Changes in Antioxidant Properties and Amounts of Bioactive Compounds during Simulated In Vitro Digestion of Wheat Bread Enriched with Plant Extracts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characteristics of Wheat Bread
2.1.1. Color, Yield and Volume of Bread
2.1.2. Phenolic Concentration in Bread
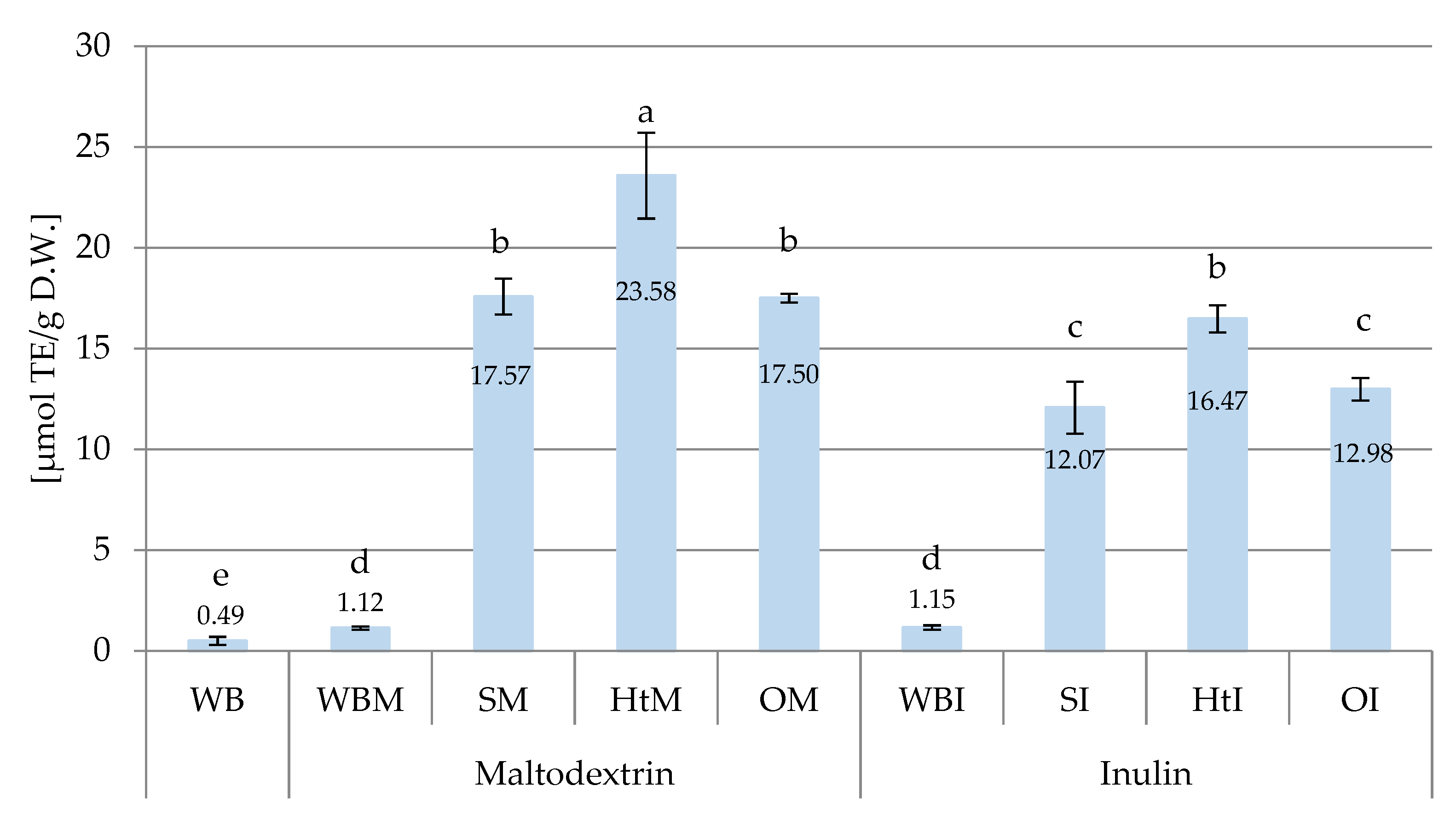
2.1.3. Antioxidant Capacity in Bread
2.2. Bioavailability of Bioactive Compounds
2.2.1. Bioactive Compounds
2.2.2. Antioxidant Capacity
3. Materials and Methods
3.1. Chemicals
3.2. Material
3.3. Obtaining the Extract of Hawthorn, Soybeans and Onion Husks
3.4. Production of Microencapsulated Extracts
3.5. Baking Procedure of Bread
3.6. Determination of Bread Yield, Volume, Color and Storage Conditions
3.7. Preparation of Bread Crumb Extracts for HPLC and Antioxidant Activity Determinations
3.8. Determination of Polyphenols by HPLC
3.9. Determination of Antioxidant Activity by ABTS+• Method
3.10. Determination of Iron Ion Reduction Force Using FRAP Method
3.11. In Vitro Digestion
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Dziki, D.; Różyło, R.; Gawlik-Dziki, U.; Świeca, M. Current trends in the enhancement of antioxidant activity of wheat bread by the addition of plant materials rich in phenolic compounds. Trends Food Sci. Technol. 2014, 40, 48–61. [Google Scholar] [CrossRef]
- Ko, E.Y.; Hariram Nile, S.; Sharma, K.; Hao Li, G.; Park, S.W. Effect of different exposed lights on quercetin and quercetin glucoside content in onion (Allium cepa L.). Saudi J. Biol. Sci. 2015, 22, 398–403. [Google Scholar] [CrossRef] [Green Version]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols—A review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Hellenbrand, N.; Sendker, J.; Lechtenberg, M.; Petereit, F.; Hensel, A. Isolation and quantification of oligomeric and polymeric procyanidins in leaves and flowers of Hawthorn (Crataegus spp.). Fitoterapia 2015, 104, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Włoch, A.; Kapusta, I.; Bielecki, K.; Oszmiański, J.; Kleszczyńska, H. Activity of hawthorn leaf and bark extracts in relation to biological membrane. J. Membrane Biol. 2013, 246, 545–556. [Google Scholar] [CrossRef] [Green Version]
- Choi, I.S.; Cho, E.J.; Moon, J.-H.; Bae, H.-J. Onion skin waste as a valorization resource for the by-products quercetin and biosugar. Food Chem. 2015, 88, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Stoclet, J.C.; Chataigneau, T.; Ndiaye, M.; Oak, M.H.; El Bedoui, J.; Chataigneau, M.; Schini-Kerth, V.B. Vascular protection by dietary polyphenols. Eur. J. Pharmacol. 2004, 500, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Zdunczyk, Z.; Frejnagel, S.; Wróblewska, M.; Jukiewicz, J.; Oszmiański, J.; Estrella, I. Biological activity of polyphenol extracts from different plant sources. Food Res. Int. 2002, 35, 183–186. [Google Scholar] [CrossRef]
- Rodríguez-Roque, M.J.; Rojas-Graü, M.A.; Elez-Martínez, P.; Martín-Belloso, O. In vitro bioaccessibility of health-related compounds from a blended fruit juice-soymilk beverage: Influence of the food matrix. J. Funct. Foods 2014, 7, 161–169. [Google Scholar] [CrossRef]
- Chethan, S.; Malleshi, N.G. Finger millet polyphenols: Optimization of extraction and the effect of pH on their stability. Food Chem. 2007, 105, 862–870. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Effect of spray drying and storage on the stability of bayberry polyphenols. Food Chem. 2011, 129, 1139–1147. [Google Scholar] [CrossRef]
- Sokół-Łętowska, A.; Kucharska, A.Z.; Wińska, K.; Szumny, A.; Nawirska-Olszańska, A.; Mizgier, P.; Wyspiańska, D. Composition and antioxidant activity of red fruit liqueurs. Food Chem. 2014, 157, 533–539. [Google Scholar] [CrossRef]
- Pérez-Gregorio, M.R.; García-Falcón, M.S.; Simal-Gándara, J. Flavonoids changes in fresh-cut onions during storage in different packaging systems. Food Chem. 2011, 124, 652–658. [Google Scholar] [CrossRef]
- Ioannou, I.; Hafsa, I.; Hamdi, S.; Charbonnel, C.; Ghoul, M. Review of the effects of food processing and formulation on flavonol and anthocyanin behaviour. J. Food Eng. 2012, 111, 208–217. [Google Scholar] [CrossRef]
- Patras, A.; Brunton, N.P.; O’Donnell, C.; Tiwari, B.K. Effect of thermal processing on anthocyanin stability in foods; mechanisms and kinetics of degradation. Trends Food Sci. Technol. 2010, 21, 3–11. [Google Scholar] [CrossRef]
- Faller, A.L.K.; Fialho, E. The antioxidant capacity and polyphenol content of organic and conventional retail vegetables after domestic cooking. Food Res. Int. 2009, 42, 210–215. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Wijngaard, H.; Arendt, E.K.; Gallagher, E. Polyphenol composition and in vitro antioxidant activity of amaranth, quinoa buckwheat and wheat as affected by sprouting and baking. Food Chem. 2010, 119, 770–778. [Google Scholar] [CrossRef]
- Czaja, A.; Czubaszek, A.; Wyspiańska, D.; Sokół-Łętowska, A.; Kucharska, A.Z. Quality of wheat bread enriched with onion extract and polyphenols content and antioxidant activity changes during bread storage. Int. J. Food Sci. Technol. 2020, 55, 1725–1734. [Google Scholar] [CrossRef]
- Li, F.M.; Li, T.; Li, W.; De Yang, L. Changes in antioxidant capacity, levels of soluble sugar, total polyphenol, organosulfur compound and constituents in garlic clove during storage. Ind. Crop. Prod. 2015, 69, 137–142. [Google Scholar] [CrossRef]
- Islek, M.; Nilufer-Erdil, D.; Knuthsen, P. Changes in flavonoids of sliced and fried yellow onions (Allium cepa L. var. zittauer) during storage at different atmospheric, temperature and light conditions. J. Food Proc. Preserv. 2014, 39, 357–368. [Google Scholar] [CrossRef]
- Zheng, G.; Deng, J.; Wen, L.; You, L.; Zhao, Z.; Zhou, L. Release of phenolic compounds and antioxidant capacity of Chinese hawthorn “Crataegus pinnatifida” during in vitro digestion. J. Funct. Foods 2018, 40, 76–85. [Google Scholar] [CrossRef]
- Ottaviani, J.I.; Borges, G.; Momma, T.Y.; Spencer, J.P.E.; Keen, C.L.; Crozier, A.; Schroeter, H. The metabolome of [2-14C](-)-epicatechin in humans: Implications for the assessment of efficacy, safety, and mechanisms of action of polyphenolic bioactives. Sci. Rep. 2016, 6, 29034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Świeca, M.; Gawlik-Dziki, U.; Dziki, D.; Baraniak, B.; Czyż, J. The influence of protein-flavonoid interactions on protein digestibility in vitro and the antioxidant quality of breads enriched with onion skin. Food Chem. 2013, 141, 451–458. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Dziki, D.; Baraniak, B.; Lin, R. The effect of simulated digestion in vitro on bioactivity of wheat bread with Tartary buckwheat flavones addition. LWT—Food Sci. Technol. 2009, 42, 137–143. [Google Scholar] [CrossRef]
- Perez-Moral, N.; Saha, S.; Philo, M.; Hart, D.J.; Winterbone, M.S.; Hollands, W.J.; Spurr, M.; Bows, J.; van der Velpen, V.; Kroon, P.A.; et al. Comparative bio-accessibility, bioavailability and bioequivalence of quercetin, apigenin, glucoraphanin and carotenoids from freeze-dried vegetables incorporated into a baked snack versus minimally processed vegetables: Evidence from in vitro models and a human bioavailability study. J. Funct. Foods 2018, 48, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Roque, M.J.; Rojas-Graü, M.A.; Elez-Martínez, P.; Martín-Belloso, O. Soymilk phenolic compounds, isoflavones and antioxidant activity as affected by in vitro gastrointestinal digestion. Food Chem. 2013, 136, 206–212. [Google Scholar] [CrossRef]
- Nemeth, K.; Piskula, M.K. Food content, processing, absorption and metabolism of onion flavonoids. Critic. Rev. Food Sci. Nutr. 2007, 47, 397–409. [Google Scholar] [CrossRef]
- Chávez-Santoscoy, R.A.; Gutiérrez-Uribe, J.A.; Serna-Saldivar, S.O.; Perez-Carrillo, E. Production of maize tortillas and cookies from nixtamalized flour enriched with anthocyanins, flavonoids and saponins extracted from black bean (Phaseolus vulgaris) seed coats. Food Chem. 2016, 192, 90–97. [Google Scholar] [CrossRef]
- Shoaib, M.; Shehzad, A.; Omar, M.; Rakha, A.; Raza, H.; Sharif, H.R.; Shakeel, A.; Ansari, A.; Niazi, S. Inulin: Properties, health benefits and food applications. Carb. Poly. 2016, 147, 444–454. [Google Scholar] [CrossRef]
- Salinas, M.V.; Zuleta, A.; Ronayne, P.; Puppo, M.C. Wheat bread enriched with organic calcium salts and inulin. A bread quality study. J. Food Sci. Technol. 2016, 53, 491–500. [Google Scholar] [CrossRef] [Green Version]
- Karolini-Skaradzińska, Z.; Bihuniak, P.; Piotrowska, E.; Wdowik, L. Properties of dough and qualitative characteristics of wheat bread with addition inulin. Polish J. Food Nutr. Sci. 2007, 57, 267–270, eISSN:2083-6007. [Google Scholar]
- Morris, C.; Morris, G.A. The effect of inulin and fructo-oligosaccharide supplementation on the textural, rheological and sensory properties of bread and their role in weight management: A review. Food Chem. 2012, 133, 237–248. [Google Scholar] [CrossRef] [Green Version]
- Karolini-Skaradzińska, Z.; Czubaszek, A.; Stanisławska, M.; Szewców, P. Zmiany właściwości wypiekowych mąki pszennej pod wpływem dodatku maltodekstryn. Żywność Nauka Technol. Jakość 2012, 4, 108–121. (In Polish) [Google Scholar]
- Witczak, M.; Korus, J.; Ziobro, R.; Juszczak, L. The effects of maltodextrins on gluten-free dough and quality of bread. J. Food Eng. 2010, 96, 258–265. [Google Scholar] [CrossRef]
- Pycia, K.; Jaworska, G.; Telega, J.; Sudoł, I.; Kuźniar, P. Effect of adding potato maltodextrins on baking properties of triticale flour and quality of bread. LWT—Food Sci. Technol. 2018, 96, 199–204. [Google Scholar] [CrossRef]
- Wang, H.Y.; Qian, H.; Yao, W.R. Melanoidins produced by the Maillard reaction: Structure and biological activity. Food Chem. 2011, 128, 573–584. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.-O.O.; Chung, S.-J.J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Comp. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Wang, R.; Zhou, W.; Isabelle, M. Comparison study of the effect of green tea extract (GTE) on the quality of bread by instrumental analysis and sensory evaluation. Food Res. Int. 2007, 40, 470–479. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Świeca, M.; Dziki, D.; Baraniak, B.; Tomiło, J.; Czyż, J. Quality and antioxidant properties of breads enriched with dry onion (Allium cepa L.) skin. Food Chem. 2013, 138, 1621–1628. [Google Scholar] [CrossRef]
- Michalak-Majewska, M.; Sołowiej, B.; Sławińska, A. Antioxidant activity, technological and rheological properties of baked rolls containing dried onions (Allium cepa L.). J. Food Proc. Preserv. 2017, 41, 1–8. [Google Scholar] [CrossRef]
- Sivam, A.S.; Sun-Waterhouse, D.; Perera, C.O.; Waterhouse, G.I.N. Exploring the interactions between blackcurrant polyphenols, pectin and wheat biopolymers in model breads; A FTIR and HPLC investigation. Food Chem. 2012, 131, 802–810. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Sivam, A.S.; Cooney, J.; Zhou, J.; Perera, C.O.; Waterhouse, G.I.N. Effects of added fruit polyphenols and pectin on the properties of finished breads revealed by HPLC/LC-MS and Size-Exclusion HPLC. Food Res. Int. 2011, 44, 3047–3056. [Google Scholar] [CrossRef]
- Goh, R.; Gao, J.; Ananingsih, V.K.; Ranawana, V.; Henry, C.J.; Zhou, W. Green tea catechins reduced the glycaemic potential of bread: An in vitro digestibility study. Food Chem. 2015, 180, 203–210. [Google Scholar] [CrossRef]
- Lin, J.; Zhou, W. Role of quercetin in the physicochemical properties, antioxidant and antiglycation activities of bread. J. Funct. Foods. 2018, 40, 299–306. [Google Scholar] [CrossRef]
- Lee, J.H.; Cho, K.M. Changes occurring in compositional components of black soybeans maintained at room temperature for different storage periods. Food Chem. 2012, 131, 161–169. [Google Scholar] [CrossRef]
- Prabhakaran, M.P.; Hui, L.S.; Perera, C.O. Evaluation of the composition and concentration of isoflavones in soy based supplements, health products and infant formulas. Food Res. Int. 2006, 39, 730–738. [Google Scholar] [CrossRef]
- Harris, S.; Brunton, N.; Tiwari, U.; Cummins, E. Human exposure modelling of quercetin in onions (Allium cepa L.) following thermal processing. Food Chem. 2015, 187, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Mitchell, A.E. Pharmacokinetics of quercetin absorption from apples and onions in healthy humans. J. Agric. Food Chem. 2012, 60, 3874–3881. [Google Scholar] [CrossRef] [PubMed]
- Araujo-Díaz, S.B.; Leyva-Porras, C.; Aguirre-Bañuelos, P.; Álvarez-Salas, C.; Saavedra-Leos, Z. Evaluation of the physical properties and conservation of the antioxidants content, employing inulin and maltodextrin in the spray drying of blueberry juice. Carb. Polym. 2017, 167, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Bąkowska-Barczak, A.M.; Kołodziejczyk, P.P. Black currant polyphenols: Their storage stability and microencapsulation. Ind. Crops Prod. 2011, 34, 1301–1309. [Google Scholar] [CrossRef]
- Ersus, S.; Yurdagel, U. Microencapsulation of anthocyanin pigments of black carrot (Daucus carota L.) by spray drier. J. Food Eng. 2007, 80, 805–812. [Google Scholar] [CrossRef]
- Tolun, A.; Altintas, Z.; Artik, N. Microencapsulation of grape polyphenols using maltodextrin and gum arabic as two alternative coating materials: Development and characterization. J. Biotechnol. 2016, 239, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Ma, J.; Cheng, K.W.; Jiang, Y.; Chen, F.; Wang, M. The effects of grape seed extract fortification on the antioxidant activity and quality attributes of bread. Food Chem. 2010, 119, 49–53. [Google Scholar] [CrossRef]
- Chen, G.L.; Chen, S.G.; Zhao, Y.Y.; Luo, C.X.; Li, J.; Gao, Y.Q. Total phenolic contents of 33 fruits and their antioxidant capacities before and after in vitro digestion. Ind. Crops Prod. 2014, 57, 150–157. [Google Scholar] [CrossRef]
- Embuscado, M.E. Spices and herbs: Natural sources of antioxidants—A mini review. J. Funct. Foods. 2015, 18, 811–819. [Google Scholar] [CrossRef]
- Lacerda, E.C.Q.; de Araújo Calado, V.M.; Monteiro, M.; Finotelli, P.V.; Torres, A.G.; Perrone, D. Starch, inulin and maltodextrin as encapsulating agents affect the quality and stability of jussara pulp microparticles. Carb. Polym. 2016, 151, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.V.D.B.; Borges, S.V.; Botrel, D.A. Gum arabic/starch/maltodextrin/inulin as wall materials on the microencapsulation of rosemary essential oil. Carb. Polym. 2014, 101, 524–532. [Google Scholar] [CrossRef]
- Wyspiańska, D.; Kucharska, A.Z.; Sokół-Łętowska, A.; Kolniak-Ostek, J. Effect of microencapsulation on concentration of isoflavones during simulated in vitro digestion of isotonic drink. Food Sci. Nutr. 2019, 7, 1–12. [Google Scholar] [CrossRef]
- Zhu, Q.Y.; Holt, R.R.; Lazarus, S.A.; Ensunsa, J.L.; Hammerstone, J.F.; Schmitz, H.H.; Keen, C.L. Stability of the flavan-3-ols epicatechin and catechin and related dimeric procyanidins derived from cocoa. J. Agric. Food Chem. 2002, 50, 1700–1705. [Google Scholar] [CrossRef]
- Lai, F.; Franceschini, I.; Corrias, F.; Sala, M.C.; Cilurzo, F.; Sinico, C.; Pini, E. Maltodextrin fast dissolving films for quercetin nanocrystal delivery. A feasibility study. Carb. Polym. 2015, 121, 217–223. [Google Scholar] [CrossRef]
- Wyspiańska, D.; Kucharska, A.Z.; Sokół-Łętowska, A.; Kolniak-Ostek, J. Physico-chemical, antioxidant, and anti-inflammatory properties and stability of hawthorn (Crataegus monogyna Jacq.) procyanidins microcapsules with inulin and maltodextrin. J. Sci. Food Agri. 2017, 97, 669–678. [Google Scholar] [CrossRef]
- Wanezaki, S.; Araki, H. High Solubility Composition with High Isoflavone Concentration and Process of Producing Same. U.S. Patent 7,560,131, 14 July 2009. [Google Scholar]
- Kucharska, A.Z.; Sokół-Łętowska, A.; Oszmiański, J.; Piórecki, N.; Fecka, I. Iridoids, phenolic compounds and antioxidant activity of edible honeysuckle berries (Lonicera caerulea var. kamtschatica Sevast). Molecules 2017, 22, 405. [Google Scholar] [CrossRef] [Green Version]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of „antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Gawlik-Dziki, U. Zmiany poziomu związków fenolowych i właściwości przeciwutleniających płynów uzyskanych po trawieniu in vitro chleba pszennego. Żywność Nauka Technol. Jakość 2007, 3, 184–192. (In Polish) [Google Scholar]




| Carrier of Extract | In Vitro Digestion Step | Isoflavones (mg/mL) * | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Daidzin | Glycitin | Genistin | Malonyl-daidzin | Malonyl-glycitin | Malonyl-genistin Isomer | Malonyl-genistein | Daidzein | Genistein | Total | ||
| Maltodextrin | I | 0.23 | nd | 0.40 | 0.33 | 0.08 | 0.39 | 0.47 | nd | 0.35 | 2.26 a |
| II | 0.14 | nd | 0.34 | 0.23 | nd | 0.32 | 0.48 | nd | 0.31 | 1.83 b | |
| III | 0.08 | nd | 0.19 | 0.17 | nd | 0.28 | 0.34 | nd | 0.30 | 1.37 c | |
| IV | 0.06 | nd | 0.11 | 0.08 | 0.01 | 0.09 | 0.16 | nd | 0.12 | 0.62 d | |
| Inulin | I | 0.14 | nd | 0.25 | 0.38 | 0.08 | 0.42 | 0.32 | nd | 0.21 | 1.79 a |
| II | 0.13 | nd | 0.24 | 0.18 | nd | 0.24 | 0.43 | nd | 0.36 | 1.57 b | |
| III | 0.06 | nd | 0.12 | 0.14 | nd | 0.20 | 0.29 | nd | 0.29 | 1.10 c | |
| IV | 0.04 | nd | 0.07 | 0.10 | 0.01 | 0.11 | 0.16 | nd | 0.13 | 0.62 d | |
| Carrier of Extract | In Vitro Digestion Step | Procyanidins (mg/mL) * | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Procyanidin Dimer B2 | Procyanidin Dimer B6 | (−)-Epicatechin | Procyanidin Trimer C1 | Procyanidin Tetramer I | Procyanidin Tetramer II | Procyanidin Trimer | Procyanidin Tetramer III | Total | ||
| Maltodextrin | I | 0.06 | 0.02 | 0.53 | 0.53 | 0.02 | 0.02 | nd | nd | 1.18 a |
| II | 0.05 | 0.03 | 0.3 | 0.28 | 0.06 | 0.01 | nd | 0.03 | 0.75 b | |
| III | nd | nd | 0.3 | 0.02 | nd | nd | nd | nd | 0.32 c | |
| IV | nd | nd | 0.11 | nd | nd | nd | nd | nd | 0.11 d | |
| Inulin | I | 0.06 | 0.02 | 0.43 | 0.52 | 0.03 | 0.02 | nd | nd | 1.09 a |
| II | 0.05 | 0.04 | 0.39 | 0.35 | 0.07 | 0.01 | 0.01 | 0.04 | 0.97 b | |
| III | nd | nd | 0.3 | 0.02 | nd | nd | nd | nd | 0.32 c | |
| IV | nd | nd | 0.11 | nd | nd | nd | nd | nd | 0.11 d | |
| Carrier of Extract | In Vitro Digestion Step | Flavonols (mg/mL) * | ||||||
|---|---|---|---|---|---|---|---|---|
| Quercetin 7,4-diglucoside | Quercetin 3,4-diglucoside | Quercetin 3-glucoside | Quercetin 4-glucoside | Isorhamnetin 4-glucoside | Quercetin | Total | ||
| Maltodextrin | I | nd | nd | nd | nd | nd | 0.01 | 0.01 a |
| II | nd | nd | nd | 0.01 | nd | 0.03 | 0.03 a | |
| III | nd | nd | nd | nd | nd | nd | nd | |
| IV | nd | nd | nd | nd | nd | nd | nd | |
| Inulin | I | nd | nd | nd | nd | nd | 0.01 | 0.01 a |
| II | nd | nd | nd | nd | nd | 0.02 | 0.02 a | |
| III | nd | nd | nd | nd | nd | nd | nd | |
| IV | nd | nd | nd | nd | nd | nd | nd | |
| Carrier of Extract | In Vitro Digestion Step | ABTS (μmol TE/mL D.W) | ||
|---|---|---|---|---|
| Soy | Hawthorn | Onion | ||
| Maltodextrin | I | 0.51 ± 0.12 b | 0.69 ± 0.08 c | 1.33 ± 0.18 a |
| II | 0.99 ± 0.06 a | 2.60 ± 0.17 a | 1.49 ± 0.15 a | |
| III | 0.47 ± 0.13 b | 2.87 ± 0.14 a | 1.16 ± 0.10 b | |
| IV | 0.83 ± 0.03 a | 1.04 ± 0.10 b | 0.32 ± 0.17 c | |
| Inulin | I | 0.31 ± 0.07 c | 0.27 ± 0.10 d | 0.43 ± 0.07 c |
| II | 0.69 ± 0.07 a | 3.19 ± 0.19 a | 1.62 ± 0.09 b | |
| III | 0.49 ± 0.07 b | 2.29 ± 0.24 b | 1.50 ± 0.16 b | |
| IV | 0.61 ± 0.02 a | 1.50 ± 0.13 c | 1.82 ± 0.19 a | |
| Carrier of Extract | In Vitro Digestion Step | FRAP (μmol TE/mL D.W) | ||
|---|---|---|---|---|
| Soy | Hawthorn | Onion | ||
| Maltodextrin | I | 0.15 ± 0.01 a | 1.43 ± 0.06 a | 0.40 ± 0.02 a |
| II | 0.13 ± 0.02 a | 1.16 ± 0.06 a | 0.49 ± 0.03 a | |
| III | 0.09 ± 0.02 a | 0.50 ± 0.05 b | 0.33 ± 0.02 b | |
| IV | 0.03 ± 0.01 b | 0.21 ± 0.02 c | 0.03 ± 0.01 c | |
| Inulin | I | 0.16 ± 0.02 a | 1.32 ± 0.08 a | 0.38 ± 0.04 a |
| II | 0.13 ± 0.01 a | 1.13 ± 0.02 a | 0.37 ± 0.04 a | |
| III | 0.06 ± 0.02 b | 0.58 ± 0.07 b | 0.27 ± 0.02 b | |
| IV | 0.01 ± 0.01 b | 0.13 ± 0.08 c | 0.33 ± 0.02 b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czubaszek, A.; Czaja, A.; Sokół-Łętowska, A.; Kolniak-Ostek, J.; Kucharska, A.Z. Changes in Antioxidant Properties and Amounts of Bioactive Compounds during Simulated In Vitro Digestion of Wheat Bread Enriched with Plant Extracts. Molecules 2021, 26, 6292. https://doi.org/10.3390/molecules26206292
Czubaszek A, Czaja A, Sokół-Łętowska A, Kolniak-Ostek J, Kucharska AZ. Changes in Antioxidant Properties and Amounts of Bioactive Compounds during Simulated In Vitro Digestion of Wheat Bread Enriched with Plant Extracts. Molecules. 2021; 26(20):6292. https://doi.org/10.3390/molecules26206292
Chicago/Turabian StyleCzubaszek, Anna, Anna Czaja, Anna Sokół-Łętowska, Joanna Kolniak-Ostek, and Alicja Z. Kucharska. 2021. "Changes in Antioxidant Properties and Amounts of Bioactive Compounds during Simulated In Vitro Digestion of Wheat Bread Enriched with Plant Extracts" Molecules 26, no. 20: 6292. https://doi.org/10.3390/molecules26206292
APA StyleCzubaszek, A., Czaja, A., Sokół-Łętowska, A., Kolniak-Ostek, J., & Kucharska, A. Z. (2021). Changes in Antioxidant Properties and Amounts of Bioactive Compounds during Simulated In Vitro Digestion of Wheat Bread Enriched with Plant Extracts. Molecules, 26(20), 6292. https://doi.org/10.3390/molecules26206292







