Influence of Freeze-Dried Phenolic-Rich Plant Powders on the Bioactive Compounds Profile, Antioxidant Activity and Aroma of Different Types of Chocolates
Abstract
:1. Introduction
2. Results and Discussion
2.1. Water Content and Activity and Color of Chocolates
2.2. Profile and Concentrations of Bioactive Compounds
2.3. Antioxidant Activity
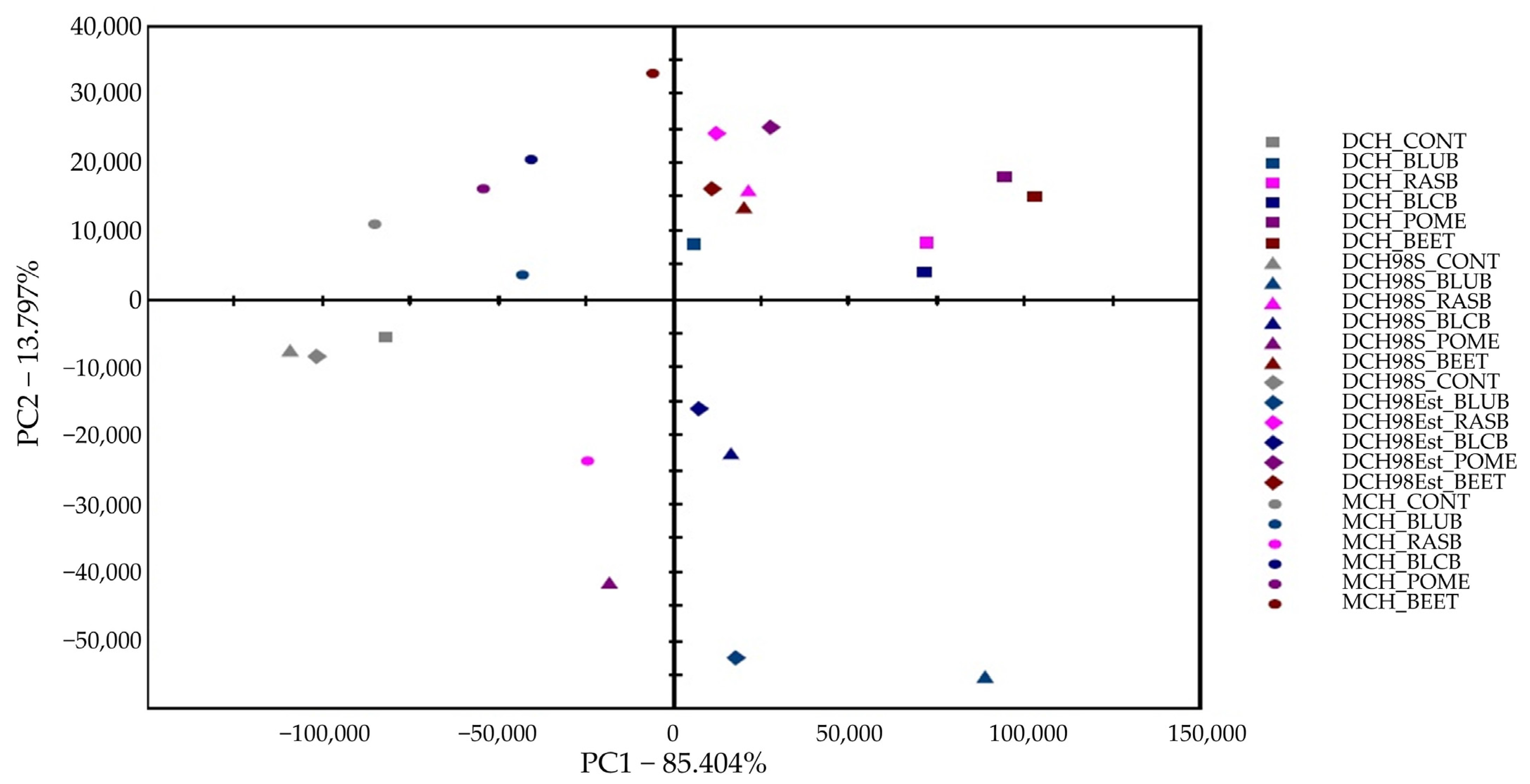
2.4. Electronic Nose Analysis of Chocolates
3. Materials and Methods
3.1. Materials
3.2. Chemicals and Reagents
3.3. Lyophilization of Fruits and Vegetables
3.4. Preparation of Chocolates
3.5. Water Content and Water Activity Determination
3.6. Color Determination
3.7. UHPLC-DAD-ESI–MS/MS Analysis of Phenolic Compounds
3.8. Free Radical Scavenging Assay
3.9. Ferric Reducing Antioxidant Power Assay
3.10. Electronic Nose Analysis of Tested Chocolates
3.11. Organoleptic Evaluation of Chocolates
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BEET | beetroots |
| BLCB | blackberries |
| BLUB | blueberries |
| Cat | catechin |
| CONT | control chocolates without the addition of freeze-dried phenolic-rich plant powders |
| Cy-3-(6″-Mal-Glu) | cyanidin-3-(6″-malonyl)-glucoside |
| Cy-3,5-diGlu | cyanidin-3,5-O-diglucoside |
| Cy-3-Glu | cyanidin-3-O-glucoside |
| Cy-3-Rut | cyanidin-3-O-rutinoside |
| Cy-3-Xyl | cyanidin-3-O-xyloside |
| DCH | dark chocolate with 53% cocoa and the total fat content of 35% (w/w) sweetened with sucrose |
| DCH98Est | dark chocolate with 98% cocoa and the total fat content of 51% (w/w) sweetened with erythritol with stevia |
| DCH98S | dark chocolate with 98% cocoa and the total fat content of 51% (w/w) sweetened with sucrose |
| Del-3,5-diGlu | delphinidin-3,5-O-diglucoside |
| Del-3-Glu | delphinidin-3-O-glucoside |
| Ecat | epicatechin |
| GA | gallic acid |
| MCH | milk chocolate with the total fat content of 36% (w/w) sweetened with sucrose |
| PA | protocatechuic acid |
| PC B2 | procyanidin B2 |
| PC C1 | procyanidin C1 |
| PCA | principal component analysis |
| Pel-3,5-diGlu | pelargonidin-3,5-O-diglucoside |
| p-HBA | p-hydroxybenzoic acid |
| POME | pomegranates pomace |
| RASP | raspberries |
| VCs | volatile compounds |
References
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.J.; Li, H.B. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- Valls, J.; Millán, S.; Marti, M.P.; Borràs, E.; Arola, L. Advanced separation methods of food anthocyanins, isoflavones, and flavanols. J. Chromatogr. A 2009, 1216, 7143–7172. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.J.; Xu, D.P.; Zhou, T.; Zhou, Y.; Li, S.; Li, H. Bioactivities and health benefits of wild fruits. Int. J. Mol. Sci. 2016, 17, 1258. [Google Scholar] [CrossRef] [Green Version]
- Minatel, I.O.; Borges, C.V.; Ferreira, M.I.; Gomez, H.A.G.; Chen, C.Y.O.; Lima, G.P.P. Phenolic Compounds: Functional properties, impact of processing and bioavailability. In Phenolic Compounds—Biological Activity; Soto-Hernandez, M., Palma-Tenango, M., del Rosario Garcia-Mateos, M., Eds.; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef] [Green Version]
- Cienfuegos-Jovellanos, E.; Quiñones, M.M.; Muguerza, B.; Moulay, L.; Miguel, M.; Aleixandre, A. Antihypertensive effect of a polyphenol-rich cocoa powder industrially processed to preserve the original flavonoids of the cocoa beans. J. Agric. Food Chem. 2009, 57, 6156–6162. [Google Scholar] [CrossRef] [PubMed]
- Arranz, S.; Valderas-Martinez, P.; Chiva-Blanch, G.; Casas, R.; Urpi-Sarda, M.; Lamuela-Raventos, R.M.; Estruch, R. Cardioprotective effects of cocoa: Clinical evidence from randomized clinical intervention trials in humans. Mol. Nutr. Food Res. 2013, 57, 936–947. [Google Scholar] [CrossRef] [PubMed]
- Ioannone, F.; Di Mattia, C.D.; De Gregorio, M.; Sergi, M.; Serafini, M.; Sacchetti, G. Flavanols, proanthocyanidins and antioxidant activity changes during cocoa (Theobroma cacao L.) roasting as affected by temperature and time of processing. Food Chem. 2015, 174, 256–262. [Google Scholar] [CrossRef]
- Fernández-Romero, E.; Chavez-Quintana, S.G.; Siche, R.; Castro-Alayo, E.M.; Cardenas-Toro, F.P. The kinetics of total phenolic content and monomeric flavan-3-ols during the roasting process of Criollo cocoa. Antioxidants 2020, 9, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medeiros, N.D.; Marder, R.K.; Wohlenberg, M.F.; Funchal, C.; Dani, C. Total phenolic content and antioxidant activity of different types of chocolate, milk, semisweet, dark, and soy, in cerebral cortex, hippocampus, and cerebellum of wistar rats. Biochem. Res. Int. 2015, 2015, 294659. [Google Scholar] [CrossRef] [Green Version]
- Wollgast, J.; Anklam, E. Review on polyphenols in Theobroma cacao: Changes in composition during the manufacture of chocolate and methodology for identification and quantification. Food Res. Int. 2000, 33, 423–447. [Google Scholar] [CrossRef]
- Tomas-Barberan, F.A.; Cienfuegos-Jovellanos, E.; Marín, A.; Muguerza, B.; Gil-Izquierdo, A.; Cerda, B.; Zafrilla, P.; Morillas, J.; Mulero, J.; Ibarra, A.; et al. A new process to develop a cocoa powder with higher flavonoid monomer content and enhanced bioavailability in healthy humans. J. Agric. Food Chem. 2007, 55, 3926–3935. [Google Scholar] [CrossRef]
- Aprotosoaie, A.C.; Miron, A.; Trifan, A.; Luca, V.S.; Costache, I.I. The cardiovascular effects of cocoa polyphenols—An overview. Diseases 2016, 4, 39. [Google Scholar] [CrossRef] [Green Version]
- Andres-Lacueva, C.; Monagas, M.; Khan, N.; Izquierdo-Pulido, M.; Urpi-Sarda, M.; Permanyer, J.; Lamuela-Raventtos, R.M. Flavanol and flavonol contents of cocoa powder products: Influence of manufacturing process. J. Agric. Food Chem. 2008, 56, 3111–3117. [Google Scholar] [CrossRef] [PubMed]
- Suazo, Y.; Davidov-Pardo, G.; Arozarena, I. Effect of fermentation and roasting on the phenolic concentration and antioxidant activity of cocoa from Nicaragua. J. Food Qual. 2014, 37, 50–56. [Google Scholar] [CrossRef]
- Voigt, J.; Lieberei, R. Biochemistry of cocoa fermentation. In Cocoa and Coffee Fermentation; CRC Press: Boca Raton, FL, USA, 2014; pp. 193–227. [Google Scholar]
- Oracz, J.; Żyżelewicz, D.; Nebesny, E. The content of polyphenolic compounds in cocoa beans (Theobroma cacao L.), depending on variety, growing region and processing operations: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1176–1192. [Google Scholar] [CrossRef] [PubMed]
- Counet, C.; Ouwerx, C.; Rosoux, D.; Collin, S. Relationship between procyanidin and flavor contents of cocoa liquors from different origins. J. Agric. Food Chem. 2004, 52, 6243–6249. [Google Scholar] [CrossRef]
- Schinella, G.; Mosca, S.; Cienfuegos-Jovellanos, E.; Pasamar, M.A.; Muguerza, B.; Ramon, D.; Rios, J.L. Antioxidant properties of polyphenol-rich cocoa products industrially processed. Food Res. Int. 2010, 43, 1614–1623. [Google Scholar] [CrossRef] [Green Version]
- Afoakwa, E. Roasting effects on phenolic content and free radical scavenging activities of pulp preconditioned and fermented (Theobroma cacao) beans. Afr. J. Food Agric. Nutr. Dev. 2015, 15, 9635–9650. [Google Scholar]
- Teh, Q.T.M.; Tan, G.L.Y.; Loo, S.M.; Azhar, F.Z.; Menon, A.S.; Hii, C.L. The drying kinetics and polyphenol degradation of cocoa beans: Cocoa drying and polyphenol degradation. J. Food Proc. Eng. 2016, 39, 484–491. [Google Scholar] [CrossRef]
- Sacchetti, G.; Ioannone, F.; De Gregorio, M.; Di Mattia, C.; Serafini, M.; Mastrocola, D. Non enzymatic browning during cocoa roasting as affected by processing time and temperature. J. Food Eng. 2016, 169, 44–52. [Google Scholar] [CrossRef]
- Niemenak, N.; Rohsius, C.; Elwers, S.; Omokolo Ndoumou, D.; Lieberei, R. Comparative study of different cocoa (Theobroma cacao L.) clones in terms of their phenolics and anthocyanins contents. J. Food Compost. Anal. 2006, 19, 612–619. [Google Scholar] [CrossRef]
- Jumnongpon, R.; Chaiseri, S.; Hongsprabhas, P.; Healy, J.P.; Meade, S.J.; Gerrard, J.A. Cocoa protein crosslinking using Maillard chemistry. Food Chem. 2012, 134, 375–380. [Google Scholar] [CrossRef]
- Belščak, A.; Komes, D.; Horzic, D.; Kovacević Ganić, K.; Karlović, D. Comparative study of commercially available cocoa products in terms of their bioactive composition. Food Res. Int. 2009, 42, 707–716. [Google Scholar] [CrossRef]
- Żyżelewicz, D.; Krysiak, W.; Oracz, J.; Sosnowska, D.; Budryn, G.; Nebesny, E. The influence of the roasting process conditions on the polyphenol content in cocoa beans, nibs and chocolates. Food Res. Int. 2016, 89, 918–929. [Google Scholar] [CrossRef]
- Di Mattia, C.D.; Sacchetti, G.; Mastrocola, D.; Serafini, M. From cocoa to chocolate: The impact of processing on in vitro antioxidant activity and the effects of chocolate on antioxidant markers in vivo. Front. Immunol. 2017, 8, 1207. [Google Scholar] [CrossRef] [Green Version]
- Mursu, J.; Voutilainen, S.; Nurmi, T.; Rissanen, T.H.; Virtanen, J.K.; Kaikkonen, J.; Nyyssönen, K.; Salonen, J.T. Dark chocolate consumption increases HDL cholesterol concentration and chocolate fatty acids may inhibit lipid peroxidation in healthy humans. Free Radic. Biol. Med. 2004, 37, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Żyżelewicz, D.; Budryn, G.; Oracz, J.; Antolak, H.; Kręgiel, D.; Kaczmarska, M. The effect on bioactive components and characteristics of chocolate by functionalization with raw cocoa beans. Food Res. Int. 2018, 113, 234–244. [Google Scholar] [CrossRef]
- González-Barrio, R.; Nuñez-Gomez, V.; Cienfuegos-Jovellanos, E.; García-Alonso, F.J.; Periago-Castón, M.J. Improvement of the flavanol profile and the antioxidant capacity of chocolate using a phenolic rich cocoa powder. Foods 2020, 9, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muhammad, D.M.; Saputro, A.D.; Rottiers, H.; Van de Walle, D.; Dewettinck, K. Physicochemical properties and antioxidant activities of chocolates enriched with engineered cinnamon nanoparticles. Eur. Food Res. Tech. 2018, 244, 1185–1202. [Google Scholar] [CrossRef]
- Belščak-Cvitanović, A.; Komes, D.; Benković, M.; Karlović, S.; Hečimović, D.J.; Bauman, I. Innovative formulations of chocolates enriched with plant polyphenols from Rubus idaeus L. leaves and characterization of their physical, bioactive and sensory properties. Food Res. Int. 2012, 48, 820–830. [Google Scholar] [CrossRef]
- Lončarević, I.; Pajin, B.; Tumbas Šaponjac, V.; Petrović, J.; Vulić, J.; Fišteš, A.; Jovanović, P. Physical, sensorial and bioactive characteristics of white chocolate with encapsulated green tea extract. J. Sci. Food Agric. 2019, 99, 5834–5841. [Google Scholar] [CrossRef]
- Bolenz, S.; Glöde, L. Technological and nutritional aspects of milk chocolate enriched with grape pomace products. Eur. Food Res. Tech. 2021, 247, 623–636. [Google Scholar] [CrossRef]
- Godočiková, L.; Ivanišová, E.; Noguera-Artiaga, L.; Carbonell-Barrachina, Á.A.; Kačániová, M. Biological activity, antioxidant capacity and volatile profile of enriched Slovak chocolates. J. Sci. Food Agric. 2019, 58, 283–293. [Google Scholar]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín, J.; Kuskoski, E.M.; Navas, J.M.; Asuero, A.G. Antioxidant capacity of anthocyanin pigments. In Flavonoids—From Biosynthesis to Human Health; Justino, G.C., Ed.; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef] [Green Version]
- European Council Directive 2000/36/EG Relating to Cocoa and Chocolate Products Intended for Human Consumption. Off. J. Eur. Communities 2000, L 197/19. Available online: https://eur-lex.europa.eu/eli/dir/2000/36/oj (accessed on 3 October 2021).
- Toker, O.S.; Zorlucan, F.T.; Konar, N.; Daglıoglu, O.; Sagdic, O.; Sener, S. Investigating the effect of production process of ball mill refiner on some physical quality parameters of compound chocolate: Response surface methodology approach. Int. J. Food Sci. Tech. 2017, 52, 788–799. [Google Scholar] [CrossRef]
- Miller, K.B.; Stuart, D.A.; Smith, N.L.; Lee, C.Y.; Mc Hale, N.L.; Flanagan, J.A.; Ou, B.; Hurst, W.J. Antioxidant activity and polyphenol and procyanidin contents of selected commercially available cocoa-containing and chocolate products in the United States. J. Agric. Food Chem. 2006, 54, 4062–4068. [Google Scholar] [CrossRef]
- Todorovic, V.; Radojcic, I.; Todorovic, Z.; Jankovic, G.; Dodevska, M.; Sobajic, S. Polyphenols, methylxanthines, and antioxidant capacity of chocolates produced in Serbia. J. Food Compost. Anal. 2015, 41, 137–143. [Google Scholar] [CrossRef]
- Godočiková, L.; Ivanišová, E.; Kačániová, M. The influence of fortification of dark chocolate with sea buckthorn and mulberry on the content of biologically active substances. Adv. Res. Life Sci. 2017, 1, 26–31. [Google Scholar] [CrossRef] [Green Version]
- Martini, S.; Conte, A.; Tagliazucchi, D. Comprehensive evaluation of phenolic profile in dark chocolate and dark chocolate enriched with Sakura green tea leaves or turmeric powder. Food Res. Int. 2018, 112, 1–16. [Google Scholar] [CrossRef]
- Batista, N.N.; de Andrade, D.P.; Ramos, C.L.; Dias, D.R.; Schwan, R.F. Antioxidant capacity of cocoa beans and chocolate assessed by FTIR. Food Res. Int. 2016, 90, 313–319. [Google Scholar] [CrossRef]
- Nowicka, P.; Wojdyło, A. Bioactive compounds and sensory attributes of sour cherry puree sweetened with natural sweeteners. Int. J. Food Sci. Technol. 2015, 50, 585–591. [Google Scholar] [CrossRef]
- Shalaby, E.A.; Mahmoud, G.I.; Shanab, S.M.M. Suggested mechanism for the effect of sweeteners on radical scavenging activity of phenolic compounds in black and green tea. Front. Life Sci. 2016, 9, 241–251. [Google Scholar] [CrossRef] [Green Version]
- Belščak-Cvitanović, A.; Komes, D.; Dujmović, M.; Karlović, S.; Biškić, M.; Brnčić, M.; Ježek, D. Physical, bioactive and sensory quality parameters of reduced sugar chocolates formulated with natural sweeteners as sucrose alternatives. Food Chem. 2015, 167, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Bertazzo, A.; Comai, S.; Brunato, I.; Zancato, M.; Costa, C.V.L. The content of protein and non-protein (free and protein-bound) tryptophan in Theobroma cacao beans. Food Chem. 2011, 124, 93–96. [Google Scholar] [CrossRef]
- Aprotosoaie, A.C.; Luca, S.V.; Miron, A. Flavor chemistry of cocoa and cocoa products—An overview. Compr. Rev. Food Sci. Food Saf. 2016, 15, 73–91. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Campos, J.; Escalona-Buendía, H.B.; Orozco-Avila, I.; Lugo-Cervantes, E.; Jaramillo-Flores, M.E. Dynamics of volatile and non-volatile compounds in cocoa (Theobroma cacao L.) during fermentation and drying processes using principal components analysis. Food Res. Int. 2011, 44, 250–258. [Google Scholar] [CrossRef]
- Rottiers, H.; Sosa, D.A.T.; Van de Vyver, L.; Hinneh, M.; Everaert, H.; De Wever, J.; Messens, K.; Dewettinck, K. Discrimination of cocoa liquors based on their odor fingerprint: A fast GC electronic nose suitability study. Food Anal. Methods 2019, 12, 475–488. [Google Scholar] [CrossRef]
- Żyżelewicz, D.; Krysiak, W.; Nebesny, E.; Budryn, G. Application of various methods for determination of the color of cocoa beans roasted under variable process parameters. Eur. Food Res. Tech. 2014, 238, 549–563. [Google Scholar] [CrossRef] [Green Version]
- Oracz, J.; Nebesny, E.; Żyżelewicz, D. Identification and quantification of free and bound phenolic compounds contained in the high-molecular weight melanoidin fractions derived from two different types of cocoa beans by UHPLC-DAD-ESI-HR-MSn. Food Res. Int. 2019, 115, 135–149. [Google Scholar] [CrossRef]
- Fischer, U.A.; Carle, R.; Kammerer, D.R. Identification and quantification of phenolic compounds from pomegranate (Punica granatum L.) peel, mesocarp, aril and differently produced juices by HPLC-DAD-ESI/MS. Food Chem. 2011, 127, 807–821. [Google Scholar] [CrossRef]
- Yang, H.; Kim, H.W.; Kwon, Y.S.; Kim, H.K.; Sung, S.H. Fast and simple discriminative analysis of anthocyanins-containing berries using LC/MS spectral data. Phytochem. Anal. 2017, 28, 416–423. [Google Scholar] [CrossRef]
- Oracz, J.; Żyżelewicz, D. In vitro antioxidant activity and FTIR characterization of high-molecular weight melanoidin fractions from different types of cocoa beans. Antioxidants 2019, 8, 560. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Chocolate Type | Functional Enrichment | Water Content (%) | Water Activity | CIE L*a*b* Color Parameters | Organoleptic Assessment (Point) | ||
|---|---|---|---|---|---|---|---|
| L* | a* | b* | |||||
| DCH | CONT | 1.15 ± 0.04 b | 0.408 ± 0.003 b | 27.79 ± 0.12 a | 5.30 ± 0.10 a | 1.15 ± 0.09 b | 4.8 ± 0.2 a |
| BLUB | 1.63 ± 0.02 c | 0.426 ± 0.002 c | 29.88 ± 0.11 e | 5.33 ± 0.09 b | 1.63 ± 0.02 c | 4.9 ± 0.1 a | |
| RASB | 2.19 ± 0.09 f | 0.446 ± 0.005 d | 29.17 ± 0.11 b | 5.54 ± 0.05 d | 2.19 ± 0.06 f | 4.8 ± 0.2 a | |
| BLCB | 1.73 ± 0.08 d | 0.490 ± 0.002 f | 29.78 ± 0.12 d | 5.39 ± 0.09 c | 1.73 ± 0.02 d | 4.8 ± 0.2 a | |
| POME | 1.83 ± 0.05 e | 0.400 ± 0.007 a | 29.58 ± 0.13 c | 5.50 ± 0.11 d | 1.83 ± 0.03 e | 4.7 ± 0.2 a | |
| BEET | 0.93 ± 0.04 a | 0.455 ± 0.003 e | 31.69 ± 0.10 f | 5.39 ± 0.08 c | 0.93 ± 0.04 a | 4.3 ± 0.3 b | |
| DCH98S | CONT | 1.63 ± 0.02 b | 0.409 ± 0.001 b | 27.70 ± 0.11 b | 3.75 ± 0.09 e | 1.63 ± 0.08 b | 4.5 ± 0.1 a |
| BLUB | 1.97 ± 0.01 f | 0.378 ± 0.002 a | 27.74 ± 0.12 b | 3.51 ± 0.10 c | 1.97 ± 0.07 f | 4.6 ± 0.1 a | |
| RASB | 1.69 ± 0.04 c | 0.447 ± 0.003 d | 28.79 ± 0.14 e | 3.34 ± 0.12 a | 1.69 ± 0.02 c | 4.6 ± 0.2 a | |
| BLCB | 1.74 ± 0.07 d | 0.433 ± 0.006 c | 27.82 ± 0.11 c | 3.60 ± 0.03 d | 1.74 ± 0.06 d | 4.5 ± 0.2 a | |
| POME | 1.26 ± 0.02 a | 0.408 ± 0.003 b | 27.98 ± 0.13 d | 3.53 ± 0.07 c | 1.26 ± 0.04 a | 4.5 ± 0.2 a | |
| BEET | 1.86 ± 0.08 e | 0.445 ± 0.005 d | 27.57 ± 0.12 a | 3.40 ± 0.09 b | 1.86 ± 0.02 e | 4.1 ± 0.3 b | |
| DCH98ESt | CONT | 1.83 ± 0.05 c | 0.408 ± 0.002 b | 27.22 ± 0.10 a | 3.36 ± 0.07 e | 1.83 ± 0.03 c | 4.4 ± 0.2 a |
| BLUB | 1.58 ± 0.04 a | 0.394 ± 0.003 a | 27.96 ± 0.10 c | 3.25 ± 0.10 d | 1.58 ± 0.02 a | 4.5 ± 0.1 a | |
| RASB | 1.60 ± 0.02 a | 0.443 ± 0.001 d | 28.78 ± 0.12 e | 3.11 ± 0.09 b | 1.60 ± 0.01 a | 4.5 ± 0.2 a | |
| BLCB | 2.03 ± 0.03 d | 0.445 ± 0.002 d | 29.31 ± 0.11 f | 3.03 ± 0.05 a | 2.03 ± 0.06 d | 4.4 ± 0.2 a,b | |
| POME | 1.81 ± 0.01 c | 0.392 ± 0.004 a | 27.85 ± 0.09 b | 3.49 ± 0.07 f | 1.81 ± 0.03 c | 4.3 ± 0.1 a,b | |
| BEET | 1.70 ± 0.04 b | 0.436 ± 0.003 c | 28.35 ± 0.13 d | 3.18 ± 0.09 c | 1.70 ± 0.02 b | 3.9 ± 0.3 c | |
| MCH | CONT | 1.22 ± 0.06 c | 0.449 ± 0.004 b | 32.99 ± 0.10 b | 7.54 ± 0.06 b | 1.22 ± 0.05 b | 4.8 ± 0.2 a |
| BLUB | 1.24 ± 0.03 c | 0.393 ± 0.003 a | 34.01 ± 0.15 f | 7.25 ± 0.08 a | 1.24 ± 0.03 c | 4.9 ± 0.1 a | |
| RASB | 1.17 ± 0.04 b | 0.441 ± 0.002 b | 33.16 ± 0.12 d | 7.60 ± 0.11 b | 1.17 ± 0.02 b | 4.8 ± 0.2 a | |
| BLCB | 1.19 ± 0.05 b | 0.475 ± 0.001 c | 32.29 ± 0.11 a | 7.55 ± 0.06 b | 1.19 ± 0.05 b | 4.8 ± 0.2 a | |
| POME | 0.94 ± 0.04 a | 0.396 ± 0.002 a | 33.37 ± 0.11 e | 7.99 ± 0.07 d | 0.94 ± 0.02 a | 4.6 ± 0.1 b | |
| BEET | 1.24 ± 0.07 c | 0.446 ± 0.003 b | 33.08 ± 0.12 c | 7.70 ± 0.09 c | 1.24 ± 0.08 c | 3.8 ± 0.3 c | |
| Compounds and Antioxidant Activity | BLUB | RASB | BLCB | POME | BEET |
|---|---|---|---|---|---|
| Phenolic Content | |||||
| Flavan-3-ols (mg/100 g DM) | |||||
| Cat | 35.41 ± 0.12 e | 30.70 ± 0.14 d | 10.09 ± 0.09 a | 11.56 ± 0.11 b | 21.87 ± 0.15 c |
| Ecat | 57.45 ± 0.24 d | 52.23 ± 0.19 b | 48.34 ± 0.18 a | 55.34 ± 0.26 c | 58.50 ± 0.31 e |
| PC B2 | 39.34 ± 0.17 d | 20.46 ± 0.19 c | 43.24 ± 0.21 e | 16.78 ± 0.13 b | 13.65 ± 0.10 a |
| PC C1 | 11.98 ± 0.08 e | 0.06 ± 0.02 a | 0.12 ± 0.03 c | 0.09 ± 0.02 b | 0.05 ± 0.02 a |
| Total | 144.18 ± 0.56 e | 103.45 ± 0.25 d | 101.79 ± 0.21 c | 83.77 ± 0.14 a | 94.07 ± 0.18 b |
| Anthocyanins (mg/100 g DM) | |||||
| Cy-3-Glu | nd | 3730.65 ± 10.87 a | 4370.09 ± 12.44 b | nd | nd |
| Cy-3-Rut | nd | nd | 59.76 ± 0.21 | nd | nd |
| Cy-3,5-diGlu | 10,280.60 ± 32.51 b | nd | nd | 98.69 ± 0.36 a | nd |
| Cy-3-Xyl | 4195.67 ± 14.41 b | nd | 164.86 ± 0.41 a | nd | nd |
| Cy-3-(6″-Mal-Glu) | nd | nd | 315.46 ± 0.65 b | nd | nd |
| Del-3,5-diGlu | 7697.48 ± 26.54 b | nd | nd | 31.56 ± 0.21 a | nd |
| Del-3-Glu | nd | nd | nd | 8.49 ± 0.07 | nd |
| Pel-3,5-diGlu | 10,759.88 ± 36.38 | nd | nd | nd | nd |
| Total | 32,933.63 ± 61.89 d | 3730.65 ± 10.87 b | 4910.17 ± 13.70 c | 138.74 ± 0.39 a | nd |
| Phenolic Acids (mg/100 g DM) | |||||
| GA | 9.36 ± 0.09 b | nd | 2.57 ± 0.05 a | nd | nd |
| PA | 6.14 ± 0.05 c | 3.19 ± 0.05 b | 2.24 ± 0.04 a | nd | 26.48 ± 0.11 d |
| p-HBA | 1.83 ± 0.03 a | 10.29 ± 0.08 b | 18.48 ± 0.12 d | 11.80 ± 0.06 c | 70.40 ± 0.19 e |
| Total | 17.33 ± 0.25 c | 13.48 ± 0.08 b | 23.29 ± 0.22 d | 11.80 ± 0.05 a | 96.88 ± 0.36 e |
| Total phenolics (mg/100 g DM) | 33,095.14 ± 62.70 e | 3847.58 ± 11.20 c | 5035.25 ± 14.13 d | 234.31 ± 0.58 b | 190.95 ± 0.54 a |
| Antioxidant Activity | |||||
| DPPH EC50 (mg/mg DPPH) | 0.15 ± 0.02 a | 0.62 ± 0.03 b | 1.17 ± 0.05 c | 1.49 ± 0.04 d | 0.15 ± 0.02 a |
| FRAP (μmol TE/g DM) | 761.27 ± 0.13 e | 646.86 ± 0.16 c | 629.61 ± 0.09 b | 671.25 ± 0.12 d | 498.34 ± 0.18 a |
| Phenolic Compounds | Functional Enrichment | |||||
|---|---|---|---|---|---|---|
| CONT | BLUB | RASB | BLCB | POME | BEET | |
| DCH | ||||||
| Flavan-3-ols | ||||||
| Cat | 14.15 ± 0.09 a | 18.66 ± 0.08 e | 16.60 ± 0.07 d | 14.18 ± 0.11 b | 15.16 ± 0.12 c | 17.89 ± 0.09 e |
| Ecat | 77.23 ± 0.48 a | 97.32 ± 0.43 f | 85.24 ± 0.47 c | 80.68 ± 0.38 b | 88.67 ± 0.34 d | 96.54 ± 0.51 e |
| PC B2 | 23.30 ± 0.11 a | 43.19 ± 0.15 d | 43.35 ± 0.16 d | 45.49 ± 0.12 e | 40.41 ± 0.18 c | 34.89 ± 0.15 b |
| PC C1 | 10.47 ± 0.06 a | 17.79 ± 0.07 e | 11.80 ± 0.08 b | 14.71 ± 0.09 d | 11.86 ± 0.07 b | 14.40 ± 0.06 c |
| Total flavan-3-ols | 125.15 ± 0.54 a | 176.96 ± 0.59 d | 156.99 ± 0.66 b | 156.06 ± 0.65 b | 156.10 ± 0.58 b | 163.72 ± 0.63 c |
| Anthocyanins | ||||||
| Cy-3-Glu | nd | nd | 3.73 ± 0.05 a | 4.37 ± 0.04 b | nd | nd |
| Cy-3-Rut | nd | nd | nd | 0.06 ± 0.01 a | nd | nd |
| Cy-3,5-diGlu | nd | 10.28 ± 0.15 b | nd | nd | 0.10 ± 0.04 a | nd |
| Cy-3-Xyl | nd | 4.20 ± 0.07 b | nd | 0.16 ± 0.07 a | nd | nd |
| Cy-3-(6″-Mal-Glu) | nd | nd | nd | 0.32 ± 0.03 | nd | nd |
| Del-3,5-diGlu | nd | 7.70 ± 0.10 b | nd | nd | 0.03 ± 0.05 a | nd |
| Del-3-Glu | nd | nd | nd | nd | 0.01 ± 0.01 | nd |
| Pel-3,5-diGlu | nd | 10.76 ± 0.09 | nd | nd | nd | nd |
| Total anthocyanins | nd | 32.93 ± 0.31 d | 3.73 ± 0.05 b | 4.91 ± 0.15 c | 0.14 ± 0.09 a | nd |
| Phenolic acids | ||||||
| GA | 7.23 ± 0.09 b | 8.77 ± 0.11 c | 7.42 ± 0.10 b | 7.12 ± 0.12 a | 9.25 ± 0.10 d | 8.88 ± 0.11 c |
| PA | 1.34 ± 0.04 a | 4.24 ± 0.10 e | 2.86 ± 0.07 c | 2.41 ± 0.06 b | 2.91 ± 0.08 c | 3.07 ± 0.05 d |
| p-HBA | 7.69 ± 0.09 a | 8.25 ± 0.11 c | 8.60 ± 0.05 d | 7.90 ± 0.09 b | 7.96 ± 0.10 b | 8.90 ± 0.03 e |
| Total phenolic acids | 16.26 ± 0.21 a | 21.26 ± 0.24 f | 18.88 ± 0.21 c | 17.43 ± 0.18 b | 20.12 ± 0.26 d | 20.85 ± 0.19 e |
| Total phenolics | 141.41 ± 0.74 a | 231.16 ± 0.63 d | 179.60 ± 0.64 b | 177.40 ± 0.70 b | 176.36 ± 0.71 b | 184.57 ± 0.84 e |
| DCH98S | ||||||
| Flavan-3-ols | ||||||
| Cat | 28.48 ± 0.10 c | 31.73 ± 0.08 d | 28.21 ± 0.14 c | 23.14 ± 0.12 a | 25.77 ± 0.09 b | 33.00 ± 0.14 e |
| Ecat | 152.29 ± 0.51 a | 165.44 ± 0.45 e | 164.91 ± 0.31 d | 154.30 ± 0.39 b | 155.17 ± 0.43 c | 173.70 ± 0.23 f |
| PC B2 | 39.61 ± 0.12 a | 63.42 ± 0.11 e | 53.69 ± 0.14 c | 48.43 ± 0.09 b | 70.96 ± 0.10 f | 59.39 ± 0.19 d |
| PC C1 | 21.20 ± 0.08 b | 29.25 ± 0.10 e | 20.05 ± 0.06 a | 27.95 ± 0.07 c | 21.23 ± 0.09 b | 28.73 ± 0.11 d |
| Total flavan-3-ols | 241.58 ± 0.78 a | 289.84 ± 0.69 e | 266.86 ± 0.58 c | 253.82 ± 0.72 b | 273.13 ± 0.73 d | 294.82 ± 0.63 f |
| Anthocyanins | ||||||
| Cy-3-Glu | nd | nd | 3.73 ± 0.06 a | 4.37 ± 0.05 b | nd | nd |
| Cy-3-Rut | nd | nd | nd | 0.06 ± 0.02 | nd | nd |
| Cy-3,5-diGlu | nd | 10.28 ± 0.11 b | nd | nd | 0.10 ± 0.02 a | nd |
| Cy-3-Xyl | nd | 4.20 ± 0.08 b | nd | 0.16 ± 0.04 a | nd | nd |
| Cy-3-(6″-Mal-Glu) | nd | nd | nd | 0.32 ± 0.03 | nd | nd |
| Del-3,5-diGlu | nd | 7.70 ± 0.12 b | nd | nd | 0.03 ± 0.01 a | nd |
| Del-3-Glu | nd | nd | nd | nd | 0.01 ± 0.01 | nd |
| Pel-3,5-diGlu | nd | 10.76 ± 0.17 | nd | nd | nd | nd |
| Total anthocyanins | nd | 32.94 ± 0.34 d | 3.73 ± 0.06 b | 4.91 ± 0.14 c | 0.14 ± 0.04 a | nd |
| Phenolic acids | ||||||
| GA | 11.29 ± 0.11 a | 14.91 ± 0.09 e | 12.61 ± 0.10 b | 13.53 ± 0.12 d | 16.28 ± 0.11 f | 12.95 ± 0.08 c |
| PA | 2.58 ± 0.04 a | 7.21 ± 0.06 f | 4.86 ± 0.05 d | 4.59 ± 0.06 c | 5.20 ± 0.07 e | 3.72 ± 0.05 b |
| p-HBA | 9.78 ± 0.10 b | 14.02 ± 0.12 f | 10.41 ± 0.08 c | 11.21 ± 0.11 d | 13.53 ± 0.10 e | 8.59 ± 0.07 a |
| Total phenolic acids | 23.65 ± 0.24 a | 36.14 ± 0.27 f | 27.88 ± 0.23 c | 29.33 ± 0.28 d | 35.01 ± 0.28 e | 25.26 ± 0.20 b |
| Total phenolics | 265.23 ± 1.02 a | 358.92 ± 1.32 f | 298.47 ± 0.87 c | 288.06 ± 0.94 b | 308.28 ± 0.95 d | 320.08 ± 0.82 e |
| DCH98Est | ||||||
| Flavan-3-ols | ||||||
| Cat | 27.73 ± 0.13 d | 26.90 ± 0.12 c | 25.92 ± 0.14 b | 25.62 ± 0.11 a | 25.62 ± 0.13 a | 27.97 ± 0.11 e |
| Ecat | 149.37 ± 0.49 d | 140.24 ± 0.40 b | 139.79 ± 0.35 a | 139.84 ± 0.47 a | 141.54 ± 0.21 b | 147.25 ± 0.31 c |
| PC B2 | 33.18 ± 0.17 a | 53.76 ± 0.16 e | 45.52 ± 0.15 c | 41.05 ± 0.14 b | 60.15 ± 0.21 f | 50.35 ± 0.16 d |
| PC C1 | 17.76 ± 0.15 b | 24.80 ± 0.11 e | 17.00 ± 0.10 a | 23.70 ± 0.12 d | 18.00 ± 0.13 c | 24.35 ± 0.10 d |
| Total flavan-3-ols | 228.04 ± 0.66 a | 245.70 ± 0.55 c | 228.23 ± 0.61 a | 230.21 ± 0.59 b | 245.31 ± 0.69 c | 249.92 ± 0.53 d |
| Anthocyanins | ||||||
| Cy-3-Glu | nd | nd | 3.74 ± 0.05 a | 4.38 ± 0.04 b | nd | nd |
| Cy-3-Rut | nd | nd | nd | 0.06 ± 0.02 | nd | nd |
| Cy-3,5-diGlu | nd | 10.30 ± 0.12 b | nd | nd | 0.10 ± 0.01 a | nd |
| Cy-3-Xyl | nd | 4.20 ± 0.09 b | nd | 0.17 ± 0.03 a | nd | nd |
| Cy-3-(6″-Mal-Glu) | nd | nd | nd | 0.32 ± 0.04 | nd | nd |
| Del-3,5-diGlu | nd | 7.71 ± 0.11 b | nd | nd | 0.03 ± 0.01 a | nd |
| Del-3-Glu | nd | nd | nd | nd | 0.01 ± 0.01 | nd |
| Pel-3,5-diGlu | nd | 10.78 ± 0.09 | nd | nd | nd | nd |
| Total anthocyanins | nd | 32.99 ± 0.35 d | 3.74 ± 0.05 b | 4.93 ± 0.13 c | 0.14 ± 0.03 a | nd |
| Phenolic acids | ||||||
| GA | 11.14 ± 0.08 c | 12.64 ± 0.10 e | 10.69 ± 0.09 a | 11.47 ± 0.11 d | 13.80 ± 0.09 f | 10.98 ± 0.09 b |
| PA | 2.16 ± 0.04 a | 6.11 ± 0.03 f | 4.12 ± 0.05 d | 3.89 ± 0.06 c | 4.41 ± 0.07 e | 3.15 ± 0.05 b |
| p-HBA | 10.70 ± 0.09 c | 11.89 ± 0.10 f | 11.74 ± 0.06 e | 9.50 ± 0.08 b | 11.47 ± 0.11 d | 7.29 ± 0.06 a |
| Total phenolic acids | 24.00 ± 0.21 b | 30.64 ± 0.23 f | 26.55 ± 0.22 d | 24.86 ± 0.24 c | 29.68 ± 0.19 e | 21.42 ± 0.20 a |
| Total phenolics | 252.04 ± 0.86 a | 309.33 ± 0.83 e | 258.52 ± 0.72 b | 260.00 ± 0.81 b | 275.13 ± 0.90 d | 271.34 ± 0.73 c |
| MCH | ||||||
| Flavan-3-ols | ||||||
| Cat | 4.44 ± 0.03 a | 5.54 ± 0.04 b | 11.83 ± 0.03 d | 11.35 ± 0.04 c | 12.01 ± 0.05 e | 14.65 ± 0.11 f |
| Ecat | 45.58 ± 0.16 a | 60.00 ± 0.17 e | 58.72 ± 0.18 d | 56.92 ± 0.18 b | 58.21 ± 0.21 c | 64.64 ± 0.16 f |
| PC B2 | 23.41 ± 0.12 a | 31.59 ± 0.11 e | 31.03 ± 0.10 d | 27.55 ± 0.13 b | 28.40 ± 0.11 c | 34.05 ± 0.14 f |
| PC C1 | 6.23 ± 0.05 c | 18.90 ± 0.04 f | 5.90 ± 0.07 b | 7.36 ± 0.06 d | 15.93 ± 0.07 e | 2.20 ± 0.05 a |
| Total flavan-3-ols | 79.66 ± 0.36 a | 116.03 ± 0.36 f | 107.48 ± 0.38 c | 103.18 ± 0.41 b | 114.55 ± 0.44 d | 115.54 ± 0.46 e |
| Anthocyanins | ||||||
| Cy-3-Glu | nd | nd | 3.77 ± 0.03 a | 4.40 ± 0.04 b | nd | nd |
| Cy-3-Rut | nd | nd | nd | 0.60 ± 0.02 | nd | nd |
| Cy-3,5-diGlu | nd | 10.38 ± 0.15 b | nd | nd | 0.10 ± 0.02 a | nd |
| Cy-3-Xyl | nd | 4.24 ± 0.04 b | nd | 0.17 ± 0.03 a | nd | nd |
| Cy-3-(6″-Mal-Glu) | nd | nd | nd | 0.32 ± 0.02 | nd | nd |
| Del-3,5-diGlu | nd | 7.77 ± 0.12 b | nd | nd | 0.03 ± 0.01 a | nd |
| Del-3-Glu | nd | nd | nd | nd | 0.01 ± 0.01 | nd |
| Pel-3,5-diGlu | nd | 10.08 ± 0.13 | nd | nd | nd | nd |
| Total anthocyanins | nd | 32.47 ± 0.24 d | 3.77 ± 0.03 b | 5.49 ± 0.11 c | 0.14 ± 0.04 a | nd |
| Phenolic acids | ||||||
| GA | 6.08 ± 0.10 a | 7.18 ± 0.11 b | 7.56 ± 0.12 c | 7.99 ± 0.09 d | 7.34 ± 0.13 b | 8.09 ± 0.05 d |
| PA | 1.52 ± 0.04 a | 2.04 ± 0.05 c | 1.87 ± 0.03 b | 2.00 ± 0.03 c | 1.81 ± 0.05 b | 2.53 ± 0.10 d |
| p-HBA | 1.98 ± 0.06 a | 2.96 ± 0.07 c | 3.05 ± 0.02 d | 3.01 ± 0.05 d | 2.81 ± 0.04 b | 3.92 ± 0.04 e |
| Total phenolic acids | 9.58 ± 0.20 a | 12.18 ± 0.23 b | 12.48 ± 0.17 b,c | 13.00 ± 0.17 c | 11.96 ± 0.22 b | 14.54 ± 0.19 d |
| Total phenolics | 89.24 ± 0.56 a | 160.68 ± 0.83 f | 123.73 ± 0.58 c | 121.67 ± 0.69 b | 126.65 ± 0.70 d | 130.08 ± 0.65 e |
| Volatile Compounds | Functional Enrichment | |||||
|---|---|---|---|---|---|---|
| CONT | BLUB | RASB | BLCB | POME | BEET | |
| DCH | ||||||
| Alcohols and Phenols | ||||||
| 2,3-Butanediol | 0.25 ± 0.04 a | 0.69 ± 0.05 e | 0.43 ± 0.03 c | 0.58 ± 0.06 d | 0.37 ± 0.08 b | 0.40 ± 0.02 c |
| 2-Phenylethanol | 0.03 ± 0.01 a | 0.11 ± 0.03 b | 0.04 ± 0.02 a | 0.04 ± 0.01 a | 0.03 ± 0.02 a | 0.03 ± 0.02 a |
| Aldehydes and Ketones | ||||||
| 2-Methylpropanal | 3.49 ± 0.03 c | 2.84 ± 0.06 a | 4.09 ± 0.04 e | 3.68 ± 0.02 d | 2.99 ± 0.02 b | 4.66 ± 0.04 f |
| Benzaldehyde | 18.91 ± 0.13 d | 12.74 ± 0.14 a | 19.93 ± 0.10 e | 17.55 ± 0.15 c | 15.50 ± 0.13 b | 22.39 ± 0.19 f |
| Butan-2-one | 0.47 ± 0.06 b | 0.54 ± 0.02 c | 0.45 ± 0.03 b | 0.56 ± 0.04 c | 0.46 ± 0.06 b | 0.40 ± 0.02 a |
| 3-Methylbutanal | 1.01 ± 0.07 e | 0.88 ± 0.09 d | 0.70 ± 0.06 b | 0.90 ± 0.04 d | 0.81 ± 0.03 c | 0.52 ± 0.04 a |
| 2,3-Pentanedione | 0.63 ± 0.04 a | 1.18 ± 0.10 e | 0.87 ± 0.04 c | 1.03 ± 0.04 d | 0.76 ± 0.04 b | 0.84 ± 0.05 c |
| Pentanal | 0.08 ± 0.02 a | 0.67 ± 0.06 c | 0.29 ± 0.04 b | 1.13 ± 0.04 d | 0.11 ± 0.04 a | 0.09 ± 0.04 a |
| (Z)-4-Heptenal | 0.08 ± 0.01 a | 0.10 ± 0.05 a | 0.08 ± 0.02 a | 0.06 ± 0.01 a | 0.08 ± 0.02 a | 0.07 ± 0.03 a |
| Octanal | 0.02 ± 0.01 a | 0.10 ± 0.06 b | 0.02 ± 0.01 a | 0.02 ± 0.01 a | 0.01 ± 0.01 a | 0.01 ± 0.01 a |
| Butanal | 0.04 ± 0.01 b | 0.08 ± 0.03 c | 0.03 ± 0.01 a | 0.03 ± 0.01 a | 0.03 ± 0.01 a | 0.01 ± 0.01 a |
| Nonan-2-one | 0.06 ± 0.03 | 0.06 ± 0.01 | 0.07 ± 0.01 | 0.05 ± 0.04 | 0.07 ± 0.01 | 0.07 ± 0.02 |
| (Z)-2-Nonenal | 0.10 ± 0.04 b | 0.08 ± 0.02 a | 0.07 ± 0.02 a | 0.07 ± 0.02 a | 0.11 ± 0.04 b | 0.06 ± 0.02 a |
| (E,E)-2,4-Nonadienal | 0.02 ± 0.01 a | 0.09 ± 0.02 c | 0.06 ± 0.04 b | 0.05 ± 0.04 b | 0.03 ± 0.01 a | 0.07 ± 0.01 b |
| (Z)-2-Decenal | 0.04 ± 0.01 a | 0.57 ± 0.10 c | 0.10 ± 0.02 b | 0.02 ± 0.01 a | 0.04 ± 0.01 a | 0.04 ± 0.01 a |
| Vanillin | 0.06 ± 0.02 | 0.06 ± 0.01 | 0.07 ± 0.02 | 0.06 ± 0.01 | 0.06 ± 0.02 | 0.07 ± 0.02 |
| Acids | ||||||
| Pentanoic acid | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.04 ± 0.02 | 0.02 ± 0.01 |
| Acetic acid | 60.86 ± 0.13 b | 69.34 ± 0.14 e | 60.13 ± 0.11 b | 62.73 ± 0.16 c | 66.99 ± 0.09 d | 57.29 ± 0.12 a |
| Phenylacetic acid | 0.49 ± 0.02 d | 0.40 ± 0.03 b | 0.46 ± 0.01 c | 0.30 ± 0.02 a | 0.51 ± 0.03 d | 0.50 ± 0.04 d |
| Furfurals | ||||||
| 2-Furfural | 2.17 ± 0.04 d | 1.11 ± 0.05 a | 1.77 ± 0.07 b | 1.14 ± 0.03 a | 1.92 ± 0.05 c | 1.92 ± 0.04 c |
| Pyrazines | ||||||
| 2,5-Dimethylpyrazine | 10.85 ± 0.12 | 7.54 ± 0.11 | 9.80 ± 0.10 | 9.47 ± 0.13 | 8.60 ± 0.11 | 10.01 ± 0.12 |
| Trimethylpyrazine | 0.08 ± 0.03 b | 0.07 ± 0.02 b | 0.05 ± 0.01 a | 0.06 ± 0.02 a,b | 0.05 ± 0.02 a | 0.04 ± 0.01 a |
| Tetramethylpyrazine | 0.06 ± 0.01 a | 0.15 ± 0.05 c | 0.07 ± 0.02 a,b | 0.09 ± 0.03 b | 0.06 ± 0.02 a | 0.05 ± 0.01 a |
| Esters | ||||||
| Ethyl octanoate | 0.03 ± 0.01 a | 0.05 ± 0.01 a | 0.07 ± 0.02 a,b | 0.07 ± 0.03 a,b | 0.07 ± 0.02 a,b | 0.08 ± 0.01 b |
| Phenylethylacetate | 0.13 ± 0.03 b | 0.31 ± 0.06 c | 0.16 ± 0.04 b | 0.07 ± 0.02 a | 0.13 ± 0.03 b | 0.16 ± 0.04 b |
| Lactones | ||||||
| γ-Nonalactone | nd | 0.08 ± 0.02 | 0.08 ± 0.02 | 0.11 ± 0.04 | 0.08 ± 0.03 | 0.10 ± 0.05 |
| Sulfur Compounds | ||||||
| Dimethyl trisulfide | 0.02 ± 0.01 a | 0.13 ± 0.03 c | 0.08 ± 0.01 b | 0.09 ± 0.02 b | 0.08 ± 0.03 b | 0.10 ± 0.04 b |
| DCH98S | ||||||
| Alcohols and Phenols | ||||||
| 2,3-Butanediol | 0.28 ± 0.05 a | 1.13 ± 0.03 e | 0.61 ± 0.04 c | 0.92 ± 0.07 d | 0.50 ± 0.05 b,c | 0.47 ± 0.03 b |
| 2-Phenylethanol | 0.06 ± 0.02 b | 0.19 ± 0.05 c | 0.05 ± 0.02 a,b | 0.06 ± 0.02 b | 0.03 ± 0.01 a | 0.03 ± 0.01 a |
| Aldehydes and Ketones | ||||||
| 2-Methylpropanal | 3.57 ± 0.07 c | 2.19 ± 0.06 a | 4.69 ± 0.08 e | 3.87 ± 0.03 d | 2.48 ± 0.07 b | 5.24 ± 0.08 f |
| Benzaldehyde | 17.34 ± 0.15 d | 6.58 ± 0.09 a | 20.95 ± 0.17 e | 16.18 ± 0.09 c | 12.09 ± 0.11 b | 24.13 ± 0.18 f |
| Butan-2-one | 0.35 ± 0.04 a | 0.60 ± 0.07 c | 0.43 ± 0.05 b | 0.65 ± 0.06 c | 0.46 ± 0.03 b | 0.36 ± 0.05 a |
| 3-Methylbutanal | 0.51 ± 0.03 c | 0.75 ± 0.05 e | 0.40 ± 0.06 b | 0.80 ± 0.05 e | 0.61 ± 0.04 d | 0.28 ± 0.03 a |
| 2,3-Pentanedione | 0.93 ± 0.07 b | 1.72 ± 0.08 e | 1.10 ± 0.05 c | 1.42 ± 0.09 d | 0.88 ± 0.08 a | 0.93 ± 0.07 b |
| Pentanal | 0.13 ± 0.06 b | 1.26 ± 0.07 d | 0.50 ± 0.03 c | 2.18 ± 0.04 e | 0.13 ± 0.03 b | 0.09 ± 0.03 a |
| (Z)-4-Heptenal | 0.06 ± 0.02 b | 0.11 ± 0.05 c | 0.07 ± 0.02 b | 0.04 ± 0.01 a | 0.07 ± 0.02 b | 0.07 ± 0.02 b |
| Octanal | 0.02 ± 0.01 a | 0.18 ± 0.03 b | 0.03 ± 0.01 a | 0.03 ± 0.01 a | 0.01 ± 0.01 a | 0.01 ± 0.01 a |
| Butanal | 0.02 ± 0.01 a | 0.13 ± 0.04 b | 0.02 ± 0.01 a | 0.03 ± 0.01 a | 0.03 ± 0.01 a | 0.01 ± 0.01 a |
| Nonan-2-one | 0.02 ± 0.01 a | 0.07 ± 0.02 b | 0.07 ± 0.01 b | 0.04 ± 0.02 a | 0.08 ± 0.02 b | 0.08 ± 0.03 b |
| (Z)-2-Nonenal | 0.02 ± 0.01 a | 0.06 ± 0.02 b | 0.04 ± 0.02 a,b | 0.02 ± 0.01 a | 0.12 ± 0.04 c | 0.03 ± 0.01 a |
| (E,E)-2,4-Nonadienal | 0.12 ± 0.05 c | 0.16 ± 0.06 d | 0.11 ± 0.03 b,c | 0.08 ± 0.02 b | 0.03 ± 0.01 a | 0.10 ± 0.03 b,c |
| (Z)-2-Decenal | 0.03 ± 0.01 a | 1.10 ± 0.04 d | 0.17 ± 0.05 c | 0.02 ± 0.01 a | 0.04 ± 0.01 a,b | 0.05 ± 0.02 b |
| Vanillin | 0.06 ± 0.02 | 0.06 ± 0.02 | 0.07 ± 0.02 | 0.06 ± 0.02 | 0.06 ± 0.01 | 0.07 ± 0.03 |
| Acids | ||||||
| Pentanoic acid | 0.02 ± 0.01 a | 0.01 ± 0.01 a | 0.02 ± 0.01 a | 0.02 ± 0.01 a | 0.06 ± 0.02 b | 0.03 ± 0.01 a |
| Acetic acid | 67.02 ± 0.12 d | 77.90 ± 0.15 f | 59.48 ± 0.11 b | 64.68 ± 0.10 c | 73.20 ± 0.13 e | 55.54 ± 0.16 a |
| Phenylacetic acid | 0.02 ± 0.01 a | 0.32 ± 0.05 c | 0.43 ± 0.04 d | 0.10 ± 0.03 b | 0.53 ± 0.07 e | 0.50 ± 0.06 e |
| Furfurals | ||||||
| 2-Furfural | 1.63 ± 0.04 d | 0.05 ± 0.01 a | 1.36 ± 0.09 c | 0.11 ± 0.03 b | 1.68 ± 0.07 d | 1.79 ± 0.05 e |
| Pyrazines | ||||||
| 2,5-Dimethylpyrazine | 7.60 ± 0.10 c | 4.14 ± 0.09 a | 8.67 ± 0.07 e | 8.01 ± 0.08 d | 6.28 ± 0.11 b | 9.54 ± 0.14 f |
| Trimethylpyrazine | 0.02 ± 0.01 a | 0.06 ± 0.02 b | 0.03 ± 0.01 a | 0.04 ± 0.02 a | 0.03 ± 0.01 a | 0.03 ± 0.01 a |
| Tetramethylpyrazine | 0.06 ± 0.02 a | 0.25 ± 0.06 d | 0.09 ± 0.03 b | 0.13 ± 0.04 c | 0.06 ± 0.02 a | 0.05 ± 0.02 a |
| Esters | ||||||
| Ethyl octanoate | 0.03 ± 0.01 a | 0.08 ± 0.02 b | 0.10 ± 0.03 c | 0.12 ± 0.04 c | 0.11 ± 0.03 c | 0.11 ± 0.02 c |
| Phenylethylacetate | 0.02 ± 0.01 a | 0.49 ± 0.07 d | 0.19 ± 0.05 c | 0.02 ± 0.01 a | 0.13 ± 0.03 b | 0.17 ± 0.05 c |
| Lactones | ||||||
| γ-Nonalactone | nd | 0.16 ± 0.03 a | 0.16 ± 0.05 a | 0.21 ± 0.07 b | 0.16 ± 0.05 a | 0.15 ± 0.03 a |
| Sulfur Compounds | ||||||
| Dimethyl trisulfide | 0.08 ± 0.03 a | 0.25 ± 0.06 c | 0.15 ± 0.02 b | 0.16 ± 0.03 b | 0.15 ± 0.04 b | 0.13 ± 0.02 b |
| DCH98ESt | ||||||
| Alcohols and Phenols | ||||||
| 2,3-Butanediol | 0.29 ± 0.05 a | 0.71 ± 0.06 d | 0.53 ± 0.04 b | 0.60 ± 0.03 c | 0.51 ± 0.03 b | 0.50 ± 0.04 b |
| 2-Phenylethanol | 0.06 ± 0.02 b | 0.12 ± 0.03 c | 0.04 ± 0.02 a | 0.06 ± 0.02 b | 0.04 ± 0.01 a | 0.03 ± 0.01 a |
| Aldehydes And Ketones | ||||||
| 2-Methylpropanal | 2.78 ± 0.04 b | 2.48 ± 0.04 a | 4.14 ± 0.06 d | 3.32 ± 0.04 c | 3.31 ± 0.05 c | 4.69 ± 0.05 e |
| Benzaldehyde | 17.67 ± 0.11 d | 12.12 ± 0.10 a | 19.05 ± 0.09 e | 16.93 ± 0.11 c | 15.57 ± 0.12 b | 21.59 ± 0.12 f |
| Butan-2-one | 0.31 ± 0.02 a | 0.46 ± 0.03 c | 0.42 ± 0.04 b | 0.48 ± 0.04 c | 0.44 ± 0.05 b,c | 0.39 ± 0.04 b |
| 3-Methylbutanal | 0.43 ± 0.03 b | 0.59 ± 0.03 d | 0.43 ± 0.03 b | 0.61 ± 0.04 d | 0.52 ± 0.05 c | 0.35 ± 0.03 a |
| 2,3-Pentanedione | 1.48 ± 0.03 c | 1.60 ± 0.04 d | 0.98 ± 0.03 b | 1.45 ± 0.04 c | 0.93 ± 0.04 a | 0.95 ± 0.04 a,b |
| Pentanal | 1.59 ± 0.05 d | 1.42 ± 0.04 c | 0.24 ± 0.03 b | 1.89 ± 0.05 e | 0.18 ± 0.04 a | 0.17 ± 0.03 a |
| (Z)-4-Heptenal | 0.04 ± 0.01 a | 0.08 ± 0.04 b | 0.07 ± 0.04 b | 0.04 ± 0.01 a | 0.07 ± 0.04 b | 0.07 ± 0.02 b |
| Octanal | 0.02 ± 0.01 a | 0.10 ± 0.02 b | 0.01 ± 0.01 a | 0.02 ± 0.01 a | 0.01 ± 0.01 a | 0.01 ± 0.01 a |
| Butanal | 0.02 ± 0.01 a | 0.08 ± 0.02 b | 0.02 ± 0.01 a | 0.03 ± 0.01 a | 0.02 ± 0.01 a | 0.01 ± 0.01 a |
| Nonan-2-one | 0.02 ± 0.01 a | 0.04 ± 0.01 a | 0.08 ± 0.01 b | 0.03 ± 0.01 a | 0.08 ± 0.01 b | 0.08 ± 0.02 b |
| (Z)-2-Nonenal | 0.02 ± 0.01 a | 0.04 ± 0.01 a,b | 0.06 ± 0.01 a | 0.03 ± 0.01a | 0.09 ± 0.02 c | 0.05 ± 0.01 a |
| (E,E)-2,4-Nonadienal | 0.03 ± 0.01 a | 0.10 ± 0.03 b | 0.08 ± 0.02 b | 0.05 ± 0.02 a | 0.05 ± 0.01 a | 0.09 ± 0.01 b |
| (Z)-2-Decenal | 0.04 ± 0.01 a | 0.57 ± 0.05 c | 0.09 ± 0.02 b | 0.02 ± 0.01 a | 0.07 ± 0.02 b | 0.07 ± 0.02 b |
| Vanillin | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.07 ± 0.02 | 0.06 ± 0.02 | 0.06 ± 0.01 | 0.07 ± 0.01 |
| Acids | ||||||
| Pentanoic acid | 0.02 ± 0.01 a | 0.01 ± 0.01 a | 0.04 ± 0.01 a,b | 0.01 ± 0.01 a | 0.05 ± 0.01 b | 0.03 ± 0.01 a |
| Acetic acid | 67.46 ± 0.14 d | 72.69 ± 0.15 e | 62.74 ± 0.17 b | 66.08 ± 0.15 c | 67.97 ± 0.17 d | 59.14 ± 0.16 a |
| Phenylacetic acid | 0.03 ± 0.01 a | 0.17 ± 0.03 b | 0.49 ± 0.04 c | 0.07 ± 0.01 b | 0.51 ± 0.06 c | 0.49 ± 0.05 c |
| Furfurals | ||||||
| 2-Furfural | 0.06 ± 0.01 a | 0.05 ± 0.01 a | 1.61 ± 0.06 b | 0.08 ± 0.02 a | 1.64 ± 0.05 b | 1.70 ± 0.08 c |
| Pyrazines | ||||||
| 2,5-Dimethylpyrazine | 7.38 ± 0.13 b | 5.69 ± 0.12 a | 8.16 ± 0.14 d | 7.62 ± 0.12 c | 7.22 ± 0.12 b | 8.85 ± 0.13 e |
| Trimethylpyrazine | 0.02 ± 0.01 | 0.04 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 |
| Tetramethylpyrazine | 0.06 ± 0.01 a | 0.15 ± 0.03 c | 0.07 ± 0.02 a | 0.09 ± 0.01 a,b | 0.06 ± 0.01 a | 0.06 ± 0.01 a |
| Esters | ||||||
| Ethyl octanoate | 0.02 ± 0.01 a | 0.05 ± 0.01 b | 0.11 ± 0.03 c | 0.07 ± 0.01 b | 0.11 ± 0.02 c | 0.11 ± 0.01 c |
| Phenylethylacetate | 0.03 ± 0.01 a | 0.26 ± 0.07 c | 0.16 ± 0.03 b | 0.02 ± 0.01 a | 0.15 ± 0.03 b | 0.17 ± 0.04 b |
| Lactones | ||||||
| γ-Nonalactone | nd | 0.15 ± 0.02 | 0.16 ± 0.03 | 0.18 ± 0.04 | 0.16 ± 0.03 | 0.15 ± 0.03 |
| Sulfur Compounds | ||||||
| Dimethyl trisulfide | 0.07 ± 0.02 a | 0.16 ± 0.03 b | 0.14 ± 0.03 b | 0.12 ± 0.02 b | 0.15 ± 0.03 b | 0.14 ± 0.02 b |
| MCH | ||||||
| Alcohols and Phenols | ||||||
| 2,3-Butanediol | 0.29 ± 0.03 a | 0.79 ± 0.06 e | 0.53 ± 0.06 d | 0.58 ± 0.08 d | 0.36 ± 0.04 b | 0.41 ± 0.03 c |
| 2-Phenylethanol | 0.03 ± 0.01 a | 0.10 ± 0.02 b | 0.05 ± 0.01 a | 0.04 ± 0.01 a | 0.03 ± 0.01 a | 0.03 ± 0.01 a |
| Aldehydes and Ketones | ||||||
| 2-Methylpropanal | 3.42 ± 0.07 b | 2.83 ± 0.05 a | 4.37 ± 0.07 c | 3.41 ± 0.06 b | 3.10 ± 0.04 b | 4.63 ± 0.07 d |
| Benzaldehyde | 17.96 ± 0.10 c | 11.88 ± 0.08 a | 20.20 ± 0.12 d | 16.08 ± 0.11 b | 16.00 ± 0.09 b | 22.07 ± 0.10 e |
| Butan-2-one | 0.45 ± 0.04 b | 0.55 ± 0.05 c | 0.43 ± 0.05 b | 0.53 ± 0.06 c | 0.45 ± 0.04 b | 0.39 ± 0.03 a |
| 3-Methylbutanal | 0.69 ± 0.05 c,d | 0.72 ± 0.06 d | 0.47 ± 0.05 b | 0.71 ± 0.07 d | 0.66 ± 0.07 c | 0.41 ± 0.05 a |
| 2,3-Pentanedione | 1.22 ± 0.06 c | 1.40 ± 0.07 d | 1.13 ± 0.06 b | 1.24 ± 0.07 c | 1.11 ± 0.06 b | 1.04 ± 0.05 a |
| Pentanal | 0.30 ± 0.02 c | 1.03 ± 0.05 e | 0.45 ± 0.03 d | 1.02 ± 0.06 e | 0.24 ± 0.03 b | 0.16 ± 0.03 a |
| (Z)-4-Heptenal | 0.11 ± 0.03 b | 0.09 ± 0.03 a | 0.08 ± 0.02 a | 0.07 ± 0.04 a | 0.09 ± 0.03 a | 0.08 ± 0.02 a |
| Octanal | 0.02 ± 0.01 a | 0.08 ± 0.04 b | 0.02 ± 0.01 a | 0.02 ± 0.01 a | 0.01 ± 0.01 a | 0.01 ± 0.01 a |
| Butanal | 0.03 ± 0.01 a | 0.07 ± 0.04 b | 0.02 ± 0.01 a | 0.03 ± 0.01 a | 0.03 ± 0.01 a | 0.01 ± 0.01 a |
| Nonan-2-one | 0.06 ± 0.03 | 0.06 ± 0.03 | 0.07 ± 0.03 | 0.06 ± 0.04 | 0.07 ± 0.04 | 0.07 ± 0.03 |
| (Z)-2-Nonenal | 0.11 ± 0.04 c | 0.08 ± 0.03 a,b | 0.06 ± 0.03 a | 0.08 ± 0.04 a,b | 0.11 ± 0.04 c | 0.06 ± 0.03 a |
| (E,E)-2,4-Nonadienal | 0.02 ± 0.01 a | 0.09 ± 0.04 b | 0.08 ± 0.03 b | 0.04 ± 0.01 a | 0.02 ± 0.01 a | 0.07 ± 0.03 b |
| (Z)-2-Decenal | 0.15 ± 0.05 b | 0.48 ± 0.07 c | 0.17 ± 0.04 b | 0.07 ± 0.02 a | 0.11 ± 0.06 a | 0.08 ± 0.03 a |
| Vanillin | 0.06 ± 0.02 | 0.06 ± 0.02 | 0.07 ± 0.04 | 0.06 ± 0.04 | 0.06 ± 0.03 | 0.07 ± 0.04 |
| Acids | ||||||
| Pentanoic acid | 0.05 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.04 ± 0.02 | 0.05 ± 0.03 | 0.04 ± 0.02 |
| Acetic acid | 64.84 ± 0.17 b | 71.71 ± 0.19 e | 60.82 ± 0.15 a | 66.45 ± 0.16 c | 67.64 ± 0.15 d | 58.64 ± 0.12 a |
| Phenylacetic acid | 0.15 ± 0.04 a | 0.29 ± 0.04 c | 0.36 ± 0.05 b | 0.21 ± 0.04 b | 0.28 ± 0.04 c | 0.38 ± 0.05 b |
| Furfurals | ||||||
| 2-Furfural | 1.93 ± 0.08 e | 0.76 ± 0.07 a | 1.51 ± 0.08 c | 1.15 ± 0.07 b | 1.85 ± 0.09 d | 1.84 ± 0.04 d |
| Pyrazines | ||||||
| 2,5-Dimethylpyrazine | 7.44 ± 0.12 c | 6.00 ± 0.11 a | 8.37 ± 0.11 d | 7.44 ± 0.10 c | 7.06 ± 0.09 b | 8.83 ± 0.12 e |
| Trimethylpyrazine | 0.02 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 |
| Tetramethylpyrazine | 0.06 ± 0.01 a | 0.16 ± 0.03 b | 0.08 ± 0.01 a | 0.08 ± 0.02 a | 0.06 ± 0.02 a | 0.05 ± 0.01 a |
| Esters | ||||||
| Ethyl octanoate | 0.03 ± 0.01 | 0.08 ± 0.01 b | 0.08 ± 0.02 b | 0.08 ± 0.02 b | 0.05 ± 0.01 a | 0.08 ± 0.01 b |
| Phenylethylacetate | 0.49 ± 0.05 d | 0.32 ± 0.04 b,c | 0.26 ± 0.04 b | 0.22 ± 0.04 a | 0.37 ± 0.05 c | 0.28 ± 0.04 b |
| Lactones | ||||||
| γ-Nonalactone | nd | 0.14 ± 0.03 b | 0.12 ± 0.02 b | 0.12 ± 0.01 b | 0.05 ± 0.01 a | 0.11 ± 0.02 b |
| Sulfur Compounds | ||||||
| Dimethyl trisulfide | 0.08 ± 0.01 a | 0.18 ± 0.02 c | 0.13 ± 0.03 b | 0.13 ± 0.02 b | 0.10 ± 0.01 a,b | 0.12 ± 0.02 b |
| Raw Material | Content (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Control DCH | DCH | Control DCH98S | DCH98S | Control DCH98ESt | DCH98ESt | Control MCH | MCH | |
| Cocoa liquor | 40.00 | 40.00 | 92.00 | 92.00 | 92.00 | 92.00 | 20.00 | 20.00 |
| Milk powder | - | - | - | - | - | - | 20.00 | 20.00 |
| Cocoa butter | 13.40 | 13.40 | 0.80 | 0.80 | 0.80 | 0.80 | 19.80 | 19.80 |
| Alkalized cocoa powder | - | - | 5.00 | 5.00 | 5.00 | 5.00 | - | - |
| Lecithin | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| PGPR | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Ethyl vanillin | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Sugar (sucrose) | 45.59 | 44.59 | 1.19 | 0.19 | - | - | 39.19 | 38.19 |
| Erythritol+stevia | - | - | - | - | 1.19 | 0.19 | - | - |
| Lyophilizate of fruits or vegetables | - | 1.00 | - | 1.00 | - | 1.00 | - | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żyżelewicz, D.; Oracz, J.; Bilicka, M.; Kulbat-Warycha, K.; Klewicka, E. Influence of Freeze-Dried Phenolic-Rich Plant Powders on the Bioactive Compounds Profile, Antioxidant Activity and Aroma of Different Types of Chocolates. Molecules 2021, 26, 7058. https://doi.org/10.3390/molecules26227058
Żyżelewicz D, Oracz J, Bilicka M, Kulbat-Warycha K, Klewicka E. Influence of Freeze-Dried Phenolic-Rich Plant Powders on the Bioactive Compounds Profile, Antioxidant Activity and Aroma of Different Types of Chocolates. Molecules. 2021; 26(22):7058. https://doi.org/10.3390/molecules26227058
Chicago/Turabian StyleŻyżelewicz, Dorota, Joanna Oracz, Martyna Bilicka, Kamila Kulbat-Warycha, and Elżbieta Klewicka. 2021. "Influence of Freeze-Dried Phenolic-Rich Plant Powders on the Bioactive Compounds Profile, Antioxidant Activity and Aroma of Different Types of Chocolates" Molecules 26, no. 22: 7058. https://doi.org/10.3390/molecules26227058
APA StyleŻyżelewicz, D., Oracz, J., Bilicka, M., Kulbat-Warycha, K., & Klewicka, E. (2021). Influence of Freeze-Dried Phenolic-Rich Plant Powders on the Bioactive Compounds Profile, Antioxidant Activity and Aroma of Different Types of Chocolates. Molecules, 26(22), 7058. https://doi.org/10.3390/molecules26227058