxopyrrolidin-1-yl-5-((4-(Cyclobut-2-en-1-Ylthio)Phenyl)Amino)-5-Oxopentanoate (17)
A mixture of compound 16 (6.2 mg, 0.021 mmol, 1.0 equiv), N,N-disuccinimidyl carbonate (DSC) (5.4 mg, 0.021 mmol, 1.0 equiv), and TEA (4.5 μL, 0.032 mmol, 1.5 equiv) was dissolved in tetrahydrofuran (THF) (0.5 mL) under Ar. The resulting mixture was stirred at room temperature for 4 h. After reaction completion, the reaction was quenched with water and extracted with ethyl acetate. The combined organic phases were washed with brine, dried over anhydrous Na2SO4, and evaporated in vacuo. Similarly to analogue 16, the residue was purified by thin-layer chromatography to afford the desired product 17 as a white solid in 51% yield (4.2 mg). 1H–NMR (400 MHz, CDCl3) δ 8.02 (s, 1H), 7.47 (d, J = 8.5 Hz, 2H), 7.30 (d, J = 8.6 Hz, 2H), 6.09 (dd, J = 21.8, 2.5 Hz, 2H), 4.26 (d, J = 3.5 Hz, 1H), 3.02 (dd, J = 13.8, 3.6 Hz, 1H), 2.88 (s, 4H), 2.75–2.68 (m, 2H), 2.47 (dd, J = 12.9, 5.8 Hz, 3H), 2.23–2.12 (m, 2H). 13C–NMR (101 MHz, CDCl3) δ 170.2, 169.7, 168.5, 138.0, 137.6, 136.9, 131.7, 130.9, 120.3, 47.0, 40.0, 35.5, 30.0, 25.8, 21.2. HRMS [M+Na]+ m/z calcd. for [C19H20N2NaO5S]+ 411.0985, found 411.0989.