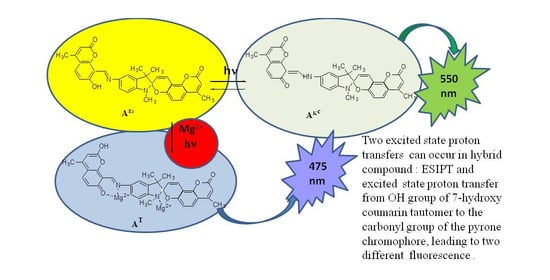
Excited State Proton Transfers in Hybrid Compound Based on Indoline Spiropyran of the Coumarin Type and Azomethinocoumarin in the Presence of Metal Ions
Abstract
1. Introduction
2. Results
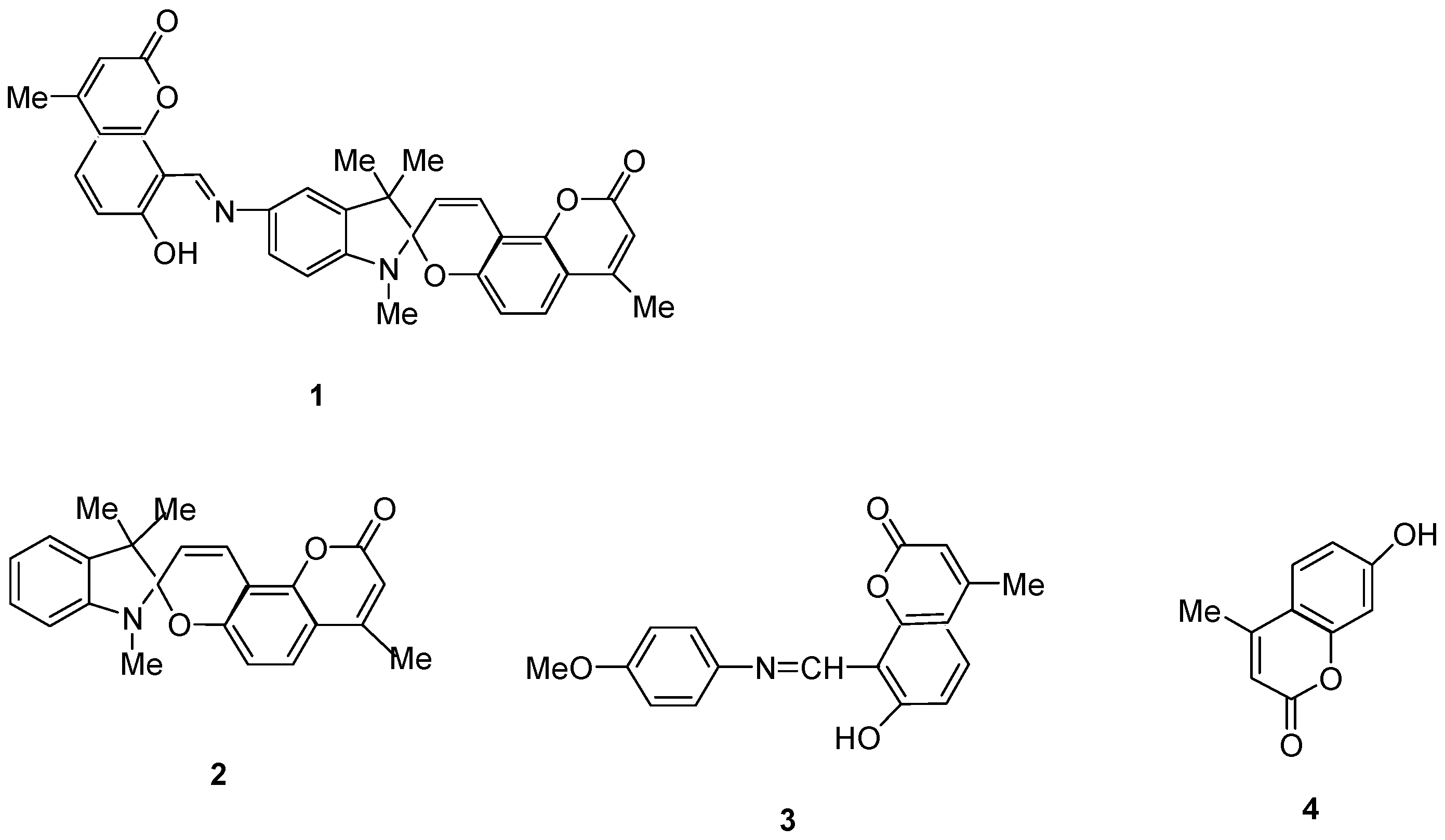
2.1. Design and Synthesis
2.2. Abbreviations
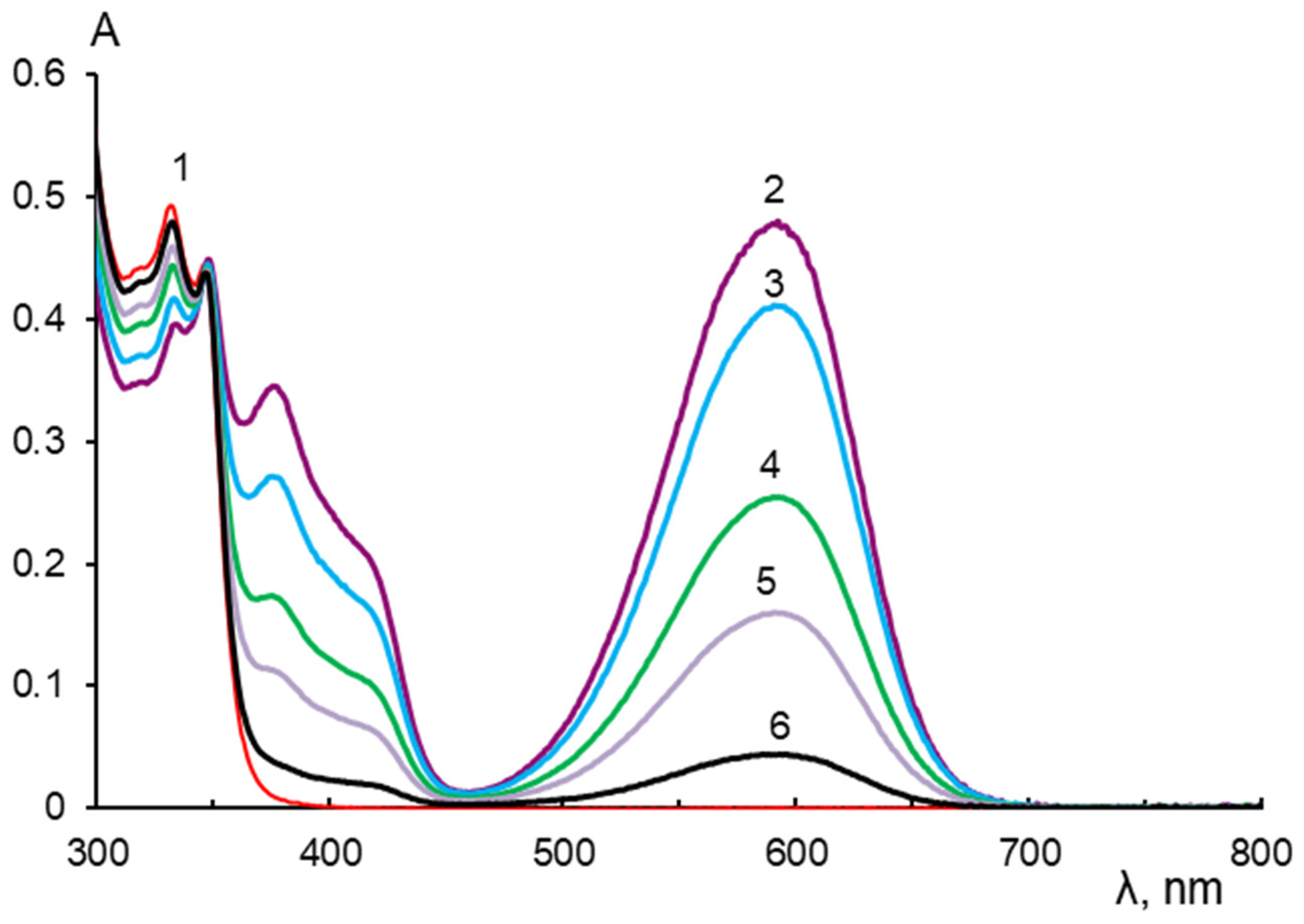
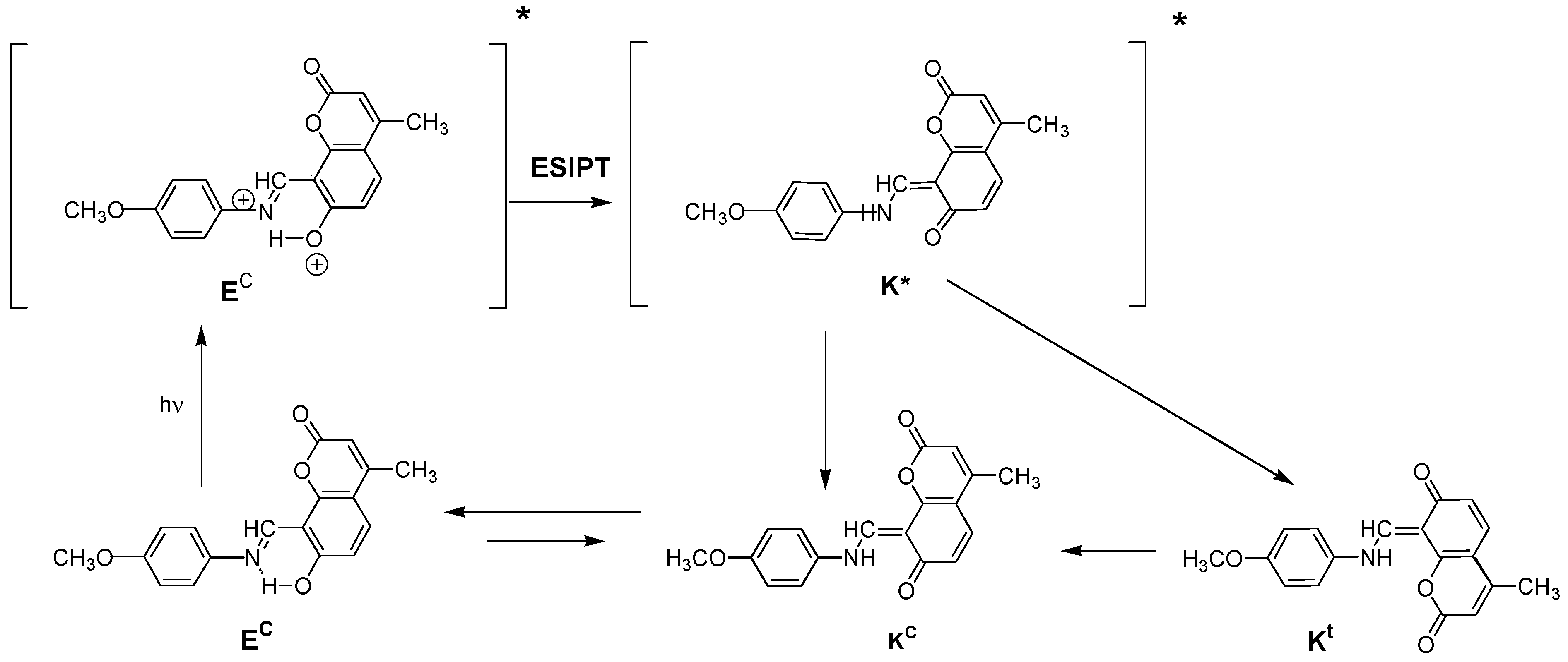
2.3. Spectral-Luminescent Properties of Model Compounds
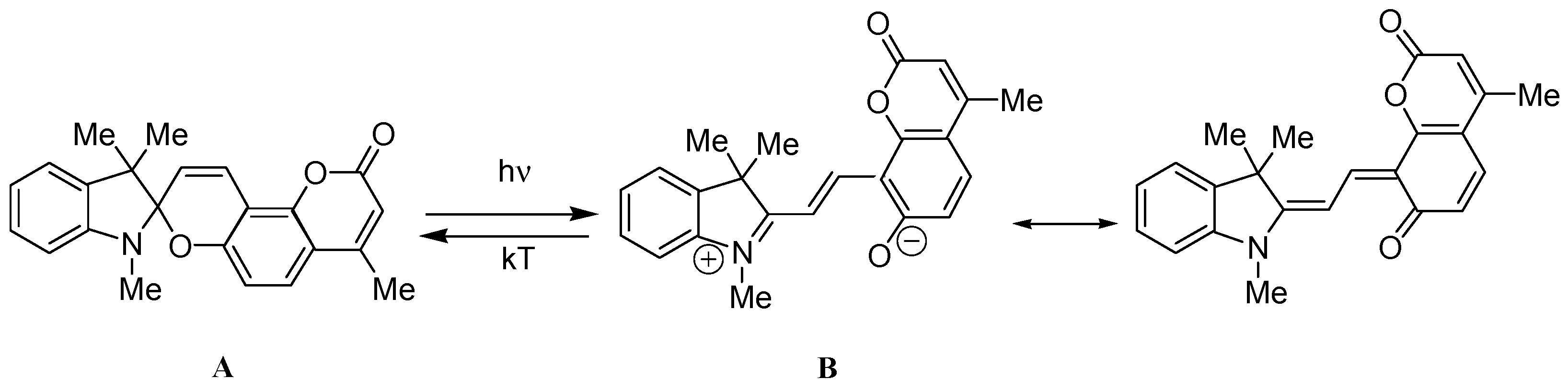
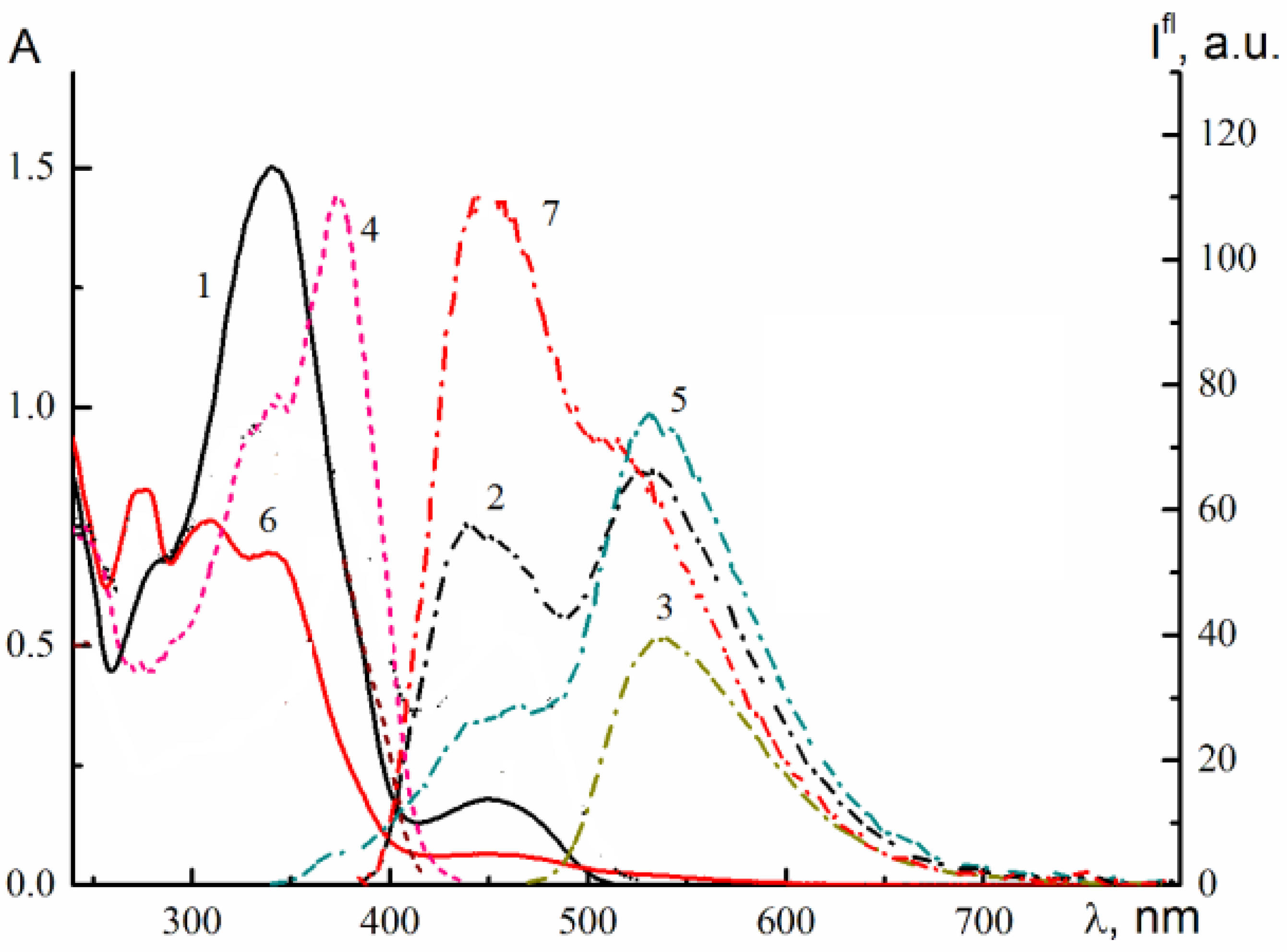
2.4. Spectral-Luminescent Properties of the Hybrid Compound 1
3. Materials and Methods
3.1. Chemicals and Instrumentation
3.2. Synthesis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhao, J.; Ji, S.; Chen, Y.; Gou, H.; Yang, P. Excited state intramolecular proton transfer (ESIPT):from principal photophysics to the development of new chromophores and applications in fluorescent molecular probes and luminescent materials. Phys. Chem. Chem. Phys. 2012, 14, 8803–8817. [Google Scholar] [CrossRef] [PubMed]
- Padalkar, V.S.; Seki, S. Excited-state intramolecular proton-transfer (ESIPT)—inspired solid state emitters. Chem. Soc. Rev. 2016, 45, 169–202. [Google Scholar] [CrossRef]
- Joshi, H.C.; Antonov, L. Excited-state intramolecular proton transfer: A short introductory review. Molecules 2021, 26, 1475. [Google Scholar] [CrossRef] [PubMed]
- Tasaki, S.; Momotake, A.; Kanna, Y.; Sato, T.; Nishimura, Y.; Arai, T. Producing a dual-fluorescent molecule by tuning the energetics of excited-state intramolecular proton transfer. Photochem. Photobiol. Sci. 2015, 14, 1864–1871. [Google Scholar] [CrossRef] [PubMed]
- Levin, P.P.; Liubimov, A.V.; Shashkov, A.S.; Mardaleishvili, I.R.; Venidiktova, O.V.; Shienok, A.I.; Koltsova, L.S.; Astafiev, A.A.; Barachevsky, V.A.; Zaichenko, N.L. Multiple fluorescence of tetraarylimidazole and azomethinocoumarin dyad with dual excited-state intramolecular proton transfer. Dyes Pigment 2020, 183, 108716. [Google Scholar] [CrossRef]
- Levin, P.P.; Tatikolov, A.S.; Zaichenko, N.L.; Shienok, A.I.; Koltsova, L.S.; Oskina, O.Y.; Mardaleishvili, I.R.; Popov, L.D.; Levchenkov, S.I.; Berlin, A.A. Kinetics of photochemical reactions of multifunctional hybrid compounds based on spironaphthoxazines upon photoexcitation with light of different wavelengths. J. Photochem. Photobiol. A. Chem. 2013, 251, 141–147. [Google Scholar] [CrossRef]
- Levin, P.P.; Tatikolov, A.S.; Zaichenko, N.L.; Shienok, A.I.; Koltsova, L.S.; Sherbakova, I.M.; Mardaleishvili, I.R.; Berlin, A.A. Kinetics of photochemical reactions of biphotochromic compounds based on spironaphthopyran—Conjugation effect. Photochem. Photobiol. Sci. 2016, 15, 382–388. [Google Scholar] [CrossRef]
- Zaichenko, N.L.; Shienok, A.I.; Kol’tsova, L.S.; Lyubimov, A.V.; Mardaleishvili, I.R.; Retivov, V.M.; Belus, S.K.; Ait, A.O. Synthesis of triarylimidazole hybrid compound with switchable luminescence. Rus. J. Gen. Chem. 2016, 86, 1022–1027. [Google Scholar] [CrossRef]
- Berkovic, G.; Krongauz, V.; Weiss, V. Spiropyrans and spirooxazines for memories and switches. Chem. Rev. 2000, 100, 1741–1754. [Google Scholar] [CrossRef]
- Kadowaki, S. Photochromic Lens for Eye Glasses. U.S. Patent 9,335,566, 10 May 2016. [Google Scholar]
- Xie, X.; Crespo, G.A.; Mistlberger, G.; Bakker, E. Photocurrent generation based on a light-driven proton pump in an artificial liquid membrane. Natur. Chem. 2014, 6, 202–207. [Google Scholar] [CrossRef]
- Xie, X.; Bakker, E. Creating electrochemical gradients by light: From bio-inspired concepts to photoelectric conversion. Phys. Chem. Chem. Phys. 2014, 16, 19781–19789. [Google Scholar] [CrossRef]
- Xie, X.; Mistlberger, G.N.; Bakker, E. Reversible photodynamic chloride-selective sensor based on photochromic spiropyran. J. Amer.Chem. Soc. 2012, 134, 16929–16932. [Google Scholar] [CrossRef] [PubMed]
- Florea, L.; Hennart, A.; Diamond, D.; Benito-Lopez, F. Synthesis and characterisation of spiropyran-polymer brushes in micro-capillaries: Towards an integrated optical sensor for continuous flow analysis. Sens. Actuators B Chem. 2012, 175, 92–99. [Google Scholar] [CrossRef]
- Dunne, A.; Delaney, C.; McKeon, A.; Nesterenko, P.; Paull, B.; Benito-Lopez, F.; Diamond, D.; Florea, L. Micro-Capillary Coatings Based on Spiropyran Polymeric Brushes for Metal Ion Binding, Detection, and Release in Continuous Flow. Sensors 2018, 18, 1083. [Google Scholar] [CrossRef] [PubMed]
- Liubimov, A.V.; Venidiktova, O.V.; Valova, T.M.; Shienok, A.I.; Koltsova, L.S.; Liubimova, G.V.; Popov, L.D.; Zaichenko, N.L.; Barachevsky, V.A. Photochromic and luminescence properties of a hybrid compound based on indoline spiropyran of the coumarin type and azomethinocoumarin. Photochem. Photobiol. Sci. 2018, 17, 1365–1375. [Google Scholar] [CrossRef]
- Chibisov, A.K.; Gorner, H. Complexes of spiropyran-derived merocyanins with metal ions: Relaxation kinetics, photochrmistry and solvent effect. Chem. Phys. 1998, 257, 425–442. [Google Scholar] [CrossRef]
- Paramonov, S.V.; Lokshin, V.; Fedorova, O.A. Spiropyran. Chromene or spirooxazine ligands: Insight into mutual relations between complexing and photochromic properties. J. Photochem. Photobiol. C 2011, 12, 209–236. [Google Scholar] [CrossRef]
- Barachevsky, V.A. Advances in photonics of organic photochromism. J. Photochem. Photobiol. A Chem. 2018, 354, 61–69. [Google Scholar] [CrossRef]
- Devaraj, S.; Tsui, Y.-K.; Chiang, C.-Y.; Yen, Y.-P. A new dual functional sensor: Highly selective colorimetric chemosensor for Fe3+ and fluorescent sensor for Mg2+. Spectrochim. Acta A 2012, 96, 594–599. [Google Scholar] [CrossRef]
- Dong, Y.; Li, J.; Jiang, X.; Song, F.; Cheng, Y.; Zhu, C. Na+ Triggered Fluorescence Sensors for Mg2+ Detection Based on a Coumarin Salen Moiety. Org. Lett. 2011, 13, 2252–2255. [Google Scholar] [CrossRef]
- Mylonas-Margaritis, I.; Maniaki, D.; Mayans, J.; Ciammaruchi, L.; Bekiari, V.; Raptopoulou, C.P.; Psycharis, V.; Christodoulou, S.; Escue, A.; Perlepes, S.P. Mononuclear Lanthanide(III)-Salicylideneaniline, Complexes: Synthetic, Structural, Spectroscopic, Magnetic Studies. Magnetochemistry 2018, 4, 45. [Google Scholar] [CrossRef]
- Matozzo, P.; Colombo, A.; Dragonetti, C.; Righetto, S.; Roberto, D.; Biagini, P.; Fantacci, S.; Marinotto, D. A Chiral Bis(salicylaldiminato)zinc(II) Complex with Second-Order Nonlinear Optical and Luminescent Properties in Solution. Inorganics 2020, 8, 25. [Google Scholar] [CrossRef]
- Kulkarni, A.; Avaji, P.G.; Bagihalli, G.B.; Patil, S.A.; Badami, P.S. Synthesis, spectral, electrochemical and biological studies of Co(II), Ni(II) and Cu(II) complexes with Schiff bases of 8-formyl-7-hydroxy-4-methyl coumarin. J. Coord. Chem. 2013, 62, 481–492. [Google Scholar] [CrossRef]
- Prabhakara, C.T.; Patil, S.A.; Toragalmath, S.S.; Kinnal, S.M.; Badami, P.S. Synthesis, characterization and biological approach of metal chelates of some first row transition metal ions with halogenated bidentate coumarin Schiff bases containing N and O donor atoms. J. Photochem. Photobiol. B 2016, 157, 1–14. [Google Scholar] [CrossRef]
- Traven, V.F.; Miroshnikov, V.S.; Chibisova, T.A.; Barachevsky, V.A.; Venidiktova, O.V.; Strokach, Y.P. Synthesis and structure of indoline spiropyrans of the coumarin series. Rus. Chem. Bull. 2005, 54, 2417–2424. [Google Scholar] [CrossRef]
- Barachevsky, V.A. Photochromic spirocompounds and chromenes for sensing metal ions. Rev. J. Chem. 2013, 3, 52–94. [Google Scholar] [CrossRef]
- Zgierski, M.Z.; Grabowska, A. Photochromism of salicylideneaniline (SA). How the photochromic transient is created: A theoretical approach J. Chem. Phys. 2000, 112, 6329–6337. [Google Scholar] [CrossRef]
- Fabian, W.M.; Antonov, L.; Nedeltcheva, D.; Kamounah, F.S.; Taylor, P.J. Tautomerism in hydroxynaphthaldehyde anils and azo analogues: A combined experimental and computational study. J. Chem. Phys. A 2004, 108, 7603–7612. [Google Scholar] [CrossRef]
- Ziolek, M.; Gil, M.; Organero, J.A.; Douhal, A. What is the difference between the dynamics of anion- and keto-type of photochromic salicylaldehyde azine? Physi. Chem. Chem. Phys. 2019, 12, 2107–2115. [Google Scholar] [CrossRef]
- Shulman, S.G.; Rosenberg, L.S. Tautomerization kinetics of 7-hydroxy-4-methylcoumarin in the excited singlet state. J. Phys. Chem. 1979, 83, 447–451. [Google Scholar] [CrossRef]
- Nizomov, N.; Kholov, A.U.; Ishchenko, A.A.; Ishchenko, V.V.; Khilya, V.P. Electronic structure and spectral fluorescence properties of umbelliferone and herniarin. J. Appl. Spectrosc. 2007, 74, 626–634. [Google Scholar] [CrossRef]
- De Silva, N.; Minezava, N.; Gordon, M.S. Excited-state hydrogen atom transfer reaction in solvated 7-hydroxy-4methylcoumarin. J. Phys. Chem. B 2017, 117, 15386–15394. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moriya, T. Excited-state reaction of coumarins. VII. The solvent-dependent fluorescence of 7-hydroxycoumarins. Bull. Chem. Soc. Jpn. 1988, 61, 1873–1886. [Google Scholar] [CrossRef]
- Foresman, J.B.; Frisch, A. Exploring Chemistry with Electronic Structure Methods; Gaussian; Inc.: Wallingford, CT, USA, 2015. [Google Scholar]
- Cances, E.; Mennucci, B.; Tomasi, J. A new integral equation formalism for the polarizable continuum model: Theoretical background and applications to isotropic and anisotropic dielectrics. Chem. Phys. 1997, 107, 3032–3041. [Google Scholar] [CrossRef]
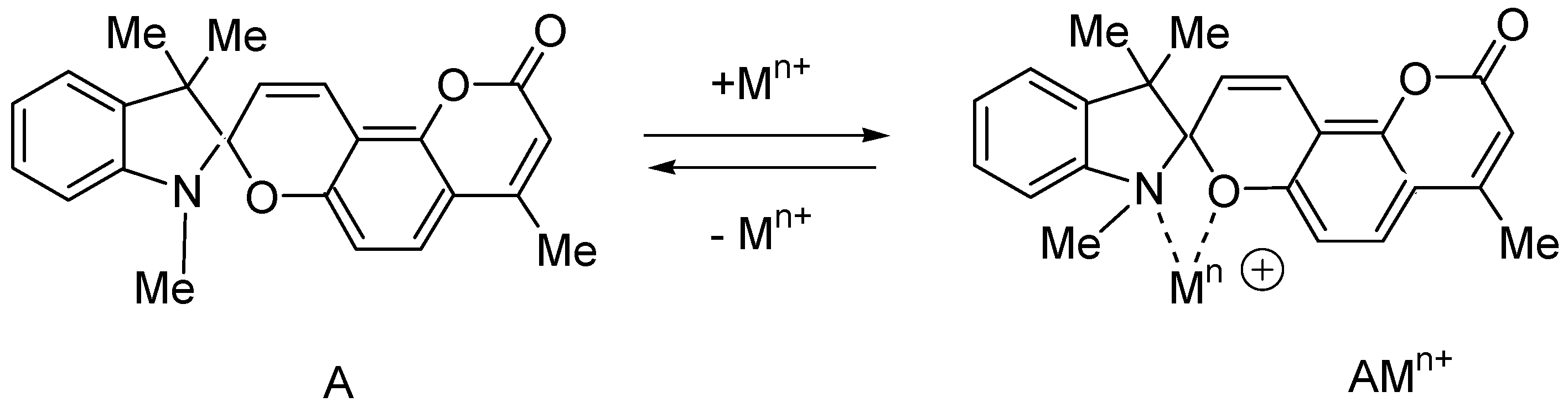
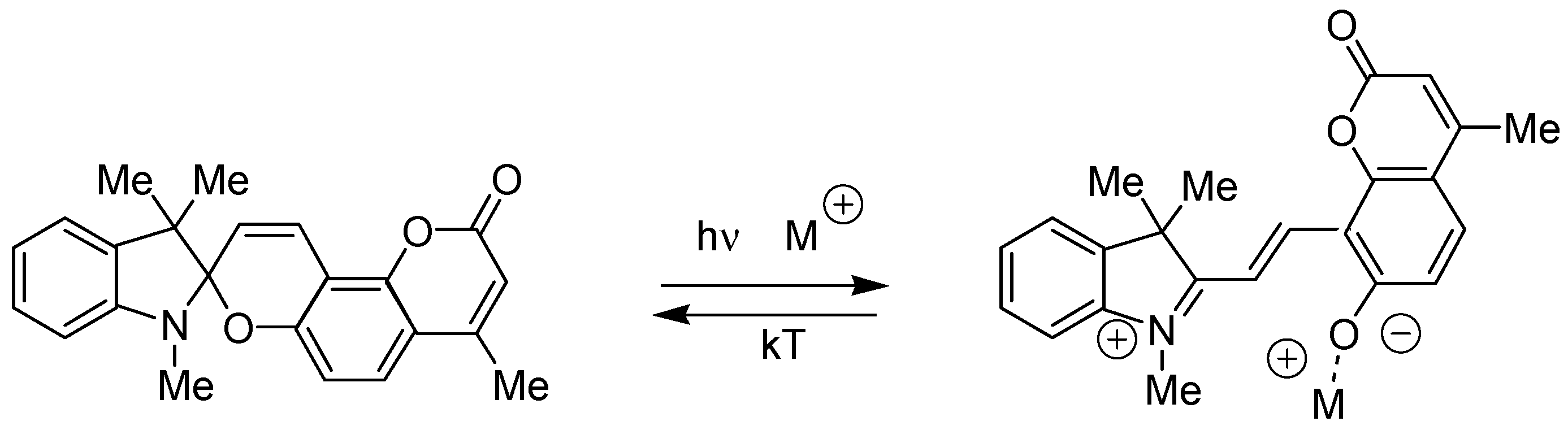
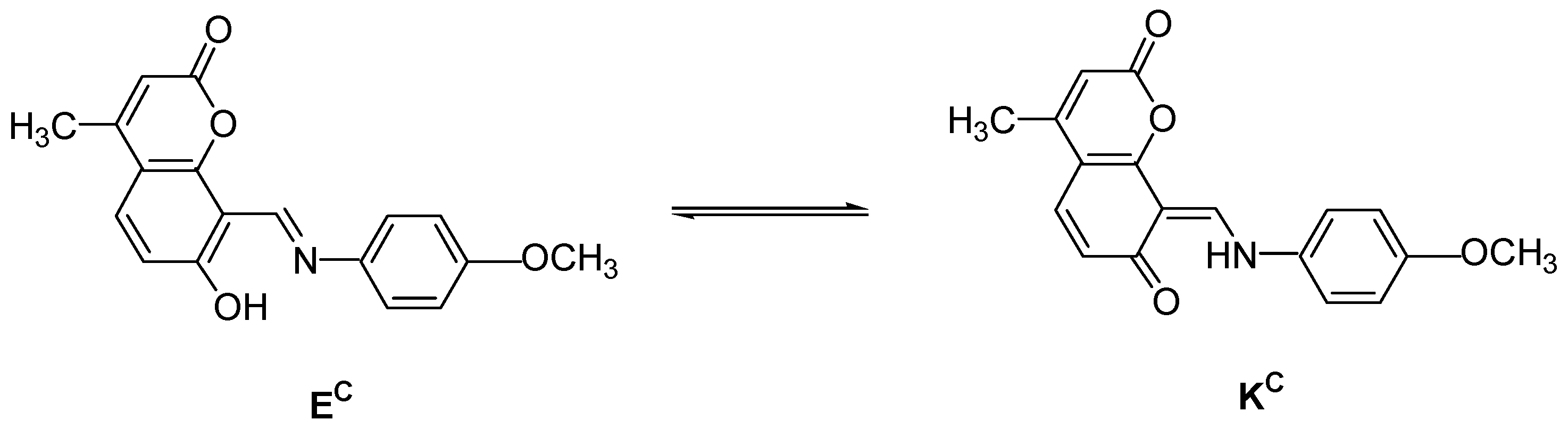
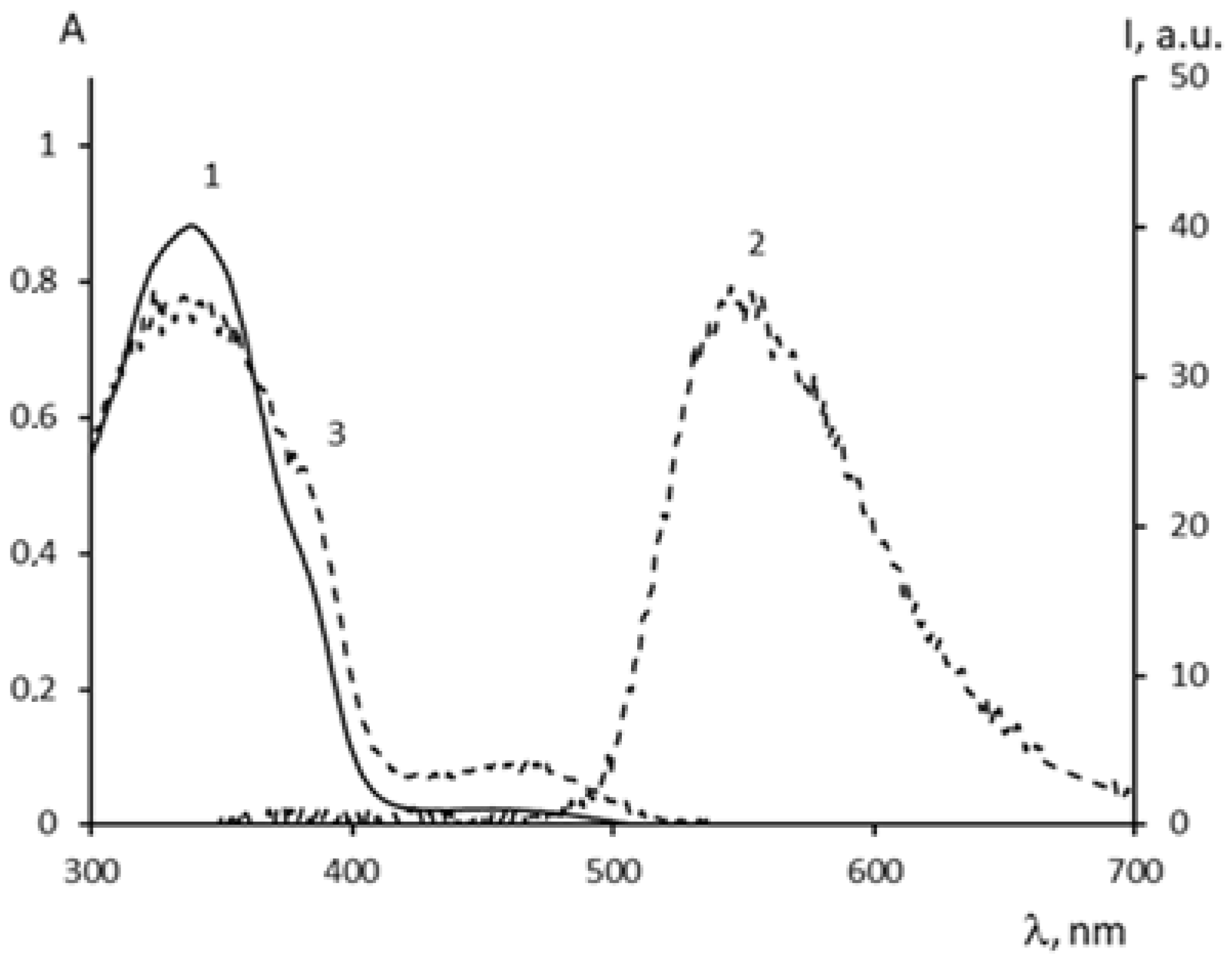
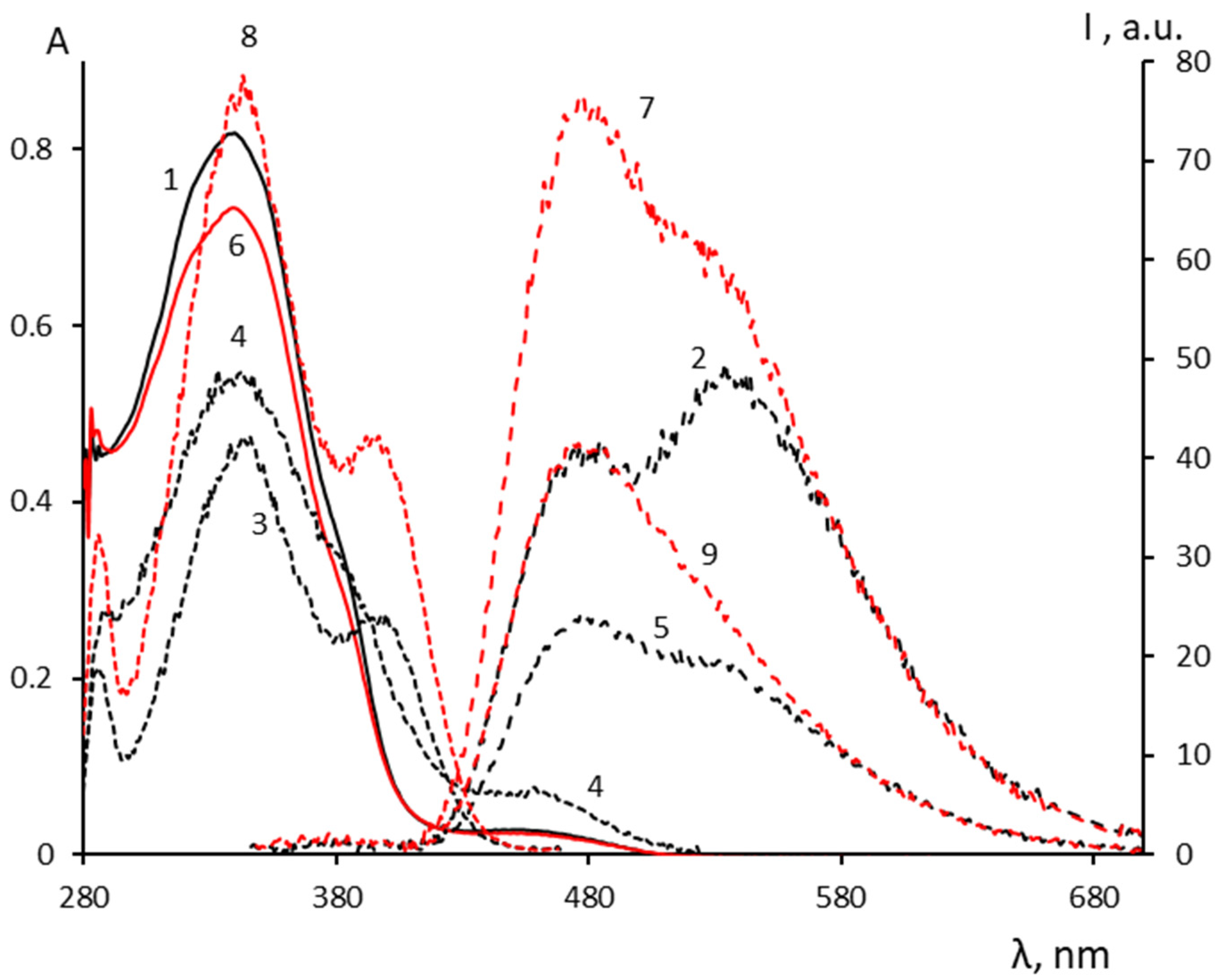
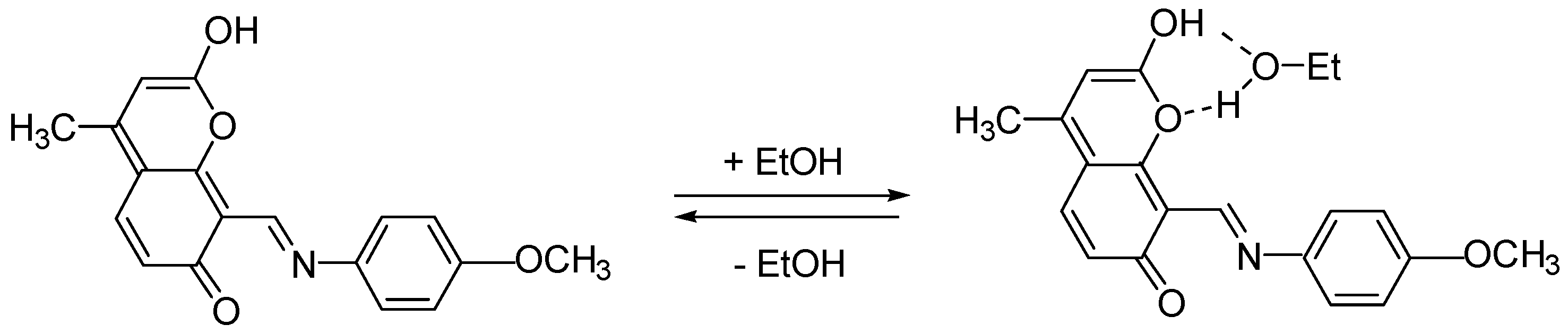
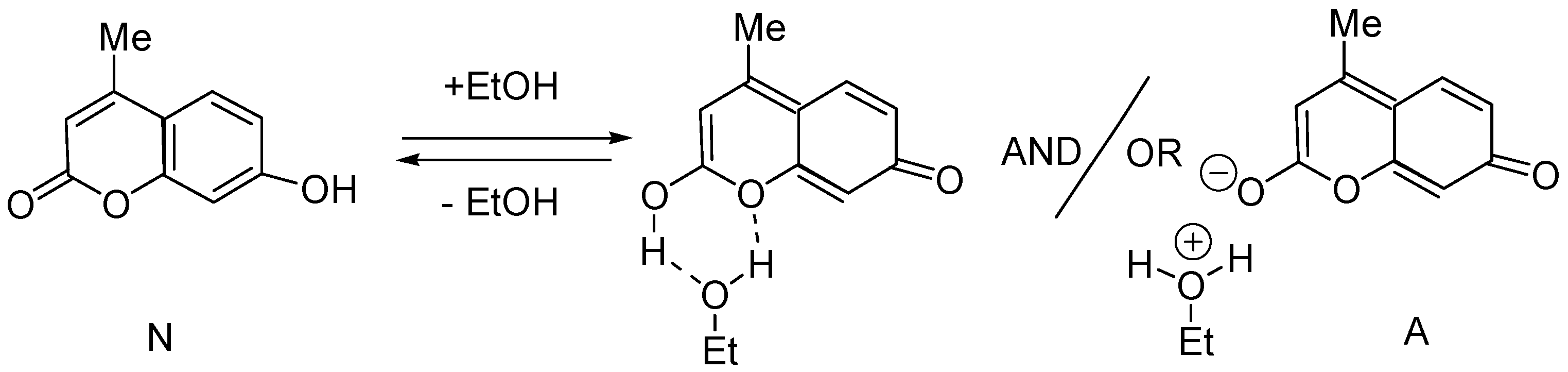

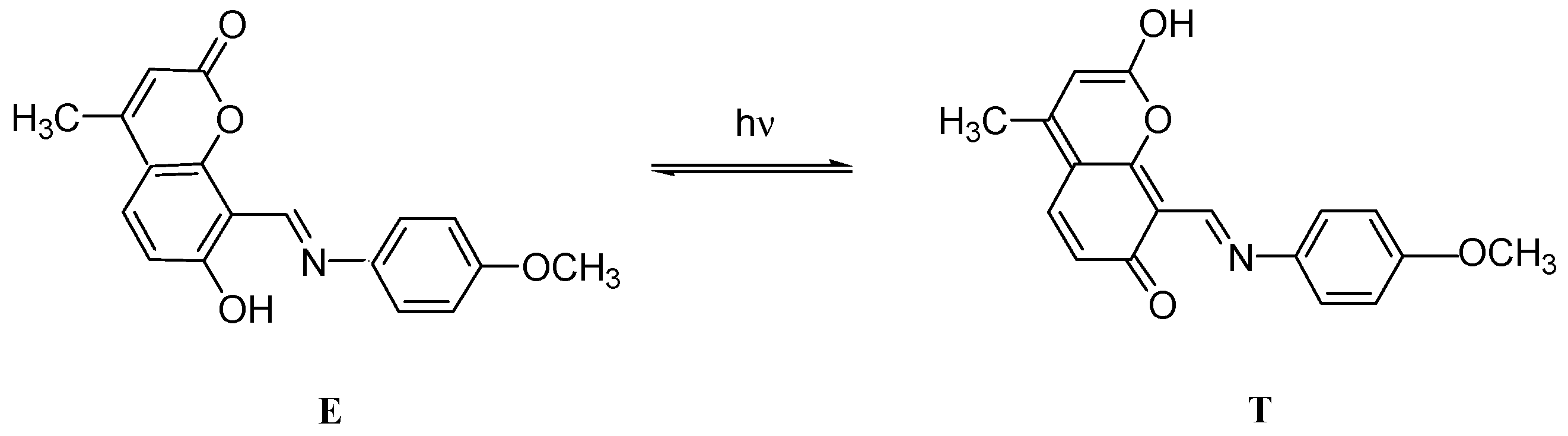
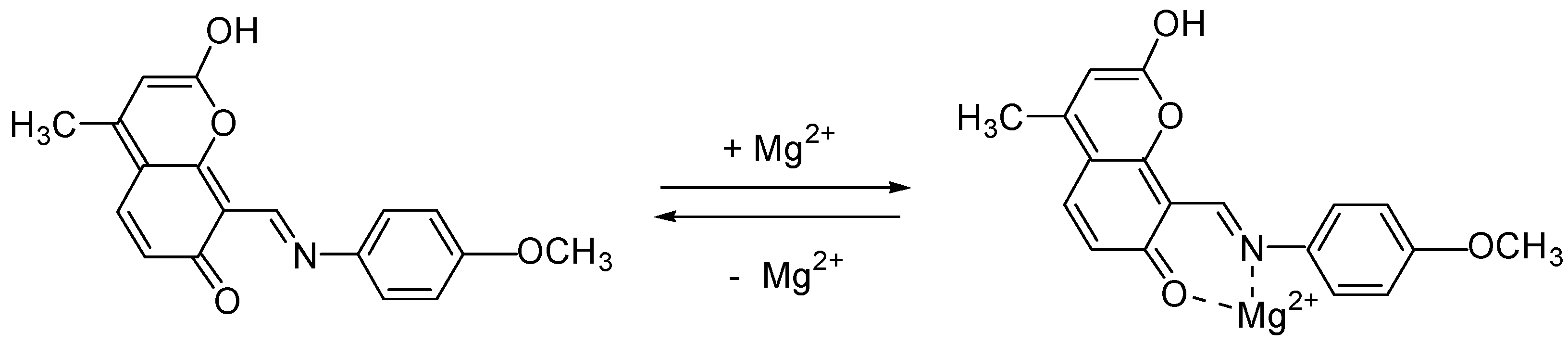
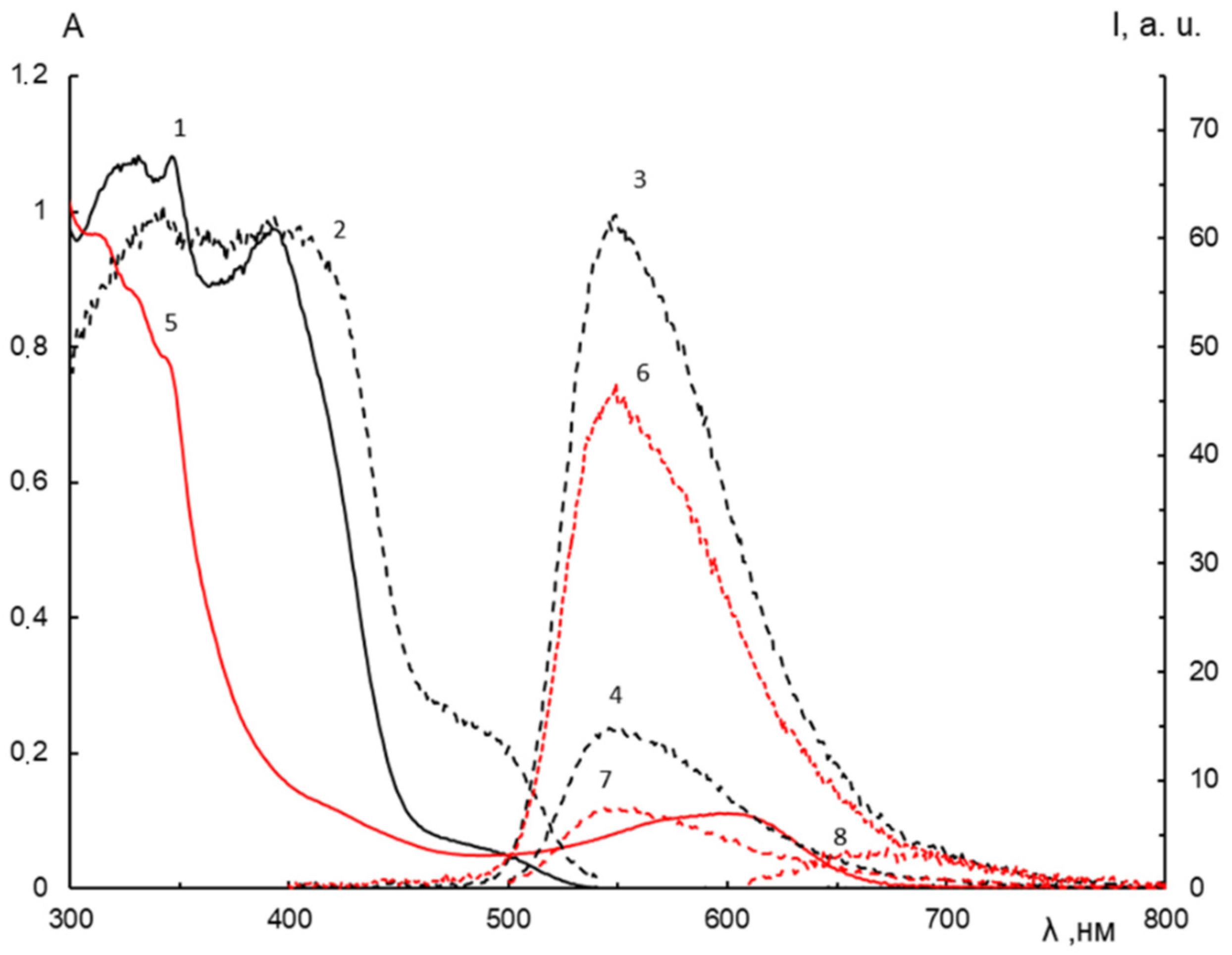

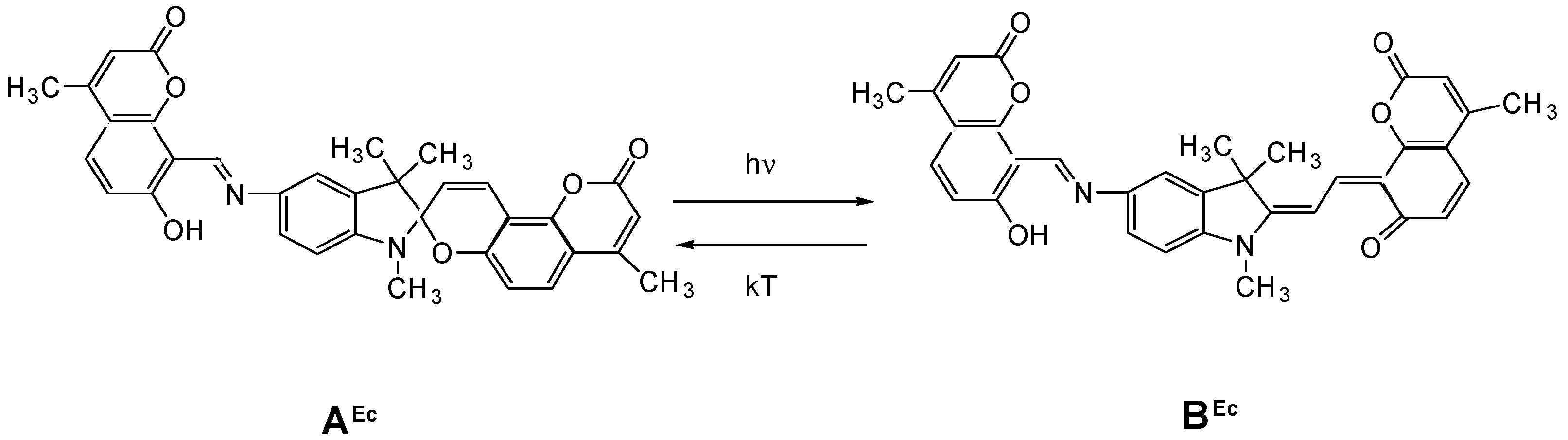
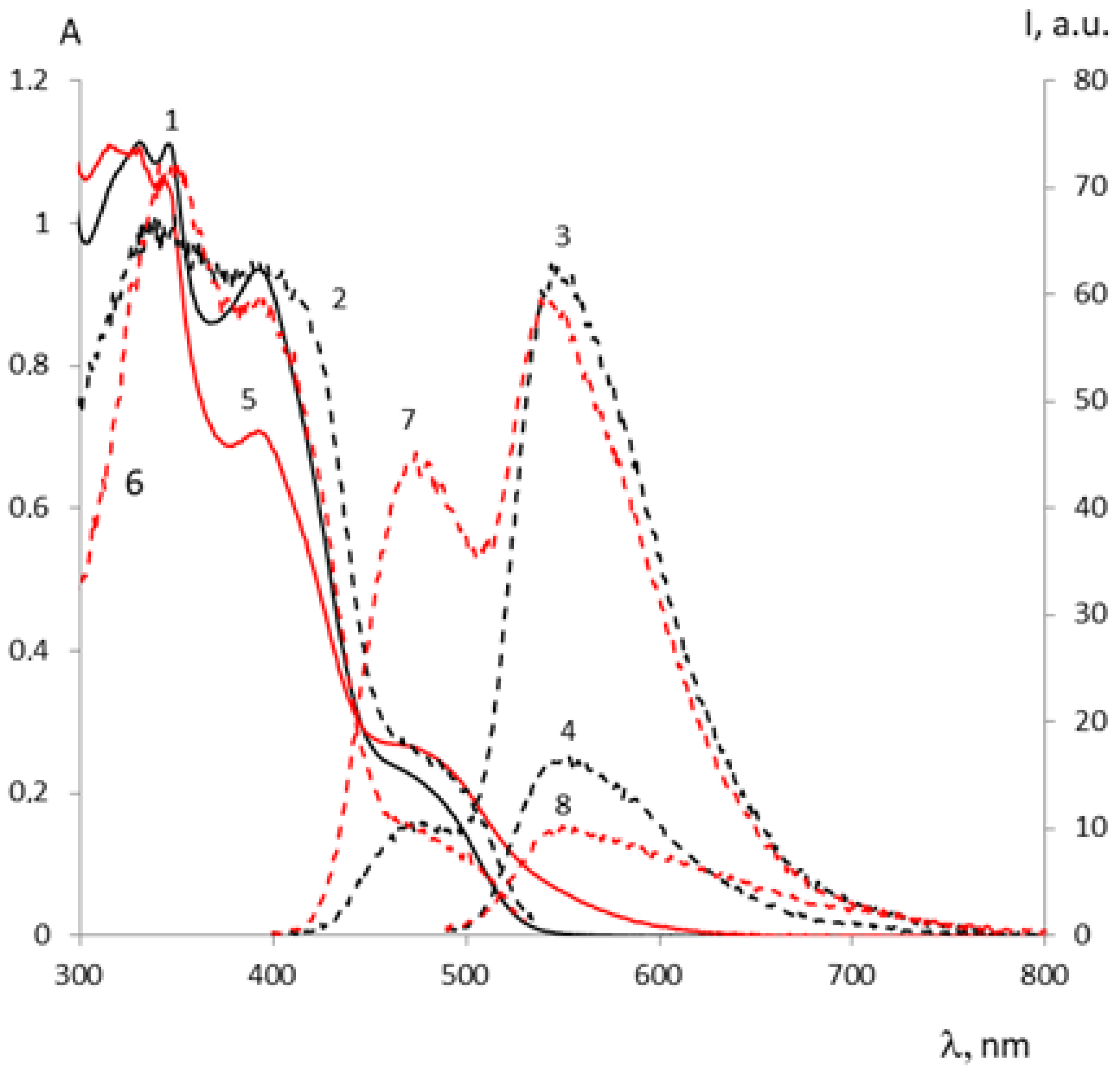
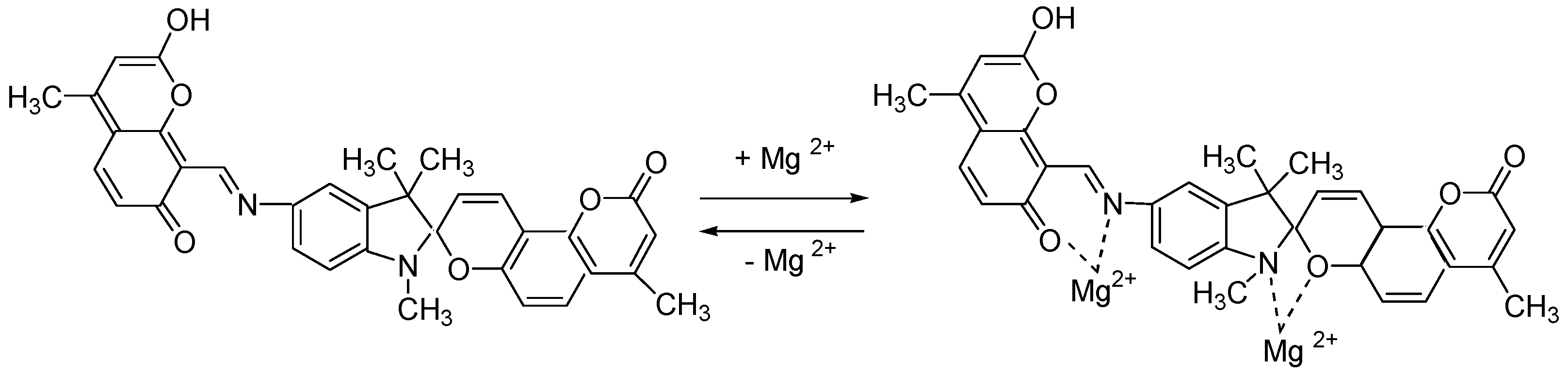

| Metal | λAmax, nm (DAmax) | λex.fl, nm | λflmax, nm before UV (Iflmax, a.u.) | λflmax, nm after UV (Iflmax, a.u.) |
|---|---|---|---|---|
| - | 338 (0.88), 380 (0.41 sh), 457 (0.02) | 338 | 545 (36) | 545 (31) |
| Li+ | 339 (0.81), 379 (0.3 sh), 461 (0.02) | 338 | 548 (36) | 548 (31) |
| Ca2+ | 339 (0.92), 382 (0.41 sh), 459 (0.04) | 338 | 545 (36) | 545 (33) |
| Zn2+ | 317 (0.49), 341 (0.48), 457 (0.02) | 338 | 485 (29 sh) | 485 (25 sh) |
| 338 | 528 (39) | 528 (35) | ||
| Mg2+ | 338 (0.82), 386 (0.30 sh), 463 (0.03) | 338 | 477 (41) 534 (50) | 477 (77) 528 (62 sh) |
| 399 | 477 (2) 528 (20 sh) | 477 (42) 528 (35) |
| λAmax, nm (DAmax) | λB max, nm | DBphot | ± ΔλB, nm | |
|---|---|---|---|---|
| - | 330 (1.08), 345 (1.08), 390 (0.97), 480 (0.07 sh) | 600 | 0.11 | - |
| Li+ | 330 (1.10), 345 (1.10), 390 (0.99), 480 (0.07 sh) | 580 | 0.01 | −20 |
| Ca2+ | 330 (1.13), 345 (1.15), 390 (0.93), 460 (0.29 sh) | 560 | 0.02 | −40 |
| Mg2+ | 330 (1.10), 345 (1.10), 390 (0.93), 480 (0.23 sh) | 540 | 0.07 | −60 |
| Metal | λ1fl.max, nm | I1fl.max, a. u. | λ2fl.max, nm | I2fl.max, a.u. |
|---|---|---|---|---|
| - | 550 | 61 | 550 | 46 |
| Li+ | 550 | 59 | 550 | 56 |
| Ca2+ | 550 | 55 | 550 | 53 |
| Zn2+ | 545 | 57 | 490 sh 545 | 23 35 |
| Mg2+ | 475 545 | 10 63 | 475 540 | 45 59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaichenko, N.L.; Valova, T.M.; Venidiktova, O.V.; Lyubimov, A.V.; Shienok, A.I.; Koltsova, L.S.; Ayt, A.O.; Lyubimova, G.V.; Popov, L.D.; Barachevsky, V.A. Excited State Proton Transfers in Hybrid Compound Based on Indoline Spiropyran of the Coumarin Type and Azomethinocoumarin in the Presence of Metal Ions. Molecules 2021, 26, 6894. https://doi.org/10.3390/molecules26226894
Zaichenko NL, Valova TM, Venidiktova OV, Lyubimov AV, Shienok AI, Koltsova LS, Ayt AO, Lyubimova GV, Popov LD, Barachevsky VA. Excited State Proton Transfers in Hybrid Compound Based on Indoline Spiropyran of the Coumarin Type and Azomethinocoumarin in the Presence of Metal Ions. Molecules. 2021; 26(22):6894. https://doi.org/10.3390/molecules26226894
Chicago/Turabian StyleZaichenko, Natalia L., Tatyana M. Valova, Olga V. Venidiktova, Alexander V. Lyubimov, Andrey I. Shienok, Liubov S. Koltsova, Anton O. Ayt, Galina V. Lyubimova, Leonid D. Popov, and Valery A. Barachevsky. 2021. "Excited State Proton Transfers in Hybrid Compound Based on Indoline Spiropyran of the Coumarin Type and Azomethinocoumarin in the Presence of Metal Ions" Molecules 26, no. 22: 6894. https://doi.org/10.3390/molecules26226894
APA StyleZaichenko, N. L., Valova, T. M., Venidiktova, O. V., Lyubimov, A. V., Shienok, A. I., Koltsova, L. S., Ayt, A. O., Lyubimova, G. V., Popov, L. D., & Barachevsky, V. A. (2021). Excited State Proton Transfers in Hybrid Compound Based on Indoline Spiropyran of the Coumarin Type and Azomethinocoumarin in the Presence of Metal Ions. Molecules, 26(22), 6894. https://doi.org/10.3390/molecules26226894