Bioprospecting of Coralline Red Alga Amphiroa rigida J.V. Lamouroux: Volatiles, Fatty Acids and Pigments
Abstract
1. Introduction
2. Results and Discussion
2.1. Analysis of Volatile Organic Compounds (VOCs) of A. rigida
2.1.1. The Headspace Composition of A. rigida
2.1.2. Volatile Oil Composition of A. rigida
2.2. Fatty Acids Composition of A. rigida
2.3. Pigment Composition of A. rigida
2.4. Antimicrobial Activity of A. rigida
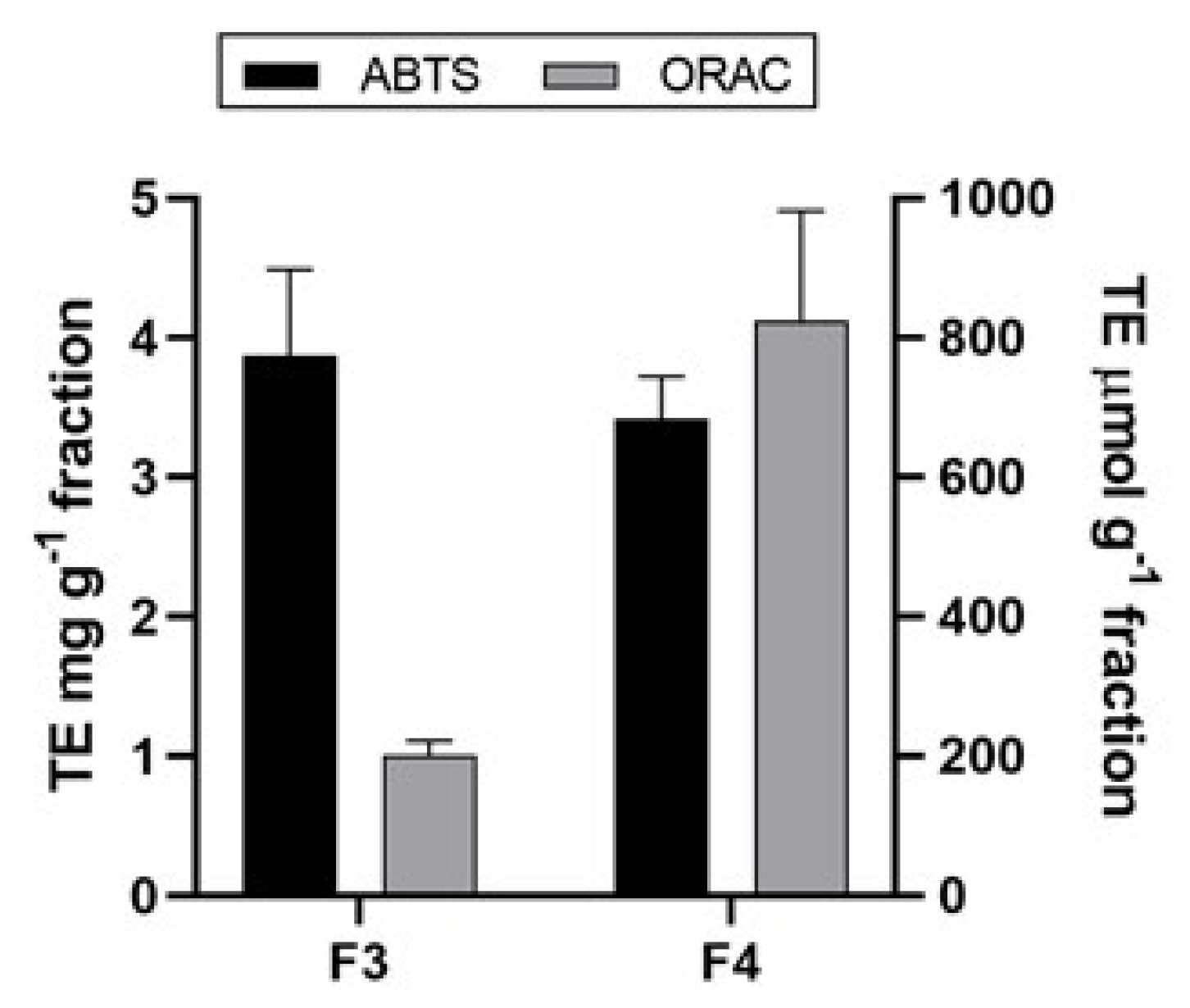
2.5. Antioxidant Activity of A. rigida
3. Materials and Methods
3.1. Chemicals
3.2. Material and Preparation Procedure
3.3. Headspace Solid-Phase Microextraction (HS-SPME)
3.4. Hydrodistillation (HD)
3.5. Gas Chromatography (GC) Analysis
3.6. Extraction and Fractionation with Solid-Phase Extraction (SPE)
3.7. Extraction and GC-FID Analysis of Fatty Acids
3.8. High Performance Liquid Chromatography (HPLC) Analysis of Pigments
3.9. Antimicrobial Activity
3.10. Antioxidant Scavenging Ability Determination
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zuo, Z. Why algae release volatile organic compounds-the emission and roles. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Ceron, M.C.; Garcia-Malea, M.C.; Rivas, J.; Acien, F.G.; Fernandez, J.M.; Del Rio, E.; Guerrero, M.G.; Molina, E. Antioxidant activity of Haematococcus pluvialis cells grown in continuous culture as a function of their carotenoid and fatty acid content. Appl. Microbiol. Biotechnol. 2007, 74, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Pereira, H.; Barreira, L.; Figueiredo, F.; Custodio, L.; Vizetto-Duarte, C.; Polo, C.; Rešek, E.; Engelen, A.; Varela, J. Polyunsaturated Fatty Acids of Marine Macroalgae: Potential for Nutritional and Pharmaceutical Applications. Mar. Drugs 2012, 10, 1920–1935. [Google Scholar] [CrossRef] [PubMed]
- Cotas, J.; Leandro, A.; Pacheco, D.; Goncalves, A.M.M.; Pereira, L. A Comprehensive Review of the Nutraceutical and Therapeutic Applications of Red Seaweeds (Rhodophyta). Life 2020, 10, 19. [Google Scholar] [CrossRef]
- Dumay, J.; Morancais, M. Proteins and Pigments. In Seaweed in Health and Disease Prevention; Fleurence, J., Levine, I., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 275–318. [Google Scholar]
- Sachindra, N.M.; Sato, E.; Maeda, H.; Hosokawa, M.; Niwano, Y.; Kohno, M.; Miyashita, K. Radical Scavenging and Singlet Oxygen Quenching Activity of Marine Carotenoid Fucoxanthin and Its Metabolites. J. Agric. Food Chem. 2007, 55, 8516–8522. [Google Scholar] [CrossRef]
- Kavalappa, Y.P.; Rudresh, D.U.; Gopal, S.S.; Shivarudrappa, A.H.; Stephen, N.M.; Rangiah, K.; Ponesakki, G. β-carotene isolated from the marine red alga, Gracillaria sp. potently attenuates the growth of human hepatocellular carcinoma (HepG2) cells by modulating multiple molecular pathways. J. Funct. Foods 2019, 52, 165–176. [Google Scholar] [CrossRef]
- Karpinski, T.M.; Adamczak, A. Fucoxanthin—An Antibacterial Carotenoid. Antioxidants 2019, 8, 239. [Google Scholar] [CrossRef]
- Choi, D.S.; Lee, I.K. On surface structures of Amphiroa (Corallinaceae, Rhodophyta). Korean J. Phycol. 1988, 3, 111–117. [Google Scholar]
- Riosmena-Rodríguez, R.; Siquelros-Beltrones, D. Taxonomy of the genus Amphiroa (Corallinales, Rhodophyta) in the southern Baja California Peninsula, Mexico. Phycologia 1996, 35, 135–147. [Google Scholar] [CrossRef]
- Woelkerling, W.J.; Campbell, S.J. An account of Southern Australian species of Uthophyllum (Corallinaceae, Rhodophyta). Bull. Brit. Mus. (Nat. Hist.) 1992, 22, 1–106. [Google Scholar] [CrossRef]
- Kim, S. Morphogenesis in Amphiroa (Corallinales, Rhodophyta): Taxonomic implications. J. Phycol. 1990, 26, 4. [Google Scholar]
- Garbary, D.J.; Johansen, H.W. Morphogenesis and Evolution in the Amphiroideae (Rhodophyta, Corallinaceae). Br. Phycol. J. 1987, 22, 1–10. [Google Scholar] [CrossRef]
- Economou-Amilli, A.; Bitis, I.; Paschou, M. Morphological variability in Amphiroa, Corallina and Jania (Rhodophyta—Corallinaceae) from Greece. Bot. Mar. 1990, 33, 261–271. [Google Scholar] [CrossRef]
- Gopu, M.; Selvam, K. Polysaccharides from marine red algae Amphiroa rigida and their biomedical potential: An in-vitro study. Biocatal. Agric. Biotechnol. 2020, 29, 101769. [Google Scholar] [CrossRef]
- Khan, S.B.; Kong, C.S.; Kim, J.A.; Kim, S.K. Protective Effect of Amphiroa dilatata on ROS Induced Oxidative Damage and MMP Expressions in HT1080 Cells. Biotechnol. Bioprocess Eng. 2010, 15, 191–198. [Google Scholar] [CrossRef]
- Boonchum, W.; Peerapornpisal, Y.; Kanjanapothi, D.; Pekkoh, J.; Amornlerdpison, D.; Pumas, C.; Sangpaiboon, P.; Vacharapiyasophon, P. Antimicrobial and Anti-inflammatory Properties of Various Seaweeds from the Gulf of Thailand. Int. J. Agric. Biol. 2011, 13, 100–104. [Google Scholar]
- Stirk, W.A.; Reinecke, D.L.; van Staden, J. Seasonal variation in antifungal, antibacterial and acetylcholinesterase activity in seven South African seaweeds. J. Appl. Phycol. 2007, 19, 271–276. [Google Scholar] [CrossRef]
- Jayasree, N.B.; Aneesh, T.P.; Prabhakar, V.; Anandan, R. GC-MS, HPLC and AAS analysis of fatty acids, amino acids and minerals in red algae Amphiroa anceps. Int. J. Pharm. Pharma. Sci. 2012, 4, 187–190. [Google Scholar]
- Fleury, B.G.; Figueiredo, L.; Marconi, M.I.; Teixeira, V.L.; Ferreira, A.B.B.; Pinto, A.C. Fatty Acids as Chemotaxonomic Markers of Marine Macrophytes from Rio de Janeiro State, Brazil. Nat. Prod. Commun. 2011, 6, 667–672. [Google Scholar] [CrossRef]
- Jerković, I.; Kranjac, M.; Marijanović, Z.; Šarkanj, B.; Cikoš, A.-M.; Aladić, K.; Pedisić, S.; Jokić, S. Chemical diversity of Codium bursa (Olivi) C. Agardh headspace compounds, volatiles, fatty acids and insight into its antifungal activity. Molecules 2019, 24, 842. [Google Scholar] [CrossRef]
- Jerković, I.; Kranjac, M.; Marijanović, Z.; Roje, M.; Jokić, S. Chemical diversity of headspace and volatile oil composition of two brown algae (Taonia atomaria and Padina pavonica) from the Adriatic Sea. Molecules 2019, 24, 495. [Google Scholar] [CrossRef]
- Jerković, I.; Marijanović, Z.; Roje, M.; Kuś, P.M.; Jokić, S.; Čož-Rakovac, R. Phytochemical study of the headspace volatile organic compounds of fresh algae and seagrass from the Adriatic Sea (single point collection). PLoS ONE 2018, 13, e0196462. [Google Scholar] [CrossRef] [PubMed]
- Youngblood, W.W.; Blumer, I.M.; Guillard, R.L.; Fiore, F. Saturated and unsaturated hydrocarbons in marine benthic algae. Mar. Biol. 1971, 8, 190–201. [Google Scholar] [CrossRef]
- Youngblood, W.W.; Blumer, M. Alkanes and alkenes in marine benthic algae. Mar. Biol. 1973, 21, 163–172. [Google Scholar] [CrossRef]
- Clark, R.C., Jr.; Blumer, M. Distribution of n-paraffins in marine organisms and sediments. Limnol. Oceanogr. 1967, 12, 79–87. [Google Scholar] [CrossRef]
- Berneira, L.; da Silva, C.; Poletti, T.; Ritter, M.; dos Santos, M.; Colepicolo, P.; de Pereira, C.M.P. Evaluation of the volatile composition and fatty acid profile of seven Antarctic macroalgae. J. Appl. Phycol. 2020, 32, 3319–3329. [Google Scholar] [CrossRef]
- Han, J.; Chan, H.W.S.; Calvin, M. Biosynthesis of alkanes in Nostoc muscorum. J. Am. Chem. Soc. 1969, 91, 5156–5159. [Google Scholar] [CrossRef]
- McInnes, A.G.; Walter, J.A.; Wright, J.L.C. Biosynthesis of hydrocarbons by algae: Decarboxylation of stearic acid to n-heptadecane in Anacystis nidulans determined by 13C- and 2H-labeling and 13C nuclear magnetic resonance. Lipids 1980, 15, 609–615. [Google Scholar] [CrossRef]
- Boonprab, K.; Matsuia, K.; Yoshida, M.; Akakabe, Y.; Chirapart, A.; Kajiwara, T. C6-Aldehyde formation by fatty acid hydroperoxide lyase in the brown alga Laminaria angustata. Z. Naturforsc. C J. Biosi. 2014, 58, 207–214. [Google Scholar] [CrossRef]
- Boonprab, K.; Matsui, K.; Akakabea, Y.; Yotsukurab, N.; Kajiwara, T. Hydroperoxy-arachidonic acid mediated n-hexanal and (Z)-3- and (E)-2-nonenal formation in Laminaria angustata. Phytochemistry 2003, 63, 669–678. [Google Scholar] [CrossRef]
- Le Pape, M.A.; Grua-Priol, J.; Prost, C.; Demaimay, M. Optimization of dynamic headspace extraction of the edible red algae Palmaria palmata and identification of the volatile components. J. Agric. Food Chem. 2004, 52, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Boonprab, K.; Matsui, K.; Akakabe, Y.; Yoshida, M.; Yotsukura, N.; Chirapart, A.; Kajiwara, T. Formation of aldehyde flavor (n-hexanal, 3Z-nonenal and 2E-nonenal) in the brown alga, Laminaria angustata. J. Appl. Phycol. 2006, 18, 409–412. [Google Scholar] [CrossRef]
- Azarbad, M.H.; Jelen, H. Determination of hexanal-an indicator of lipid oxidation by static headspace gas chromatography (SHS-GC) in fat-rich food matrices. Food Anal. Methods 2015, 8, 1727–1733. [Google Scholar] [CrossRef]
- Takahashi, H.; Sumitani, H.; Inada, Y.; Mori, D. Identification of volatile compounds of Kombu (Laminaria spp.) and their odor description. Nippon. Kagaku Kaishi 2002, 49, 228–237. [Google Scholar] [CrossRef]
- Sugisawa, H.; Nakamura, K.; Tamura, H. The aroma profile of the volatiles in marine green algae Ulva pertusa. Food Rev. Int. 1990, 6, 573–589. [Google Scholar] [CrossRef]
- Lee, G.-H.; Suriyaphan, O.; Cadwallader, K.R. Aroma components of cooked tail meat of American lobster (Homarus americanus). J. Agric. Food Chem. 2001, 49, 4324–4332. [Google Scholar] [CrossRef]
- Pennarun, A.-L.; Prost, C.; Demaimay, M. Identification and origin of the character-impact compounds of raw oyster Crassostrea gigas. J. Sci. Food Agric. 2002, 82, 1652–1660. [Google Scholar] [CrossRef]
- Pohnert, G. Diatom/copepod interactions in plankton: The indirect chemical defense of unicellular algae biogeneration of volatile compounds via oxylipins in edible seaweeds. Chem. Biochem. 2005, 6, 946–959. [Google Scholar]
- Sun, S.-M.; Chung, G.-H.; Shin, T.-S. Volatile compounds of the green alga, Capsosiphon fulvescens. J. Appl. Phycol. 2012, 24, 1003–1013. [Google Scholar] [CrossRef]
- Ishida, B.K.; Bartley, G.E. Carotenoids: Chemistry, Sources, and Physiology. Encyclopedia of Human Nutrition, 2nd ed.; Caballero, B., Allen, L., Prentice, A., Eds.; Academic Press: Cambridge, MA, USA, 2005; Chapter C; Volume 1, pp. 330–338. [Google Scholar]
- Jüttner, F. Physiology and biochemistry of odorous compounds from freshwater cyanobacteria and algae. Water Sci. Technol. 1995, 31, 69–78. [Google Scholar] [CrossRef]
- Silva Ferreira, A.C.; Monteiro, J.; Oliveira, C.; Guedes de Pinho, P. Study of major aromatic compounds in port wines from carotenoid degradation. Food Chem. 2008, 110, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Ikawa, M.; Sasner, J.J.; Haney, J.F. Activity of cyanobacterial and algal odor compounds found in lake waters on green alga Chlorella pyrenoidosa growth. Hydrobiologia 2001, 443, 19–22. [Google Scholar] [CrossRef]
- Chang, D.W.; Hsieh, M.L.; Chen, Y.M.; Lin, T.F.; Chang, J.S. Kinetics of cell lysis for Microcystis aeruginosa and Nitzschia palea in the exposure to b-cyclocitral. J. Hazard. Mater. 2011, 185, 1214–1220. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, K.; Ohta, A.; Iwata, C.; Horikawa, A.; Tsuji, K.; Ito, E.; Ikai, Y.; Harada, K. Lysis of cyanobacteria with volatile organic compounds. Chemosphere 2008, 71, 1531–1538. [Google Scholar] [CrossRef]
- Newman, K.A.; Gschwend, P.M. A method for quantitative determination of volatile organic compounds in marine macroalgae. Limnol. Oceanogr. 1987, 32, 702–708. [Google Scholar] [CrossRef]
- La Barre, S.; Potin, P.; Leblanc, C.; Delage, L. The halogenated metabolism of brown algae (Phaeophyta), its biological importance and its environmental significance. Mar. Drugs 2010, 8, 988–1010. [Google Scholar] [CrossRef]
- Malin, G.; Kirst, G.O. Algal production of dimethyl sulfide and its atmospheric role. J. Phycol. 1997, 33, 889–896. [Google Scholar] [CrossRef]
- Gu, S.Q.; Wang, X.C.; Tao, N.P.; Wu, N. Characterization of volatile compounds in different edible parts of steamed Chinese mitten crab (Eriocheir sinensis). Food Res. Int. 2013, 54, 81–92. [Google Scholar] [CrossRef]
- Fors, S. Sensory Properties of Volatile Maillard Reaction Products and Related Compounds, The Maillard Reaction in Foods and Nutrition; Waller, G., Feather, M., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1983; pp. 185–286. [Google Scholar]
- De Souza, N.J.; Nes, W.R. The presence of phytol in brown and blue-green algae and its relationship to evolution. Phytochemistry 1969, 8, 819–822. [Google Scholar] [CrossRef]
- Yin, S.-W.; Wang, C.-Y.; Li, X.-M.; Wang, B.-G. A new clerosterol derivative, trans-phytol, and related metabolites from marine green alga Codium fragile (Codiaceae) and their chemotaxonomic significance. Biochem. Syst. Ecol. 2005, 33, 1288–1292. [Google Scholar] [CrossRef]
- Bianchi, T.S.; Kautsky, L.; Argyrou, M. Dominant chlorophylls and carotenoids in macroalgae of the Baltic Sea (Baltic proper): Their use as potential biomarkers. Sarsia 1997, 82, 55–62. [Google Scholar] [CrossRef]
- Rontani, J.-F.; Volkman, J.K. Phytol degradation products as biogeochemical tracers in aquatic environments. Org. Geochem. 2003, 34, 1–35. [Google Scholar] [CrossRef]
- De-Paula, J.C.; Bueno, L.B.; Cavalcanti, D.N.; Yoneshigue-Valentin, Y.; Teixeira, V.L. Diterpenes from the brown alga Dictyota crenulata. Molecules 2008, 13, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Galloway, A.W.E.; Britton-Simmons, K.H.; Duggins, D.O.; Gabrielson, P.W.; Brett, M.T. Fatty acid signatures differentiate marine macrophytes at ordinal and family ranks. J. Phycol. 2012, 48, 956–965. [Google Scholar] [CrossRef]
- Li, X.; Fan, X.; Han, L.; Lou, Q. Fatty acids of some algae from the Bohai Sea. Phytochem. 2002, 59, 157–161. [Google Scholar] [CrossRef]
- Schmid, M.; Guiheneuf, F.; Stengel, D.B. Fatty acid contents and profiles of 16 macroalgae collected from the Irish Coast at two seasons. J. Appl. Phycol. 2014, 26, 451–463. [Google Scholar] [CrossRef]
- Aburai, N.; Ohkubo, S.; Miyashita, H.; Abe, K. Composition of carotenoids and identification of aerial microalgae isolated from the surface of rocks in mountainous districts of Japan. Algal. Res. 2013, 2, 237–243. [Google Scholar] [CrossRef]
- Henriques, M.; Silva, A.; Rocha, J. Extraction and quantification of pigments from a marine microalga: A simple and reproducible method. In Communicating Current Research and Educatioal Topics and Trends in Applied Microbiology; Mendez-Vilas, A., Ed.; Formatex: Badajoz, Spain, 2007; pp. 586–593. [Google Scholar]
- Takaichi, S. Carotenoids in Algae: Distributions, Biosyntheses and Functions. Mar. Drugs 2011, 9, 1101–1118. [Google Scholar] [CrossRef]
- Bjornland, T.; Aguilar-Martinez, M. Carotenoids in red algae. Phytochemistry 1976, 15, 291–296. [Google Scholar] [CrossRef]
- Schubert, N.; Garcia-Mendoza, E.; Pacheco-Ruiz, I. Carotenoid composition of marine red algae. J. Phycol. 2006, 42, 1208–1216. [Google Scholar] [CrossRef]
- Pereira, L.C.C.; da Silva, N.I.S.; da Costa, R.M.; Asp, N.E.; da Costa, K.G.; Vila-Concejo, A. Seasonal changes in oceanographic processes at an equatorial macrotidal beach in northern Brazil. Cont. Shelf Res. 2012, 43, 95–106. [Google Scholar] [CrossRef]
- Roy, S. Screening and Partial Characterization of Natural Antioxidants from Seaweeds Collected From, Rameshwaram Southeast Coast of India. J. Mari. Sci. Res. Ocean. 2020, 3, 1–12. [Google Scholar]
- Genovese, G.; Faggio, C.; Gugliandolo, C.; Torre, A.; Spano, A.; Morabito, M.; Maugeri, T.L. In vitro evaluation of antibacterial activity of Asparagopsis taxiformis from the Straits of Messina against pathogens relevant in aquaculture. Mar. Environ. Res. 2012, 73, 1–6. [Google Scholar] [CrossRef]
- Marino, F.; Di Caro, G.; Gugliandolo, C.; Spano, A.; Faggio, C.; Genovese, G.; Morabito, M.; Russo, A.; Barreca, D.; Fazio, F.; et al. Preliminary Study on the In vitro and In vivo Effects of Asparagopsis taxiformis Bioactive Phycoderivates on Teleosts. Front. Physiol. 2016, 7, 459. [Google Scholar] [CrossRef] [PubMed]
- Goiris, K.; Muylaert, K.; Fraeye, I.; Foubert, I.; De Brabanter, J.; De Cooman, L. Antioxidant potential of microalgae in relation to their phenolic and carotenoid content. J. Appl. Phycol. 2012, 24, 1477–1486. [Google Scholar] [CrossRef]
- Machu, L.; Misurcova, L.; Ambrozova, J.V.; Orsavova, J.; Mlcek, J.; Sochor, J.; Jurikova, T. Phenolic content and antioxidant capacity in algal food products. Molecules 2015, 20, 1118–1133. [Google Scholar] [CrossRef] [PubMed]
- Souza, B.W.S.; Cerqueira, M.A.; Martins, J.T.; Quintas, M.A.C.; Ferreira, A.C.S.; Teixeira, J.A.; Vicente, A.A. Antioxidant potential of two red seaweeds from the Brazilian coasts. J. Agric. Food Chem. 2011, 59, 5589–5594. [Google Scholar] [CrossRef]
- Siriwardhana, N.; Lee, K.W.; Kim, S.H.; Ha, J.W.; Jeon, Y.J. Antioxidant activity of Hizikia fusiformis on reactive oxygen species scavenging and lipid peroxidation inhibition. Food Sci. Technol. Int. 2003, 9, 339–347. [Google Scholar] [CrossRef]
- Wu, X.; Hansen, C. Antioxidant capacity, phenolic content, polysaccharide content of Lentinus edodes grown in whey permeate-based submerged culture. J. Food Sci. 2008, 73, M1–M8. [Google Scholar] [CrossRef]
- Gan, J.; Feng, Y.; He, Z.; Li, X.; Zhang, H. Correlations between antioxidant activity and alkaloids and phenols of Maca (Lepidium meyenii). Hindawi J. Food Qual. 2017, 1–10. [Google Scholar] [CrossRef]
- Antolovich, M.; Prenzler, P.D.; Patsalides, E.; McDonald, S.; Robards, K. Methods for testing antioxidant activity. Analyst 2002, 127, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Bohm, V.; Puspitasari-Nienaber, N.L.; Ferruzzi, M.G.; Schwartz, S.J. Trolox Equivalent Antioxidant Capacity of Different Geometrical Isomers of α-Carotene, β-Carotene, Lycopene, and Zeaxanthin. J. Agric. Fodd Chem. 2002, 50, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Mueller, L.; Boehm, V. Antioxidant Activity of β-Carotene Compounds in Different in Vitro Assays. Molecules 2011, 16, 1055–1069. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Standards and Technology (NIST) Chemistry WebBook. NIST Standard Reference Database Number 69. Available online: http://webbook.nist.gov/chemistry/ (accessed on 14 September 2020).
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- European Commission Regulation (EC) No 796/2002 of 6 May 2002 amending Regulation (EEC) No 2568/91 on the characteristics of olive oil and olive-pomace oil and on the relevant methods of analysis and the additional notes in the Annex to Council Regulation (EEC) No 2658/87 on the tariff and statistical nomenclature and on the Common Customs Tariff. Off. J. Eur. Comm. 2002, L128, 8–28.
- Castro-Puyana, M.; Perez-Sanchez, A.; Valdes, A.; Ibrahim, O.H.M.; Suarez-Alvarez, S.; Ferragut, J.A.; Micol, V.; Cifuentes, A.; Ibanez, E.; Garcia-Canas, V. Pressurized liquid extraction of Neochloris oleoabundans for the recovery of bioactive carotenoids with anti-proliferative activity against human colon cancer cells. Food Res. Int. 2017, 99, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 10th ed.; M07-A10; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi, 3rd ed.; M38; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- Miller, R.A.; Walker, R.D.; Baya, A.; Clemens, K.; Coles, M.; Hawke, J.P.; Henricson, B.E.; Hsu, H.M.; Mathers, J.J.; Oaks, J.L.; et al. Antimicrobial Susceptibility Testing of Aquatic Bacteria: Quality Control Disk Diffusion Ranges for Escherichia coli ATCC 25922 and Aeromonas salmonicida subsp. salmonicida ATCC 33658 at 22 and 28 °C. J. Clin. Microbiol. 2003, 41, 4318–4323. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef]
| No | Compound | RI | I Av ± SD | II Av ± SD | III Av ± SD | IV Av ± SD |
|---|---|---|---|---|---|---|
| 1. | Dimethyl sulfide | <900 | - | - | 4.38 ± 0.14 | 1.22 ± 0.02 |
| 2. | Pentanal S | <900 | 1.25 ± 0.03 | 0.79 ± 0.03 | 1.84 ± 0.05 | 0.68 ± 0.01 |
| 3. | (E)-Pent-2-enal S | <900 | 0.53 ± 0.02 | - | - | - |
| 4. | Pentan-1-ol S | <900 | 0.67 ± 0.02 | 0.86 ± 0.03 | - | - |
| 5. | (Z)-Pent-2-en-1-ol S | <900 | 1.22 ± 0.03 | - | - | - |
| 6. | Hexanal S | <900 | 6.72 ± 0.10 | 2.31 ± 0.02 | 6.31 ± 0.21 | 2.01 ± 0.02 |
| 7. | (E)-Hex-2-enal S | <900 | 7.41 ± 0.23 | 0.48 ± 0.03 | 2.47 ± 0.04 | 0.87 ± 0.03 |
| 8. | Hexan-1-ol S | <900 | 2.36 ± 0.02 | 1.09 ± 0.03 | - | - |
| 9. | Tribromomethane S | <900 | 1.52 ± 0.04 | 1.27 ± 0.02 | - | - |
| 10. | Heptanal S | 901 | 0.49 ± 0.02 | 0.47 ± 0.02 | 1.00 ± 0.03 | 0.48 ± 0.01 |
| 11. | Benzaldehyde S | 967 | 0.93 ± 0.03 | 1.60 ± 0.04 | 1.16 ± 0.05 | 1.04 ± 0.03 |
| 12. | Oct-1-en-3-ol S | 982 | 0.50 ± 0.03 | 0.40 ± 0.01 | 0.51 ± 0.03 | 0.47 ± 0.01 |
| 13. | Octane-2,3-dione | 984 | - | - | 1.21 ± 0.05 | 0.80 ± 0.03 |
| 14. | 6-Methylhept-5-en-2-one S | 988 | 0.67 ± 0.03 | 0.44 ± 0.03 | 0.90 ± 0.02 | 0.71 ± 0.02 |
| 15. | 2-Pentylfuran S | 993 | - | - | 0.98 ± 0.03 | 0.80 ± 0.04 |
| 16. | Octanal S | 1003 | 0.53 ± 0.02 | 0.74 ± 0.02 | - | - |
| 17. | (E,E)-Hepta-2,4-dienal S | 1014 | - | 0.14 ± 0.01 | - | - |
| 18. | Benzyl alcohol S | 1042 | - | - | 8.58 ± 0.10 | 9.44 ± 0.23 |
| 19. | (E)-Oct-2-enal S | 1061 | 0.69 ± 0.03 | 0.48 ± 0.05 | 0.13 ± 0.03 | 0.44 ± 0.05 |
| 20. | (E,E)-Octa-3,5-dien-2-one | 1073 | - | - | - | 1.13 ± 0.04 |
| 21. | Octan-1-ol S | 1074 | 0.34 ± 0.05 | 0.49 ± 0.02 | - | - |
| 22. | Nonanal S | 1099 | 1.27 ± 0.05 | 1.63 ± 0.03 | 0.99 ± 0.02 | 1.50 ± 0.05 |
| 23. | 6-[(1Z)-1-Butenyl]-cyclohepta-1,4-diene (Ectocarpene) | 1150 | 0.97 ± 0.02 | 0.66 ± 0.05 | - | - |
| 24. | Decanal S | 1296 | - | - | - | 0.62 ± 0.03 |
| 25. | β-Cyclocitral S | 1222 | 0.78 ± 0.05 | 0.47 ± 0.02 | - | 0.67 ± 0.01 |
| 26. | (E)-Dec-2-enal S | 1264 | - | 0.16 ± 0.02 | - | - |
| 27. | α-Cubebene | 1366 | - | 0.88 ± 0.03 | - | - |
| 28. | β-Bourbonene | 1385 | - | 0.37 ± 0.02 | 0.90 ± 0.02 | 1.53 ± 0.02 |
| 29. | Germacrene D S | 1481 | - | 1.13 ± 0.05 | 1.33 ± 0.06 | 2.66 ± 0.05 |
| 30. | (E)-β-Ionone S | 1486 | 1.55 ± 0.04 | 0.79 ± 0.04 | 0.50 ± 0.02 | 0.53 ± 0.02 |
| 31. | Pentadec-1-ene S | 1492 | 1.10 ± 0.06 | 13.05 ± 0.24 | - | - |
| 32. | (E)-Pentadec-7-ene * | 1495 | - | 6.00 ± 0.32 | - | - |
| 33. | Pentadecane S | 1500 | 5.51 ± 0.25 | 7.08 ± 0.20 | 5.21 ± 0.10 | 7.02 ± 0.23 |
| 34. | Hexadec-7-ene * | 1515 | - | 11.25 ± 0.22 | - | - |
| 35. | Ttradecan-1-ol S | 1678 | 0.96 ± 0.05 | 1.02 ± 0.06 | - | - |
| 36. | Heptadec-1-ene S | 1692 | 0.62 ± 0.05 | 1.04 ± 0.06 | - | - |
| 37. | (E)-Heptadec-8-ene | 1678 | 0.76 ± 0.05 | 0.77 ± 0.03 | 0.10 ± 0.02 | 0.28 ± 0.02 |
| 38. | Heptadecane S | 1700 | 47.77 ± 2.66 | 30.08 ± 1.67 | 50.16 ± 3.05 | 53.96 ± 4.05 |
| 39. | (E)-Heptadec-3-ene * | 1717 | 3.63 ± 0.05 | 4.79 ± 0.06 | 6.44 ± 0.25 | 7.41 ± 0.45 |
| 40. | Eicosane S | 2000 | - | 0.58 ± 0.03 | - | - |
| No | Compound | RI | I Av ± SD | II Av ± SD |
|---|---|---|---|---|
| 1. | (E)-Hex-2-enal S | <900 | 0.85 ± 0.02 | 1.55 ± 0.03 |
| 2. | Heptan-2-one S | <900 | - | 0.12 ± 0.01 |
| 3. | Heptanal S | 901 | 0.04 ± 0.01 | 0.12 ± 0.01 |
| 4. | (E)-Hept-2-enal S | 961 | - | 0.05 ± 0.01 |
| 5. | Benzaldehyde S | 967 | 0.26 ± 0.02 | 0.31 ± 0.02 |
| 6. | Oct-1-en-3-ol S | 982 | - | 0.09 ± 0.01 |
| 7. | Octan-2,3-dione | 984 | - | 0.21 ± 0.02 |
| 8. | 6-Methylhept-5-en-2-one S | 988 | 0.50 ± 0.02 | 0.36 ± 0.02 |
| 9. | Octan-2-one S | 992 | 0.05 ± 0.03 | 0.25 ± 0.04 |
| 10. | Octanal S | 1003 | 0.12 ± 0.02 | 0.21 ± 0.07 |
| 11. | (E,E)-Hepta-2,4-dienal S | 1014 | - | 0.12 ± 0.02 |
| 12. | 2-Ethylhexan-1-ol S | 1033 | 0.12 ± 0.02 | 0.12 ± 0.01 |
| 13. | 2,6,6-Trimethylcyclohexanone | 1040 | - | 0.03 ± 0.01 |
| 14. | Benzyl alcohol S | 1042 | 0.26 ± 0.02 | 0.23 ± 0.01 |
| 15. | Phenylacetaldehyde S | 1049 | 0.14 ± 0.02 | 0.17 ± 0.02 |
| 16. | (E)-Oct-2-enal S | 1061 | 0.08 ± 0.02 | 0.22 ± 0.03 |
| 17. | Acetophenone S | 1072 | - | 0.08 ± 0.01 |
| 18. | Octan-1-ol S | 1074 | 0.16 ± 0.02 | 0.23 ± 0.03 |
| 19. | Nonan-2-one S | 1091 | 0.05 ± 0.02 | 0.06 ± 0.01 |
| 20. | (E,Z)-Octa-3,5-dien-2-one | 1095 | - | 0.06 ± 0.01 |
| 21. | Nonanal S | 1099 | 0.10 ± 0.02 | 0.23 ± 0.04 |
| 22. | (E,E)-Octa-2,4-dienal | 1111 | - | 0.18 ± 0.02 |
| 23. | Phenylacetonitrile S | 1144 | 0.08 ± 0.01 | 0.01 ± 0.00 |
| 24. | 4-Ketoisophorone S | 1148 | 0.04 ± 0.01 | - |
| 25. | 6-[(1Z)-1-Butenyl]-cyclohepta-1,4-diene (Ectocarpene) | 1150 | 0.01 ± 0.00 | - |
| 26. | (E,Z)-Nona-2,6-dienal S | 1157 | - | 0.12 ± 0.01 |
| 27. | (Z)-Non-2-enal S | 1163 | - | 0.12 ± 0.02 |
| 28. | 3-Methylacetophenone S | 1187 | - | 0.06 ± 0.01 |
| 29. | Decan-2-one S | 1194 | 0.18 ± 0.02 | 0.48 ± 0.05 |
| 30. | Decanal S | 1296 | - | 0.16 ± 0.02 |
| 31. | β-Cyclocitral S | 1222 | - | 0.16 ± 0.03 |
| 32. | Benzothiazole S | 1227 | 0.10 ± 0.02 | 0.08 ± 0.01 |
| 33. | β-Cyclohomocitral | 1260 | - | 0.14 ± 0.02 |
| 34. | (E)-Dec-2-enal S | 1264 | - | 0.16 ± 0.023 |
| 35. | Decan-1-ol S | 1276 | - | 0.21 ± 0.02 |
| 36. | Undecan-2-one S | 1294 | 0.04 ± 0.01 | - |
| 37. | (E,Z)-Deca-2,4-dienal | 1294 | - | 0.14 ± 0.02 |
| 38. | Undecanal S | 1307 | - | 0.17 ± 0.01 |
| 39. | (E,E)-Deca-2,4-dienal S | 1318 | - | 0.31 ± 0.03 |
| 40. | Undecan-1-ol S | 1377 | - | 0.18 ± 0.02 |
| 41. | β-Bourbonene | 1385 | 0.05 ± 0.01 | 0.03 ± 0.01 |
| 42. | β-Cubebene | 1391 | - | 0.09 ± 0.02 |
| 43. | Tetradecane S | 1400 | 0.05 ± 0.01 | - |
| 44. | Dodecanal S | 1409 | - | 0.36 ± 0.05 |
| 45. | α-Ionone S | 1429 | - | 0.13 ± 0.06 |
| 46. | (E)-Geranylacetone S | 1455 | - | 0.85 ± 0.04 |
| 47. | Dodecan-1-ol S | 1477 | 0.44 ± 0.02 | 2.11 ± 0.20 |
| 48. | Germacrene D S | 1481 | 1.01 ± 0.06 | 0.46 ± 0.07 |
| 49. | (E)-β-Ionone S | 1486 | 0.29 ± 0.02 | 2.55 ± 0.06 |
| 50. | Pentadec-1-ene S | 1492 | 0.50 ± 0.02 | 0.39 ± 0.01 |
| 51. | (E)-Pentadec-7-ene * | 1495 | 0.04 ± 0.01 | - |
| 52. | Pentadecane S | 1500 | 2.36 ± 0.05 | 0.91 ± 0.02 |
| 53. | N,N-Dimethyldodecan-1-amine | 1504 | 0.94 ± 0.02 | - |
| 54. | Tridecanal S | 1510 | - | 0.47 ± 0.05 |
| 55. | Cubebol | 1516 | 0.59 ± 0.03 | 0.57 ± 0.01 |
| 56. | Tridecan-1-ol | 1578 | 0.29 ± 0.01 | 0.63 ± 0.01 |
| 57. | Hexadecane S | 1600 | - | 0.23 ± 0.02 |
| 58. | Tetradecanal S | 1612 | - | 0.48 ± 0.02 |
| 59. | Benzophenone S | 1627 | - | 0.66 ± 0.03 |
| 60. | Cubenol | 1644 | 0.42 ± 0.02 | 1.95 ± 0.05 |
| 61. | Tetradecan-1-ol S | 1678 | 0.48 ± 0.02 | 2.76 ± 0.09 |
| 62. | Eudesma-4(15),7-dien-1β-ol | 1686 | 1.06 ± 0.03 | 0.29 ± 0.02 |
| 63. | Heptadec-1-ene S | 1692 | 0.75 ± 0.02 | 0.82 ± 0.03 |
| 64. | (E)-Heptadec-8-ene S | 1678 | 0.31 ± 0.02 | 2.02 ± 0.03 |
| 65. | Heptadecane S | 1700 | 3.80 ± 0.21 | 12.22 ± 0.23 |
| 66. | Pentadecanal S | 1714 | 0.50 ± 0.02 | 1.07 ± 0.06 |
| 67. | (E)-Heptadec-3-ene * | 1717 | 0.29 ± 0.02 | 2.50 ± 0.06 |
| 68. | trans-Farnesol S | 1724 | 0.10 ± 0.01 | 0.29 ± 0.02 |
| 69. | (E)-2-Hexylcinnamaldehyde | 1748 | - | 0.25 ± 0.04 |
| 70. | Pentadecan-1-ol S | 1780 | 0.18 ± 0.02 | 0.95 ± 0.03 |
| 71. | 2-Ethylhexyl salicylate | 1805 | 0.64 ± 0.02 | 1.03 ± 0.09 |
| 72. | Octadecane S | 1800 | 0.22 ± 0.02 | 0.23 ± 0.02 |
| 73. | 6,10,14-Trimethylpentadecan-2-one (Phytone) | 1845 | 2.21 ± 0.09 | 7.28 ± 0.12 |
| 74. | (Z)-Hexadec-11-en-1-ol | 1861 | - | 2.02 ± 0.10 |
| 75. | Diisobutyl phthalate S | 1868 | 0.55 ± 0.02 | 0.47 ± 0.03 |
| 76. | Hexadecan-1-ol S | 1881 | 1.13 ± 0.09 | 3.79 ± 0.80 |
| 77. | Nonadecane S | 1900 | - | 0.22 ± 0.02 |
| 78. | Heptadecan-2-one S | 1900 | 0.08 ± 0.01 | 0.22 ± 0.02 |
| 79. | (E,E)-Farnesyl acetone | 1918 | - | 1.15 ± 0.08 |
| 80. | Methyl palmitate S | 1926 | - | 0.57 ± 0.02 |
| 81. | Dibutyl phthalate S | 1962 | 0.94 ± 0.04 | 1.65 ± 0.05 |
| 82. | Cyclooctasulfur | 2009 | 0.22 ± 0.02 | - |
| 83. | Octadecanal S | 2019 | - | 0.87 ± 0.02 |
| 84. | Epimanool | 2051 | 3.18 ± 0.09 | 1.77 ± 0.08 |
| 85. | (E)-Phytol S | 2112 | 41.75 ± 1.87 | 16.44 ± 0.15 |
| 86. | Pachydictyol A | 2123 | 2.97 ± 0.09 | 1.11 ± 0.04 |
| 87. | Isopachydictyol A | 2136 | 2.41 ± 0.02 | 1.75 ± 0.03 |
| 88. | Tricosane S | 2300 | - | 0.69 ± 0.02 |
| 89. | Ferruginol | 2331 | 1.45 ± 0.023 | - |
| No. | Fatty Acid | Av ± SD * (%) |
|---|---|---|
| 1. | Butanoic acid (Butyric acid) (C4:0) | 0.20 ± 0.02 |
| 2. | Hexanoic acid (Caproic acid) (C6:0) | 0.91 ± 0.08 |
| 3. | Tetradecanoic acid (Myristic acid) (C14:0) | 3.34 ± 0.15 |
| 4. | Pentadecanoic acid (Pentadecyclic acid) (C15:0) | 0.69 ± 0.01 |
| 5. | Hexadecanoic acid (Palmitic acid) (C16:0) | 42.86 ± 0.26 |
| 6. | Octadecanoic acid (Stearic acid) (C18:0) | 11.65 ± 0.10 |
| Total saturated fatty acids (SFA) | 59.65 | |
| 7. | (Z)-Hexadec-9-enoic acid (Palmitoleic acid) (C16:1) | 1.73 ± 0.09 |
| 8. | (Z)-Octadec-9-enoic acid+(E)-Octadec-9-enoic acid (cis-Oleic acid+trans-Oleic acid) (C18:1n9c+t) | 5.46 ± 0.03 |
| Total monounsaturated fatty acids (MUFA) | 7.19 | |
| 9. | (9Z,12Z)-Octadeca-9,12-dienoic acid (cis-Linoleic acid) (C18:2n6c) | 3.03 ± 0.03 |
| 10. | (9E,12E)-Octadeca-9,12-dienoic acid (trans-Linoleic acid) (C18:2n6t) | 2.22 ± 0.02 |
| 11. | (6Z,9Z,12Z)-Octadeca-6,9,12-trienoic acid (γ-Linolenic acid) (C18:3n6) | 0.46 ± 0.05 |
| 12. | (9Z,12Z,15Z)-Octadeca-9,12,15-trienoic acid (α-linolenic acid) (C18:3n3) | 0.41 ± 0.06 |
| 13. | (11Z,14Z)-Icosa-11,14-dienoic acid (Eicosadienoic acid) (C20:2n6) | 7.90 ± 0.11 |
| 14. | (5Z,8Z,11Z,14Z,17Z)-Icosa-5,8-11,14,17-pentaenoic acid (Eicosapentaenoic acid) (C20:5n3) | 19.14 ± 0.32 |
| Total polyunsaturated fatty acids (PUFA) | 33.16 | |
| Total ω3 fatty acids | 21.96 | |
| Total ω6 fatty acids | 13.61 |
| Pigments | F3 Av ± SD * | F4 Av ± SD * |
|---|---|---|
| Fucoxanthin | 0.63 ± 0.62 | n.d. |
| Lutein | 5.83 ± 0.97 | n.d. |
| Chlorophyll a | n.d. | 13.65 ± 0.81 |
| β-Carotene | n.d. | 6.18 ± 0.72 |
| α-Carotene | n.d. | n.q. |
| Fraction | mg GAE g−1 of Dry Fraction |
|---|---|
| F3 | 59.38 ± 1.62 |
| F4 | 16.71 ± 0.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cikoš, A.-M.; Flanjak, I.; Bojanić, K.; Babić, S.; Čižmek, L.; Čož-Rakovac, R.; Jokić, S.; Jerković, I. Bioprospecting of Coralline Red Alga Amphiroa rigida J.V. Lamouroux: Volatiles, Fatty Acids and Pigments. Molecules 2021, 26, 520. https://doi.org/10.3390/molecules26030520
Cikoš A-M, Flanjak I, Bojanić K, Babić S, Čižmek L, Čož-Rakovac R, Jokić S, Jerković I. Bioprospecting of Coralline Red Alga Amphiroa rigida J.V. Lamouroux: Volatiles, Fatty Acids and Pigments. Molecules. 2021; 26(3):520. https://doi.org/10.3390/molecules26030520
Chicago/Turabian StyleCikoš, Ana-Marija, Ivana Flanjak, Krunoslav Bojanić, Sanja Babić, Lara Čižmek, Rozelindra Čož-Rakovac, Stela Jokić, and Igor Jerković. 2021. "Bioprospecting of Coralline Red Alga Amphiroa rigida J.V. Lamouroux: Volatiles, Fatty Acids and Pigments" Molecules 26, no. 3: 520. https://doi.org/10.3390/molecules26030520
APA StyleCikoš, A.-M., Flanjak, I., Bojanić, K., Babić, S., Čižmek, L., Čož-Rakovac, R., Jokić, S., & Jerković, I. (2021). Bioprospecting of Coralline Red Alga Amphiroa rigida J.V. Lamouroux: Volatiles, Fatty Acids and Pigments. Molecules, 26(3), 520. https://doi.org/10.3390/molecules26030520









