Insight into Biomass Upgrade: A Review on Hydrogenation of 5-Hydroxymethylfurfural (HMF) to 2,5-Dimethylfuran (DMF)
Abstract
:1. Introduction
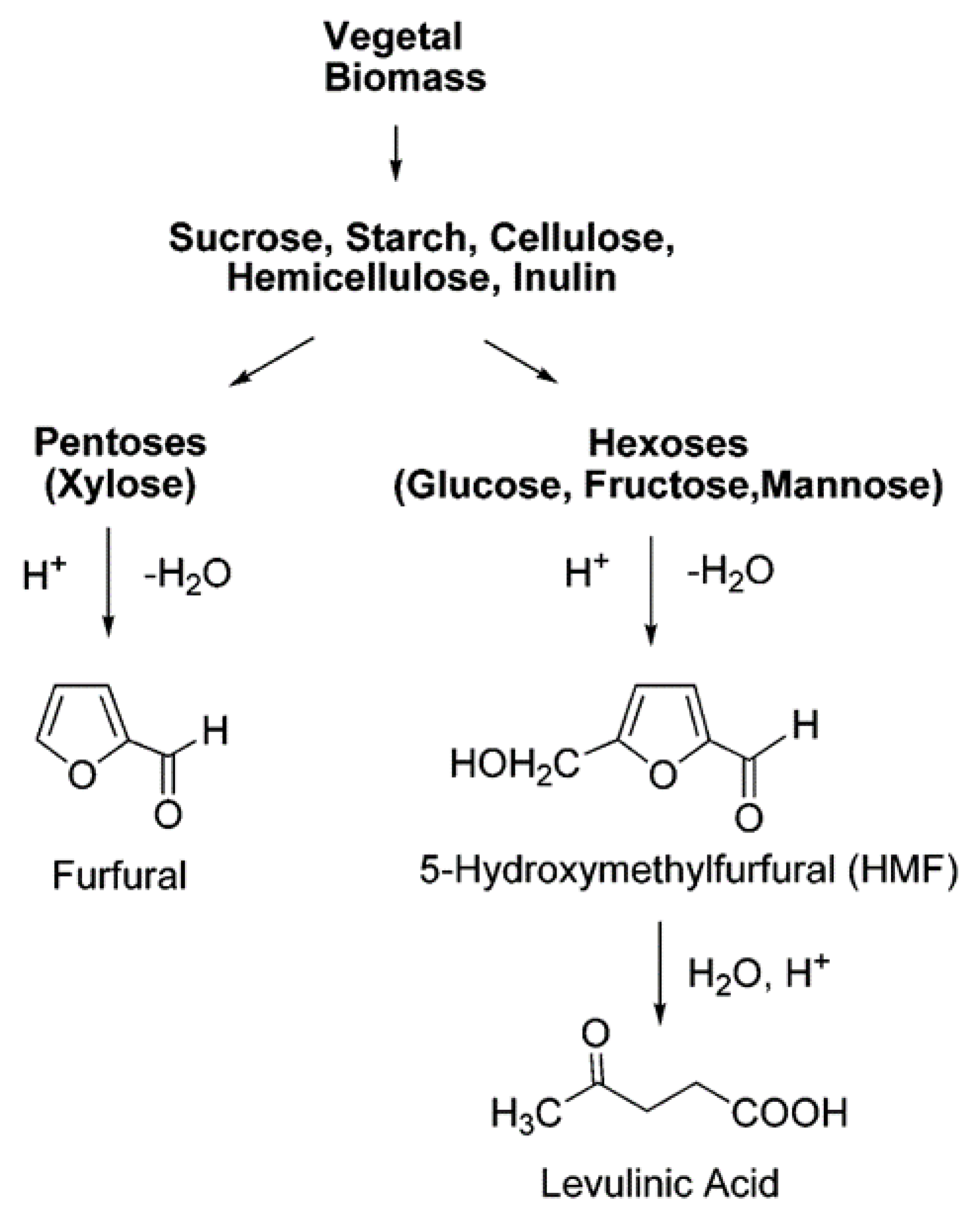
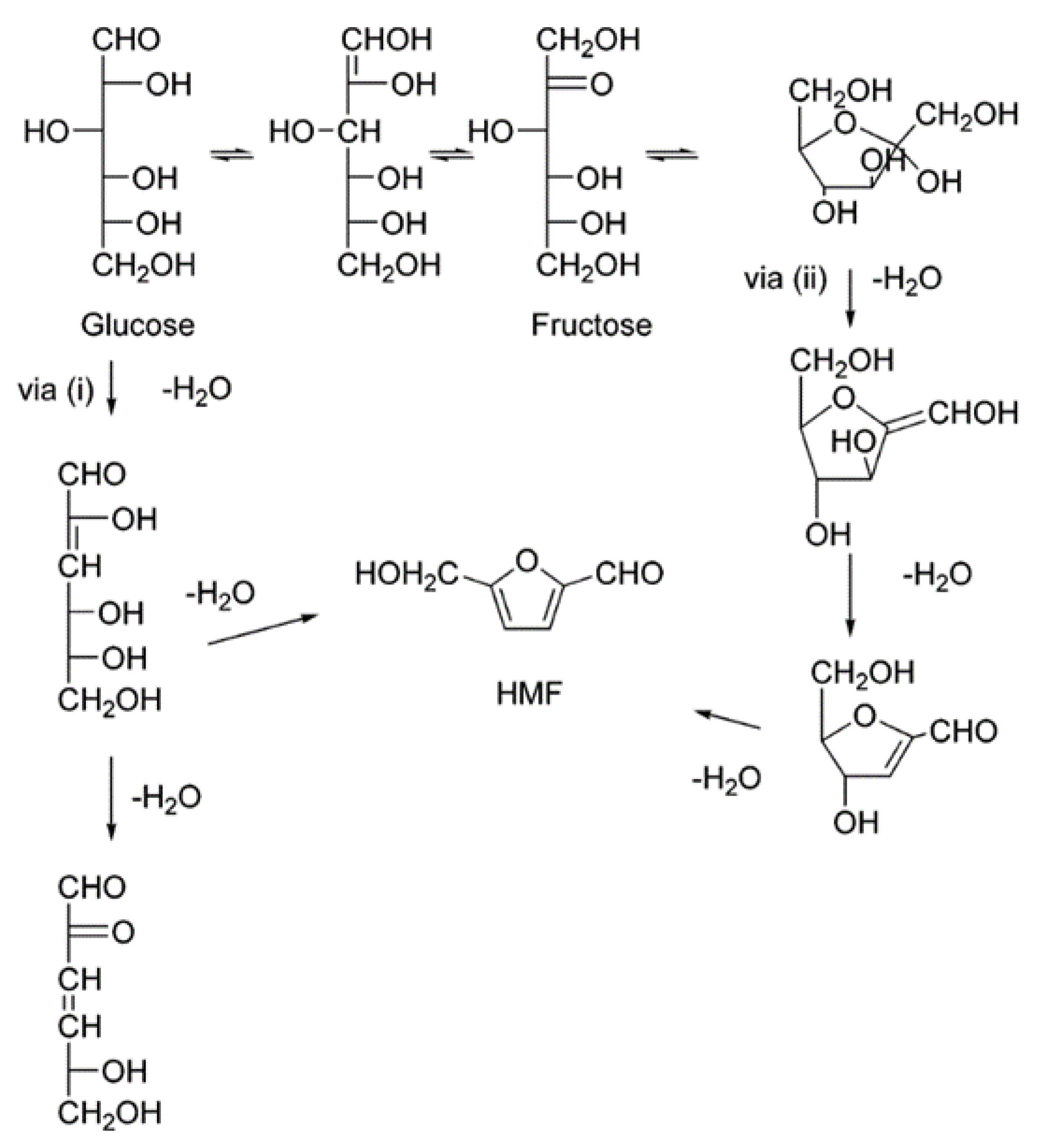
2. Production of HMF
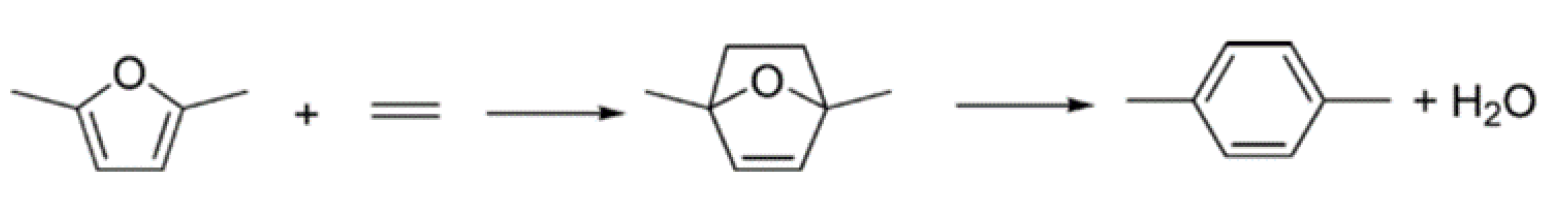
3. DMF as an Alternative Fuel
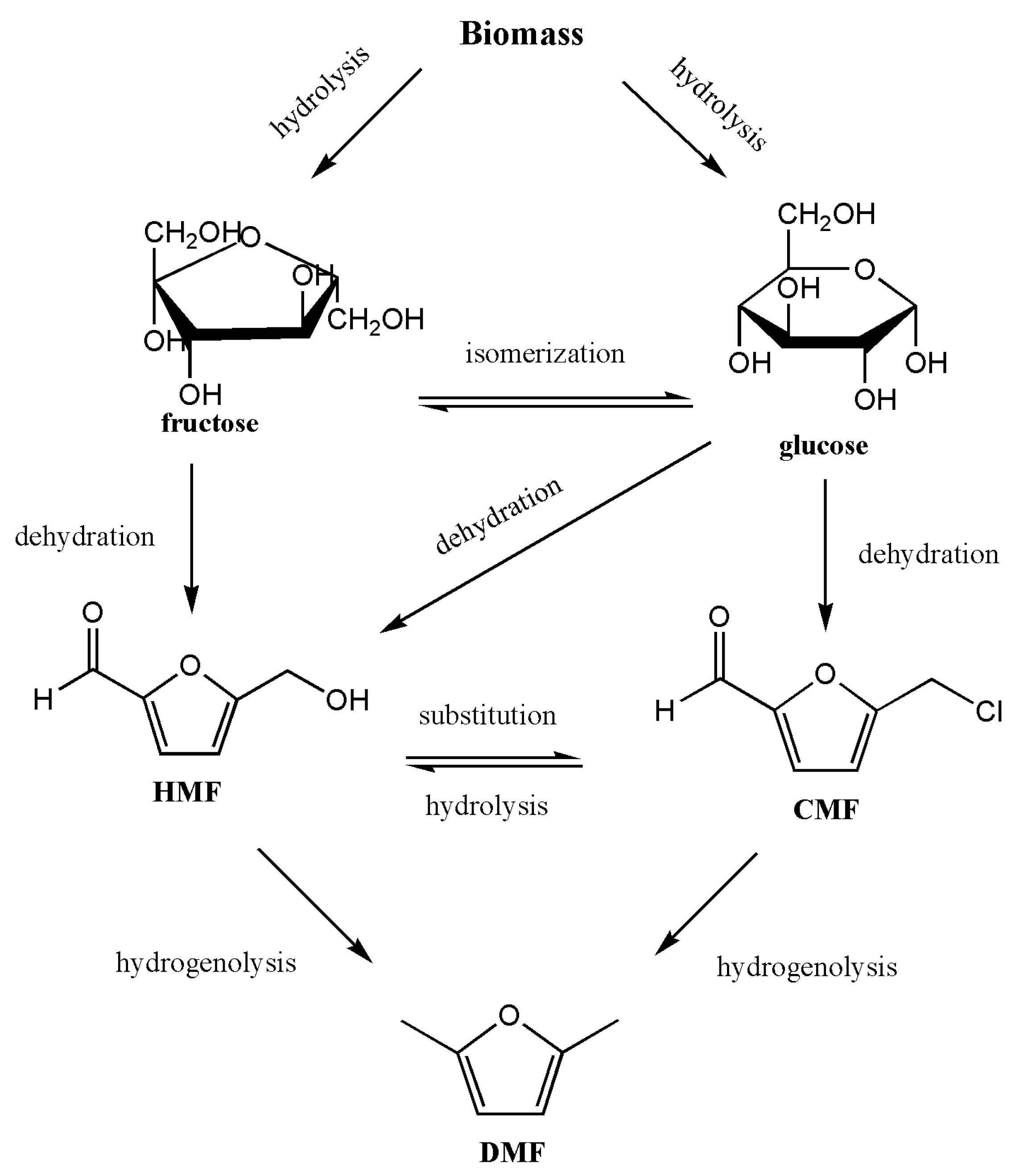
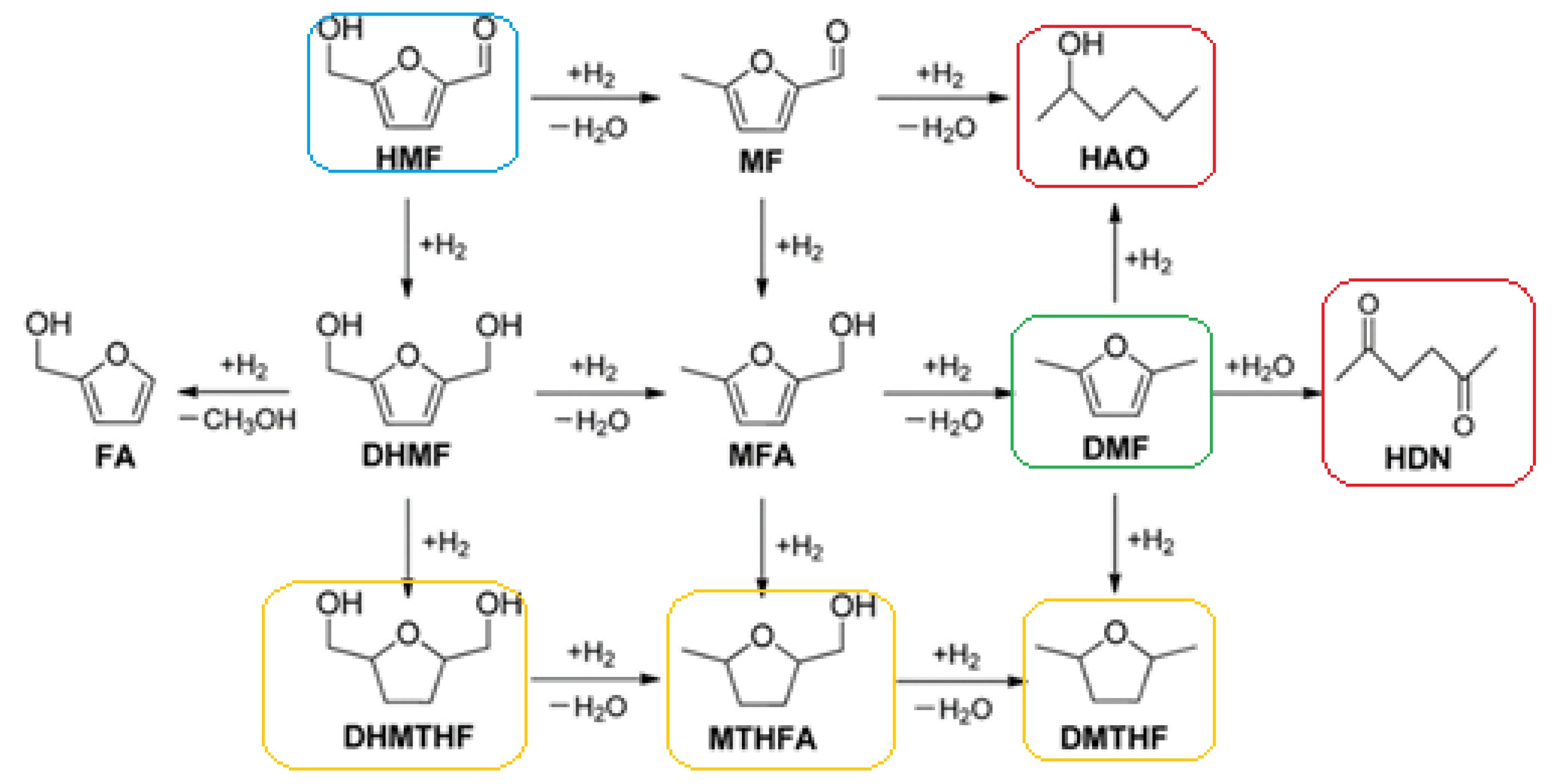
4. Production of DMF
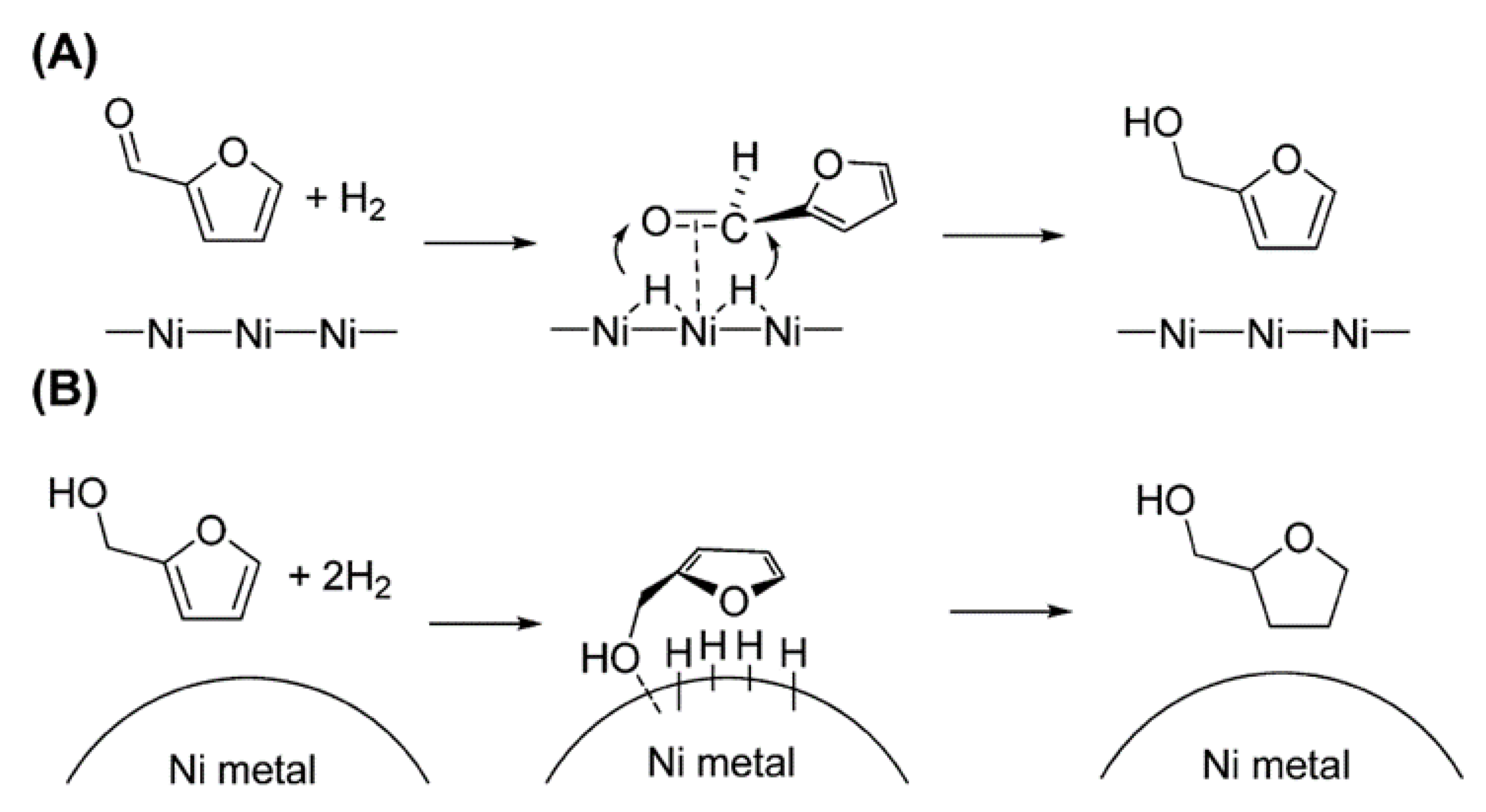
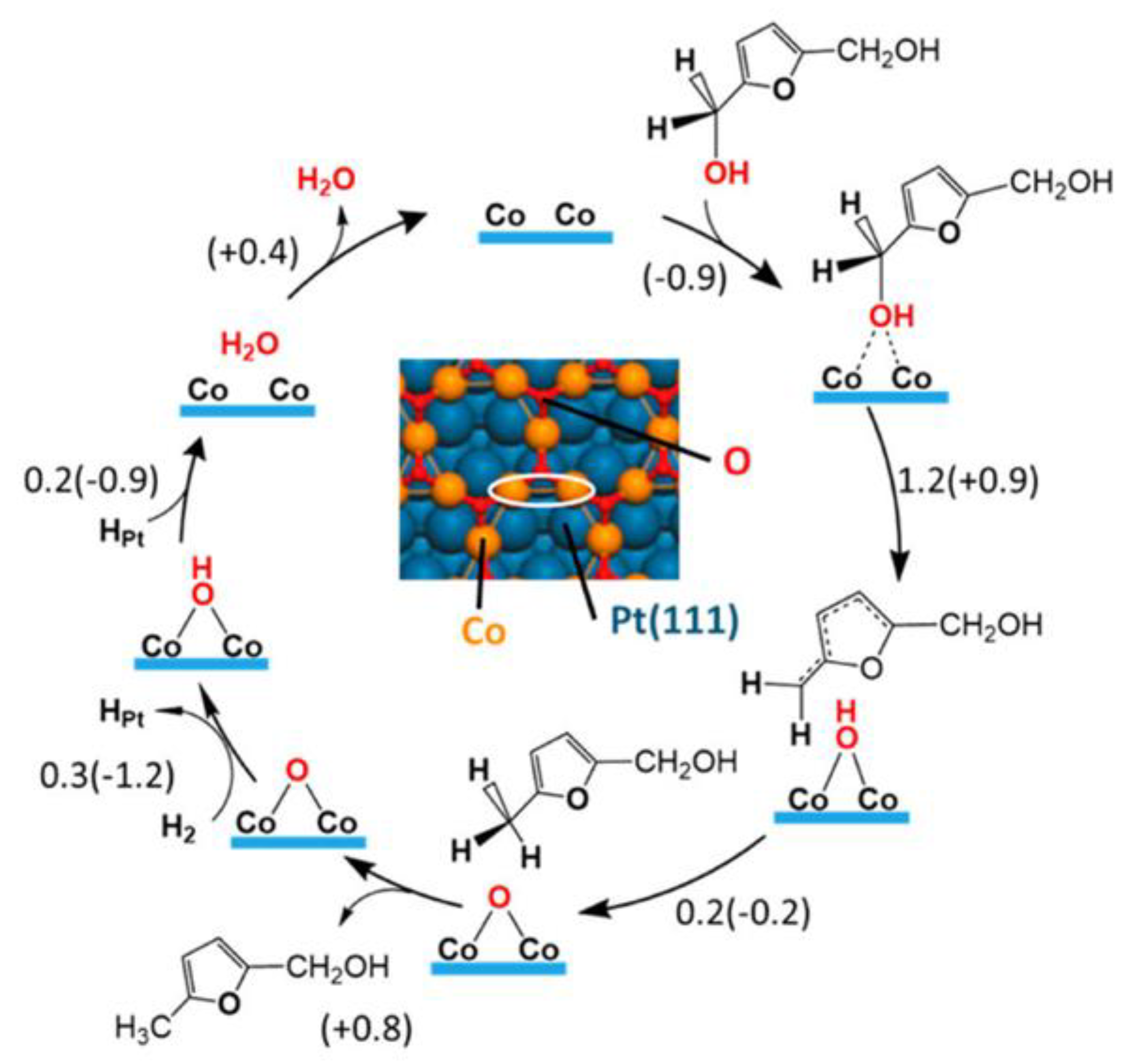
5. Mechanism of HMF Hydrogenation to DMF
6. Catalytic Conversion of HMF to DMF
6.1. Hydrogenation with Noble Metal Catalysts
6.2. Hydrogenation Reactions with a Transitional Metal Catalyst
6.3. Hydrogenation Reactions with Bimetallic Catalysts
6.4. The Role of a Support
6.5. The Role of Solvents
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, W.-Y.; Suzuki, T.; Lackner, M. Handbook of Climate Change Mitigation and Adaptation; Springer: New York, NY, USA, 2016. [Google Scholar]
- Balat, M. An overview of biofuels and policies in the European Union. Energy Sources Part B Econ. Plan. Policy 2007, 2, 167–181. [Google Scholar] [CrossRef]
- Gallezot, P. Conversion of biomass to selected chemical products. Chem. Soc. Rev. 2012, 41, 1538–1558. [Google Scholar] [CrossRef]
- Chakar, F.S.; Ragauskas, A. Review of current and future softwood kraft lignin process chemistry. Ind. Crop. Prod. 2004, 20, 131–141. [Google Scholar] [CrossRef]
- Zakzeski, J.; Bruijnincx, P.C.A.; Jongerius, A.L.; Weckhuysen, B.M. The Catalytic Valorization of Lignin for the Production of Renewable Chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef]
- Wyman, C.; Decker, S.; Himmel, M.; Brady, J.; Skopec, C.; Viikari, L. Hydrolysis of Cellulose and Hemicellulose. Polysaccharides 2004, 1, 1023–1062. [Google Scholar]
- Lewkowski, J. Synthesis, chemistry and applications of 5-hydroxymethylfurfural and its derivatives. ARKIVOC 2001, 1, 17–54. [Google Scholar] [CrossRef] [Green Version]
- Besson, M.; Gallezot, P.; Pinel, C. Conversion of Biomass into Chemicals over Metal Catalysts. Chem. Rev. 2014, 114, 1827–1870. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohy-drates-the US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Bozell, J.J. An evolution from pretreatment to fractionation will enable successful development of the integrated biorefinery. Bio. Resour. 2010, 5, 1326–1327. [Google Scholar]
- Zeitsch, K.J. The Chemistry and Technology of Furfural and Its Many by-Products; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Wettstein, S.G.; Alonso, D.M.; I Gürbüz, E.; A Dumesic, J. A roadmap for conversion of lignocellulosic biomass to chemicals and fuels. Curr. Opin. Chem. Eng. 2012, 1, 218–224. [Google Scholar] [CrossRef]
- Román-Leshkov, Y.; Barrett, C.J.; Liu, Z.Y.; Dumesic, J.A. Production of dimethylfuran for liquid fuels from biomass-derived carbohydrates. Nature 2007, 447, 982–985. [Google Scholar] [CrossRef] [PubMed]
- Rosatella, A.; Simeonov, S.; Frade, R.; Afonso, C.A.M. 5-Hydroxymethylfurfural (HMF) as a building block platform: Biological properties, synthesis and synthetic applications. Green Chem. 2011, 13, 754–793. [Google Scholar] [CrossRef]
- Shaw, P.E.; Tatum, J.H.; Berry, R.E. Acid-catalyzed degradation of d-fructose. Carbohydr. Res. 1967, 5, 266–273. [Google Scholar] [CrossRef]
- Tong, X.; Ma, Y.; Li, Y. Biomass into chemicals: Conversion of sugars to furan derivatives by catalytic processes. Appl. Catal. A Gen. 2010, 385, 1–13. [Google Scholar] [CrossRef]
- Zhao, H.; Holladay, J.E.; Brown, H.; Zhang, Z.C. Metal Chlorides in Ionic Liquid Solvents Convert Sugars to 5-Hydroxymethylfurfural. Science 2007, 316, 1597–1600. [Google Scholar] [CrossRef] [PubMed]
- Antal, M.J.; Mok, W.S.; Richards, G.N. Mechanism of formation of 5-(hydroxymethyl)-2-furaldehyde from d-fructose and sucrose. Carbohydr. Res. 1990, 199, 91–109. [Google Scholar] [CrossRef]
- Van Dam, H.; Kieboom, A.; Van Bekkum, H. The conversion of fructose and glucose in acidic media: Formation of hy-droxymethylfurfural. Starch-Stärke 1986, 38, 95–101. [Google Scholar] [CrossRef]
- Kuster, B.F.M. 5-Hydroxymethylfurfural (HMF). A Review Focussing on its Manufacture. Starch-Stärke 1990, 42, 314–321. [Google Scholar] [CrossRef] [Green Version]
- Van Putten, R.J.; van der Waal, J.C.; de Jong, E.D.; Rasrendra, C.B.; Heeres, H.J.; de Vries, J.G. Hydroxymethylfurfural, A Versatile Platform Chemical Made from Renewable Resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef]
- Cottier, L.; Descotes, G. 5-(Hydroxymethyl) furfural Syntheses and Chemical Transformations. ChemInform 1994, 25, 939–944. [Google Scholar] [CrossRef]
- Hahn-Hägerdal, B.; Galbe, M.; Gorwa-Grauslund, M.F.; Liden, G.; Zacchi, G. Bio-ethanol–the fuel of tomorrow from the residues of today. Trends Biotechnol. 2006, 24, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Balat, M.; Balat, H. Recent trends in global production and utilization of bio-ethanol fuel. Appl. Energy 2009, 86, 2273–2282. [Google Scholar] [CrossRef]
- Dodić, S.N.; Popov, S.D.; Dodić, J.M.; Ranković, J.A.; Zavargo, Z.Z. Potential contribution of bioethanol fuel to the transport sector of Vojvodina. Renew. Sustain. Energy Rev. 2009, 13, 2197–2200. [Google Scholar] [CrossRef]
- Dumesic, J.A.; Rom, Y.; Chheda, J.N. Catalytic Process for Producing Furan Derivatives in a Biphasic Reactor. US Patent 7572925 B2, 6 June 2006. [Google Scholar]
- Saha, B.; Abu-Omar, M.M. Current Technologies, Economics, and Perspectives for 2,5-Dimethylfuran Production from Biomass-Derived Intermediates. ChemSusChem 2015, 8, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Gong, J.; Huang, Z. Review on the production methods and fundamental combustion characteristics of furan derivatives. Renew. Sustain. Energy Rev. 2016, 54, 1189–1211. [Google Scholar] [CrossRef]
- Zhong, S.; Daniel, R.; Xu, H.; Zhang, J.; Turner, D.; Wyszynski, M.L.; Richards, P. Combustion and Emissions of 2,5-Dimethylfuran in a Direct-Injection Spark-Ignition Engine. Energy Fuels 2010, 24, 2891–2899. [Google Scholar] [CrossRef]
- Binder, J.B.; Raines, R. Simple Chemical Transformation of Lignocellulosic Biomass into Furans for Fuels and Chemicals. J. Am. Chem. Soc. 2009, 131, 1979–1985. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.L.; Chang, C.-C.; Do, P.; Nikbin, N.; Caratzoulas, S.; Vlachos, D.; Lobo, R.F.; Fan, W.; Dauenhauer, P.J. Cycloaddition of Biomass-Derived Furans for Catalytic Production of Renewable p-Xylene. ACS Catal. 2012, 2, 935–939. [Google Scholar] [CrossRef]
- Wang, D.; Osmundsen, C.M.; Taarning, E.; Dumesic, J.A. Selective Production of Aromatics from Alkylfurans over Solid Acid Catalysts. ChemCatChem 2013, 5, 2044–2050. [Google Scholar] [CrossRef]
- Chang, C.-C.; Green, S.K.; Williams, C.L.; Dauenhauer, P.J.; Fan, W. Ultra-selective cycloaddition of dimethylfuran for renewable p-xylene with H-BEA. Green Chem. 2013, 16, 585–588. [Google Scholar] [CrossRef]
- Rinaldi, R.; Schüth, F. Acid Hydrolysis of Cellulose as the Entry Point into Biorefinery Schemes. ChemSusChem 2010, 3, 296. [Google Scholar] [CrossRef]
- Lei, H.; Yong, S.; Lu, L. Pathways and mechanisms of liquid fuel 2, 5-dimethylfuran from biomass. Prog. Chem. 2011, 23, 2079–2084. [Google Scholar]
- Dutta, S.; Mascal, M. Novel Pathways to 2,5-Dimethylfuran via Biomass-Derived 5-(Chloromethyl)furfural. ChemSusChem 2014, 7, 3028–3030. [Google Scholar] [CrossRef]
- Mascal, M.; Nikitin, E.B. Dramatic Advancements in the Saccharide to 5-(Chloromethyl)furfural Conversion Reaction. ChemSusChem 2009, 2, 859–861. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Emel’Yanenko, V.N.; Stepurko, E.N.; Ralys, R.V.; Zaitsau, D.; Stark, A. Biomass-Derived Platform Chemicals: Thermodynamic Studies on the Conversion of 5-Hydroxymethylfurfural into Bulk Intermediates. Ind. Eng. Chem. Res. 2009, 48, 10087–10093. [Google Scholar] [CrossRef]
- De, S.; Dutta, S.; Saha, B. One-Pot Conversions of Lignocellulosic and Algal Biomass into Liquid Fuels. ChemSusChem 2012, 5, 1826–1833. [Google Scholar] [CrossRef]
- Hu, L.; Lin, L.; Liu, S. Chemoselective Hydrogenation of Biomass-Derived 5-Hydroxymethylfurfural into the Liquid Bio-fuel 2,5-Dimethylfuran. Ind. Eng. Chem. Res. 2014, 53, 9969–9978. [Google Scholar] [CrossRef]
- March, J. Reactions, mechanisms, and structure. Adv. Org. Chem. 1985, 3, 173–176. [Google Scholar]
- Lucas, N.; Kanna, N.R.; Nagpure, A.S.; Kokate, G.; Chilukuri, S. Novel catalysts for valorization of biomass to value-added chemicals and fuels. J. Chem. Sci. 2014, 126, 403–413. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Tang, X.; Xu, J.; Wu, Z.; Lin, L.; Liu, S. Selective Transformation of 5-Hydroxymethylfurfural into the Liquid Fuel 2,5-Dimethylfuran over Carbon-Supported Ruthenium. Ind. Eng. Chem. Res. 2014, 53, 3056–3064. [Google Scholar] [CrossRef]
- Mitra, J.; Zhou, X.; Rauchfuss, T. Pd/C-catalyzed reactions of HMF: Decarbonylation, hydrogenation, and hydrogenolysis. Green Chem. 2014, 17, 307–313. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, L.; Liu, S. Efficient Production of Furan Derivatives from a Sugar Mixture by Catalytic Process. Energy Fuels 2012, 26, 4560–4567. [Google Scholar] [CrossRef]
- Chidambaram, M.; Bell, A. A two-step approach for the catalytic conversion of glucose to 2,5-dimethylfuran in ionic liquids. Green Chem. 2010, 12, 1253–1262. [Google Scholar] [CrossRef]
- Thananatthanachon, T.; Rauchfuss, T.B. Efficient Production of the Liquid Fuel 2,5-Dimethylfuran from Fructose Using Formic Acid as a Reagent. Angew. Chem. Int. Ed. 2010, 49, 6616–6618. [Google Scholar] [CrossRef]
- Hansen, T.S.; Barta, K.; Anastas, P.T.; Ford, P.C.; Riisager, A. One-pot reduction of 5-hydroxymethylfurfural via hydrogen transfer from supercritical methanol. Green Chem. 2012, 14, 2457–2461. [Google Scholar] [CrossRef] [Green Version]
- Jae, J.; Zheng, W.; Lobo, R.F.; Vlachos, D.G. Production of Dimethylfuran from Hydroxymethylfurfural through Catalytic Transfer Hydrogenation with Ru-thenium Supported on Carbon. ChemSusChem 2013, 6, 1158–1162. [Google Scholar] [CrossRef]
- Chatterjee, M.; Ishizaka, T.; Kawanami, H. Hydrogenation of 5-hydroxymethylfurfural in supercritical carbon diox-ide-water: A tunable approach to dimethylfuran selectivity. Green Chem. 2014, 16, 1543–1551. [Google Scholar] [CrossRef]
- Zu, Y.; Yang, P.; Wang, J.; Liu, X.; Ren, J.; Lu, G.; Wang, Y. Efficient production of the liquid fuel 2,5-dimethylfuran from 5-hydroxymethylfurfural over Ru/Co3O4 catalyst. Appl. Catal. B Environ. 2014, 146, 244–248. [Google Scholar] [CrossRef]
- Wang, G.-H.; Hilgert, J.; Richter, F.H.; Wang, F.R.; Bongard, H.; Spliethoff, B.; Weidenthaler, C.; Schüth, F. Platinum–cobalt bimetallic nanoparticles in hollow carbon nanospheres for hydrogenolysis of 5-hydroxymethylfurfural. Nat. Mater. 2014, 13, 293–300. [Google Scholar] [CrossRef]
- Shun, N.; Naoya, I.; Kohki, E. Selective hydrogenation of biomass-derived 5-hydroxymethylfurfural (HMF) to 2,5-dimethylfuran (DMF) under atmospheric hydrogen pressure over carbon supported PdAu bimetallic catalyst. Catalysis Today 2014, 232, 89–98. [Google Scholar]
- Huang, Y.; Chen, M.-Y.; Yan, L.; Guo, Q.-X.; Fu, Y. Nickel-Tungsten Carbide Catalysts for the Production of 2,5-Dimethylfuran from Biomass-Derived Molecules. ChemSusChem 2014, 7, 1068–1072. [Google Scholar] [CrossRef] [PubMed]
- Scholz, D.; Aellig, C.; Hermans, I. Catalytic Transfer Hydrogenation/Hydrogenolysis for Reductive Upgrading of Furfural and 5-(Hydroxymethyl)furfural. ChemSusChem 2013, 7, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Kumalaputri, A.J.; Bottari, G.; Erne, P.M.; Heeres, H.J.; Barta, K. Tunable and Selective Conversion of 5-HMF to 2,5-Furandimethanol and 2,5-Dimethylfuran over Copper-Doped Porous Metal Oxides. ChemSusChem 2014, 7, 2266–2275. [Google Scholar] [CrossRef]
- Saha, B.; Bohn, C.M.; Abu-Omar, M.M. Zinc-Assisted Hydrodeoxygenation of Biomass-Derived 5-Hydroxymethylfurfural to 2,5-Dimethylfuran. ChemSusChem 2014, 7, 3095–3101. [Google Scholar] [CrossRef]
- Nagpure, A.S.; Venugopal, A.K.; Lucas, N.; Manikandan, M.; Thirumalaiswamy, R.; Chilukuri, S. Renewable fuels from biomass-derived compounds: Ru-containing hydrotalcites as catalysts for conver-sion of HMF to 2,5-dimethylfuran. Catal. Sci. Technol. 2015, 5, 1463–1472. [Google Scholar] [CrossRef]
- Yang, P.; Cui, Q.; Zu, Y.; Liu, X.; Lu, G.; Wang, Y. Catalytic production of 2,5-dimethylfuran from 5-hydroxymethylfurfural over Ni/Co3O4 catalyst. Catal. Commun. 2015, 66, 55–59. [Google Scholar] [CrossRef]
- Kong, X.; Zheng, R.; Zhu, Y.; Ding, G.; Zhu, Y.; Li, Y.-W. Rational design of Ni-based catalysts derived from hydrotalcite for selective hydrogenation of 5-hydroxymethylfurfural. Green Chem. 2015, 17, 2504–2514. [Google Scholar] [CrossRef]
- Chen, B.; Li, F.; Huang, Z.; Yuan, G. Carbon-coated Cu-Co bimetallic nanoparticles as selective and recyclable catalysts for production of biofuel 2,5-dimethylfuran. Appl. Catal. B Environ. 2017, 200, 192–199. [Google Scholar] [CrossRef]
- Shi, J.; Wang, Y.; Yu, X.; Du, W.; Hou, Z. Production of 2,5-dimethylfuran from 5-hydroxymethylfurfural over reduced graphene oxides supported Pt catalyst under mild conditions. Fuel 2016, 163, 74–79. [Google Scholar] [CrossRef]
- Luo, J.; Lee, J.D.; Yun, H.; Wang, C.; Monai, M.; Murray, C.B.; Fornasiero, P.; Gorte, R.J. Base metal-Pt alloys: A general route to high selectivity and stability in the production of biofuels from HMF. Appl. Catal. B Environ. 2016, 199, 439–446. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; He, L.; Chen, J.; Zheng, J.; Ye, L.; Lin, H.; Yuan, Y. Robust and Recyclable Nonprecious Bimetallic Nanoparticles on Carbon Nanotubes for the Hydrogenation and Hydrogenolysis of 5-Hydroxymethylfurfural. ChemCatChem 2015, 7, 1701–1707. [Google Scholar] [CrossRef]
- Nagpure, A.S.; Lucas, N.; Chilukuri, S.V. Efficient Preparation of Liquid Fuel 2,5-Dimethylfuran from Biomass-Derived 5-Hydroxymethylfurfural over Ru–NaY Catalyst. ACS Sustain. Chem. Eng. 2015, 3, 2909–2916. [Google Scholar] [CrossRef]
- Bottari, G.; Kumalaputri, A.J.; Krawczyk, K.; Feringa, B.L.; Heeres, H.J.; Barta, K. Copper-Zinc Alloy Nanopowder: A Robust Precious-Metal-Free Catalyst for the Conversion of 5-Hydroxymethylfurfural. ChemSusChem 2015, 8, 1323–1327. [Google Scholar] [CrossRef]
- Priecel, P.; Endot, N.A.; Carà, P.D.; Lopez-Sanchez, J.A. Fast Catalytic Hydrogenation of 2,5-Hydroxymethylfurfural to 2,5-Dimethylfuran with Ruthenium on Car-bon Nanotubes. Ind. Eng. Chem. Res. 2018, 57, 1991–2002. [Google Scholar] [CrossRef]
- Jae, J.; Zheng, W.; Karim, A.M.; Wei, G.; Lobo, R.F.; Vlachos, D.G. The Role of Ru and RuO2in the Catalytic Transfer Hydrogenation of 5-Hydroxymethylfurfural for the Production of 2,5-Dimethylfuran. ChemCatChem 2014, 6, 848–856. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Tamura, M.; Tomishige, K. Catalytic Reduction of Biomass-Derived Furanic Compounds with Hydrogen. ACS Catal. 2013, 3, 2655–2668. [Google Scholar] [CrossRef]
- Fouilloux, P. The nature of raney nickel, its adsorbed hydrogen and its catalytic activity for hydrogenation reactions (review). Appl. Catal. 1983, 8, 1–42. [Google Scholar] [CrossRef]
- Iriondo, A.; Mendiguren, A.; Güemez, M.; Requies, J.; Cambra, J.F. 2,5-DMF production through hydrogenation of real and synthetic 5-HMF over transition metal catalysts supported on carriers with different nature. Catal. Today 2017, 279, 286–295. [Google Scholar] [CrossRef]
- Vetere, V.; Merlo, A.B.; Ruggera, J.F.; Casella, M.L. Transition metal-based bimetallic catalysts for the chemoselective hydrogenation of furfuraldehyde. J. Braz. Chem. Soc. 2010, 21, 914–920. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, Y.; Nakazawa, H.; Watanabe, H.; Tomishige, K. Total Hydrogenation of Furfural over a Silica-Supported Nickel Catalyst Prepared by the Reduction of a Nickel Nitrate Precursor. ChemCatChem 2012, 4, 1791–1797. [Google Scholar] [CrossRef]
- Sugano, Y.; Shiraishi, Y.; Tsukamoto, D.; Ichikawa, S.; Tanaka, S.; Hirai, T. Supported Au–Cu Bimetallic Alloy Nanoparticles: An Aerobic Oxidation Catalyst with Regenerable Activity by Visible-Light Irradiation. Angew. Chem. Int. Ed. 2013, 52, 5295–5299. [Google Scholar] [CrossRef] [PubMed]
- Iglesia, E.; Soled, S.; Fiato, R.; Via, G. Bimetallic Synergy in Cobalt Ruthenium Fischer-Tropsch Synthesis Catalysts. J. Catal. 1993, 143, 345–368. [Google Scholar] [CrossRef]
- Zhu, L.; Zheng, L.; Du, K.; Fu, H.; Li, Y.; You, G.; Chen, B.H. An efficient and stable Ru-Ni/C nano-bimetallic catalyst with a comparatively low Ru loading for benzene hydrogenation under mild reaction conditions. RSC Adv. 2013, 3, 713–719. [Google Scholar] [CrossRef]
- Ruban, A.V.; Skriver, H.L.; Nørskov, J.K. Surface segregation energies in transition-metal alloys. Phys. Rev. B 1999, 59, 15990–16000. [Google Scholar] [CrossRef] [Green Version]
- Yoon, B.; Pan, H.-B.; Wai, C.M. Relative Catalytic Activities of Carbon Nanotube-Supported Metallic Nanoparticles for Room-Temperature Hydrogenation of Benzene. J. Phys. Chem. C 2009, 113, 1520–1525. [Google Scholar] [CrossRef]
- Oosthuizen, R.S.; Nyamori, V.O. Carbon Nanotubes as Supports for Palladium and Bimetallic Catalysts for Use in Hydrogenation Reactions. Platin. Met. Rev. 2011, 55, 154–169. [Google Scholar] [CrossRef]
- Li, H.; Li, H.; Deng, J.-F. Glucose hydrogenation over Ni–B/SiO2 amorphous alloy catalyst and the promoting effect of metal dopants. Catal. Today 2002, 74, 53–63. [Google Scholar] [CrossRef]
- Jordão, M.H.; Simões, V.; Cardoso, D. Zeolite supported Pt-Ni catalysts in n-hexane isomerization. Appl. Catal. A: Gen. 2007, 319, 1–6. [Google Scholar] [CrossRef]
- Sun, J.; Yang, G.; Ma, Q.; Ooki, I.; Taguchi, A.; Abe, T.; Xie, Q.; Yoneyama, Y.; Tsubaki, N. Fabrication of active Cu–Zn nanoalloys on H-ZSM5 zeolite for enhanced dimethyl ether synthesis via syngas. J. Mater. Chem. A 2014, 2, 8637–8643. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Takada, K.; Tamura, M.; Tomishige, K. Total Hydrogenation of Furfural and 5-Hydroxymethylfurfural over Supported Pd–Ir Alloy Catalyst. ACS Catal. 2014, 4, 2718–2726. [Google Scholar] [CrossRef]
- Ni, Z.-M.; Xia, M.Y.; Shi, W.; Qian, P.P. Adsorption and Decarbonylation Reaction of Furfural on Pt (111) Surface. Acta Phys. -Chim. Sinica 2013, 29, 1916–1922. [Google Scholar]
- Aramendía, M.; Borau, V.; Jiménez, C.; Marinas, J.; Porras, A.; Urbano, F.J. Selective Liquid-Phase Hydrogenation of Citral over Supported Palladium. J. Catal. 1997, 172, 46–54. [Google Scholar] [CrossRef]
- Vicente, A.; Lafaye, G.; Especel, C.; Marécot, P.; Williams, C.T. The relationship between the structural properties of bimetallic Pd–Sn/SiO 2 catalysts and their performance for selective citral hydrogenation. J. Catal. 2011, 283, 133–142. [Google Scholar] [CrossRef]
- Luo, J.; Yun, H.; Mironenko, A.V.; Goulas, K.; Lee, J.D.; Monai, M.; Wang, C.; Vorotnikov, V.; Murray, C.B.; Vlachos, D.G.; et al. Mechanisms for High Selectivity in the Hydrodeoxygenation of 5-Hydroxymethylfurfural over PtCo Nanocrystals. ACS Catalysis 2016, 6, 4095–4104. [Google Scholar] [CrossRef]
- Serp, P.; Figueiredo, J.L. (Eds.) Carbon Materials for Catalysis; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Alamillo, R.; Tucker, M.; Chia, M.; Pagan-Torres, Y.; Dumesic, J. The selective hydrogenation of biomass-derived 5-hydroxymethylfurfural using heterogeneous catalysts. Green Chem. 2012, 14, 1413–1419. [Google Scholar] [CrossRef]
- Cai, H.; Li, C.; Wang, A.; Zhang, T. Biomass into chemicals: One-pot production of furan-based diols from carbohydrates via tandem reactions. Catal. Today 2014, 234, 59–65. [Google Scholar] [CrossRef]
- Ohyama, J.; Esaki, A.; Yamamoto, Y.; Arai, S.; Satsuma, A. Selective hydrogenation of 2-hydroxymethyl-5-furfural to 2,5-bis(hydroxymethyl)furan over gold sub-nano clusters. RSC Adv. 2013, 3, 1033–1036. [Google Scholar] [CrossRef]
- Mäki-Arvela, P.; Ruiz, D.; Murzin, D.Y. Catalytic Hydrogenation/Hydrogenolysis of 5-Hydroxymethylfurfural to 2,5-Dimethylfuran. ChemSusChem. 2020, 14, 150–168. [Google Scholar] [CrossRef]
- Esteves, L.M.; Brijaldo, M.H.; Oliveira, E.G.; Martínez, J.J.; Rojas, H.; Caytuero, A.; Passos, F.B. Effect of support on selective 5-hydroxymethylfurfural hydrogenation towards 2,5-dimethylfuran over copper catalysts. Fuel 2020, 270, 117524. [Google Scholar] [CrossRef]
- Chatterjee, M.; Ishizaka, T.; Kawanami, H. Selective hydrogenation of 5-hydroxymethylfurfural to 2,5-bis-(hydroxymethyl)furan using Pt/MCM-41 in an aqueous medium: A simple approach. Green Chem. 2014, 16, 4734–4739. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, C.; Li, D.; Liu, Q.; Tan, J.; Wang, C.; Cai, C.; Ma, L. Recent advances in catalytic conversion of biomass to 5-hydroxymethylfurfural and 2, 5-dimethylfuran. Renew. Sustain. Energy Rev. 2019, 103, 227–247. [Google Scholar] [CrossRef]





| Property | DMF | Ethanol | Gasoline |
|---|---|---|---|
| Gravimetric oxygen content (%) | 16.7 | 34.8 | 0 |
| Density at 20 °C (kg·m−3) | 889.7 | 790.9 | 744.6 |
| Water miscibility at 25 °C (gL−1) | 2.3 | Miscible | Immiscible |
| Boiling point (°C) | 93 | 78 | 32–200 |
| Energy density (MJ/L) | 30 | 23.4 | 31 |
| RON | 119 | 110 | 95.8 |
| Auto-ignition temperature (°C) | 286 | 423 | 257 |
| Entry | Catalyst | Conditions | HMF Conversion (%) | DMF Yield (%) | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|
| Solvent | H2 Donor | Temperature (°C) | Time (h) | Pressure (bar) | |||||
| 1 | CuCrO4 | 1-Butanol | H2 | 220 | 10 | 6.8 | 100 | 61 | [13] |
| 2 | CuRu/C | 1-Butanol | H2 | 220 | 10 | 6.8 | 100 | 71 | [13] |
| 3 | Ru/C | THF | Formic acid | 75 | 15 | - | - | 32 | [39] |
| 4 | Ru/K-OMS-2 | 1-Butanol | H2 | 220 | 6 | 10 | 90 | 33 | [42] |
| 5 | Ru/C | THF | H2 | 200 | 2 | 20 | 100 | 94.7 | [43] |
| 6 | Pd/C | Dioxane | Formic acid | 120 | 15 | - | 100 | >95 | [44] |
| 7 | Ru/C | 1-Butanol | H2 | 260 | 1.5 | - | 99.8 | 60.3 | [45] |
| 8 | Pd/C | [EMIM]Cl | H2 | 120 | 1 | 62 | 47 | 15 | [46] |
| 9 | Pd/C/H2SO4 | THF | Formic acid | 70 | 15 | - | 100 | 95 | [47] |
| 10 | Cu-PMO | Methanol | Methanol | 300 | 0.75 | - | 100 | 34 | [48] |
| 11 | Ru/C | Isopropyl alcohol | Isopropyl alcohol | 190 | 6 | - | 100 | 81 | [49] |
| 12 | Pd/C | Water:CO2 | H2 | 80 | 2 | 10 | 100 | 100 | [50] |
| 13 | Ru/Co3O4 | THF | H2 | 130 | 24 | 7 | 99 | 93.4 | [51] |
| 14 | PtCo@HCS | 1-Butanol | H2 | 180 | 2 | 10 | 100 | 98 | [52] |
| 15 | PdAu/C | THF | H2 | 60 | 6 | atm | 100 | 96 | [53] |
| 16 | Ni-W2C/AC | THF | H2 | 180 | 3 | 40 | 100 | 96 | [54] |
| 17 | Pd/Fe2O3 | 2-Propanol | 2-Propanol | 180 | - | 15 a | 98 | 72 | [55] |
| 18 | Cu-Ru-PMO | Ethanol | H2 | 220 | 6 | 50 | 100 | 79 b | [56] |
| 19 | Pd/C/Zn | THF | H2 | 150 | 8 | 8 | >99 | 85 | [57] |
| 20 | Ru/Hydrotalcites | 2-Propanol | H2 | 220 | 4 | 10 | 100 | 58 | [58] |
| 21 | Ni/Co3O4 | THF | H2 | 130 | 24 | 10 | 99 | 76 | [59] |
| 22 | Ni-Al2O3 | Dioxane | H2 | 180 | 4 | 12 | 100 | 92 | [60] |
| 23 | Cu-Co@C | Ethanol | H2 | 180 | 8 | 50 | 100 | 99.4 | [61] |
| 24 | Pt/rGO | 1-Butanol | H2 | 120 | 2 | 30 | 100 | 73.2 | [62] |
| 25 | Pt-Ni/C | 1-Propanol | H2 | 200 | 5 c | 33 | 100 | 98 | [63] |
| 26 | Ni-Fe/CNT | n-Butanol | H2 | 200 | 3 | 30 | 100 | 91.3 d | [64] |
| 27 | Ru/NaY zeolite | THF | H2 | 220 | 1 | 15 | 100 | 78 | [65] |
| 28 | CuZn | CPME | H2 | 220 | 6 | 20 | 100 | 97 b | [66] |
| 29 | Ru/CNT | Dioxane | H2 | 150 | 1 | 20 | 100 | 83.5 | [67] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Endot, N.A.; Junid, R.; Jamil, M.S.S. Insight into Biomass Upgrade: A Review on Hydrogenation of 5-Hydroxymethylfurfural (HMF) to 2,5-Dimethylfuran (DMF). Molecules 2021, 26, 6848. https://doi.org/10.3390/molecules26226848
Endot NA, Junid R, Jamil MSS. Insight into Biomass Upgrade: A Review on Hydrogenation of 5-Hydroxymethylfurfural (HMF) to 2,5-Dimethylfuran (DMF). Molecules. 2021; 26(22):6848. https://doi.org/10.3390/molecules26226848
Chicago/Turabian StyleEndot, Nor Azam, Ramli Junid, and Mohamad Shazwan Shah Jamil. 2021. "Insight into Biomass Upgrade: A Review on Hydrogenation of 5-Hydroxymethylfurfural (HMF) to 2,5-Dimethylfuran (DMF)" Molecules 26, no. 22: 6848. https://doi.org/10.3390/molecules26226848
APA StyleEndot, N. A., Junid, R., & Jamil, M. S. S. (2021). Insight into Biomass Upgrade: A Review on Hydrogenation of 5-Hydroxymethylfurfural (HMF) to 2,5-Dimethylfuran (DMF). Molecules, 26(22), 6848. https://doi.org/10.3390/molecules26226848








