Carbon Nanotubes Hybrid Hydrogels for Environmental Remediation: Evaluation of Adsorption Efficiency under Electric Field
Abstract
:1. Introduction
2. Results and Discussion
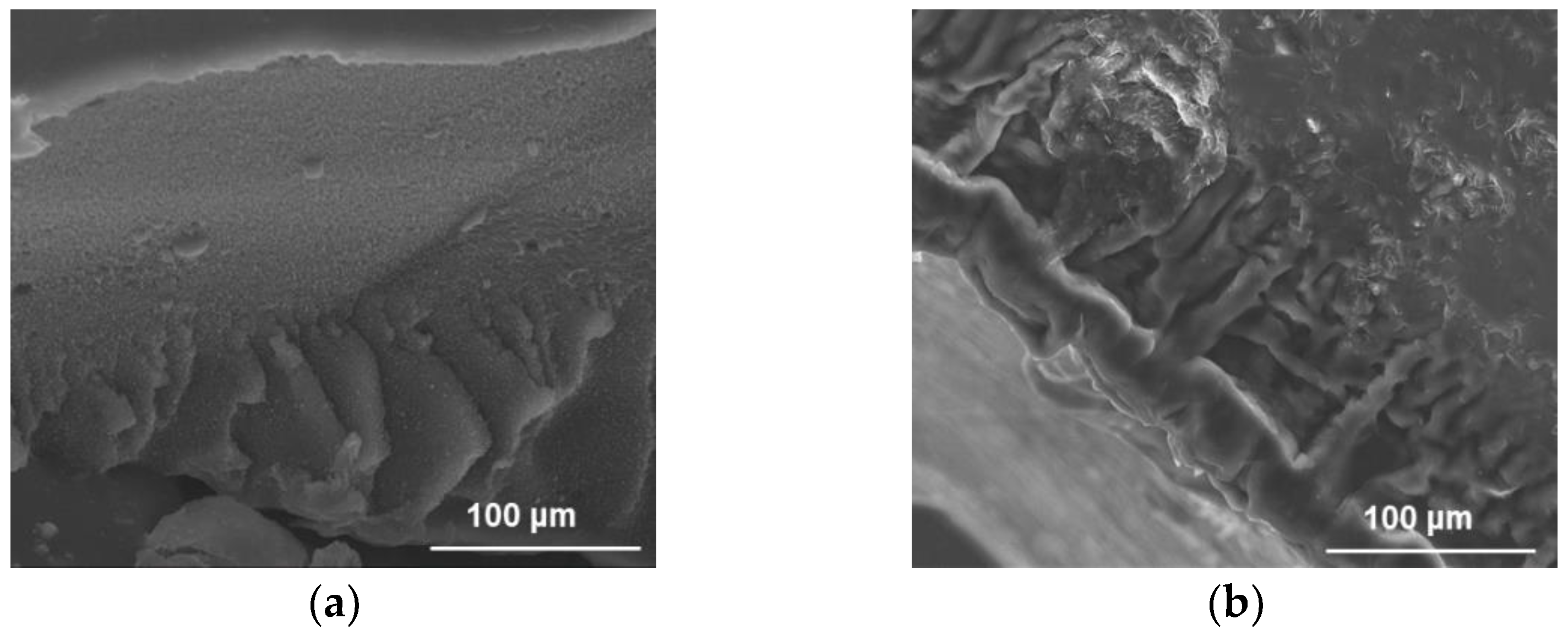
2.1. Synthesis and Characterization of Hybrid Hydrogels
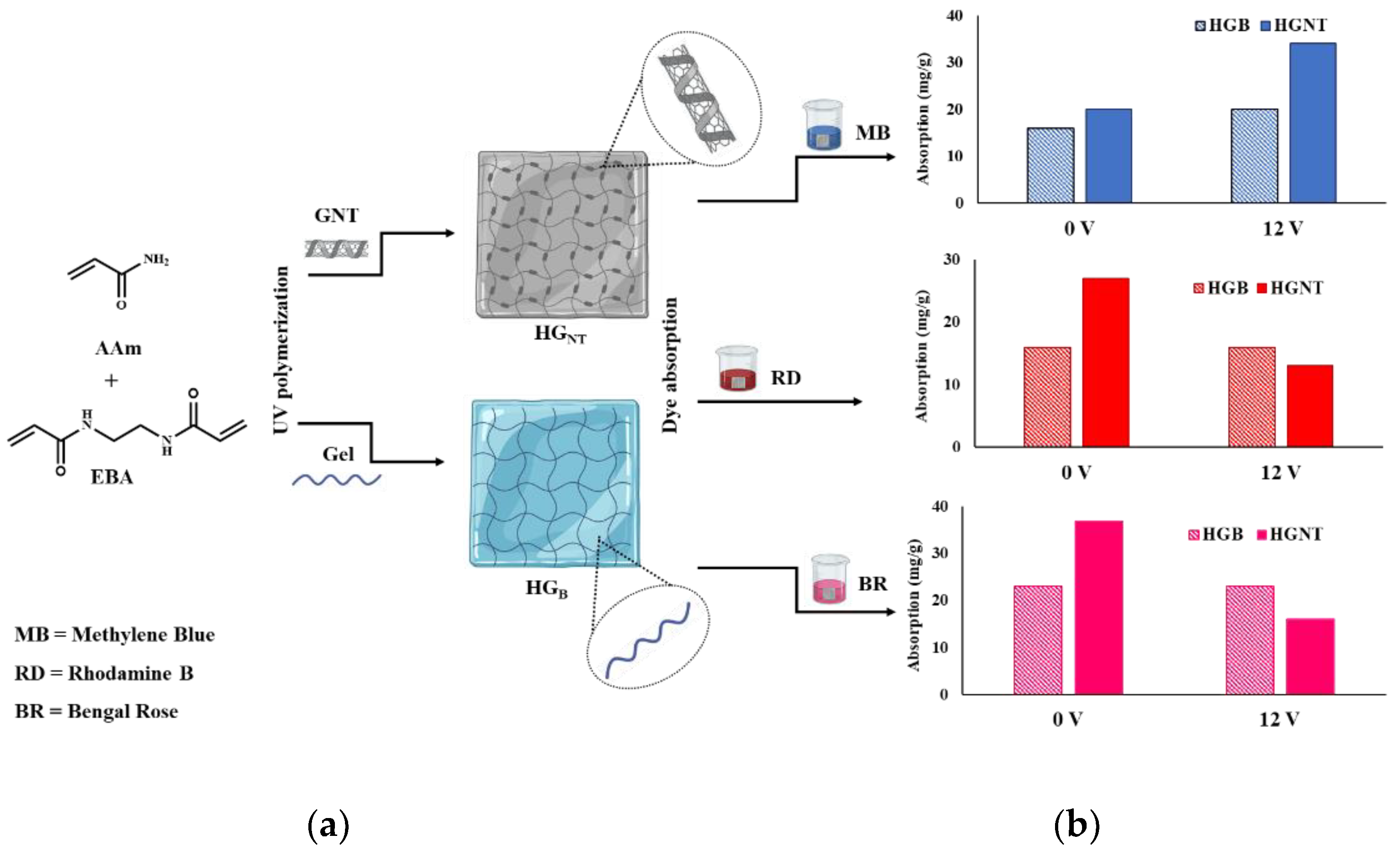
2.2. Sorption Properties of Hybrid Hydrogels
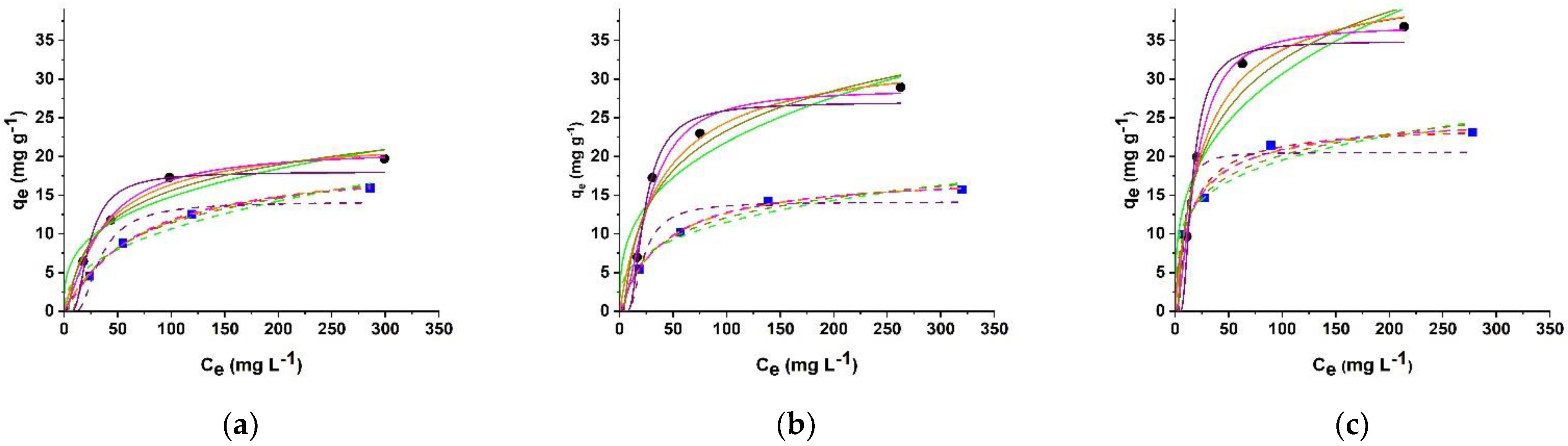
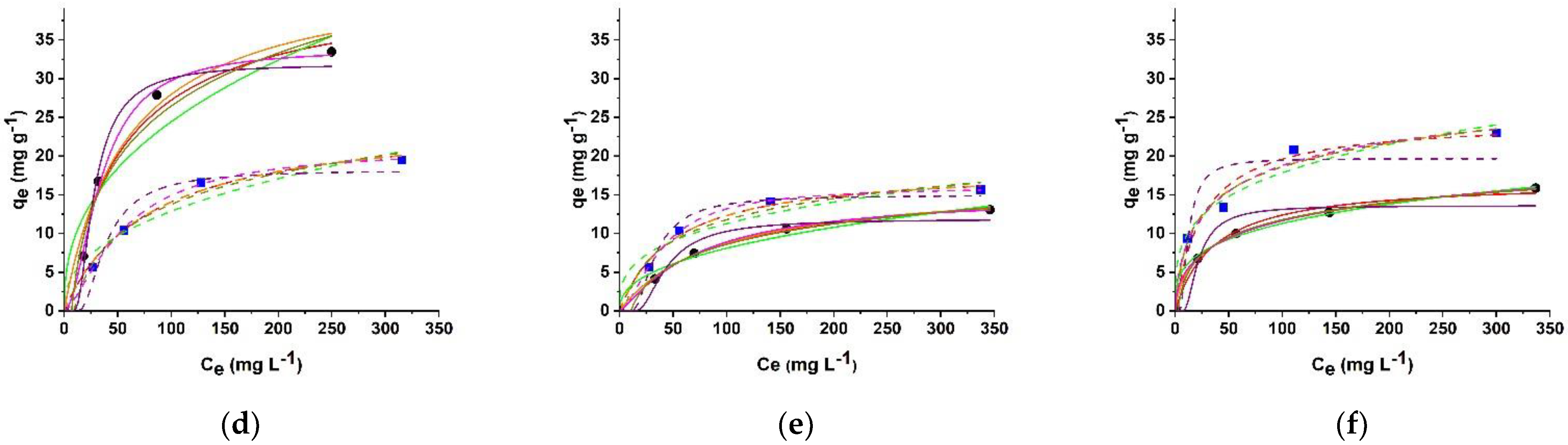
2.3. Isotherm Data Analysis
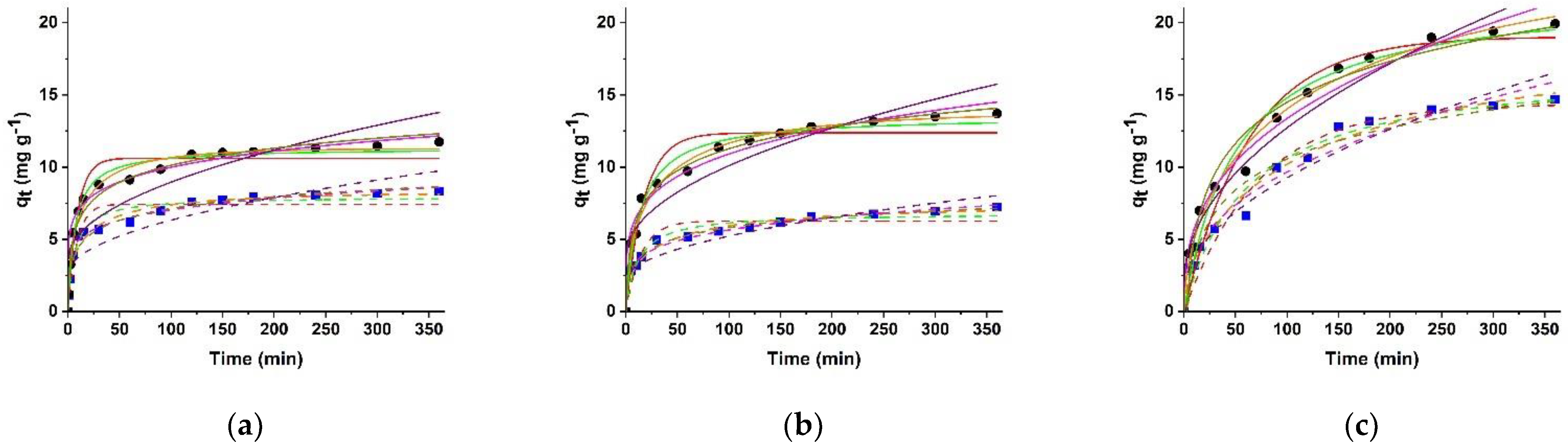
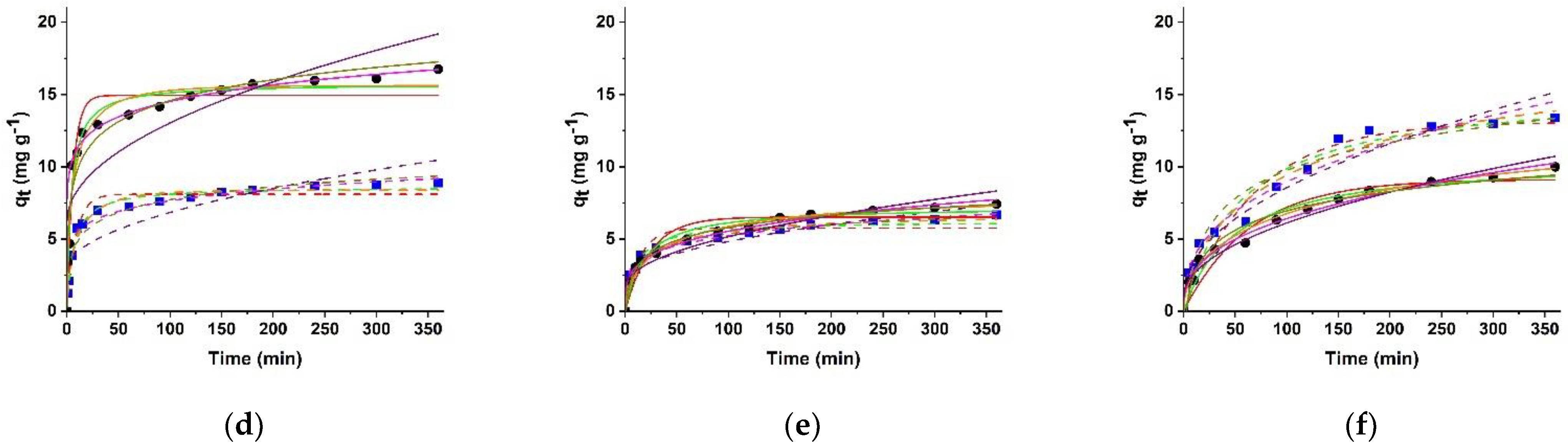
2.4. Kinetic Data Analysis
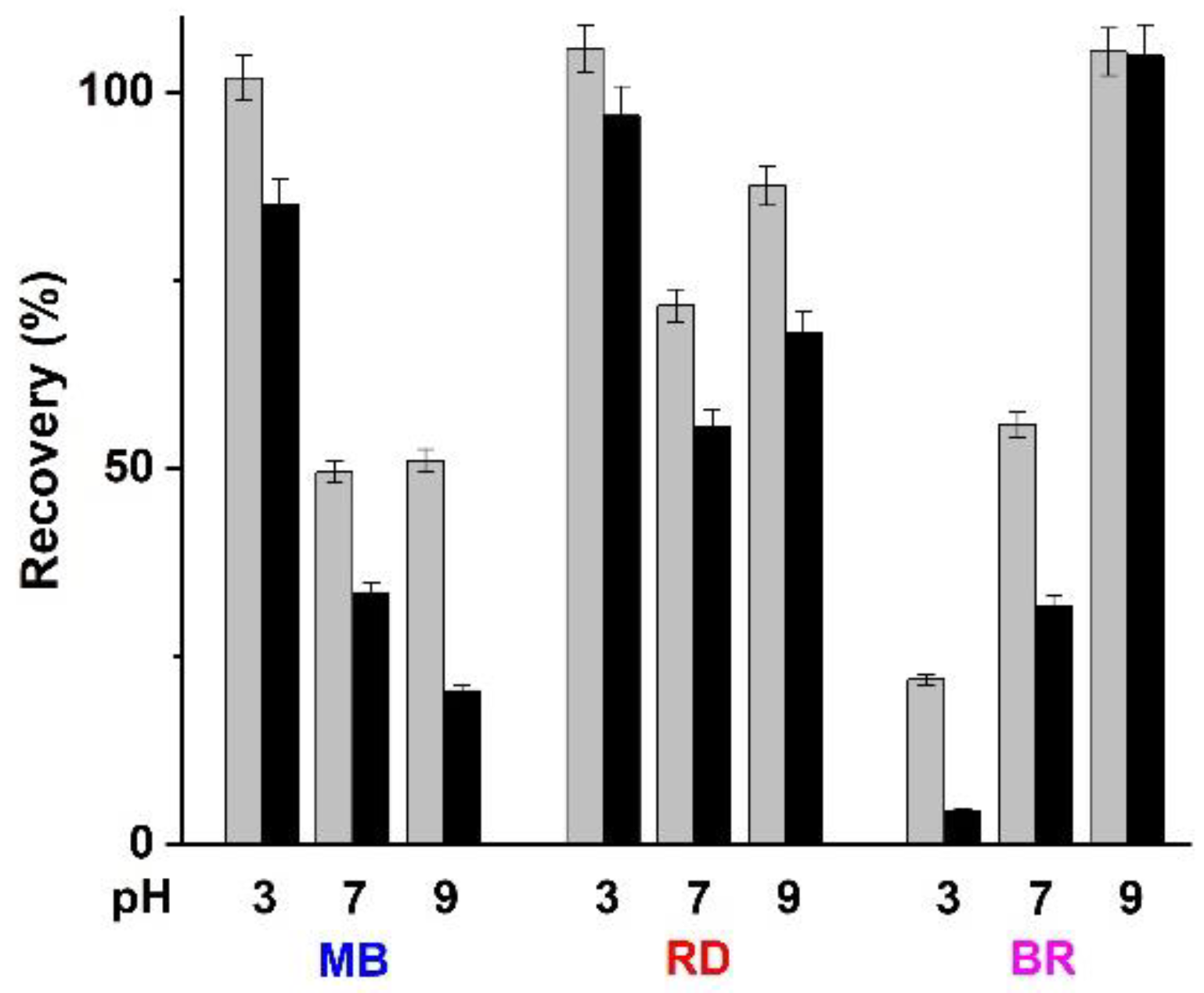
2.5. Desorption Studies
3. Materials and Methods
3.1. Synthesis of MWCNT and HGNT
3.2. Characterization Procedure
3.3. Batch Kinetics and Equilibrium Adsorption Studies
- (i)
- isothermal studies: 4.0 mg mL−1 HGNT, 0.05–0.4 mg mL−1 dye solution, contact time 1440 min, temperature 20 °C
- (ii)
- kinetics studies: 4.0 mg mL−1 HGNT, 0.1 mg mL−1 dye solution, contact time 15–1440 min, temperature 20 °C
- (iii)
- thermodynamics studies: 4.0 mg mL−1 HGNT, 0.1 mg mL−1 dye solution, contact time 1440 min, temperature range 4–50 °C
3.4. Hydrogel Regeneration
3.5. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goncalves, J.O.; da Silva, K.A.; Rios, E.C.; Crispim, M.M.; Dotto, G.L.; Pinto, L.A.D. Chitosan Hydrogel Scaffold Modified with Carbon Nanotubes and Its Application for Food Dyes Removal in Single and Binary Aqueous Systems. Int. J. Biol. Macromol. 2020, 142, 85–93. [Google Scholar] [CrossRef]
- Yao, Y.J.; Xu, F.F.; Chen, M.; Xu, Z.X.; Zhu, W. Adsorption Behavior of Methylene Blue on Carbon Nanotubes. Bioresour. Technol. 2010, 101, 3040–3046. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Goswami, A.; Nain, S. Enhanced Antibacterial Activity and Photo-Remediation of Toxic Dyes Using Ag/Swcnt/Ppy Based Nanocomposite with Core-Shell Structure. Appl. Nanosci. 2020, 10, 2255–2568. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, X.; Zhu, J.L.; Bai, J.H.; Gao, L.L.; Liu, S.F.; Jiao, T.F. Facile Preparation of Self-Assembled Chitosan-Based Composite Hydrogels with Enhanced Adsorption Performances. Colloids Surf. A-Physicochem. Eng. Asp. 2020, 598, 124860. [Google Scholar] [CrossRef]
- Gita, S.; Shukla, S.P.; Deshmukhe, G.; Choudhury, T.G.; Saharan, N.; Singh, A.K. Toxicity Evaluation of Six Textile Dyes on Growth, Metabolism and Elemental Composition (C,H,N,S) of Microalgae Spirulina Platensis: The Environmental Consequences. Bull. Environ. Contam. Toxicol. 2021, 106, 302–309. [Google Scholar] [CrossRef]
- Verma, Y. Acute Toxicity Assessment of Textile Dyes and Textile and Dye Industrial Effluents Using Daphnia Magna Bioassay. Toxicol. Ind. Health 2008, 24, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Slama, B.H.; Bouket, A.C.; Pourhassan, Z.; Alenezi, F.N.; Silini, A.; Cherif-Silini, H.; Oszako, T.; Luptakova, L.; Golinska, P.; Belbahri, L. Diversity of Synthetic Dyes from Textile Industries, Discharge Impacts and Treatment Methods. Appl. Sci. 2021, 11, 6255. [Google Scholar] [CrossRef]
- Yu, G.J.; Zhao, X.H.; Yang, H.; Chen, X.H.; Yang, Q.; Yu, L.Y.; Jiang, J.H.; Chen, X.Q. Aqueous Adsorption and Removal of Organic Contaminants by Carbon Nanotubes. Sci. Total Environ. 2014, 482, 241–251. [Google Scholar] [CrossRef]
- Zhu, M.; Miao, J.; Guan, D.Q.; Zhong, Y.J.; Ran, R.; Wang, S.B.; Zhou, W.; Shao, Z.P. Efficient Wastewater Remediation Enabled by Self-Assembled Perovskite Oxide Heterostructures with Multiple Reaction Pathways. ACS Sustain. Chem. Eng. 2020, 8, 6033–6042. [Google Scholar] [CrossRef]
- Aditya, D.; Rohan, P.; Suresh, G. Nano-Adsorbents for Wastewater Treatment: A Review. Res. J. Chem. Environ. 2011, 15, 1033–1040. [Google Scholar]
- Akter, M.; Bhattacharjee, M.; Dhar, A.K.; Rahman, F.B.; Haque, S.; Rashid, T.U.; Kabir, S.M.F. Cellulose-Based Hydrogels for Wastewater Treatment: A Concise Review. Gels 2021, 7, 30. [Google Scholar] [CrossRef]
- Liu, J.; Su, D.H.; Yao, J.R.; Huang, Y.F.; Shao, Z.Z.; Chen, X. Soy Protein-Based Polyethylenimine Hydrogel and Its High Selectivity for Copper Ion Removal in Wastewater Treatment. J. Mater. Chem. A 2017, 5, 4163–4171. [Google Scholar] [CrossRef]
- Tanan, W.; Panichpakdee, J.; Saengsuwan, S. Novel Biodegradable Hydrogel Based on Natural Polymers: Synthesis, Characterization, Swelling/Reswelling and Biodegradability. Eur. Polym. J. 2019, 112, 678–687. [Google Scholar] [CrossRef]
- Tran, V.V.; Park, D.; Lee, Y.C. Hydrogel Applications for Adsorption of Contaminants in Water and Wastewater Treatment. Environ. Sci. Pollut. Res. 2018, 25, 24569–24599. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Wang, X.M.; Zhao, L.L.; Li, M.; Yang, W. Effective Removal of Dyes from Aqueous Solutions by a Gelatin Hydrogel. J. Polym. Environ. 2021, 29, 3497–3508. [Google Scholar] [CrossRef]
- Shafiee, M.R.N.; Parhizkar, J.; Radfar, S. Removal of Rhodamine B by G-C3n4/Co3o4/Mwcnt Composite Stabilized in Hydrogel Via the Synergy of Adsorption and Photocatalysis under Visible Light. J. Mater. Sci.-Mater. Electron. 2019, 30, 12475–12486. [Google Scholar] [CrossRef]
- Mansoori, S.; Davarnejad, R.; Matsuura, T.; Ismail, A.F. Membranes Based on Non-Synthetic (Natural) Polymers for Wastewater Treatment. Polym. Test. 2020, 84, 106381. [Google Scholar] [CrossRef]
- Hu, S.X.; Liang, R.; Li, J.; Liu, Z.P.; Sun, G.X. Mechanically Strong Hydrogels Achieved by Designing Homogeneous Network Structure. Mater. Des. 2019, 163, 107547. [Google Scholar] [CrossRef]
- Sinha, V.; Chakma, S. Advances in the Preparation of Hydrogel for Wastewater Treatment: A Concise Review. J. Environ. Chem. Eng. 2019, 7, 103295. [Google Scholar] [CrossRef]
- Jing, H.G.; Wang, L.; Yu, H.J.; Amer, W.A.; Zhang, L. Recent Progress on Study of Hybrid Hydrogels for Water Treatment. Colloids Surf. A-Physicochem. Eng. Asp. 2013, 416, 86–94. [Google Scholar] [CrossRef]
- Dehne, H.; Hecht, F.M.; Bausch, A.R. The Mechanical Properties of Polymer-Colloid Hybrid Hydrogels. Soft Matter 2017, 13, 4786–4790. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Ji, J.; Cai, Y.F.; Li, H.; Ran, R. Robust, Anti-Fatigue, and Self-Healing Graphene Oxide/Hydrophobically Associated Composite Hydrogels and Their Use as Recyclable Adsorbents for Dye Wastewater Treatment. J. Mater. Chem. A 2015, 3, 17445–17458. [Google Scholar] [CrossRef]
- Subhan, H.; Alam, S.; Shah, L.A.; Ali, M.W.; Farooq, M. Sodium Alginate Grafted Poly(N-Vinyl Formamide-Co-Acrylic Acid)-Bentonite Clay Hybrid Hydrogel for Sorptive Removal of Methylene Green from Wastewater. Colloids Surf. A-Physicochem. Eng. Asp. 2021, 611, 125853. [Google Scholar] [CrossRef]
- Bahgat, M.; Farghali, A.A.; el Rouby, W.M.A.; Khedr, M.H. Synthesis and Modification of Multi-Walled Carbon Nano-Tubes (Mwcnts) for Water Treatment Applications. J. Anal. Appl. Pyrolysis 2011, 92, 307–313. [Google Scholar] [CrossRef]
- Hosseinzadeh, H. Synthesis of Carrageenan/Multi-Walled Carbon Nanotube Hybrid Hydrogel Nanocomposite for Adsorption of Crystal Violet from Aqueous Solution. Pol. J. Chem. Technol. 2015, 17, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Navrotskaya, G.A.; Aleksandrova, D.D.; Krivoshapkina, E.F.; Sillanpaa, M.; Krivoshapkin, P.V. Hybrid Materials Based on Carbon Nanotubes and Nanofibers for Environmental Applications. Front. Chem. 2020, 8, 546. [Google Scholar] [CrossRef] [PubMed]
- Makhado, E.; Hato, M.J. Preparation and Characterization of Sodium Alginate-Based Oxidized Multi-Walled Carbon Nanotubes Hydrogel Nanocomposite and Its Adsorption Behavior for Methylene Blue Dye. Front. Chem. 2021, 9, 576913. [Google Scholar] [CrossRef]
- Vo, S.T.; Vo, T.T.B.C.; Suk, J.W.; Kim, K. Recycling Performance of Graphene Oxide-Chitosan Hybrid Hydrogels for Removal of Cationic and Anionic Dyes. Nano Converg. 2020, 7, 4. [Google Scholar] [CrossRef] [Green Version]
- Yan, L.; Chang, P.R.; Zheng, P.W.; Ma, X.F. Characterization of Magnetic Guar Gum-Grafted Carbon Nanotubes and the Adsorption of the Dyes. Carbohydr. Polym. 2012, 87, 1919–1924. [Google Scholar] [CrossRef]
- Curcio, M.; Spizzirri, U.G.; Cirillo, G.; Vittorio, O.; Picci, N.; Nicoletta, F.P.; Iemma, F.; Hampel, S. On Demand Delivery of Ionic Drugs from Electro-Responsive Cnt Hybrid Films. RSC Adv. 2015, 5, 44902–44911. [Google Scholar] [CrossRef]
- Mhatre, S.; Simon, S.; Sjoblom, J. Experimental Evidence of Enhanced Adsorption Dynamics at Liquid-Liquid Interfaces under an Electric Field. Anal. Chem. 2020, 92, 12860–12870. [Google Scholar] [CrossRef]
- Benavidez, E.T.; Torrente, D.; Marucho, M.; Garcia, C.D. Adsorption of Soft and Hard Proteins onto Otces under the Influence of an External Electric Field. Langmuir 2015, 31, 2455–2462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stasaid, M.; Boutemak, K.; Ibtissem, L.; Flahaut, E.; Hadj-Ziane-Zafour, A. Synthesis of Carboxymethyl Xanthan/Double-Walled Carbon Nanotube Hybrid Hydrogel Nanocomposite for Transdermal Release of Drug. Soft Mater. 2021, 1–15. [Google Scholar] [CrossRef]
- Cirillo, G.; Hampel, S.; Spizzirri, U.G.; Parisi, O.I.; Picci, N.; Iemma, F. Carbon Nanotubes Hybrid Hydrogels in Drug Delivery: A Perspective Review. Biomed Res. Int. 2014, 2014, 825017. [Google Scholar] [CrossRef]
- Shakeri, A.; Nakhjiri, M.T.; Salehi, H.; Ghorbani, F.; Khankeshipour, N. Preparation of Polymer-Carbon Nanotubes Composite Hydrogel and Its Application as Forward Osmosis Draw Agent. J. Water Process. Eng. 2018, 24, 42–48. [Google Scholar] [CrossRef]
- Cirillo, G.; Caruso, T.; Hampel, S.; Haase, D.; Puoci, F.; Ritschel, M.; Leonhardt, A.; Curcio, M.; Iemma, F.; Khavrus, V.M.; et al. Novel Carbon Nanotube Composites by Grafting Reaction with Water-Compatible Redox Initiator System. Colloid Polym. Sci. 2013, 291, 699–708. [Google Scholar] [CrossRef]
- Ahmed, S.D.; Haider, A.J.; Mohammad, M.R. Comparesion of Functionalization of Multi-Walled Carbon Nanotubes Treated by Oil Olive and Nitric Acid and Their Characterization. Energy Procedia 2013, 36, 1111–1118. [Google Scholar] [CrossRef] [Green Version]
- Tugulu, S.; Barbey, R.; Harms, M.; Fricke, M.; Volkmer, D.; Rossi, A.; Klok, H.A. Synthesis of Poly(Methacrylic Acid) Brushes Via Surface-Initiated Atom Transfer Radical Polymerization of Sodium Methacrylate and Their Use as Substrates for the Mineralization of Calcium Carbonate. Macromolecules 2007, 40, 168–177. [Google Scholar] [CrossRef]
- Cirillo, G.; Curcio, M.; Spizzirri, U.G.; Vittorio, O.; Tucci, P.; Picci, N.; Iemma, F.; Hampel, S.; Nicoletta, F.P. Carbon Nanotubes Hybrid Hydrogels for Electrically Tunable Release of Curcumin. Eur. Polym. J. 2017, 90, 1–12. [Google Scholar] [CrossRef]
- Rashid, R.; Shafiq, I.; Akhter, P.; Iqbal, M.J.; Hussain, M. A State-of-the-Art Review on Wastewater Treatment Techniques: The Effectiveness of Adsorption Method. Environ. Sci. Pollut. Res. 2021, 28, 9050–9066. [Google Scholar] [CrossRef]
- Wawrzkiewicz, M.; Polska-Adach, E.; Hubicki, Z. Application of Titania Based Adsorbent for Removal of Acid, Reactive and Direct Dyes from Textile Effluents. Adsorpt. J. Int. Adsorpt. Soc. 2019, 25, 621–630. [Google Scholar] [CrossRef] [Green Version]
- Anil, I.; Gunday, S.T.; Bozkurt, A.; Alagha, O. Design of Crosslinked Hydrogels Comprising Poly(Vinylphosphonic Acid) and Bis[2-(Methacryloyloxy)Ethyl] Phosphate as an Efficient Adsorbent for Wastewater Dye Removal. Nanomaterials 2020, 10, 131. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.C.; Xu, W.X.; Nan, Y.; Wang, Y.G.; Hu, Y.X.; Gao, C.J.; Chen, X.H. Fabrication and Characterization of a High Performance Polyimide Ultrafiltration Membrane for Dye Removal. J. Colloid Interface Sci. 2020, 562, 589–597. [Google Scholar] [CrossRef]
- Khatri, J.; Nidheesh, P.V.; Singh, S.A.; Kumar, M.S. Advanced Oxidation Processes Based on Zero-Valent Aluminium for Treating Textile Wastewater. Chem. Eng. J. 2018, 348, 67–73. [Google Scholar] [CrossRef]
- Mallakpour, S.; Behranvand, V.; Mallakpour, F. Adsorptive Performance of Alginate/Carbon Nanotube-Carbon Dot-Magnesium Fluorohydroxyapatite Hydrogel for Methylene Blue-Contaminated Water. J. Environ. Chem. Eng. 2021, 9, 105170. [Google Scholar] [CrossRef]
- Mallakpour, S.; Tabesh, F. Green and Plant-Based Adsorbent from Tragacanth Gum and Carboxyl-Functionalized Carbon Nanotube Hydrogel Bionanocomposite for the Super Removal of Methylene Blue Dye. Int. J. Biol. Macromol. 2021, 166, 722–729. [Google Scholar] [CrossRef]
- Kulkarni, R.A.; Soppimath, K.S.; Aminabhavi, T.M.; Dave, A.M.; Mehta, M.H. Glutaraldehyde Crosslinked Sodium Alginate Beads Containing Liquid Pesticide for Soil Application. J. Control. Release 2000, 63, 97–105. [Google Scholar] [CrossRef]
- Tsai, C.H.; Lin, J.Y.; Maryani, F.; Huang, C.C.; Imae, T. Drug-Loading Capacity and Nuclear Targeting of Multiwalled Carbon Nanotubes Grafted with Anionic Amphiphilic Copolymers. Int. J. Nanomed. 2013, 8, 4427–4440. [Google Scholar] [CrossRef] [Green Version]
- Hilder, A.T.; Hill, J.M. Modeling the Loading and Unloading of Drugs into Nanotubes. Small 2009, 5, 300–308. [Google Scholar] [CrossRef]
- Peighambardoust, J.S.; Aghamohammadi-Bavil, O.; Foroutan, R.; Arsalani, N. Removal of Malachite Green Using Carboxymethyl Cellulose-G- Polyacrylamide/Montmorillonite Nanocomposite Hydrogel. Int. J. Biol. Macromol. 2020, 159, 1122–1131. [Google Scholar] [CrossRef]
- Salam, A.M.; El-Shishtawy, R.M.; Obaid, A.Y. Synthesis of Magnetic Multi-Walled Carbon Nanotubes/Magnetite/Chitin Magnetic Nanocomposite for the Removal of Rose Bengal from Real and Model Solution. J. Ind. Eng. Chem. 2014, 20, 3559–3567. [Google Scholar] [CrossRef]
- Fan, C.J.; Shi, Z.X.; Lian, M.; Li, H.; Yin, J. Mechanically Strong Graphene Oxide/Sodium Alginate/Polyacrylamide Nanocomposite Hydrogel with Improved Dye Adsorption Capacity. J. Mater. Chem. A 2013, 1, 7433–7443. [Google Scholar] [CrossRef]
- Rajabi, M.; Mahanpoor, K.; Moradi, O. Removal of Dye Molecules from Aqueous Solution by Carbon Nanotubes and Carbon Nanotube Functional Groups: Critical Review. RSC Adv. 2017, 7, 47083–47090. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.Z.; Li, Y.X.; Liu, Z. Novel Adsorption Materials Based on Graphene Oxide/Beta Zeolite Composite Materials and Their Adsorption Performance for Rhodamine B. J. Alloy. Compd. 2017, 708, 255–263. [Google Scholar] [CrossRef]
- Tao, J.X.; Wang, S.; Li, Z.W. Ultrasound-Assisted Bottom-up Synthesis of Ni-Graphene Hybrid Composites and Their Excellent Rhodamine B Removal Properties. J. Environ. Manag. 2020, 255, 109834. [Google Scholar] [CrossRef]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 2017, 3039817. [Google Scholar] [CrossRef]
- Kamga, F.T. Modeling Adsorption Mechanism of Paraquat onto Ayous (Triplochiton Scleroxylon) Wood Sawdust. Appl. Water Sci. 2019, 9, 1. [Google Scholar] [CrossRef] [Green Version]
- Sharma, K.A.; Priya; Kaith, B.S.; Bajaj, S.; Bhatia, J.K.; Panchal, S.; Sharm, N.; Tanwar, V. Efficient Capture of Eosin Yellow and Crystal Violet with High Performance Xanthan-Acacia Hybrid Super-Adsorbent Optimized Using Response Surface Methodology. Colloids Surf. B-Biointerfaces 2019, 175, 314–323. [Google Scholar] [CrossRef]
- Pashaei-Fakhri, S.; Peighambardoust, S.J.; Foroutan, R.; Arsalani, N.; Ramavandi, B. Crystal Violet Dye Sorption over Acrylamide/Graphene Oxide Bonded Sodium Alginate Nanocomposite Hydrogel. Chemosphere 2021, 270, 129419. [Google Scholar] [CrossRef] [PubMed]
- Belhachemi, M.; Addoun, F. Comparative Adsorption Isotherms and Modeling of Methylene Blue onto Activated Carbons. Appl. Water Sci. 2011, 1, 111–117. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Jin, X.Y.; Lin, H.F.; Chen, Z.L. Synthesis, Characterization and Potential Application of Organobentonite in Removing 2,4-Dcp from Industrial Wastewater. Chem. Eng. J. 2011, 166, 176–183. [Google Scholar] [CrossRef]
- Qin, G.P.; Yang, Y.X.; Zhang, X.T.; Niu, J.H.; Yang, H.; Tian, S.F.; Zhu, J.H.; Lu, M.H. Highly Efficient, Rapid, and Simultaneous Removal of Cationic Dyes from Aqueous Solution Using Monodispersed Mesoporous Silica Nanoparticles as the Adsorbent. Nanomaterials 2018, 8, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, C.Z.; Hu, C.S.; Dichiara, A.B.; Jiang, W.H.; Gu, J. Cellulose Nanofibril/Carbon Nanomaterial Hybrid Aerogels for Adsorption Removal of Cationic and Anionic Organic Dyes. Nanomaterials 2020, 10, 169. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, H.; Tanaka, A.; Nishimura, M.; Koyano, K.; Tatsumi, T.; Anpo, M. Photochemical Properties of Rhodamine-B Dye Molecules Included within Mesoporous Molecular Sieves. Mesoporous Mol. Sieves 1998, 177, 551–558. [Google Scholar]
- Daraio, M.E.; San Roman, E. Aggregation and Photophysics of Rose Bengal in Alumina-Coated Colloidal Suspensions. Helv. Chim. Acta 2001, 84, 2601–2614. [Google Scholar] [CrossRef]
- Hanbali, M.; Holail, H.; Hammud, H. Remediation of Lead by Pretreated Red Algae: Adsorption Isotherm, Kinetic, Column Modeling and Simulation Studies. Green Chem. Lett. Rev. 2014, 7, 342–358. [Google Scholar] [CrossRef]
- Gurses, A.; Dogar, C.; Yalcin, M.; Acikyildiz, M.; Bayrak, R.; Karaca, S. The Adsorption Kinetics of the Cationic Dye, Methylene Blue, onto Clay. J. Hazard. Mater. 2006, 131, 217–228. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Khraisheh, M.A.M.; Ahmad, M.N.M.; Allen, S. Adsorption Behavior of Methylene Blue onto Jordanian Diatomite: A Kinetic Study. J. Hazard. Mater. 2009, 165, 589–598. [Google Scholar] [CrossRef]
- Malash, G.F.; El-Khaiary, M.I. Methylene Blue Adsorption by the Waste of Abu-Tartour Phosphate Rock. J. Colloid Interface Sci. 2010, 348, 537–545. [Google Scholar] [CrossRef]
- Yu, Y.; Murthy, B.N.; Shapter, J.G.; Constantopoulos, K.T.; Voelcker, N.H.; Ellis, A.V. Benzene Carboxylic Acid Derivatized Graphene Oxide Nanosheets on Natural Zeolites as Effective Adsorbents for Cationic Dye Removal. J. Hazard. Mater. 2013, 260, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Vargas, M.A.M.; Cazetta, A.L.; Kunita, M.H.; Silva, T.L.; Almeida, V.C. Adsorption of Methylene Blue on Activated Carbon Produced from Flamboyant Pods (Delonix Regia): Study of Adsorption Isotherms and Kinetic Models. Chem. Eng. J. 2011, 168, 722–730. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Puad, N.A.A.; Bello, O.S. Kinetic, Equilibrium and Thermodynamic Studies of Synthetic Dye Removal Using Pomegranate Peel Activated Carbon Prepared by Microwave-Induced Koh Activation. Water Resour. Ind. 2014, 6, 18–35. [Google Scholar] [CrossRef] [Green Version]


| Dye | HGB | HGNT | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | ΔH0 | ΔS0 | R2 | ΔH0 | ΔS0 | |||||||||
| MB | 0.9143 | −23.38 | −27.72 | −30.28 | −33.77 | −36.65 | 217.48 | 0.9283 | −25.31 | −28.28 | −31.49 | −36.05 | −37.11 | 223.97 |
| RD | 0.9485 | −25.77 | −27.56 | −29.42 | −31.17 | −6.35 | 115.74 | 0.9514 | −26.35 | −28.29 | −30.63 | −32.79 | −12.14 | 138.42 |
| BR | 0.9492 | −29.59 | −32.40 | −34.83 | −36.57 | −12.39 | 152.01 | 0.9098 | −30.73 | −33.94 | −36.03 | −38.22 | −13.18 | 159.21 |
| Dye | HGB | HGNT | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MB | 15.94 ± 2.3 | 17.22 ± 2.2 | 19.51 ± 1.9 | 8.03 | 22.39 | 19.72 ± 1.9 | 22.55 ± 1.4 | 33.45 ± 2.4 | 14.35 | 69.62 |
| RD | 15.71 ± 1.8 | 15.67 ± 2.5 | 15.68 ± 2.4 | 0.25 | 0.19 | 28.93 ± 2.0 | 25.47 ± 1.7 | 13.06 ± 1.6 | 11.96 | 54.86 |
| BR | 23.12 ± 2.6 | 23.01 ± 2.6 | 22.96 ± 2.5 | 0.48 | 0.69 | 36.75 ± 2.3 | 30.74 ± 2.0 | 15.85 ± 2.0 | 16.35 | 56.87 |
| Model/Parameter | MB | RD | BR | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HGB | HGNT | HGB | HGNT | HGB | HGNT | ||||||||
| 0 V | 12 V | 0 V | 12 V | 0 V | 12 V | 0 V | 12 V | 0 V | 12 V | 0 V | 12 V | ||
| Langmuir | R2 | 0.9984 | 0.9893 | 0.9912 | 0.9688 | 0.9985 | 0.9874 | 0.9621 | 0.9981 | 0.9879 | 0.9650 | 0.9768 | 0.9874 |
| qmax | 20.19 | 25.01 | 23.01 | 42.17 | 18.00 | 18.51 | 33.91 | 16.59 | 24.18 | 24.69 | 42.64 | 16.87 | |
| kL (10−2) | 1.35 | 1.28 | 2.46 | 1.82 | 2.38 | 1.99 | 2.57 | 1.11 | 7.27 | 3.84 | 3.80 | 2.75 | |
| RL | 0.16 | 0.16 | 0.09 | 0.12 | 0.10 | 0.11 | 0.09 | 0.18 | 0.03 | 0.06 | 0.06 | 0.08 | |
| Freundlich | R2 | 0.9283 | 0.8665 | 0.8191 | 0.8004 | 0.8869 | 0.8116 | 0.8387 | 0.9285 | 0.9173 | 0.9354 | 0.9067 | 0.9867 |
| kF | 1.54 | 1.95 | 3.46 | 3.74 | 2.68 | 2.60 | 4.58 | 1.20 | 7.13 | 5.12 | 7.02 | 2.99 | |
| 1/nF | 0.42 | 0.41 | 0.32 | 0.41 | 0.32 | 0.32 | 0.34 | 0.41 | 0.22 | 0.27 | 0.32 | 0.29 | |
| Red–Pet | R2 | 0.9974 | 0.9839 | 0.9875 | 0.9477 | 0.9977 | 0.9811 | 0.9432 | 0.9969 | 0.9858 | 0.9636 | 0.9652 | 0.9994 |
| kRP | 0.27 | 0.32 | 0.57 | 0.76 | 0.43 | 0.37 | 0.87 | 0.18 | 2.24 | 1.90 | 1.62 | 1.14 | |
| αRP (10−2) | 1.34 | 1.28 | 2.46 | 1.72 | 2.38 | 1.98 | 2.57 | 1.09 | 12.88 | 18.87 | 3.80 | 21.75 | |
| g | 0.90 | 0.75 | 0.86 | 0.58 | 0.06 | 0.80 | 0.34 | 0.88 | 0.06 | 0.84 | 0.22 | 0.80 | |
| qRP | 20.22 | 24.98 | 23.15 | 44.11 | 18.07 | 18.70 | 33.89 | 16.47 | 17.40 | 10.07 | 42.60 | 5.24 | |
| Sips | R2 | 0.9994 | 0.9989 | 0.9969 | 0.9939 | 0.9981 | 0.9995 | 0.9747 | 0.9994 | 0.9894 | 0.9691 | 0.9970 | 0.9988 |
| qmax | 18.62 | 21.13 | 21.00 | 33.86 | 17.59 | 16.07 | 28.67 | 15.07 | 26.36 | 31.60 | 36.87 | 26.79 | |
| kS (10−2) | 0.86 | 0.27 | 0.93 | 0.15 | 2.01 | 0.25 | 0.27 | 0.59 | 11.86 | 8.57 | 0.57 | 7.16 | |
| nS | 1.15 | 1.47 | 1.33 | 1.85 | 1.06 | 1.63 | 1.78 | 1.19 | 0.76 | 0.62 | 1.75 | 0.52 | |
| Dub–Rad | R2 | 0.9202 | 0.9450 | 0.9369 | 0.9826 | 0.9285 | 0.9810 | 0.9771 | 0.9397 | 0.8759 | 0.8833 | 0.9818 | 0.9026 |
| qmax | 14.16 | 18.19 | 18.02 | 31.88 | 14.14 | 14.95 | 27.02 | 11.87 | 20.35 | 19.68 | 34.93 | 13.59 | |
| βDR | 0.35 | 0.48 | 0.17 | 0.25 | 0.16 | 0.35 | 0.17 | 0.60 | 0.02 | 0.05 | 0.08 | 0.15 | |
| E | 1.19 | 1.02 | 1.70 | 1.42 | 1.79 | 1.20 | 1.71 | 0.91 | 4.56 | 3.20 | 2.46 | 1.82 | |
| Temkin | R2 | 0.9977 | 0.9839 | 0.9732 | 0.9647 | 0.9890 | 0.9710 | 0.9480 | 0.9967 | 0.9835 | 0.9759 | 0.9528 | 0.9992 |
| AT | 0.12 | 0.11 | 0.26 | 0.14 | 0.26 | 0.20 | 0.23 | 0.10 | 1.69 | 0.64 | 0.37 | 0.38 | |
| B | 4.59 | 0.48 | 4.81 | 9.90 | 3.70 | 3.96 | 7.45 | 3.81 | 3.93 | 4.46 | 9.00 | 3.24 | |
| Model/Parameter | MB | RD | BR | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HGB | HGNT | HGB | HGNT | HGB | HGNT | ||||||||
| 0 V | 12 V | 0 V | 12 V | 0 V | 12 V | 0 V | 12 V | 0 V | 12 V | 0 V | 12 V | ||
| Pseudo-First | R2 | 0.9238 | 0.9654 | 0.9500 | 0.9429 | 0.9001 | 0.9050 | 0.9217 | 0.9001 | 0.9528 | 0.9386 | 0.9436 | 0.9226 |
| qe | 7.43 | 8.10 | 10.62 | 14.94 | 6.27 | 5.75 | 12.37 | 6.52 | 14.37 | 13.11 | 19.04 | 9.13 | |
| k1 (10−2) | 11.57 | 11.31 | 10.87 | 16.43 | 6.71 | 7.21 | 5.60 | 4.10 | 1.38 | 1.43 | 1.57 | 1.55 | |
| 10.63 | 7.61 | 11.76 | 21.72 | 15.22 | 14.98 | 14.26 | 12.45 | 0.63 | 0.58 | 4.04 | 8.05 | ||
| Pseudo-Second | R2 | 0.9699 | 0.9881 | 0.9845 | 0.9740 | 0.9592 | 0.9622 | 0.9739 | 0.9639 | 0.9667 | 0.9561 | 0.9682 | 0.9573 |
| qe | 7.94 | 8.61 | 11.32 | 15.76 | 6.82 | 6.24 | 13.56 | 7.27 | 17.17 | 15.45 | 22.26 | 10.60 | |
| k2 (10−2) | 1.95 | 1.82 | 1.33 | 1.49 | 1.30 | 1.52 | 0.54 | 0.73 | 0.09 | 0.11 | 0.09 | 0.19 | |
| 1.81 | 0.83 | 1.53 | 6.16 | 2.64 | 3.10 | 0.13 | 0.31 | 36.38 | 27.73 | 24.60 | 3.53 | ||
| Avrami | R2 | 0.9711 | 0.9762 | 0.9702 | 0.9614 | 0.9756 | 0.9762 | 0.9889 | 0.9837 | 0.9972 | 0.9732 | 0.9869 | 0.9940 |
| qe | 8.27 | 11.31 | 8.38 | 15.63 | 7.34 | 6.55 | 13.89 | 7.70 | 17.83 | 16.70 | 24.89 | 13.47 | |
| kA (10−2) | 5.59 | 8.64 | 6.90 | 11.74 | 2.88 | 3.37 | 3.08 | 1.92 | 0.77 | 0.73 | 0.72 | 0.49 | |
| n | 0.47 | 0.63 | 0.54 | 0.54 | 0.47 | 0.50 | 0.54 | 0.55 | 0.62 | 0.59 | 0.57 | 0.50 | |
| 0.03 | 3.02 | 1.64 | 7.98 | 0.13 | 0.27 | 0.26 | 1.04 | 55.89 | 65.82 | 99.32 | 90.23 | ||
| Fractional power | R2 | 0.9795 | 0.9771 | 0.9915 | 0.9662 | 0.9744 | 0.9689 | 0.9514 | 0.9824 | 0.9599 | 0.9524 | 0.9701 | 0.9812 |
| qe | 8.29 | 9.08 | 11.87 | 16.36 | 7.36 | 6.71 | 14.51 | 7.73 | 15.92 | 14.53 | 21.39 | 10.26 | |
| kp | 3.76 | 4.26 | 5.36 | 8.04 | 2.17 | 2.07 | 3.91 | 1.67 | 1.61 | 1.57 | 2.46 | 1.18 | |
| V | 0.13 | 0.13 | 0.14 | 0.12 | 0.21 | 0.20 | 0.22 | 0.26 | 0.39 | 0.38 | 0.37 | 0.37 | |
| 0.01 | 0.43 | 0.15 | 0.91 | 0.19 | 0.02 | 4.51 | 1.21 | 9.67 | 9.02 | 10.19 | 0.72 | ||
| Intraparticle Diffusion | R2 | 0.8008 | 0.7391 | 0.7662 | 0.8085 | 0.8338 | 0.8205 | 0.8336 | 0.8997 | 0.9550 | 0.9466 | 0.9559 | 0.9666 |
| qe | 9.59 | 10.40 | 13.61 | 19.08 | 8.03 | 7.37 | 15.74 | 8.32 | 16.55 | 15.15 | 22.39 | 10.72 | |
| ki | 0.41 | 0.43 | 0.57 | 0.82 | 0.31 | 0.28 | 0.63 | 0.35 | 0.81 | 0.74 | 1.08 | 0.51 | |
| C | 1.80 | 2.25 | 2.78 | 3.53 | 2.10 | 1.99 | 3.80 | 1.63 | 1.13 | 1.19 | 1.94 | 0.97 | |
| 16.87 | 22.18 | 25.72 | 28.72 | 7.64 | 6.40 | 26.63 | 9.71 | 21.19 | 20.72 | 27.24 | 5.07 | ||
| Elovich | R2 | 0.9782 | 0.9660 | 0.9766 | 0.9513 | 0.9927 | 0.9923 | 0.9900 | 0.9956 | 0.9085 | 0.9121 | 0.9555 | 0.9210 |
| α | 4.89 | 5.31 | 6.39 | 21.59 | 2.73 | 2.81 | 3.75 | 1.24 | 0.85 | 0.91 | 1.35 | 0.68 | |
| Β | 0.84 | 0.78 | 0.58 | 0.48 | 0.96 | 1.06 | 0.46 | 0.79 | 0.36 | 0.42 | 0.26 | 0.57 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cirillo, G.; Curcio, M.; Madeo, L.F.; Iemma, F.; De Filpo, G.; Hampel, S.; Nicoletta, F.P. Carbon Nanotubes Hybrid Hydrogels for Environmental Remediation: Evaluation of Adsorption Efficiency under Electric Field. Molecules 2021, 26, 7001. https://doi.org/10.3390/molecules26227001
Cirillo G, Curcio M, Madeo LF, Iemma F, De Filpo G, Hampel S, Nicoletta FP. Carbon Nanotubes Hybrid Hydrogels for Environmental Remediation: Evaluation of Adsorption Efficiency under Electric Field. Molecules. 2021; 26(22):7001. https://doi.org/10.3390/molecules26227001
Chicago/Turabian StyleCirillo, Giuseppe, Manuela Curcio, Lorenzo Francesco Madeo, Francesca Iemma, Giovanni De Filpo, Silke Hampel, and Fiore Pasquale Nicoletta. 2021. "Carbon Nanotubes Hybrid Hydrogels for Environmental Remediation: Evaluation of Adsorption Efficiency under Electric Field" Molecules 26, no. 22: 7001. https://doi.org/10.3390/molecules26227001
APA StyleCirillo, G., Curcio, M., Madeo, L. F., Iemma, F., De Filpo, G., Hampel, S., & Nicoletta, F. P. (2021). Carbon Nanotubes Hybrid Hydrogels for Environmental Remediation: Evaluation of Adsorption Efficiency under Electric Field. Molecules, 26(22), 7001. https://doi.org/10.3390/molecules26227001








