Isolation, Chemical Characterization and Antioxidant Activity of Pectic Polysaccharides of Fireweed (Epilobium angustifolium L.)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation and Chemical Characteristics of Fireweed Pectins
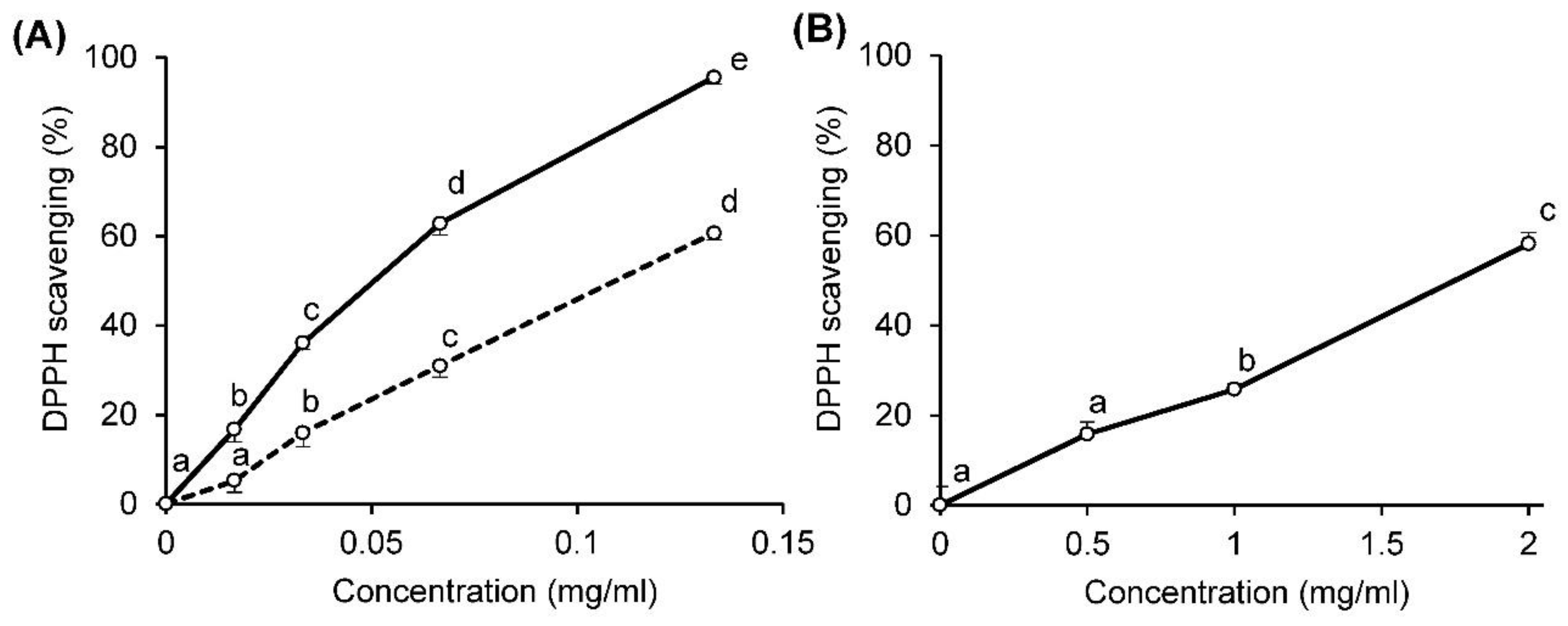
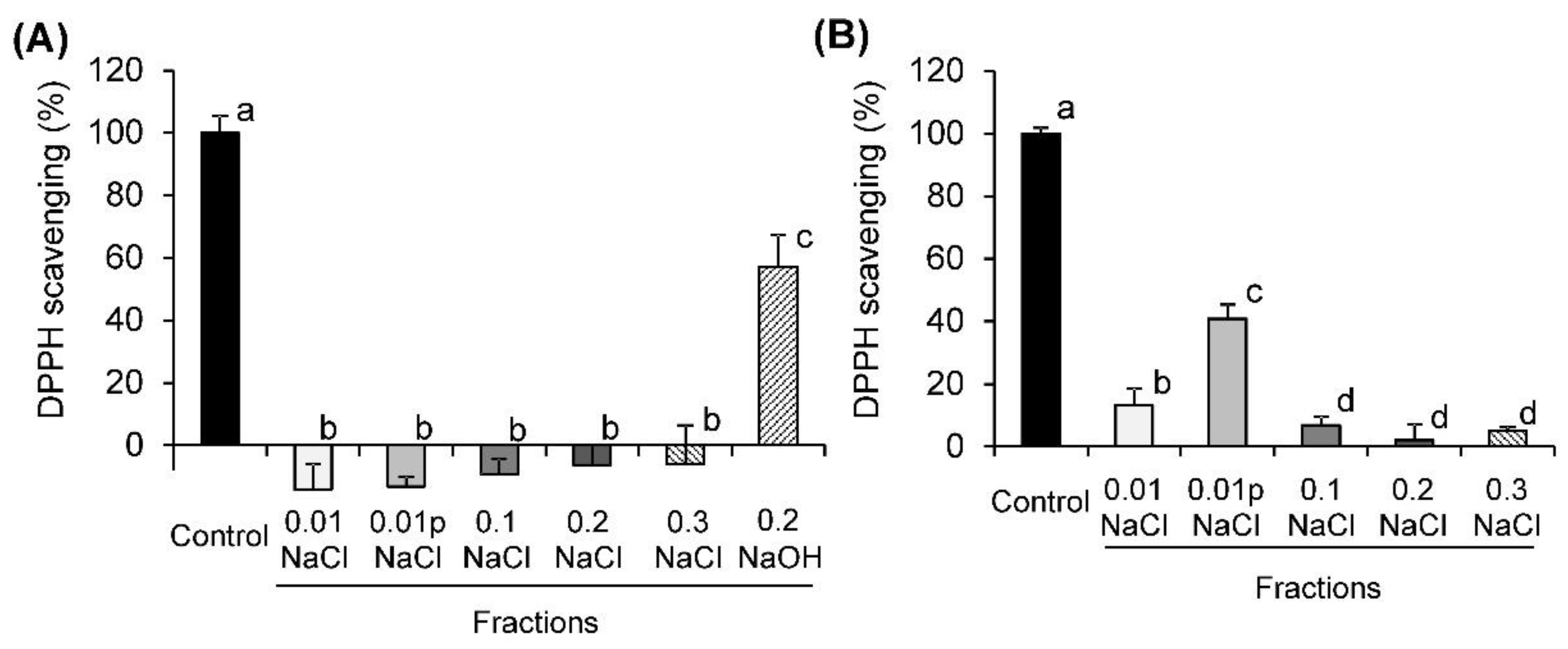
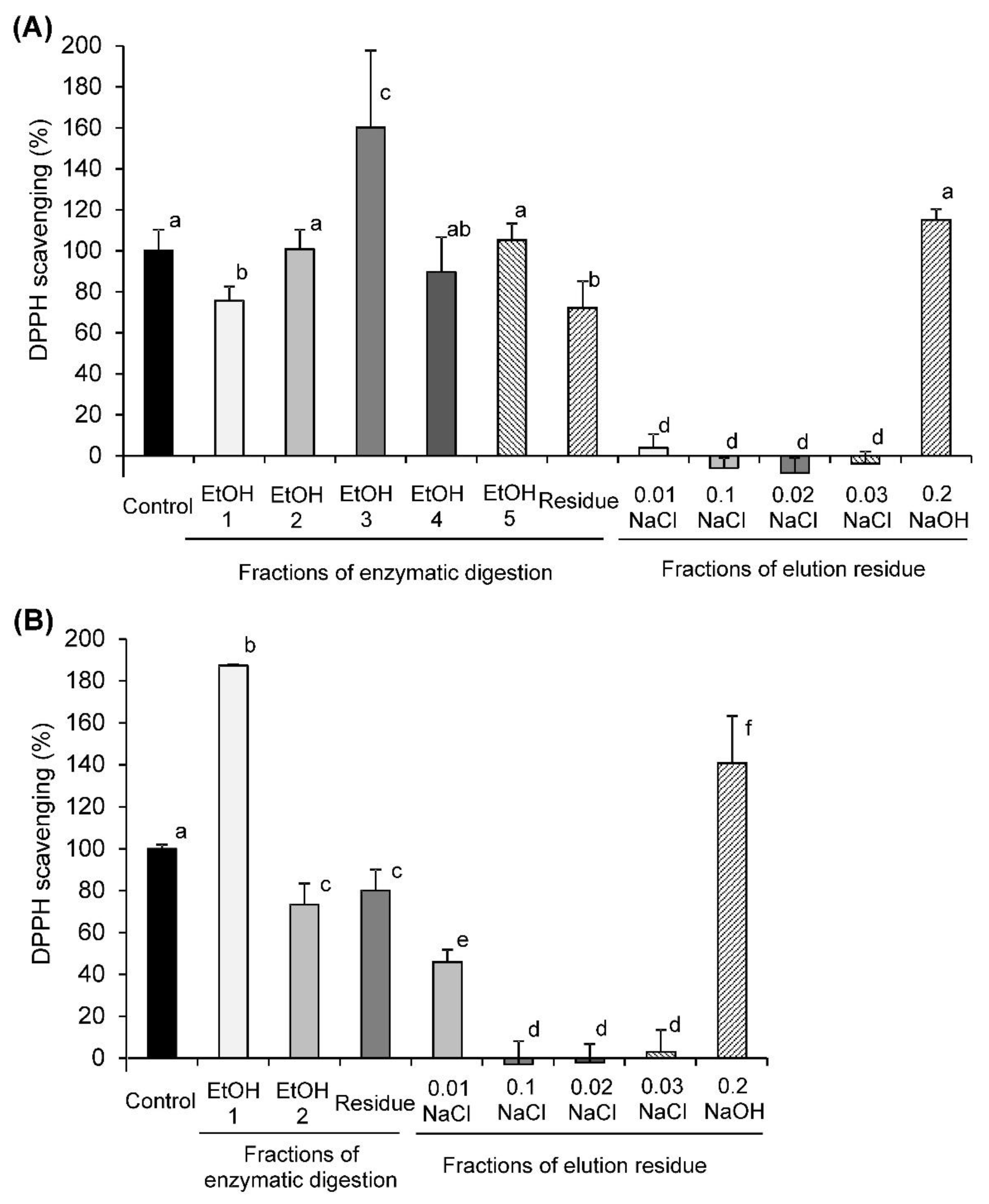
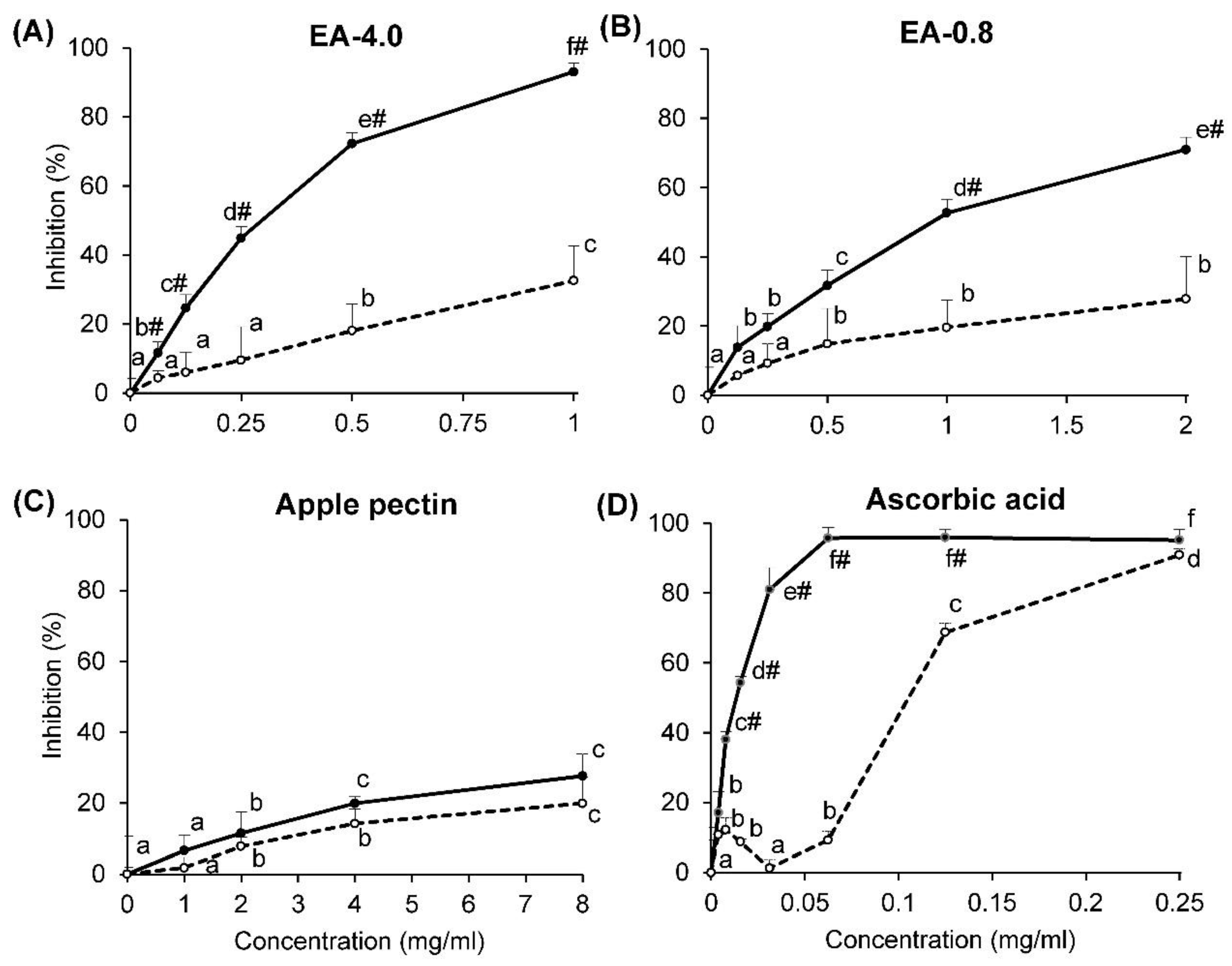
2.2. DPPH Radical-Scavenging Activity
2.3. Superoxide Radical-Scavenging Activity
3. Materials and Methods
3.1. Materials
3.2. Isolation of Pectins
3.3. General Analytical Methods
3.4. Anion-Exchange Chromatography
3.5. Enzymatic Digestion
3.6. Antioxidant Activity
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
| Ara | Arabinose |
| DM | Degree of methyl esterification |
| Gal | Galactose |
| Glc | Glucose |
| Man | Mannose |
| Rha | Rhamnose |
| Xyl | Xylose |
References
- Adamczak, A.; Dreger, M.; Seidler-Łożykowska, K.; Wielgus, K. Fireweed (Epilobium angustifolium L.): Botany, phytochemistry and traditional uses. A review. Herba Pol. 2019, 65, 51–63. [Google Scholar] [CrossRef] [Green Version]
- Lasinskas, M.; Jariene, E.; Vaitkeviciene, N.; Hallmann, E.; Najman, K. Effect of Different Durations of Solid-Phase Fermentation for Fireweed (Chamerion angustifolium (L.) Holub) Leaves on the Content of Polyphenols and Antioxidant Activity In Vitro. Molecules 2020, 25, 1011. [Google Scholar] [CrossRef] [Green Version]
- Deng, L.; Zong, W.; Tao, X.; Liu, S.; Feng, Z.; Lin, Y.; Liao, Z.; Chen, M. Evaluation of the therapeutic effect against benign prostatic hyperplasia and the active constituents from Epilobium angustifolium L. J. Ethnopharmacol. 2019, 232, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Ramstead, A.G.; Kirpotina, L.N.; Voyich, J.M.; Jutila, M.A.; Quinn, M.T. Therapeutic Potential of Polyphenols from Epilobium angustifolium (Fireweed). Phytother. Res. 2016, 30, 1287–1297. [Google Scholar] [CrossRef] [Green Version]
- Vitalone, A.; Allkanjari, O. Epilobium spp: Pharmacology and Phytochemistry. Phytother. Res. 2018, 32, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Anderson, C.T. Biosynthesis, Localisation, and Function of Pectins in Plants. In Pectin: Technological and Physiological Properties; Springer: Cham, Switzerland, 2020; pp. 1–15. [Google Scholar]
- Voragen, A.G.J.; Coenen, G.-J.; Verhoef, R.P.; Schols, H.A. Pectin, a versatile polysaccharide present in plant cell walls. Struct. Chem. 2009, 20, 263–275. [Google Scholar] [CrossRef] [Green Version]
- Minzanova, S.T.; Mironov, V.F.; Arkhipova, D.M.; Khabibullina, A.V.; Mironova, L.G.; Zakirova, Y.M.; Milyukov, V.A. Biological Activity and Pharmacological Application of Pectic Polysaccharides: A Review. Polymers 2018, 10, 1407. [Google Scholar] [CrossRef] [Green Version]
- Tan, M.; Chang, S.; Liu, J.; Li, H.; Xu, P.; Wang, P.; Wang, X.; Zhao, M.; Zhao, B.; Wang, L.; et al. Physicochemical Properties, Antioxidant and Antidiabetic Activities of Polysaccharides from Quinoa (Chenopodium quinoa Willd.) Seeds. Molecules 2020, 25, 3840. [Google Scholar] [CrossRef]
- Wu, D.-T.; Liu, W.; Han, Q.-H.; Wang, P.; Xiang, X.-R.; Ding, Y.; Zhao, L.; Zhang, Q.; Li, S.-Q.; Qin, W. Extraction Optimization, Structural Characterization, and Antioxidant Activities of Polysaccharides from Cassia Seed (Cassia obtusifolia). Molecules 2019, 24, 2817. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Zhu, Y.; Bao, X.; Zhang, L.; Li, N.; Jiang, G.; Peng, Q. Optimization of Alkali Extraction and Properties of Polysaccharides from Ziziphus jujuba cv. Residue. Molecules 2019, 24, 2221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patova, O.; Smirnov, V.; Golovchenko, V.; Vityazev, F.; Shashkov, A.; Popov, S. Structural, rheological and antioxidant properties of pectins from Equisetum arvense L. and Equisetum sylvaticum L. Carbohydr. Polym. 2019, 209, 239–249. [Google Scholar] [CrossRef]
- Zeng, F.; Chen, W.; He, P.; Zhan, Q.; Wang, Q.; Wu, H.; Zhang, M. Structural characterization of polysaccharides with potential antioxidant and immunomodulatory activities from Chinese water chestnut peels. Carbohydr. Polym. 2020, 246, 116551. [Google Scholar] [CrossRef]
- Yeung, Y.K.; Kang, Y.-R.; So, B.R.; Jung, S.K.; Chang, Y.H. Structural, antioxidant, prebiotic and anti-inflammatory properties of pectic oligosaccharides hydrolyzed from okra pectin by Fenton reaction. Food Hydrocoll. 2021, 118, 106779. [Google Scholar] [CrossRef]
- Zhang, T.; Shuai, M.; Ma, P.; Huang, J.; Sun, C.; Yao, X.; Chen, Z.; Min, X.; Yan, S. Purification, chemical analysis and antioxidative activity of polysaccharides from pH-modified citrus pectin after dialyzation. LWT 2020, 128, 109513. [Google Scholar] [CrossRef]
- Ma, X.; Yu, J.; Jing, J.; Zhao, Q.; Ren, L.; Hu, Z. Optimization of sunflower head pectin extraction by ammonium oxalate and the effect of drying conditions on properties. Sci. Rep. 2021, 11, 10616. [Google Scholar] [CrossRef]
- Ning, X.; Liu, Y.; Jia, M.; Wang, Q.; Sun, Z.; Ji, L.; Mayo, K.H.; Zhou, Y.; Sun, L. Pectic polysaccharides from Radix Sophorae Tonkinensis exhibit significant antioxidant effects. Carbohydr. Polym. 2021, 262, 117925. [Google Scholar] [CrossRef]
- Wang, W.; Ma, X.; Jiang, P.; Hu, L.; Zhi, Z.; Chen, J.; Ding, T.; Ye, X.; Liu, D. Characterization of pectin from grapefruit peel: A comparison of ultrasound-assisted and conventional heating extractions. Food Hydrocoll. 2016, 61, 730–739. [Google Scholar] [CrossRef]
- Wang, X.; Lü, X. Characterization of pectic polysaccharides extracted from apple pomace by hot-compressed water. Carbohydr. Polym. 2014, 102, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, S.; Nie, S.; Yu, Q.; Xie, M. Reviews on Mechanisms of In Vitro Antioxidant Activity of Polysaccharides. Oxid. Med. Cell. Longev. 2016, 2016, 5692852. [Google Scholar] [CrossRef] [Green Version]
- Donchenko, L.V.; Limareva, N.S.; Belousova, A.I.; Koss, A.N. Pectin-containing antioxidant drink based on extracts of grape pomace and Chamerion. IOP Conf. Ser. Earth Environ. Sci. 2021, 624, 012122. [Google Scholar] [CrossRef]
- Olawuyi, I.F.; Lee, W.Y. Structural characterization, functional properties and antioxidant activities of polysaccharide extract obtained from okra leaves (Abelmoschus esculentus). Food Chem. 2021, 354, 129437. [Google Scholar] [CrossRef] [PubMed]
- Ezzati, S.; Ayaseh, A.; Ghanbarzadeh, B.; Heshmati, M.K. Pectin from sunflower by-product: Optimization of ultrasound-assisted extraction, characterization, and functional analysis. Int. J. Biol. Macromol. 2020, 165, 776–786. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Xue, G.; Ni, Q.; Wang, Y.; Gao, Q.; Zhang, Y.; Xu, G. Physicochemical and rheological characterization of pectin-rich polysaccharides from Gardenia jasminoides J. Ellis flower. Food Sci. Nutr. 2020, 8, 3335–3345. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Khodaiyan, F.; Kazemi, M.; Najari, Z. Optimization and characterization of pectin extracted from sour orange peel by ultrasound assisted method. Int. J. Biol. Macromol. 2019, 125, 621–629. [Google Scholar] [CrossRef]
- Buathongjan, C.; Israkarn, K.; Sangwan, W.; Outrequin, T.; Gamonpilas, C.; Methacanon, P. Studies on chemical composition, rheological and antioxidant properties of pectin isolated from Riang (Parkia timoriana (DC.) Merr.) pod. Int. J. Biol. Macromol. 2020, 164, 4575–4582. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Y.; Xu, L.; Jia, Y.; Xue, Z.; Zhang, M.; Phisalaphong, M.; Chen, H. Ultrasound-assisted modified pectin from unripe fruit pomace of raspberry (Rubus chingii Hu): Structural characterization and antioxidant activities. LWT 2020, 134, 110007. [Google Scholar] [CrossRef]
- Hosseini, S.; Parastouei, K.; Khodaiyan, F. Simultaneous extraction optimization and characterization of pectin and phenolics from sour cherry pomace. Int. J. Biol. Macromol. 2020, 158, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, M.; Khodaiyan, F.; Hosseini, S.S. Utilization of food processing wastes of eggplant as a high potential pectin source and characterization of extracted pectin. Food Chem. 2019, 294, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Golbargi, F.; Gharibzahedi, S.M.T.; Zoghi, A.; Mohammadi, M.; Hashemifesharaki, R. Microwave-assisted extraction of arabinan-rich pectic polysaccharides from melon peels: Optimization, purification, bioactivity, and techno-functionality. Carbohydr. Polym. 2021, 256, 117522. [Google Scholar] [CrossRef]
- La Cava, E.L.; Gerbino, E.; Sgroppo, S.C.; Gómez-Zavaglia, A. Characterization of Pectins Extracted from Different Varieties of Pink/Red and White Grapefruits [Citrus Paradisi (Macf.)] by Thermal Treatment and Thermosonication. J. Food Sci. 2018, 83, 1613–1621. [Google Scholar] [CrossRef]
- Lefsih, K.; Delattre, C.; Pierre, G.; Michaud, P.; Aminabhavi, T.; Dahmoune, F.; Madani, K. Extraction, characterization and gelling behavior enhancement of pectins from the cladodes of Opuntia ficus indica. Int. J. Biol. Macromol. 2016, 82, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Liu, H.-M.; Lv, T.-T.; Wang, X.-D. Structure, rheological, thermal and antioxidant properties of cell wall polysaccharides from Chinese quince fruits. Int. J. Biol. Macromol. 2020, 147, 1146–1155. [Google Scholar] [CrossRef]
- Wang, W.; Liu, J. Efficient extraction, antioxidant activities and anti-inflammation of polysaccharides from Notopterygium franchetii Boiss. Carbohydr. Polym. 2020, 248, 116783. [Google Scholar] [CrossRef]
- Ke, J.; Jiang, G.; Shen, G.; Wu, H.; Liu, Y.; Zhang, Z. Optimization, characterization and rheological behavior study of pectin extracted from chayote (Sechium edule) using ultrasound assisted method. Int. J. Biol. Macromol. 2020, 147, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, Z.; Khodaiyan, F.; Kazemi, M.; Sharifan, A. Optimization of microwave-assisted extraction and structural characterization of pectin from sweet lemon peel. Int. J. Biol. Macromol. 2020, 147, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Yapo, B.M. Pectic substances: From simple pectic polysaccharides to complex pectins—A new hypothetical model. Carbohydr. Polym. 2011, 86, 373–385. [Google Scholar] [CrossRef]
- Garna, H.; Mabon, N.; Wathelet, B.; Paquot, M. New Method for a Two-Step Hydrolysis and Chromatographic Analysis of Pectin Neutral Sugar Chains. J. Agric. Food Chem. 2004, 52, 4652–4659. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, H.; Bai, X.; Liu, P.; Yang, Y.; Huang, J.; Zhou, L.; Min, X. Fractionation and antioxidant activities of the water-soluble polysaccharides from Lonicera japonica Thunb. Int. J. Biol. Macromol. 2020, 151, 1058–1066. [Google Scholar] [CrossRef]
- Li, M.; Li, T.; Hu, X.; Ren, G.; Zhang, H.; Wang, Z.; Teng, Z.; Wu, R.; Wu, J. Structural, rheological properties and antioxidant activities of polysaccharides from mulberry fruits (Murus alba L.) based on different extraction techniques with superfine grinding pretreatment. Int. J. Biol. Macromol. 2021, 183, 1774–1783. [Google Scholar] [CrossRef]
- Xia, Y.-G.; Huang, Y.-X.; Liang, J.; Kuang, H.-X. Comparable studies of two polysaccharides from leaves of Acanthopanax senticosus: Structure and antioxidation. Int. J. Biol. Macromol. 2020, 147, 350–362. [Google Scholar] [CrossRef]
- Chen, X.; Qi, Y.; Zhu, C.; Wang, Q. Effect of ultrasound on the properties and antioxidant activity of hawthorn pectin. Int. J. Biol. Macromol. 2019, 131, 273–281. [Google Scholar] [CrossRef]
- Wang, L.; Li, L.; Gao, J.; Huang, J.; Yang, Y.; Xu, Y.; Liu, S.; Yu, W. Characterization, antioxidant and immunomodulatory effects of selenized polysaccharides from dandelion roots. Carbohydr. Polym. 2021, 260, 117796. [Google Scholar] [CrossRef]
- Wikiera, A.; Grabacka, M.; Byczyński, Ł.; Stodolak, B.; Mika, M. Enzymatically Extracted Apple Pectin Possesses Antioxidant and Antitumor Activity. Molecules 2021, 26, 1434. [Google Scholar] [CrossRef]
- Yi, Y.; Lamikanra, O.; Sun, J.; Wang, L.-M.; Min, T.; Wang, H.-X. Activity diversity structure-activity relationship of polysaccharides from lotus root varieties. Carbohydr. Polym. 2018, 190, 67–76. [Google Scholar] [CrossRef]
- Li, Z.; Nie, K.; Wang, Z.; Luo, D. Quantitative Structure Activity Relationship Models for the Antioxidant Activity of Polysaccharides. PLoS ONE 2016, 11, e0163536. [Google Scholar] [CrossRef]
- Lo, T.C.-T.; Chang, C.A.; Chiu, K.-H.; Tsay, P.-K.; Jen, J.-F. Correlation evaluation of antioxidant properties on the monosaccharide components and glycosyl linkages of polysaccharide with different measuring methods. Carbohydr. Polym. 2011, 86, 320–327. [Google Scholar] [CrossRef]
- Shafie, M.H.; Gan, C.-Y. Could choline chloride-citric acid monohydrate molar ratio in deep eutectic solvent affect structural, functional and antioxidant properties of pectin? Int. J. Biol. Macromol. 2020, 149, 835–843. [Google Scholar] [CrossRef]
- Patova, O.; Golovchenko, V.V.; Vityazev, F.V.; Burkov, A.; Belyi, V.A.; Kuznetsov, S.N.; Litvinets, S.; Martinson, E.A. Physicochemical and rheological properties of gelling pectin from Sosnowskyi’s hogweed (Heracleum sosnowskyi) obtained using different pretreatment conditions. Food Hydrocoll. 2017, 65, 77–86. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Liu, X.; Renard, C.M.; Rolland-Sabaté, A.; Bureau, S.; Le Bourvellec, C. Modification of apple, beet and kiwifruit cell walls by boiling in acid conditions: Common and specific responses. Food Hydrocoll. 2021, 112, 106266. [Google Scholar] [CrossRef]
- Usov, A.I.; Bilan, M.I.; Klochkova, N.G. Polysaccharides of Algae. 48. Polysaccharide Composition of Several Calcareous Red Algae: Isolation of Alginate from Corallina pilulifera P. et R. (Rhodophyta, Corallinaceae). Bot. Mar. 1995, 38, 43–52. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ruviaro, A.R.; Barbosa, P.D.P.M.; Martins, I.M.; de Ávila, A.R.A.; Nakajima, V.M.; Dos Prazeres, A.R.; Macedo, J.A.; Macedo, G.A. Flavanones biotransformation of citrus by-products improves antioxidant and ACE inhibitory activities in vitro. Food Biosci. 2020, 38, 100787. [Google Scholar] [CrossRef]
- Golovchenko, V.V.; Naranmandakh, S.; Ganbaatar, J.; Prilepskii, A.Y.; Burygin, G.L.; Chizhov, A.O.; Shashkov, A.S. Structural investigation and comparative cytotoxic activity of water-soluble polysaccharides from fruit bodies of the medicinal fungus quinine conk. Phytochemistry 2020, 175, 112313. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.; Siddiqui, I. Determination of methanol and its application to measurement of pectin ester content and pectin methyl esterase activity. Anal. Biochem. 1971, 39, 418–428. [Google Scholar] [CrossRef]
- Houben, K.; Jolie, R.P.; Fraeye, I.; Van Loey, A.; Hendrickx, M.E. Comparative study of the cell wall composition of broccoli, carrot, and tomato: Structural characterization of the extractable pectins and hemicelluloses. Carbohydr. Res. 2011, 346, 1105–1111. [Google Scholar] [CrossRef]
- Nelson, N. A photometric adaptation of the Somogyi method for the determination of glucose. J. Biol. Chem. 1944, 153, 375–380. [Google Scholar] [CrossRef]
- Shogren, R.L.; Biswas, A. Preparation of starch–sodium lignosulfonate graft copolymers via laccase catalysis and characterization of antioxidant activity. Carbohydr. Polym. 2013, 91, 581–585. [Google Scholar] [CrossRef]
- Quick, K.L.; Hardt, J.I.; Dugan, L.L. Rapid microplate assay for superoxide scavenging efficiency. J. Neurosci. Methods 2000, 97, 139–144. [Google Scholar] [CrossRef]
| Characteristic | EA-4.0 | EA-0.8 |
|---|---|---|
| Yield, % 1 | 0.89 | 1.29 |
| Uronic acid, % 2 | 88.2 | 90.8 |
| DM 2 | 29.4 | 28.5 |
| Protein, % 3 | 2.6 | 0.3 |
| Phenolic, % 3 | 4.0 | 2.7 |
| Rha, % 2 | 1.9 | 1.9 |
| Ara, % 2 | 3.3 | 1.7 |
| Xyl, % 2 | 1.5 | 1.7 |
| Man, % 2 | 0.5 | 0.6 |
| Glc, % 2 | 1.5 | 0.8 |
| Gal, % 2 | 3.1 | 2.6 |
| Total sugar, % 3 | 86.6 | 71.8 |
| GalA/NM | 7.50 | 9.82 |
| Rha/GalA | 0.022 | 0.021 |
| Ara + Gal/Rha | 3.29 | 2.22 |
| GalA–Rha | 86.31 | 88.84 |
| 2Rha + Ara + Gal | 10.16 | 8.10 |
| Ara/Xyl | 2.15 | 0.98 |
| Xyl/GalA | 0.017 | 0.019 |
| Characteristic | EA-4.0 | EA-0.8 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.01 NaCl | 0.01p 4 NaCl | 0.1 NaCl | 0.2 NaCl | 0.3 NaCl | 0.2 NaOH | 0.01 NaCl | 0.01p 4 NaCl | 0.1 NaCl | 0.2 NaCl | 0.3 NaCl | 0.2 NaOH | |
| Yield, % 1 | 12.33 | 19.97 | 11.04 | 29.50 | 5.82 | 1.68 | 11.48 | 13.94 | 16.39 | 34.01 | 7.78 | 1.10 |
| Mw, kDa | 254 | 323 | 88 | 100 | 102 | n.d. | 172 | 232 | 61 | 90 | 93 | n.d. |
| PDI | 4.67 | 3.73 | 4.72 | 2.28 | 2.21 | n.d. | 4.26 | 3.05 | 3.86 | 2.42 | 2.55 | n.d. |
| Uronic acid, % 2 | 86.9 | 91.3 | 93.4 | 91.5 | 93.8 | 76.0 | 84.9 | 89.0 | 93.4 | 97.0 | 97.4 | 39.4 |
| Protein, % 3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | n.d. |
| Phenolic, % 3 | 0.5 | 0.5 | 0.5 | 0.9 | 0.6 | 4.1 | 1.1 | 1.5 | 0.4 | 0.3 | 0.3 | n.d. |
| Rha, % 2 | 1.9 | 1.6 | 1.3 | 1.9 | 1.7 | 1.8 | 3.1 | 2.4 | 1.3 | 0.7 | 0.4 | 7.4 |
| Ara, % 2 | 4.7 | 1.9 | 1.6 | 2.8 | 1.7 | 6.2 | 2.4 | 1.6 | 1.6 | 0.6 | 0.4 | 9.7 |
| Xyl, % 2 | 0.4 | 0.6 | 0.6 | 0.4 | 0.2 | 1.2 | 1.0 | 0.8 | 0.6 | 0.5 | 0.7 | 5.9 |
| Man, % 2 | 0.9 | 0.8 | 0.4 | 0.8 | 0.2 | 0.8 | 1.6 | 1.4 | 0.4 | 0.3 | 0.2 | 5.3 |
| Glc, % 2 | 1.3 | 1.0 | 0.5 | 0.5 | 1.0 | 4.4 | 1.7 | 1.4 | 0.5 | 0.4 | 0.6 | 13.9 |
| Gal, % 2 | 3.9 | 2.8 | 2.1 | 2.1 | 1.5 | 9.6 | 5.3 | 3.6 | 2.1 | 0.6 | 0.4 | 18.4 |
| Total sugar, % 3 | 54.82 | 65.29 | 61.60 | 69.71 | 68.07 | 37.57 | 49.54 | 55.16 | 56.84 | 71.09 | 70.72 | 40.84 |
| GalA/NM | 6.61 | 10.49 | 14.06 | 10.78 | 15.05 | 3.17 | 5.62 | 8.07 | 14.06 | 31.79 | 37.02 | 0.65 |
| Rha/GalA | 0.022 | 0.018 | 0.014 | 0.020 | 0.018 | 0.024 | 0.037 | 0.026 | 0.014 | 0.007 | 0.005 | 0.187 |
| Ara + Gal/Rha | 4.57 | 2.93 | 2.92 | 2.61 | 1.93 | 8.77 | 2.45 | 2.20 | 2.92 | 1.79 | 1.62 | 3.81 |
| GalA–Rha | 84.97 | 89.68 | 92.07 | 89.63 | 92.09 | 74.20 | 81.75 | 86.63 | 92.07 | 96.29 | 96.92 | 32.05 |
| 2Rha + Ara + Gal | 12.41 | 7.98 | 6.35 | 8.62 | 6.56 | 19.38 | 13.97 | 9.87 | 6.35 | 2.50 | 1.63 | 42.82 |
| Ara/Xyl | 10.70 | 3.02 | 2.55 | 6.60 | 8.10 | 5.10 | 2.51 | 2.08 | 2.55 | 1.10 | 0.53 | 1.63 |
| Xyl/GalA | 0.005 | 0.007 | 0.007 | 0.005 | 0.002 | 0.016 | 0.011 | 0.009 | 0.007 | 0.005 | 0.007 | 0.150 |
| Characteristic | Enzymatic Digestion | Elution of Residue | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| EtOH-1 | EtOH-2 | EtOH-3 | EtOH-4 | EtOH-5 | Residue | 0.01 NaCl | 0.1 NaCl | 0.2 NaCl | 0.3 NaCl | 0.2 NaOH | |
| Yield, % 1 | 20.03 | 1.70 | 1.53 | 4.11 | 2.36 | 53.37 | 5.52 | 35.42 | 11.56 | 3.97 | 4.02 |
| Mw, kDa | 3 | n.d. | 6 | n.d. | n.d. | 82 | 178 | 39 | 65 | 72 | n.d. |
| PDI | 1.39 | n.d. | 2.00 | n.d. | n.d. | 4.37 | 2.72 | 2.31 | 1.66 | 2.27 | n.d. |
| Uronic acid,% 2 | 90.8 | 46.9 | 88.3 | 67.7 | 72.4 | 83.2 | 69.1 | 90.1 | 95.4 | 95.1 | 64.5 |
| Protein, % 3 | 0.0 | 4.4 | 4.4 | 6.5 | 5.9 | 1.6 | 0.1 | 0.0 | 0.0 | 0.0 | 5.4 |
| Phenolic, % 3 | 3.0 | 3.8 | 7.5 | 3.2 | 3.8 | 3.3 | 1.0 | 0.3 | 0.5 | 0.6 | 7.3 |
| Rha, % 2 | 0.4 | 1.1 | 0.9 | 1.2 | 1.1 | 2.9 | 6.4 | 2.6 | 1.0 | 1.1 | 4.4 |
| Ara, % 2 | 1.8 | 4.9 | 1.7 | 6.3 | 7.0 | 2.5 | 4.0 | 1.9 | 0.5 | 0.9 | 7.2 |
| Xyl, % 2 | 2.5 | 7.1 | 2.2 | 10.5 | 8.7 | 3.1 | 1.4 | 1.2 | 1.0 | 1.0 | 2.0 |
| Man, % 2 | 0.0 | 1.3 | 0.0 | 0.0 | 0.0 | 1.1 | 4.4 | 0.7 | 0.4 | 0.3 | 1.0 |
| Glc, % 2 | 3.6 | 35.8 | 5.4 | 11.2 | 8.4 | 3.1 | 3.5 | 0.6 | 0.8 | 0.8 | 8.9 |
| Gal, % 2 | 1.1 | 2.9 | 1.6 | 3.1 | 2.5 | 4.2 | 11.2 | 2.8 | 1.0 | 0.9 | 12.0 |
| Total sugar, % 3 | 96.25 | 62.20 | 62.02 | 46.80 | 52.90 | 75.74 | 50.75 | 66.21 | 64.55 | 62.54 | 20.38 |
| GalA/NM | 9.81 | 0.88 | 7.58 | 2.10 | 2.62 | 4.93 | 2.23 | 9.14 | 20.51 | 19.37 | 1.82 |
| Rha/GalA | 0.004 | 0.023 | 0.010 | 0.018 | 0.015 | 0.035 | 0.093 | 0.028 | 0.010 | 0.011 | 0.069 |
| Ara + Gal/Rha | 7.28 | 7.27 | 3.67 | 7.81 | 8.93 | 2.28 | 2.38 | 1.86 | 1.54 | 1.65 | 4.33 |
| GalA-Rha | 90.36 | 45.82 | 87.45 | 66.50 | 71.33 | 80.22 | 62.66 | 87.59 | 94.36 | 94.05 | 60.05 |
| 2Rha + Ara + Gal | 3.62 | 10.01 | 4.99 | 11.77 | 11.69 | 12.53 | 28.01 | 9.85 | 3.50 | 3.83 | 28.09 |
| Ara/Xyl | 0.71 | 0.69 | 0.77 | 0.60 | 0.81 | 0.80 | 2.87 | 1.56 | 0.52 | 0.84 | 3.70 |
| Xyl/GalA | 0.027 | 0.151 | 0.024 | 0.155 | 0.120 | 0.037 | 0.020 | 0.014 | 0.010 | 0.011 | 0.030 |
| Characteristic | Enzymatic Digestion | Elution of Residue | ||||||
|---|---|---|---|---|---|---|---|---|
| EtOH-1 | EtOH-2 | Residue | 0.01 NaCl | 0.1 NaCl | 0.2 NaCl | 0.3 NaCl | 0.2 NaOH | |
| Yield, % 1 | 3.49 | 29.30 | 56.64 | 8.51 | 12.97 | 30.58 | 14.70 | 2.19 |
| Mw, kDa | 21 | 4 | 71 | 170 | 20 | 46 | 83 | n.d. |
| PDI | 5.36 | 1.47 | 4.51 | 4.08 | 2.17 | 1.91 | 1.76 | n.d. |
| Uronic acid, % 2 | 75.2 | 80.9 | 85.8 | 60.2 | 94.8 | 96.0 | 98.5 | 70.4 |
| Protein, % 3 | 3.2 | 0.0 | 0.2 | 1.3 | 0.0 | 0.0 | 0.0 | 2.7 |
| Phenolic, % 3 | 5.8 | 1.9 | 2.2 | 2.1 | 0.2 | 0.3 | 0.4 | 5.8 |
| Rha, % 2 | 4.2 | 0.6 | 2.3 | 8.5 | 0.8 | 1.0 | 0.4 | 3.6 |
| Ara, % 2 | 3.0 | 1.5 | 1.7 | 5.6 | 2.0 | 0.8 | 0.3 | 5.1 |
| Xyl, % 2 | 5.0 | 7.1 | 3.4 | 1.0 | 0.3 | 0.2 | 0.1 | 2.2 |
| Man, % 2 | 0.4 | 0.1 | 1.1 | 6.1 | 0.2 | 0.7 | 0.0 | 1.4 |
| Glc, % 2 | 8.0 | 7.9 | 2.4 | 4.6 | 0.3 | 0.3 | 0.2 | 7.1 |
| Gal, % 2 | 4.2 | 1.8 | 3.2 | 14.0 | 1.7 | 1.1 | 0.5 | 10.2 |
| Total sugar, % 3 | 70.49 | 68.58 | 77.22 | 54.52 | 65.93 | 79.39 | 78.49 | 23.48 |
| GalA/NM | 3.04 | 4.23 | 6.04 | 1.51 | 18.38 | 23.69 | 64.80 | 2.38 |
| Rha/GalA | 0.056 | 0.008 | 0.027 | 0.141 | 0.008 | 0.010 | 0.004 | 0.051 |
| Ara + Gal/Rha | 1.70 | 5.14 | 2.10 | 2.31 | 4.71 | 2.03 | 2.02 | 4.27 |
| GalA–Rha | 71.02 | 80.24 | 83.46 | 51.67 | 94.06 | 94.98 | 98.08 | 66.84 |
| 2Rha + Ara + Gal | 15.61 | 4.64 | 9.60 | 36.64 | 5.23 | 3.91 | 1.65 | 22.43 |
| Ara/Xyl | 0.60 | 0.21 | 0.50 | 5.69 | 7.00 | 5.19 | 3.40 | 2.27 |
| Xyl/GalA | 0.066 | 0.088 | 0.040 | 0.016 | 0.003 | 0.002 | 0.001 | 0.032 |
| Pectin/Standard | IC50 (mg/mL) |
|---|---|
| EA-4.0 | 0.050 ± 0.003 a |
| EA-0.8 | 0.109 ± 0.003 b |
| AP (control) | 1.961 ± 0.363 c |
| Trolox (standard) | 0.005 ± 0.000 d |
| Second Variable ** | R | p | Second Variable | R | p |
|---|---|---|---|---|---|
| GalA | −0.55 | 0.001 | Mw * | −0.40 | 0.053 |
| GalA–Rha | −0.53 | 0.002 | Carbohydrate | −0.18 | 0.338 |
| GalA/NM | −0.47 | 0.006 | Man | −0.06 | 0.737 |
| Ara/Xyl | −0.44 | 0.011 | Rha | 0.18 | 0.325 |
| (Ara + Gal)/Rha | 0.36 | 0.042 | Rha/GalA | 0.20 | 0.267 |
| Ara | 0.44 | 0.011 | PDI * | 0.21 | 0.313 |
| Glc | 0.50 | 0.004 | Gal | 0.26 | 0.146 |
| Xyl/GalA | 0.55 | 0.001 | 2Rha + Ara + Gal | 0.30 | 0.096 |
| Xyl | 0.58 | 0.000 | |||
| Protein | 0.72 | 0.000 | |||
| Phenolic | 0.93 | 0.000 |
| Variable | β | Standard Error of β | Parameter Estimate | Standard Error | p Value | |
|---|---|---|---|---|---|---|
| Dependent | Independent | |||||
| DPPH scavenging activity | Phenolics | 0.85 | 0.07 | 2.27 | 0.17 | 0.000 |
| Xyl/GalA | 0.20 | 0.07 | 280.73 | 91.11 | 0.004 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popov, S.; Smirnov, V.; Kvashninova, E.; Khlopin, V.; Vityazev, F.; Golovchenko, V. Isolation, Chemical Characterization and Antioxidant Activity of Pectic Polysaccharides of Fireweed (Epilobium angustifolium L.). Molecules 2021, 26, 7290. https://doi.org/10.3390/molecules26237290
Popov S, Smirnov V, Kvashninova E, Khlopin V, Vityazev F, Golovchenko V. Isolation, Chemical Characterization and Antioxidant Activity of Pectic Polysaccharides of Fireweed (Epilobium angustifolium L.). Molecules. 2021; 26(23):7290. https://doi.org/10.3390/molecules26237290
Chicago/Turabian StylePopov, Sergey, Vasily Smirnov, Elizaveta Kvashninova, Victor Khlopin, Fedor Vityazev, and Victoria Golovchenko. 2021. "Isolation, Chemical Characterization and Antioxidant Activity of Pectic Polysaccharides of Fireweed (Epilobium angustifolium L.)" Molecules 26, no. 23: 7290. https://doi.org/10.3390/molecules26237290
APA StylePopov, S., Smirnov, V., Kvashninova, E., Khlopin, V., Vityazev, F., & Golovchenko, V. (2021). Isolation, Chemical Characterization and Antioxidant Activity of Pectic Polysaccharides of Fireweed (Epilobium angustifolium L.). Molecules, 26(23), 7290. https://doi.org/10.3390/molecules26237290






