Spectral Characteristic, Storage Stability and Antioxidant Properties of Anthocyanin Extracts from Flowers of Butterfly Pea (Clitoria ternatea L.)
Abstract
:1. Introduction
2. Results and Discussion
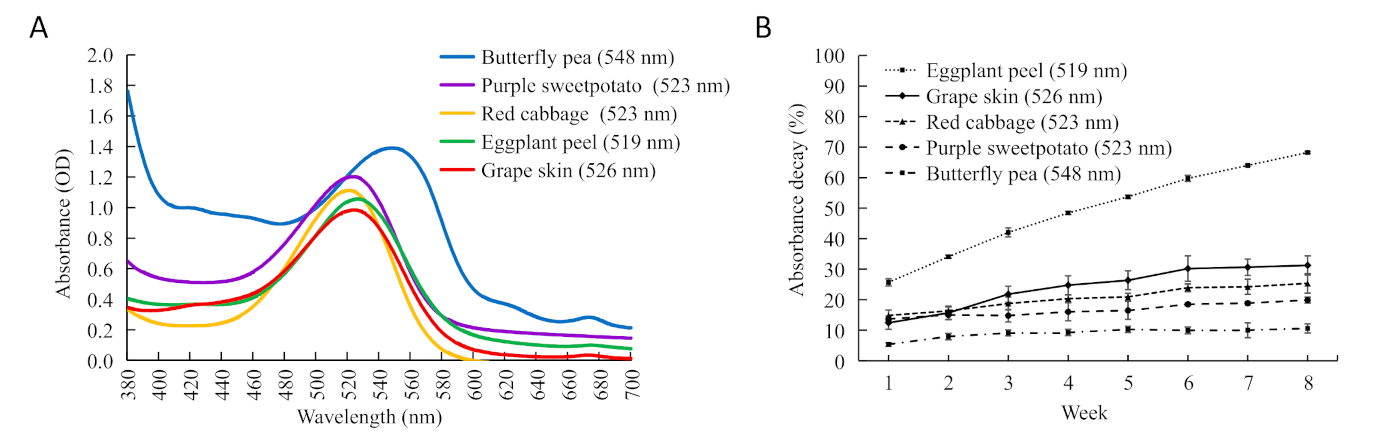
2.1. Stability Comparison of Anthocyanin Extracts from Five Plants
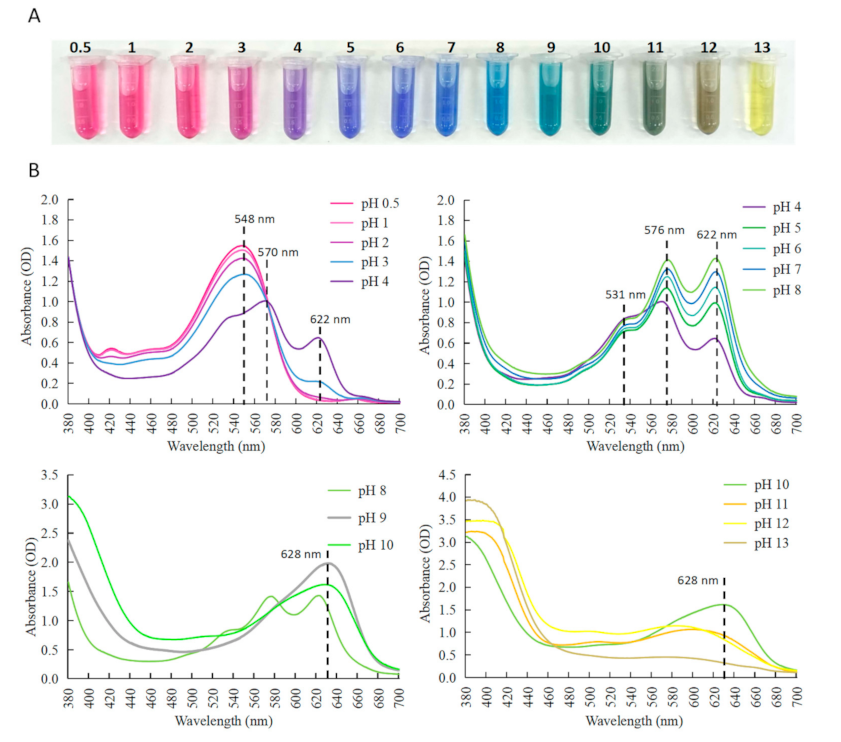
2.2. Color and Spectral Characteristic of Anthocyanin Extract from C. ternatea Blue Flowers
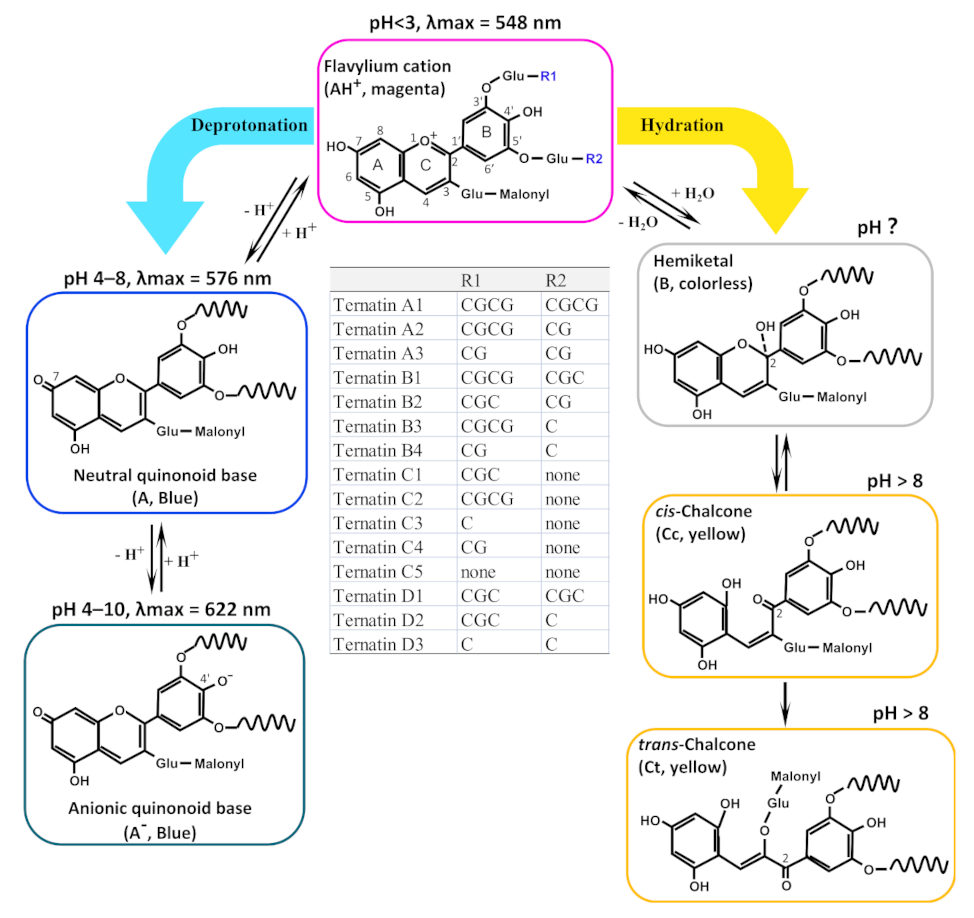
2.3. Speculated Structural Transformation of Ternatins with pH Variations
2.4. Combined Effects of pH and Temperature on the Storage Stability of CTAEs
2.5. Kinetic Parameters of Thermal Degradation for CTAEs at Different pHs
2.6. Antioxidant Properties for Extracts from C. ternatea Flowers
3. Materials and Methods
3.1. Materials
3.2. Stability Comparison for Anthocyanin Extracts from Five Plants
3.3. Stability Analysis for Anthocyanin Extracts from C. ternatea
3.4. Thermal Degradation Kinetics
3.5. Measurement of Antioxidant Activities for Extracts of C. ternatea at Different pHs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Newsome, A.G.; Culver, C.A.; van Breemen, R.B. Nature’s palette: The search for natural blue colorants. J. Agric. Food Chem. 2014, 62, 6498–6511. [Google Scholar] [CrossRef] [PubMed]
- Houghton, A.; Appelhagen, I.; Martin, C. Natural blues: Structure meets function in anthocyanins. Plants 2021, 10, 726. [Google Scholar] [CrossRef] [PubMed]
- Grotewold, E. The genetics and biochemistry of floral pigments. Annu. Rev. Plant Biol. 2006, 57, 761–780. [Google Scholar] [CrossRef] [PubMed]
- Lazr, N.; Croitoru, C.; Enachi, E.; Bahrim, G.E.; Rpeanu, G. Eggplant peels as a valuable source of anthocyanins: Extraction, thermal stability and biological activities. Plants 2021, 10, 577. [Google Scholar] [CrossRef]
- Wu, H.Y.; Yang, K.M.; Chiang, P.Y. Roselle anthocyanins: Antioxidant properties and stability to heat and pH. Molecules 2018, 23, 1357. [Google Scholar] [CrossRef] [Green Version]
- Escher, G.B.; Wen, M.; Zhang, L.; Rosso, N.D.; Granato, D. Phenolic composition by UHPLC-Q-TOF-MS/MS and stability of anthocyanins from Clitoria ternatea L. (butterfly pea) blue petals. Food Chem. 2020, 331, 127341. [Google Scholar] [CrossRef]
- Deng, J.; Wu, D.; Shi, J.; Balfour, K.; Wang, H.; Zhu, G.; Liu, Y.; Wang, J.; Zhu, Z. Multiple MYB activators and repressors collaboratively regulate the juvenile red fading in leaves of sweetpotato. Front. Plant Sci. 2020, 11, 941. [Google Scholar] [CrossRef]
- Butelli, E.; Titta, L.; Giorgio, M.; Mock, H.P.; Matros, A.; Peterek, S.; Schijlen, E.G.; Hall, R.D.; Bovy, A.G.; Luo, J.; et al. Enrichment of tomato fruit with health-promoting anthocyanins by expression of select transcription factors. Nat. Biotechnol. 2008, 26, 1301–1308. [Google Scholar] [CrossRef]
- Park, S.H.; Hanning, I.; Gilbert, W.; Munro, M.; Devareddy, L.; Ricke, S.C. Feeding mice aged and fresh blackberries powder supplements result in shifts in the gastrointestinal microflora. Food Biosci. 2013, 1, 66–72. [Google Scholar] [CrossRef]
- Zang, Z.; Chou, S.; Tian, J.; Lang, Y.; Shen, Y.; Ran, X.; Gao, N.; Li, B. Effect of whey protein isolate on the stability and antioxidant capacity of blueberry anthocyanins: A mechanistic and in vitro simulation study. Food Chem. 2021, 336, 127700. [Google Scholar] [CrossRef]
- Dini, C.; Zaro, M.J.; Rolny, N.; Caputo, M.; Boido, E.; Dellacassa, E.; Viña, S.Z. Characterization and stability analysis of anthocyanins from Pachyrhizus ahipa (Wedd) Parodi roots. Food Biosci. 2020, 34, 100534. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, S.; Zhao, G.; Ye, F. Destabilisation and stabilisation of anthocyanins in purple-fleshed sweet potatoes: A review. Trends Food Sci. Technol. 2021, 116, 1141–1154. [Google Scholar] [CrossRef]
- Kazuma, K.; Noda, N.; Suzuki, M. Flavonoid composition related to petal color in different lines of Clitoria ternatea. Phytochemistry 2003, 64, 1133–1139. [Google Scholar] [CrossRef]
- Dangles, O.; Fenger, J.A. The chemical reactivity of anthocyanins and its consequences in food science and nutrition. Molecules 2018, 23, 1970. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, R.; Lee, P.M.; Kong, H.L. Multiple color and pH stability of floral anthocyanin extract: Clitoria ternatea. In Proceedings of the 2010 International Conference on Science and Social Research (CSSR 2010), Kuala Lumpur, Malaysia, 5–7 December 2010. [Google Scholar] [CrossRef]
- Terahara, N.; Saito, N.; Honda, T.; Toki, K.; Osajima, Y. Structure of ternatin D1, an acylated anthocyanin from Clitoria ternatea flowers. Tetrahedron Lett. 1989, 30, 5305–5308. [Google Scholar] [CrossRef]
- Terahara, N.; Oda, M.; Matsui, T.; Osajima, Y.; Saito, N.; Toki, K.; Honda, T. Five new anthocyanins, ternatins A3, B4, B3, B2, and D2, from Clitoria ternatea flowers. J. Nat. Prod. 1996, 59, 139–144. [Google Scholar] [CrossRef]
- Terahara, N.; Toki, K.; Saito, N.; Honda, T.; Matsui, T.; Osajima, Y. Eight new anthocyanins, ternatins C1-C5 and D3 and preternatins A3 and C4 from young Clitoria ternatea flowers. J. Nat. Prod. 1998, 61, 1361–1367. [Google Scholar] [CrossRef]
- Chusak, C.; Thilavech, T.; Henry, C.J.; Adisakwattana, S. Acute effect of Clitoria ternatea flower beverage on glycemic response and antioxidant capacity in healthy subjects: A randomized crossover trial. BMC Complement. Altern. Med. 2018, 18, 6. [Google Scholar] [CrossRef]
- Bujak, T.; Zagórska-Dziok, M.; Ziemlewska, A.; Nizioł-Łukaszewska, Z.; Wasilewski, T.; Hordyjewicz-Baran, Z. Antioxidant and cytoprotective properties of plant extract from dry flowers as functional dyes for cosmetic products. Molecules 2021, 26, 2809. [Google Scholar] [CrossRef]
- Marpaung, A.M.; Andarwulan, N.; Hariyadi, P.; Nur Faridah, D. The colour degradation of anthocyanin-rich extract from butterfly pea (Clitoria ternatea L.) petal in various solvents at pH 7. Nat. Prod. Res. 2017, 31, 2273–2280. [Google Scholar] [CrossRef]
- Faezah, S.; My, M.L.; Atika, A.; Muhammad, H.R.; Oa, M.Z.; Ariff, A.; Py, K. A Comparative analysis of Clitoria ternatea Linn. (butterfly pea) flower extract as natural liquid pH indicator and natural pH paper. Dhaka Univ. J. Pharm. Sci. 2018, 17, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Dangles, O.; Saito, N.; Brouillard, R. Anthocyanin intramolecular copigment effect. Phytochemistry 1993, 34, 119–124. [Google Scholar] [CrossRef]
- Song, H.N.; Ji, S.A.; Park, H.R.; Kim, H.H.; Hogstrand, C. Impact of various factors on color stability of fresh blueberry juice during storage. Prev. Nutr. Food Sci. 2018, 23, 46–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Li, G.; Long, C.; Xu, J.; Cen, J.; Yang, X. The antioxidant activity and genotoxicity of isogarcinol. Food Chem. 2018, 253, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Fu, Q.; Zhang, Y. Composition of anthocyanins in pomegranate flowers and their antioxidant activity. Food Chem. 2011, 127, 1444–1449. [Google Scholar] [CrossRef]
- GÜder, A.; Engin, M.S.; Yolcu, M.; GÜr, M. Effect of processing temperature on the chemical composition and antioxidant activity of Vaccinium arctostaphylos fruit and their jam. J. Food Process. Preserv. 2014, 38, 1696–1704. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, R.; Shi, W.; Li, L.; Liu, H.; Liu, Z.; Wu, L. The multifunctional benefits of naturally occurring delphinidin and its glycosides. J. Agric. Food. Chem. 2019, 67, 11288–11306. [Google Scholar] [CrossRef]
- Ruenroengklin, N.; Zhong, J.; Duan, X.; Yang, B.; Li, J.; Jiang, Y. Effects of various temperatures and pH values on the extraction yield of phenolics from litchi fruit pericarp tissue and the antioxidant activity of the extracted anthocyanins. Int. J. Mol. Sci. 2008, 9, 1333–1341. [Google Scholar] [CrossRef]
- Kirca, A.; Özkan, M.; Cemeroğlu, B. Effects of temperature, solid content and pH on the stability of black carrot anthocyanins. Food Chem. 2007, 101, 212–218. [Google Scholar] [CrossRef]


| Temperature (°C) | R2 | k (d−1) | k (min−1) | t1/2 (d) |
|---|---|---|---|---|
| pH 0.5 (548 nm) | ||||
| 4 °C | 0.9684 | 1.36 × 10−2 | 9.45 × 10−6 | 50.9 |
| 25 °C | 0.9132 | 2.47 × 10−2 | 17.14 × 10−6 | 28.1 |
| 37 °C | 0.9801 | 3.93 × 10−2 | 27.27 × 10−6 | 17.7 |
| 50 °C | 0.9949 | 12.46 × 10−2 | 86.54 × 10−6 | 5.6 |
| pH 7 (576 nm) | ||||
| 4 °C | 0.9012 | 0.21 × 10−2 | 1.44 × 10−6 | 334.2 |
| 25 °C | 0.9652 | 1.47 × 10−2 | 10.22 × 10−6 | 47.1 |
| 37 °C | 0.9140 | 2.67 × 10−2 | 18.52 × 10−6 | 26.0 |
| 50 °C | 0.9604 | 3.75 × 10−2 | 26.04 × 10−6 | 18.5 |
| pH 7 (622 nm) | ||||
| 4 °C | 0.9072 | 0.67 × 10−2 | 4.66 × 10−6 | 103.4 |
| 25 °C | 0.9209 | 1.93 × 10−2 | 13.37 × 10−6 | 36.0 |
| 37 °C | 0.9121 | 3.37 × 10−2 | 23.38 × 10−6 | 20.6 |
| 50 °C | 0.9608 | 4.27 × 10−2 | 29.65 × 10−6 | 16.2 |
| pH 10 (628 nm) | ||||
| 4 °C | 0.9707 | 1.63 × 10−2 | 11.32 × 10−6 | 42.5 |
| 25 °C | 0.9177 | 5.82 × 10−2 | 40.39 × 10−6 | 11.9 |
| 37 °C | 0.9163 | 10.34 × 10−2 | 71.82 × 10−6 | 6.7 |
| 50 °C | 0.9071 | 14.92 × 10−2 | 103.58 × 10−6 | 4.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, X.; Wu, Q.; Wang, J.; Chen, Y.; Zhu, G.; Zhu, Z. Spectral Characteristic, Storage Stability and Antioxidant Properties of Anthocyanin Extracts from Flowers of Butterfly Pea (Clitoria ternatea L.). Molecules 2021, 26, 7000. https://doi.org/10.3390/molecules26227000
Fu X, Wu Q, Wang J, Chen Y, Zhu G, Zhu Z. Spectral Characteristic, Storage Stability and Antioxidant Properties of Anthocyanin Extracts from Flowers of Butterfly Pea (Clitoria ternatea L.). Molecules. 2021; 26(22):7000. https://doi.org/10.3390/molecules26227000
Chicago/Turabian StyleFu, Xueying, Qiang Wu, Jian Wang, Yanli Chen, Guopeng Zhu, and Zhixin Zhu. 2021. "Spectral Characteristic, Storage Stability and Antioxidant Properties of Anthocyanin Extracts from Flowers of Butterfly Pea (Clitoria ternatea L.)" Molecules 26, no. 22: 7000. https://doi.org/10.3390/molecules26227000
APA StyleFu, X., Wu, Q., Wang, J., Chen, Y., Zhu, G., & Zhu, Z. (2021). Spectral Characteristic, Storage Stability and Antioxidant Properties of Anthocyanin Extracts from Flowers of Butterfly Pea (Clitoria ternatea L.). Molecules, 26(22), 7000. https://doi.org/10.3390/molecules26227000







