1. Introduction
From the perspective of the early diagnosis of tumors, the sensitivity and specificity of the diagnostic tools remain important challenges. Molecular imaging such as Positron Emission Tomography (PET) is a reference technique for the diagnosis of many diseases [
1]. PET is a technique that is beginning to be widely used in several pathologies, such as neurology, cardiology, and inflammation. Nevertheless, it remains the most prescribed exam in nuclear oncology, with [
18F]FDG as the gold standard. This imaging requires the use of radiotracers to locate and quantify specific targets or pathological processes. Although, it has been established that [
18F]FDG PET has a lower sensitivity [
2] in determining the presence, location, and number of metastases than MRI, especially for the detection of small lesions [
3]. This suggests that PET imaging can provide valuable complementary information in the evaluation of brain tumors (aggressiveness, differentiation, etc.). [
18F]FDG shows glucidic metabolism of the tumor and is not specific. For a few years, new radiotracers based on amino acids ([
18F]F-DOPA, [
18F]FET, and [
11C]-methionine) have shown better results compared to [
18F]FDG, but they are also not very specific [
4].
The use of radiolabeled bioactive macromolecules in imaging has emerged as an important field for almost four decades in nuclear medicine [
5,
6]. In particular, labeled peptides or proteins are useful for PET [
7,
8] or preclinical and clinical research as new diagnostic agents and are today considered as fully “druggable” entities [
9,
10]. Research in nuclear medicine technology and the development of new radiotracers has considerably changed these last years by using more specific radiotracers. To have efficiently labeled peptides for PET applications, isotope half-lives need to be compatible with the peptide biodistribution time, and radiolabeling conditions need to be compatible with the peptide stability. Gallium-68 is a well-adapted isotope thanks to its quick synthesis times (~15 min) and easy radiochemistry (radiochemical yield >95%) by complexation into chelating groups. Numerous gallium-68 radiotracers are described in the literature; they can be designed with monomeric or polymeric [
11] targeting peptides for a higher specificity.
In a search for greater specificity and due to the presence of peptide receptors overexpressed on tumor cells, peptides are the ligands of choice to develop targeted molecular imaging agents [
12]. Recently, new peptide-based radiotracers have shown very promising results, such as the [
68Ga]Ga-DOTATOC radiotracer used for clinical diagnosis applications, which is composed of a DOTA as the bifunctional agent, a peptide octreotide analog as the ligand able to target the somatostatin receptor [
13]. In the past decade, the work of some researchers in Nancy has led to the development of targeting strategies via peptides to treat brain tumor glioblastoma [
14]. Tumor angiogenesis is recognized as a crucial phenomenon in the progression of malignant tumors. Vascular endothelial growth factor (VEGF) is one of the most specific and important growth factors involved in the mechanisms of tumor angiogenesis. A recent evaluation has shown that angiogenesis was correlated with overexpression of the neuropilin-1 (NRP-1) receptor [
15]. NRP-1 is also expressed in tumor angiogenic vessels and some tumor cells [
16] and seems to be a target of choice for tumor imaging.
In vivo, the relevance of a peptide targeting NRP-1 overexpressed by angiogenic endothelial cells to detect the peripheral infiltrative part of the high-grade brain tumor has been clearly demonstrated; over the past ten years, we have demonstrated the interest of vascular targeting via ATWLPPR, a ligand peptide of NRP-1 (recognition domain b1b2), but also its instability in vivo [
17,
18,
19,
20]. The work of Wu et al. [
21] also demonstrated the interest of targeting NRP-1 with the ATWLPPR peptide coupled to Fluor-18. Recently, we developed and tested other peptides that had more affinity for NRP-1, such as DKPPR [
22,
23]. In this study, the KDKPPR peptide sequence was chosen to be coupled to a bifunctional chelator and a fluorescent agent. We have already demonstrated that the coupling of a porphyrin [
24] or gold nanoparticles [
14] to this sequence does not modify its affinity for NRP-1.
In this paper, we describe the synthesis and the photophysical properties of the new gallium-68 radiolabeled KDKPPR peptide to target NRP-1. KDKPPR has been slightly modified, allowing labeling with gallium-68 anchored into a bifunctional NODAGA chelating agent, as well as Cy5 fluorophore for fluorescence detection. The cytotoxicity and the molecular affinity of this new chelated radiotracer have been evaluated in vitro and in vivo on a brain model of metastasis in a nude rat using MDA-MB-231 cells (hormone-independent and triple-negative line). For this purpose, we chose two in vitro models: HUVEC (angiogenic endothelial cell model) for the targeting of the tumor vascular network and MDA-MB-231 (triple-negative breast cancer cell line) for the targeting of small metastatic sites. In vivo experiments were conducted using a nude rat model of brain metastasis with an intracarotid injection of MDA-MB-231 cells.
3. Discussion
In this paper, we designed an original radiotracer for multimodalities of PET and fluorescence imaging applications and the targeting NRP-1 receptor. This receptor is overexpressed in angiogenic endothelial cells and in some tumors like glioblastoma and breast cancer, where it is associated with a poor prognosis. In our strategy, the targeting of NRP-1 allows us to target both vascularized brain metastases and avascular tumors. In our previous studies, we have shown that the KDKPPR peptide presented a good affinity for NRP-1 [
22,
24].
For PET modalities, the gallium-68 positron emitter was chosen as the radioisotope, allowing to obtain an efficient radiochemistry by a complexation reaction. To perform this complexation, NODAGA was used as the bifunctional chelator for its capacity with radiolabeled gallium-68 at room temperature. This is interesting for thermosensitive peptides. NODAGA is derivative of the hexadentate N
3O
3 NOTA chelator family. It is able to form more stable Ga complexes compared to NOTA thanks to the additional carboxylate function, allowing the best coordination with
68Ga
3+ [
31]. The NOTA chelator is the oldest and most successful chelator of gallium-68 and is considered to be the gold standard for Ga
3+ complexation.
For fluorescent application, Cy5 was chosen due to its interesting photophysical properties, namely a fluorescent emission in the red region and a high molar extinction coefficient. In the future, we aim to perform fluorescence microscopy.
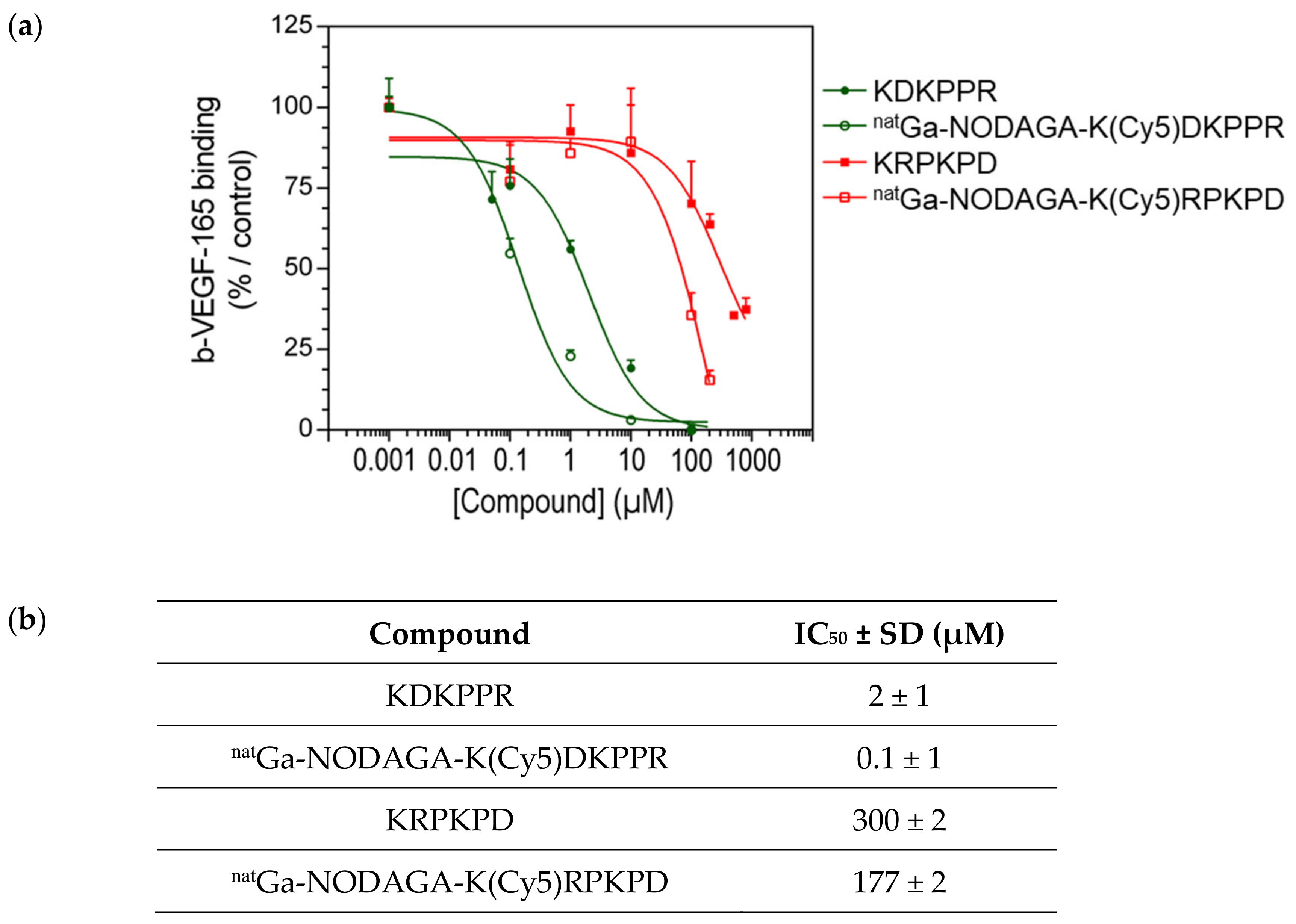
In our molecular design approach, we decided to link these 3 entities around a linker platform able to perform three links. The amino acid lysine was chosen for this role. The synthesis of the chelator–dye–peptide compound was performed with success in 6 steps on SPPS. To perform the in vitro experiment, the DKPPR peptide, scramble peptide (RPKPD), KDKPPR, peptide containing Cy5 dye K(Cy5)DKPPR and K(Cy5) RPKPD, nonradioactive reference natGa-NODAGA-K(Cy5)DKPPR, and natGa-NODAGA-K(Cy5) RPKPD were synthesized.
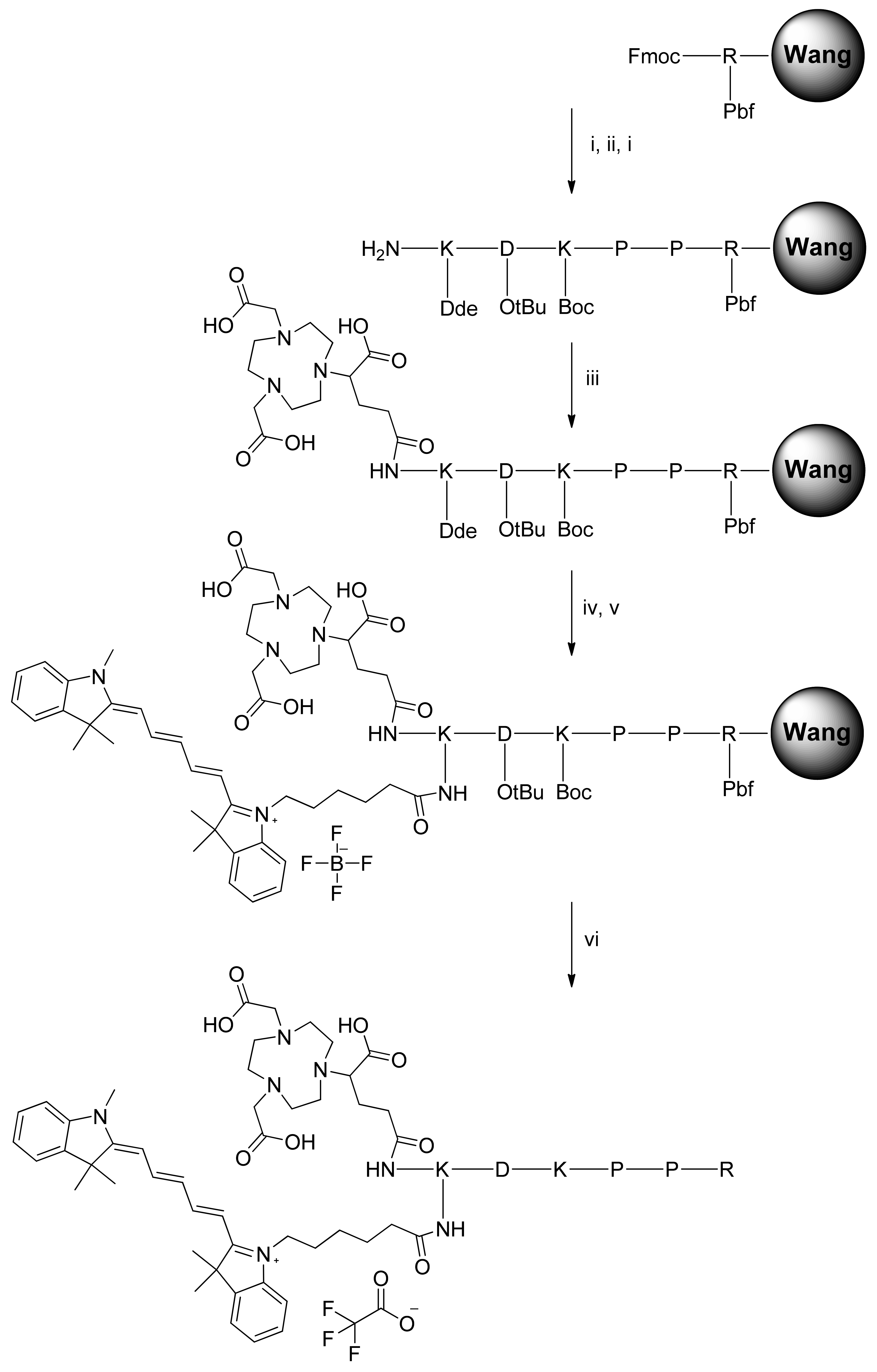
The compounds were synthesized by the Fmoc/tBu/Dde solid-phase strategy. The Dde group is an amino protective group that can be used orthogonally with both Fmoc and Boc protections [
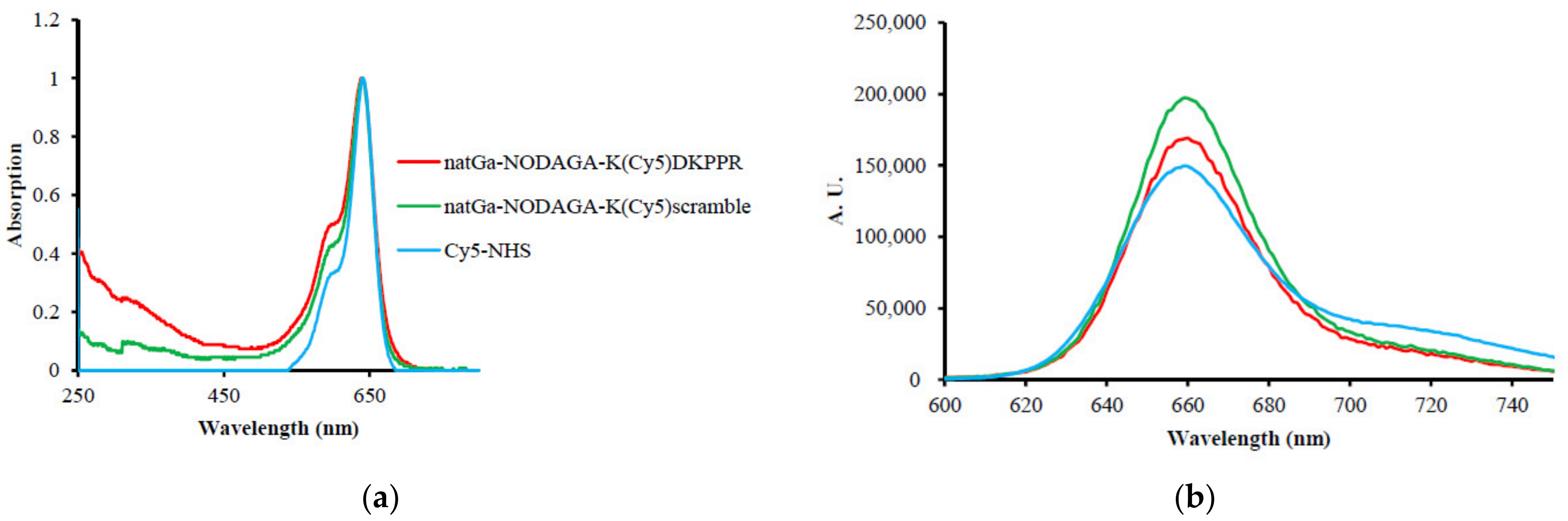
32]. The Dde group is cleaved with 3% hydrazine in DMF. Compared to Cy5-NHS, after conjugation of the peptides and NODAGA with Cy5, a decrease of ε could be observed by a factor of 4.7 for NODAGA-K(Cy5)DKPPR and 5.2 for NODAGA-K(Cy5)scramble, whereas an increase of φ
F was simultaneously observed (27% for Cy5-NHS, 61% for NODAGA-K(Cy5)DKPPR, and 79% for NODAGA-K(Cy5)scramble).
In previous studies, we have shown that molecules coupled to the ATWLPPR peptide had a good affinity for the NRP-1 protein [
17,
33]. ATWLPPR was specific for NRP-1, with an IC
50 equal to 19 μM that increased after coupling (171 μM). In order to improve the selectivity, a new DKPPR peptide was designed and optimized by our team based on the screening methodology [
23]. This peptide sequence was based on the sequence homology of the domain encoded by exon 8a of VEGF-165, and the amino acid lysine was added to allow its grafting without affecting the affinity. A docking analysis combined with biological evaluations demonstrated hydrogen bonds and π-π interactions between the NRP-1 protein and the DKPPR peptide. Using novel multimodal and multiscale approaches, we demonstrated the maintenance of the molecular affinity for the NRP-1 protein after the addition of a lysine residue at the
N-extremity (KDKPPR peptide) but, also, after grafting KDKPPR to the nanoparticles [
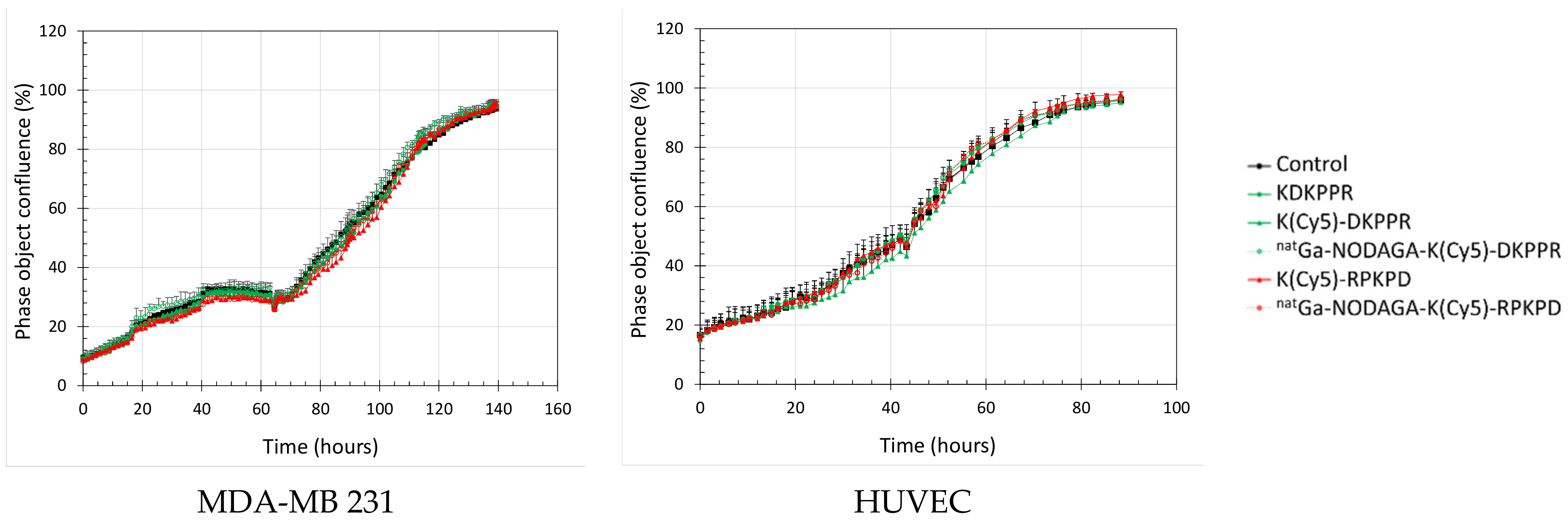
34]. No toxicity was observed in either an endothelial cell line (HUVEC) or the MDA-MB-231 cancer line, nor were any changes in the cell proliferation or metabolism observed for
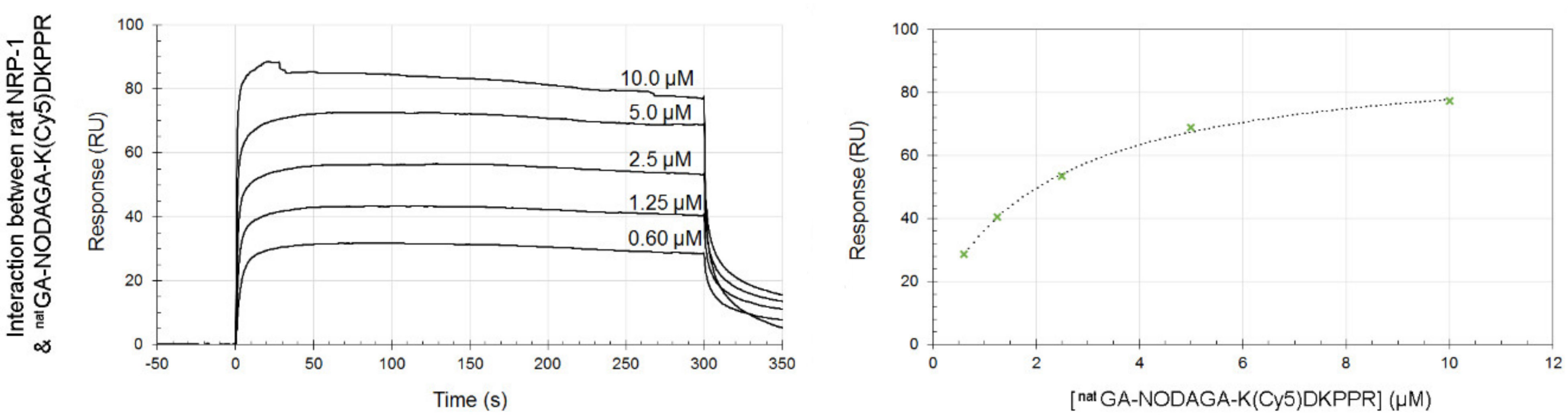
natGa-NODAGA-K(Cy5)DKPPR. Our team has already demonstrated the great interest of peptide moieties targeting the NRP-1 protein overexpressed by endothelial cells with an angiogenic phenotype. This recognition resulted in a prolonged retention time into the endothelial cells lining the vasculature of xenotransplanted human glioblastomas, followed by incorporation into the tumor tissue [
26,
27,
28,
35]. In addition, in this study, we evaluated the affinity of the KDKPPR free peptide and conjugated the
natGa-NODAGA-K(Cy5)DKPPR peptide using SPR experiments (
Table 2), SPR being a reference method.
All these in vitro results encouraged us to pursue them in vivo.
In the objective to use NODAGA-K(Cy5)DKPPR as a radiotracer, it was radiolabeled with gallium-68 in classical radiolabeling conditions (labeling at room temperature over 10 min). The optimization of radiolabeling allowed obtaining the radiotracer in a high radiochemical yield (RCY) and purity. Formation of the radioactive byproduct was limited by adding ascorbic acid due to its antioxidant rules. The radiolabeling conditions were similar to those used previously for [
68Ga]Ga-NODAGA-RGD [
36]. The process of radiosynthesis on the mAIO (Trasis
®) synthesizer was efficient, robust, and reproducible (RCY = 90.3 ± 2.5% dc;
n = 12) to produce [
68Ga]Ga-NODAGA-K(Cy5)DKPPR.
Coupling a peptide targeting NRP-1 to a fluorophore and/or radiotracer is relatively little-developed, and there are few publications in the literature. Thoreau et al. coupled the peptide ATWLPPR as an NRP-1-targeting peptide with a platform containing four RGD to target αvβ
3 integrin and Cy5-5 for fluorescent imaging application. It was evaluated in vivo in a mice model bearing a U87MG tumor. The dual targeting induced better binding and selectivity [
37].
Perret et al. were the first to use peptide ATWLPPR for nuclear medicine application. To label ATWLPPR with
99mTc for SPECT application, a benzoyl mercapto group was attached to the
N-extremity of the peptide to do a tetradentate Tc complex [
38]. A preclinical evaluation was realized on MDA-MB-231 tumor-bearing nude mice. Even if they could not obtain images due to low affinity and receptor concentrations and/or fast elimination of the peptide, this study described, for the first time, a radioligand targeting NRP-1. Wu et al. developed another radiotracer able to target both integrin αvβ
3 with RDG and NRP-1 with ATWLPPR [
21]. This heterodimeric compound was labeled with Fluor-18 by complexation into a NOTA chelator. It was evaluated by PET in vivo in a mice model bearing the U87MG tumor. The dual targeting of both integrin αvβ
3 and NRP-1 resulted in a better selectivity and uptake and retention into the tumor. In the last few years, the development of multimodal imaging PET and fluorescence has emerged as a promising tool for the development of new diagnostics and treatment agents [
39,
40,
41]. The access of fluorescent tools is very interesting for a better understanding and a better sensibility for in vivo evaluations.
4. Materials and Methods
4.1. Synthesis
All reactions involving cyanine5 compounds were performed in the dark. Preparative reverse-phase high-performance liquid chromatography (HPLC) was performed on SPOT PREP Liquid Chromatography (ARMEN Instrument, Saint-Avé, France) with a Pursuit 5-C18 column (5 μm, 21.2 × 150 mm; Varian). Pure compounds obtained were analyzed accordingly by LC-MS on a Shimadzu LCMS-2020 monitored by a Diode Array Detector SPD-M20A (Shimadzu, Marne la Vallée, France) using a Pursuit 5-C18 column (4.6 × 150 mm; Varian) (Varian, Agilent Technologies, Santa Clara, CA, USA). Absorption spectra were recorded on a Perkin-Elmer Lambda EZ210 (Perkin-Elmer, Courtaboeuf, France) double-beam UV–visible spectrophotometer. Fluorescence spectra were recorded on a Fluorolog-3 spectrofluorometer FL3-222 (Horiba Jobin Yvon, Longjumeau, France) with a thermostated cell compartment (25 °C) using a 450-W Xenon lamp. The fluorescence quantum yield (φF) was determined using tetraphenyl porphyrin (TPP) solution in toluene as the fluorescence standard (φF = 0.11).
Unless otherwise stated, all chemicals were purchased as the highest purity commercially available and were used without further purifications. Cyanine5-NHS ester was purchased from Lumiprobe GmbH (Lumiprobe Gmb, Hannover, Germany). NODAGA-NHS ester was purchased from CheMatech (CheMatech, Dijon, France). The Fmoc-Arg(Pbf)-Wang resin, Fmoc-Asp(OtBu)-Wang resin, 9-fluorenyl-methoxy-carbonyl (Fmoc)-amino acids, and N,N,N′,N′-Tetramethyl-O-(1H-benzotriazol-1-yl)uronium hexafluorophosphate) (HBTU) were purchased from Iris Biotech GmbH (Iris Biotech GmbH, Marktredwitz, Germany). N-methylmorpholine (NMM), N-methylpyrrolidinone (NMP), trifluoroacetic acid (TFA), and triisopropylsilane (TIPS) were purchased from Alfa Aesar (Alfa Aesarn Haverhill, MA, USA). Hydrazine monohydrate, diisopropylethylamine (DIPEA), and Ga(NO3)3 were obtained from Sigma-Aldrich (Sigma-Aldrich, Taufkirchen, Germany). Hydrochloric acid (HCl; 36.5−38%) was purchased from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO, USA). Only Milli-Q water (0.18 MΩ cm) was used for the aqueous solution preparation.
4.1.1. General Procedure of Peptide Synthesis
The side chains of aspartic acid and arginine were, respectively, protected by 5-tert-butyl ester (OtBu) and 2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl (Pbf). Lysine was protected either by N-tert-butyloxy carbonyl (Boc) groups or (4,4-dimethyl-2,6-dioxocyclohex-1-ylidene)ethyl (Dde). H-K(Dde)D(OtBu)K(Boc)PPR(Pbf)-Wang resin was synthesized on a multichannel peptide synthesizer (Intavis AG, Köln, Germany), according to classical Fmoc/tBu solid-phase methodology, using the Fmoc-Arg(Pbf)-Wang resin on a 100-μmol scale. Double coupling was performed using a three-fold excess of N-Fmoc amino acid and activation reagents HBTU (3 equivalents), NMP (3 equivalents), and NMM (9 equivalents) in DMF. During the coupling of cyanine 5 and all the next steps, the light exposure was minimized to limit unwanted side reactions by sealing the vessel in aluminum foil. The final Fmoc protection or Dde protection was removed using piperidine or hydrazine monohydrate, respectively. The resin obtained was dried under vacuum and then cleaved using TFA/TIPS/water (92.5/5/2.5%) for 2 h. The acidic resin was filtered and washed with 2 mL of TFA and 50 mL of CH2Cl2. The filtrate was dried under vacuum and then purified according to the appropriate method described below. Peptides were purified by preparative reversed-phase (RP) HPLC and characterized by ESI+ mass spectrometry.
Synthesis of KDKPPR
The peptide was synthesized according to the general procedure described above. The crude product was further purified by precipitation in cold diethyl ether. Pure product was isolated as a white powder (32.5 mg, 44%). (ESI): m/z calcd for C32H58N11O9 [M + H]+, 740.43; found, 740 and [M + 2H]2+, 370.71; found, 371.
Synthesis of KPRKPD (i.e., Kscramble)
The peptide was synthesized according to the general procedure described above using a Fmoc-Asp(OtBu)-Wang resin. The crude product was further purified by precipitation in cold diethyl ether. Pure product was isolated as a white powder (31 mg, 41.9%). (ESI): m/z calcd for C32H58N11O9 [M + H]+, 740.43; found, 740 and [M + 2H]2+, 370.71; found, 371.
Synthesis of Fmoc-K(Cy5)-OH
N-hydroxysuccinimide-activated cyanine5-NHS (75 mg, 0.12 mmol) and triethylamine (0.42 mmol, 50.3 μL) in 10-mL CH2Cl2 were added to Fmoc-Lys–OH and HCl (74 mg, 0.18 mmol) in a minimum of DMF in the dark under a nitrogen atmosphere. After stirring at room temperature for 24 h, the solvent was evaporated. The compound was purified by preparative HPLC with acetonitrile/(water, 0.1% TFA)(10/90) to 100% acetonitrile in 15 min, followed by isocratic acetonitrile for 10 min. Rt = 15.8 min. Pure product was isolated as a blue powder (66 mg, 65.5%). Identity and purity of the chelate were confirmed by ESI+ mass spectrometry and HPLC. (ESI): m/z calcd for C53H62N4O5 [M + H]+, 834.46; found, 834 and [M + 3H]3+, 278.82; found, 279.
Synthesis of NODAGA-K(Cy5)DKPPR
The peptide was synthesized according to the general procedure described above. After removing the Fmoc protection using piperidine, the coupling of NODAGA-NHS ester (1.2 equivalents) was realized using DIPEA (3 equivalents) in DMF for 12 h. After three cycles of Dde deprotection by hydrazine monohydrate (3% in DMF for 3 min), the coupling of cyanine5-NHS ester (1.2 equivalents) was realized using DIPEA (3 equivalents) in DMF for 12 h. After cleavage from the resin, the compound was precipitated in cold diethyl ether and purified by preparative HPLC in the same conditions as KDKPPR. Rt = 9.0 min. Pure product was isolated as a blue powder (42 mg, 27%). (ESI): m/z calcd for C79H118N16O17 [M + H]+, 1562.88; found, 1563 and [M + 2H]2+, 781.94; found, 782 and [M + 3H]3+, 521.62; found, 522 and [M + 4H]4+, 391.47; found, 391.
Synthesis of NODAGA-K(Cy5)RPKPD (i.e., NODAGA-K(Cy5)scramble)
The peptide was synthesized according to the general procedure described above using the Fmoc-Asp(OtBu)-Wang resin. After removing the Fmoc protection using piperidine, the coupling of the NODAGA-NHS ester (1.2 equivalents) was realized using DIPEA (3 equivalents) in DMF for 12 h. After three cycles of Dde deprotection by a hydrazine monohydrate (3% in DMF for 3 min, the coupling of the cyanine5–NHS ester (1.2 equivalents) was realized using DIPEA (3 equivalents) in DMF for 12 h. After cleavage from the resin, the compound was precipitated in cold diethyl ether and purified by preparative HPLC in the same conditions as KDKPPR. Rt = 11 min. Pure product was isolated as a blue powder (44 mg, 28.2%). (ESI): m/z calcd for C79H118N16O17 [M + H]+, 1562.88; found, 1563 and [M + 2H]2+, 781.94; found, 782 and [M + 3H]3+, 521.62; found, 522 and [M + 4H]4+, 391.47; found, 391.
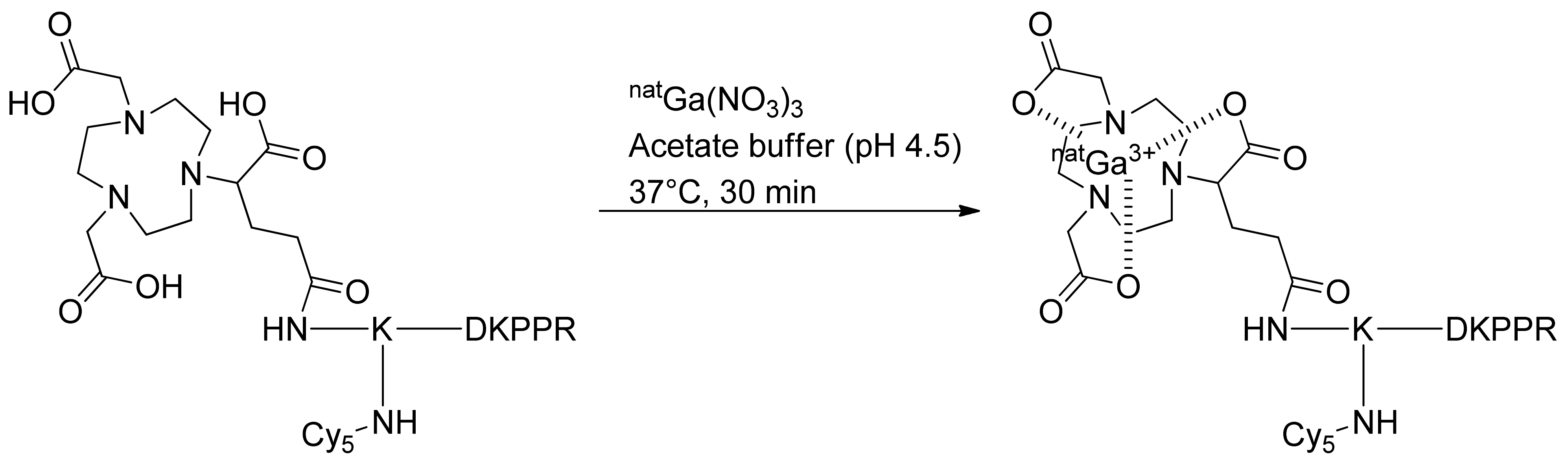
Complexation of NODAGA-K(Cy5)DKPPR with natGa for In Vitro Assay
A small amount (0.5 μmol) of NODAGA peptide was dissolved in 700 μL of an ammonium acetate solution (0.1 M in H2O). Three hundred microliters of a natGa(NO3)3 solution (0.04 M in H2O) were added and the pH adjusted to 4.5 with diluted HCl. The solution was then incubated for 30 min at 37 °C and directly purified by preparative HPLC using acetonitrile/water (0.1% TFA; 10/90) to a 100% acetonitrile gradient in 15 min, followed by isocratic acetonitrile for 10 min. Rt = 11.6 min. Pure product was isolated as a blue powder (83.17%). Identity and purity of the chelate were confirmed by ESI+ mass spectrometry and HPLC. (ESI): m/z calcd for C79H115GaN16O17 [M + H]+, 1628.78; found, 1629 and [M + 2H]2+, 814.89; found, 815 and [M + 3H]3+, 543.59; found, 544 and [M + 4H]4+, 407.94; found, 408.
Complexation of NODAGA-K(Cy5)RPKPD with natGa for In Vitro Assay
The same procedure was followed to complex NODAGA-K(Cy5)RPKPD with Ga. Rt = 11.7 min. Pure product was isolated as a blue powder (82%). Identity and purity of the chelate were confirmed by ESI+ mass spectrometry and HPLC. (ESI): m/z calcd for C79H115GaN16O17 [M + H]+, 1628.78; found, 1629 and [M + 2H]2+, 814.89; found, 815 and [M + 3H]3+, 543.59; found, 544 and [M + 4H]4+, 407.94; found, 408.
Synthesis of K(Cy5)DKPPR
The peptide was synthesized according to the general procedure described above. Fmoc-Lys(Boc)-OH was replaced by Fmoc-Lys(Cy5)-OH. The crude product was further purified by preparative HPLC in the same conditions as KDKPPR. Rt = 14.5 min. Pure product was isolated as a blue powder (20 mg, 16.6%). Identity and purity of the chelate were confirmed by ESI+ mass spectrometry and HPLC. (ESI): m/z calcd for C64H95N13O10 [M + H]+, 1205.72; found, 1205 and [M + H]2+, 603.36; found, 603 and [M + 3H]3+, 402.57; found, 402.
Synthesis of K(Cy5)RPKPD (i.e., K(Cy5)scramble)
The peptide was synthesized according to the general procedure described above using the Fmoc-Asp(OtBu)-Wang resin. Fmoc-Lys(Boc)-OH was replaced by Fmoc-Lys(Cy5)-OH. The crude product was further purified by preparative HPLC in the same conditions as KDKPPR. Rt = 14.5 min. Pure product was isolated as a blue powder (18 mg, 15%). Identity and purity of the chelate were confirmed by ESI+ mass spectrometry and HPLC. (ESI): m/z calcd for C64H95N13O10 [M + H]+, 1205.72; found, 1205 and [M + H]2+, 603.36; found, 603 and [M + 3H]3+, 402.57; found, 402.
4.2. Radiochemistry
HPLC analyses were run on a Waters system (2695eb pump, auto sampler injector, 2998 PDA detector, and NaI detector from Berthold (Bad Wildbad, Germany)) controlled by Empower Software (Orlando, FL, USA). Analyses were performed on a Pursuit XRs 5C18 (5 μm, 250 × 4.6 mm) from Agilent with an ACN/H2O/TFA mixture (proportions given in brackets) at 1 mL/min.
Approximatively 800 MBq of 68GaCl3 was eluted from a 68Ge/68Ga generator (Galliapharm; Eckert & Ziegler Europe) and eluted with 5 mL of a 0.1-N HCl solution. Radiosynthesis was carried out on a miniAllInOne (miniAIO) module from Trasis®.
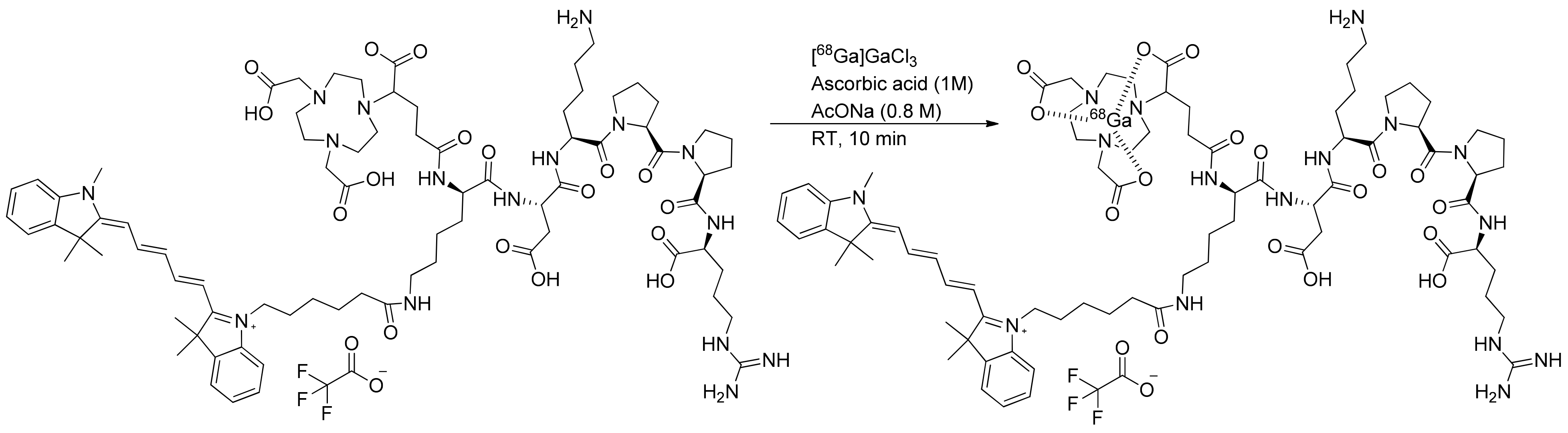
4.2.1. Radiosynthesis
The precursor NODAGA-K(Cy5)DKPPR kept in a stock solution of 1 mg/mL in deionized water. [
68Ga]Ga-NODAGA-K(Cy5)DKPPR was prepared on the mAIO (Trasis, Ans, Belgium) synthesizer with a
68Ge/
68Ga generator from Eckert and Ziegler eluted with 5 mL of the 0.1-M HCl solution and used without pre-purification of the eluate (
Figure S11). To determine the radiolabeling efficiency, different parameters were screened, such as the amount of precursor, duration, and temperature (see
Supplementary Data Figure S12). The best radiolabeling conditions found were the use of 50 µg of precursor NODAGA-K(Cy5)DKPPR with 10 µL of ascorbic acid 1 M diluted in a 0.8-M AcONa solution (1 mL) at 30 °C for 10 min. After incubation, this crude solution was purified by a HLB (Oasis) cartridge to isolate [
68Ga]Ga-NODAGA-K(Cy5)DKPPR, formulated for injection in EtOH/NaCl 0.9% (10/90
v/v) and submitted for quality control. [
68Ga]NODAGA-K(Cy5)DKPPR was produced with a decay-corrected (dc.) radiochemical yield (RCY) of 90.3 ± 2.5% (
n = 12).
The radiochemical purity and specific activity of [68Ga]Ga-NODAGA-K(Cy5)DKPPR were evaluated with HPLC analyses using a reversed-phase C18 column (Pursuit XRs 5C18). The eluent consisted of 0.1% TFA in Milli-Q water (solvent A) and an acetonitrile (solvent B) gradient of 90–0% A and 10–100% B used over a period of 15 min with a flow rate of 1.0 mL/min.
4.2.2. In Vitro Stability
The stability of [68Ga]Ga-NODAGA-K(Cy5)DKPPR was performed in various media, final formulated media, and plasma.
The final [68Ga]Ga-NODAGA-K(Cy5)DKPPR was kept at room temperature and analyzed by radio-HPLC at the end of the synthesis and 1 h, 2 h, and 3 h after manufacturing.
For the stability in plasma, 100 µL of [68Ga]Ga-NODAGA-K(Cy5)DKPPR was added to 400 µL of fresh rodent plasma and was incubated at 37 °C for 0, 30, 60, and 120 min. After incubation, the solutions were treated with 400 µL of acetonitrile to precipitate the proteins, vortexed for 1 min, and then centrifuged (2500 rpm, 980 G, 5 min). The supernatant layer was analyzed by analytical radio-HPLC.
4.2.3. Determination of Distribution Coefficient (log D)
A sample of [68Ga]Ga-NODAGA-K(Cy5)DKPPR (50 µL, ~2 MBq) was added in a vial containing 1000 µL of 1-octanol and 950 µL of PBS. The vial was vortexed vigorously during 1 min and then centrifuged for 5 min for phase separation. The defined volume of each layer was measured with a γ-counter (Perkin Elmer, Wizard 2470). The distribution coefficient was expressed as the logarithm of the ration of the counts per minute measured in the 1-octanol phase to the counts per minute measured in the PBS phase in the same volume. The values reported are the average of 3 independent measurements (+/−SD), each performed in quintuplet.
4.3. Binding to Recombinant NRP-1 Protein
4.3.1. Competitive Binding Assay
The binding of compounds for the NRP-1 protein was evaluated in terms of the half-maximal inhibitory concentration (IC
50 values) through a competitive assay, as previously described [
24]. VEGF-165, the natural ligand of NRP-1, was used as the positive control. Briefly, the surfaces of Maxisorp microplates (Thermo Fisher Scientific, France) were coated with NRP-1 recombinant chimeric proteins (Bio-techne, France). The binding of compounds to NRP-1 was assessed using biotinylated VEGF-165 (b-VEGF-165) (AcroBioSystem, Interchim, France) in the presence of heparin (Bio-techne, France) in competition or not with various concentrations of compounds or unlabeled VEGF-165 (Bio-techne, France) as the positive control. After 2 h of incubation at room temperature, the plates were washed, and the amount of bound b-VEGF-165 was stained with streptavidin horseradish peroxidase conjugate (Bio-techne, France) and assayed. The optical densities were measured at 450 nm using a Multiskan Ascent plate reader (Thermo Fisher Scientific, Finland). The results were expressed as the mean of the relative absorbance percentage to wells containing only blocking buffers of triplicate measurements. The affinities were estimated as the IC
50 values (i.e., the concentration of compounds that displaced 50% of β-VEGF-165 binding) calculated using the one-site fit log-IC
50 nonlinear analysis regression of GraphPad Prism 6 software (v 6.05, USA).
4.3.2. Surface Plasmon Resonance (SPR)
The equilibrium dissociation constant (K
D) was evaluated using surface plasmon resonance, based on Gries et al. [
34]. All experiments were performed at 25 °C using the BIACORE T200 instrument (GE Healthcare Biacore, Uppsala, Sweden). Sensor surfaces and other Biacore consumables were purchased from GE Healthcare Biacore (USA). IgG1 and recombinant human/rat NRP-1 proteins were purchased from Bio-techne (France).
The CM5 Sensor Chip was activated by using sulfo-NHS/EDC (1-ethyl-3-[3-dimethylaminopropyl] carbodiimide) solution (0.2 µM) in PBS (phosphate-buffered saline) with 0.05% (v/v) P20 surfactant, pH 7.4. Ligands were subsequently immobilized on the chip: recombinant rat NRP-1 protein (MW: 119 kDa) in formate buffer (pH 3.0) or IgG1 (as the reference surface, MW: 26.6 kDa) in sodium acetate buffer (pH 4.0) at a concentration of 50 μg/mL and a flow rate of 5 μL/min during 10 min. The immobilization levels were 4000 resonance units (RU) for IgG1 and 10,000 RU for rat NRP-1 proteins. The chip was then blocked with 1 M of ethanolamine, pH 8.5.
natGa-NODAGA-Cy5, KDKPPR, and KRPKPD (4 mM) were dissolved in ultrapure water and diluted in running buffer (0.60–10 µM). They flowed at a rate of 30 µL/min for 300 s at 25 °C. The regeneration of surfaces was achieved using two injections of HCl 5 mM at 100 µL/min during 5 s, followed by a 300-s buffer flow. Sensorgrams were corrected for signals from the reference surface (blank subtracted sensorgram). The equilibrium response was recorded 5 s before the end of the compound injection (time window: 5 s). VEGF-165, a natural ligand of NRP-1, was used as the positive control. The KD value was determined by fitting the response (RU) versus analyte concentration (µM) curve to a simple 1:1 interaction model with the BiaEvaluation 3.2 software (GE Healthcare). KD values represented the mean of two independent experiments.
4.4. Cell Culture
MDA-MB-231 (ATCC® HTB-26™) (hormone-independent and triple-negative breast cancer cell line) was grown in RPMI 1640 medium (Merck, France) supplemented with 2.5-mM L-Glu, 100-U·mL−1 penicillin, 100-μg·mL−1 streptomycin, and 10% FCS.
HUVEC (LONZA, Belgium) (Human Umbilical Vein Endothelial Cells) was grown and used until passage 5 in endothelial basal medium supplemented with the EGM™-2 SingleQuots® supplement containing FBS, hFGF-B, VEGF, R3-IGF, rhEGF, ascorbic acid, hydrocortisone, heparin, and GA (Gentamicin/Amphotericin-B) (Lonza, Belgium).
Cell cultures were performed under standard conditions (5% CO2 at 37 °C under 80% humidified air), and cells were negative for the mycoplasma detection assay (MycoSensor QPCR assay, Agilent, France).
4.4.1. NRP-1 Expression of Cells
MDA-MB-231 and HUVEC were grown until subconfluence. Briefly cells were washed 3 times with ice-cold PBS before lysis using the RIPA buffer supplemented by complete (Roche, Germany) and antiprotease cocktails 2 and 3 (Sigma-Aldrich, St. Quentin Fallavier, France). After 30 min of incubation at 4 °C, cell lysates were harvested and passed through a 23 G needle. Protein extracts were kept at −80 °C before the evaluation of the protein concentration using the bicinchoninic acid method (Pierce, France). Fifty micrograms of protein were loaded onto 10% polyacrylamide gel electrophoresis, and migration was performed at 50 V over 30 min, followed by 2 h at 110 V. Protein transfer onto a PVDF membrane was performed using the “Mixed MW turbo” program of the transblot system (Bio-Rad, France) (7 min/2.5 A/25 V). The membrane was cut into 2 pieces to separate low MW protein and high MW protein after rouge ponceau labeling. Membranes were blocked with TBST + 5% nonfat milk during 1 h and incubated overnight at 4 °C with 1/1000 diluted anti-NRP-1 rabbit (D39A5, Ozyme, France) or anti-actin rabbit (4970S, Ozyme, France) antibodies. Secondary HRP-linked antibody (7074S, Ozyme, France) was diluted to 1/1000, and the revelation was performed using the ECL Western blotting detection system (Sigma-Aldrich, France) following the instructions of the manufacturer. Images were recorded on LAS-4000 (Fujifilm Life Science, USA), and the optical density of each band was evaluated using Multigauge 3.0 software (Fujifilm Life Science, USA).
4.4.2. Metabolic Activity
Cell survival of MDA-MB-231 and HUVEC incubated with the NRP-1-targeting or nontargeting peptide or natGa-NODAGA-conjugated compounds were investigated using a MTS assay. Briefly, cells were plated into 96-well plates at a density of 10,000 or 3500 cells.cm−2, respectively, and incubated at 37 °C for 2/3 days. Old culture media were discarded, and cells were exposed to 0–10 µM of the compounds. After 4 h or 24 h, cells were washed three times with HBSS and were further incubated in culture medium for 24 h. The metabolic activity of the cells was evaluated using [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] salt according to the supplier’s recommendations (CellTiter 96® AQueous One Solution Reagent, Promega). The absorbance of formazan crystals solubilized in culture media was evaluated at 492 nm using a Multiskan Ascent plate reader (Thermo Fisher Scientific, Finland). The result was given as the mean of 3 independent experiments performed in triplicate and expressed as a percentage of variation from control cells.
4.4.3. Proliferation Assay
The proliferation of MDA-MB-231 and HUVEC cells were investigated using a label-free, noninvasive IncuCyte® S3 live-cell imaging system (Sartorius, Germany). Cells were plated as described for metabolic activity placed in an incubator (Binder) at 37 °C. Cells were photographed at 2-h intervals using a 20× objective over several days. After 3 days, cells were exposed to 0–10 µM of the compounds until the end of the experiment. Phase–object confluence was calculated at each time point based on the segmentation of high-definition phase-contrast images using IncuCyte S3 2018B software and expressed in percentages. Experiments were performed in triplicate.
Cell doubling time (
DT) was calculated using the following Equation (1):
where
Tstart/
Tend are the times at the start/end of the exponential growth phase.
Cstart/
Cend are the percentage of confluence of the cells at the start/end of the exponential growth phase.
4.5. Animal Care
All in vivo experiments were performed in accordance with the European Community animal care guidelines (Directive 2010/63/EU) for the use of experimental animals with respect to the 3 Rs’ requirements for animal welfare and carried out by competent and authorized persons in a registered establishment (establishment number D-54-547-03 issued by the Departments of Veterinary Services). All animal protocols were submitted to an ethics committee (Comité d’Ethique Lorrain en Matière d’Expérimentation Animale, CELMEA, French Ethical Committee number 66) for evaluation in accordance with the national regulations (research project APAFIS #19041 was approved by the French Ministry of Research).
Animals were kept under standard conditions (T = 24 °C ± 1 °C, hygrometry 50% ± 10%, a controlled 12-h light–dark cycle with ventilated cages, including filter tops) and had free access to standard food and water.
Eight-week-old nude female rats (Hsd: RH-Foxn1rnu) weighing 100–110 g were purchased from Charles River Laboratories (Charles River Laboratories, Saint Germain Nuelles, France).
4.5.1. Brain Metastasis Model
Breast cells from the MDA-MB-231 lineage (MDA-MB-231 (ATCC® HTB-26)) were induced by the vascular injection of 2.5 × 105 cells resuspended in a volume of 50 µL of RPMI medium (Merck, Molsheim, France) into the animal’s internal carotid.
4.5.2. MRI Experiment
Weekly longitudinal follow-ups of the tumor growth were conducted by MRI to detect metastatic outbreaks in the animal’s brain. The evolution of the metastatic focus was monitored by MRI imaging on a weekly basis for 8 weeks. MRI was initiated 10 days after tumor cell injection and followed a week later to detect brain lesions. A classic T2-weighted (T2w) morphological sequence was performed to validate the tumor development. This examination was done under general gassing anesthesia.
The MRI was performed on a 3T scanner (Prisma, Siemens Healthineers®, Erlangen, Germany) using an 8-channel volume coil dedicated to rats (Rapid Biomedical GmbH®, Rimpar, Germany). The MRI was initiated 10 days after tumor cell injection and followed a week later to detect brain lesions. Animals were anesthetized with isoflurane, and respiration was monitored and maintained constant throughout the experiment. Anatomical T2w images, covering from the frontal lobe to the posterior fossa, were acquired using a Turbo Spin-Echo (TSE) sequence (repetition time (TR)/echo-time (TE) = 2500/61 ms, Echo Train Length (ETL) = 6, flip angle = 90°, 20 slices, field of view (FOV) = 49 × 49 mm2, matrix size = 192 × 192, voxel size = 255 × 255 × 1000 μm3, Number of Scan Averages (NSA) = 8, and scan time = 10 min 37 s).
4.5.3. PET Acquisition and Analysis
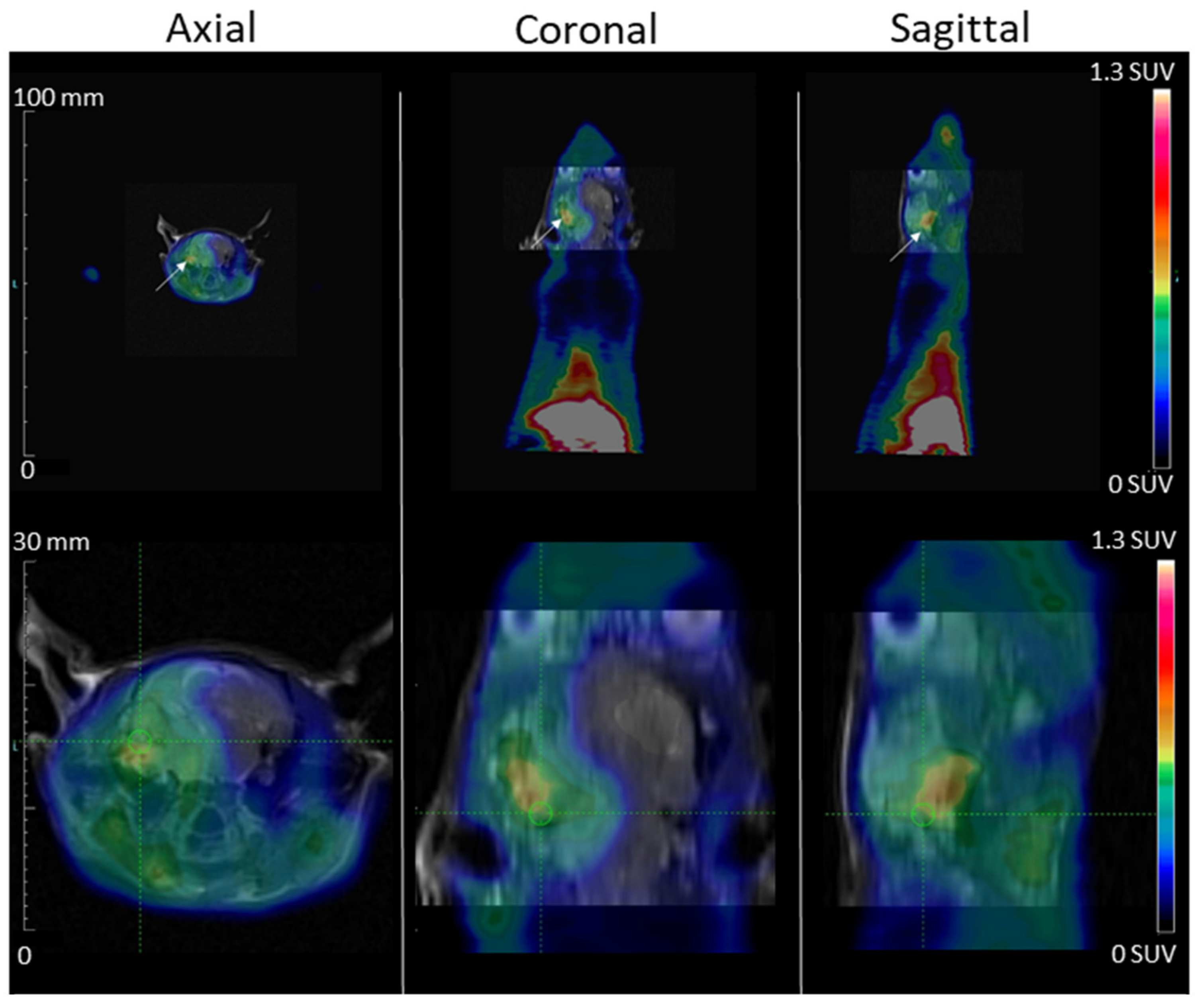
PET recordings were obtained with a camera dedicated to small animal studies (Inveon, Siemens Preclinical Solutions, Knoxville, TN, USA). After rats anesthetized with isoflurane, 50 MBq of [68Ga]Ga-NODAGA-K(Cy5)DKPPR (1st day) were injected as a bolus via a lateral tail vein.
For [68Ga]Ga-NODAGA-K(Cy5)DKPPR: 120-min list-mode acquisitions were initiated a few seconds prior to tracer injection, and the acquired PET data were subsequently reconstructed in 27 consecutive frames (5 frames of 120-s duration followed by 22 frames of 5-min duration) using the 3D ordered-subsets expectation maximization algorithm (OSEM3D, 4 iterations, 16 subsets, and 1 zoom) together with scatter and attenuation corrections based on transmission source measurements. No post-filter or regularization was applied after the reconstruction. The final voxel size was 0.8 × 0.8 × 0.8 mm3.
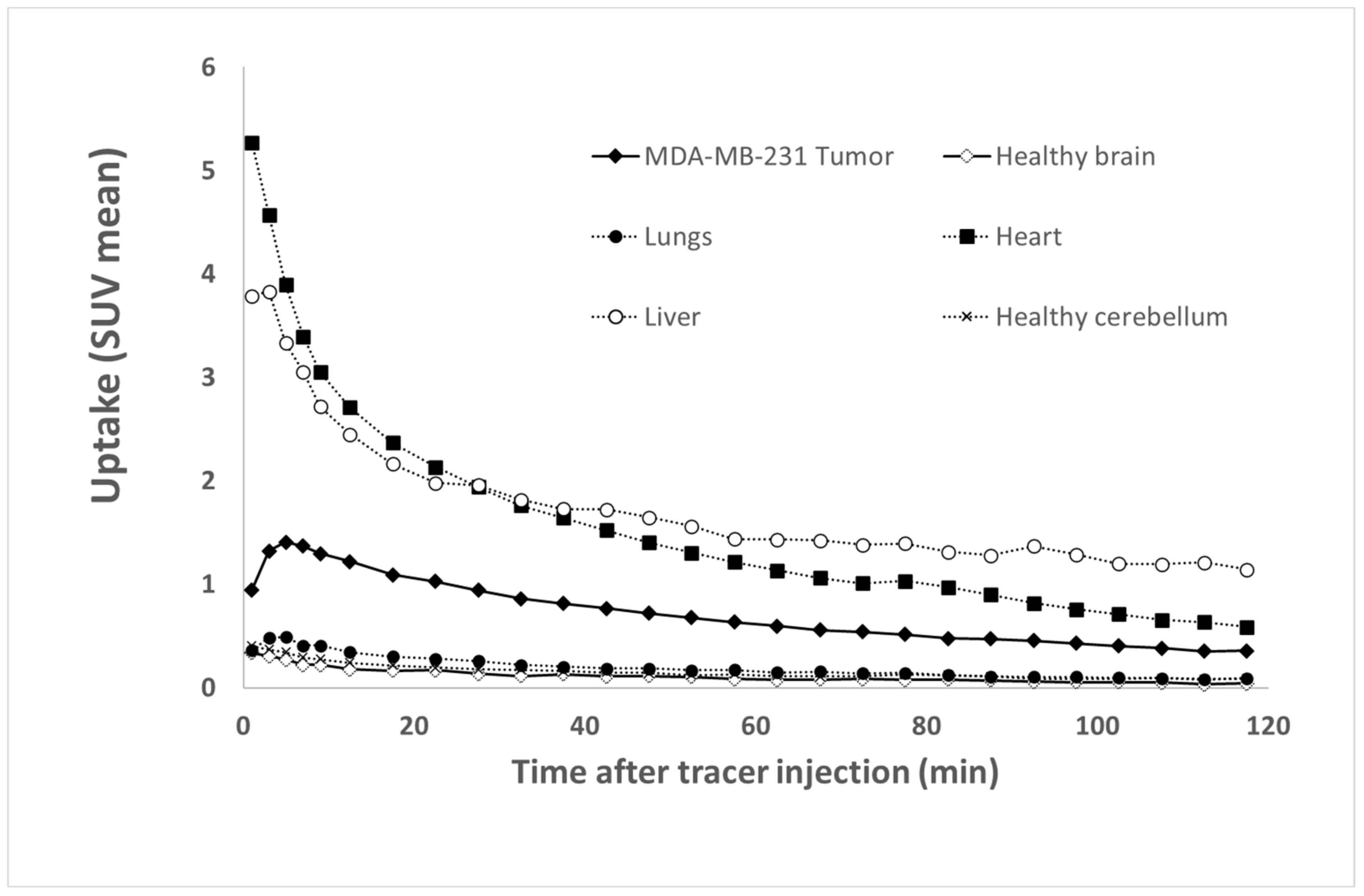
The uptake values are expressed in SUV (Standardized Uptake Values), which are standardized fixation values relative to the animal’s weight and injected activity. Tissue time activity curves (TTACs) for tumors and various organ activities were determined by average SUVs in volumes of interest (VOI), which were drawn with dedicated software (Inveon Research Workplace 4.1, Siemens
®, Knoxville, USA) on static images encompassing the whole acquisition [
35,
36]. Ellipsoid VOIs were drawn within the tumor, contralateral healthy brain (a same symmetrical VOI in the tumor and contralateral healthy brain), cerebellum (area with a low uptake of tracers used to make a normalization ratio), lungs, heart, and liver. The tumor VOI was automatically segmented with 50% of the maximum voxel value threshold [
35,
36]. At last, the tumor and healthy brain were normalized to the cerebellum to assess the contrast evolution of the segmented areas.
4.5.4. Immunohistological Analysis
Just after PET imaging, the rat was euthanized, and the tumor in the brain tissue was fixed in formalin and embedded into paraffin. On 5-µm tumor sections, anti-NRP-1 (rabbit monoclonal, 1/800, ab81321) Abcam staining was performed to detect the tumoral cell region and to confirm NRP-1 receptor expression. Each primary antibody was incubated with the tumor sections one night at 4 °C. The tumor sections were incubated with a secondary antibody (N-Histofine Universal Immuno-peroxidase Polymer Anti-Rabbit Ref414341F, Nichirei Biosciences) that was revealed by a peroxidase Kit (ImmPACT Novared Peroxidase substrate kit HRP SK-4805). Finally, the sections were counterstained with Mayer’s hematoxylin. Images of the tumors were captured using a Nikon Model Eclipse E600 microscope.
4.6. Statistical Analysis
All in vitro experiments were realized in triplicate and expressed as the percentage of variation from control cells. The results were given as the mean ± standard deviation (SD). Differences among the groups were evaluated using a variance analysis (ANOVA) followed by Bonferroni’s test (GraphPad Prism 6.0 software, San Diego, CA, USA). A value of p < 0.05 was considered statistically significant. * Versus control cells.