Progress in the Medicinal Value, Bioactive Compounds, and Pharmacological Activities of Gynostemma pentaphyllum
Abstract
:1. Introduction
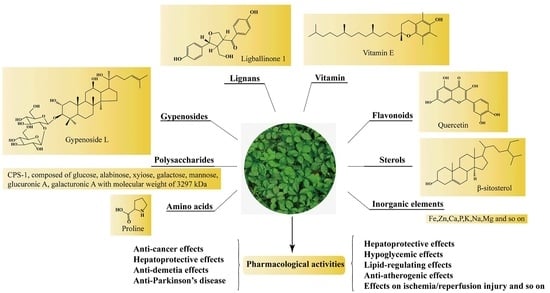
2. Chemical Constituents
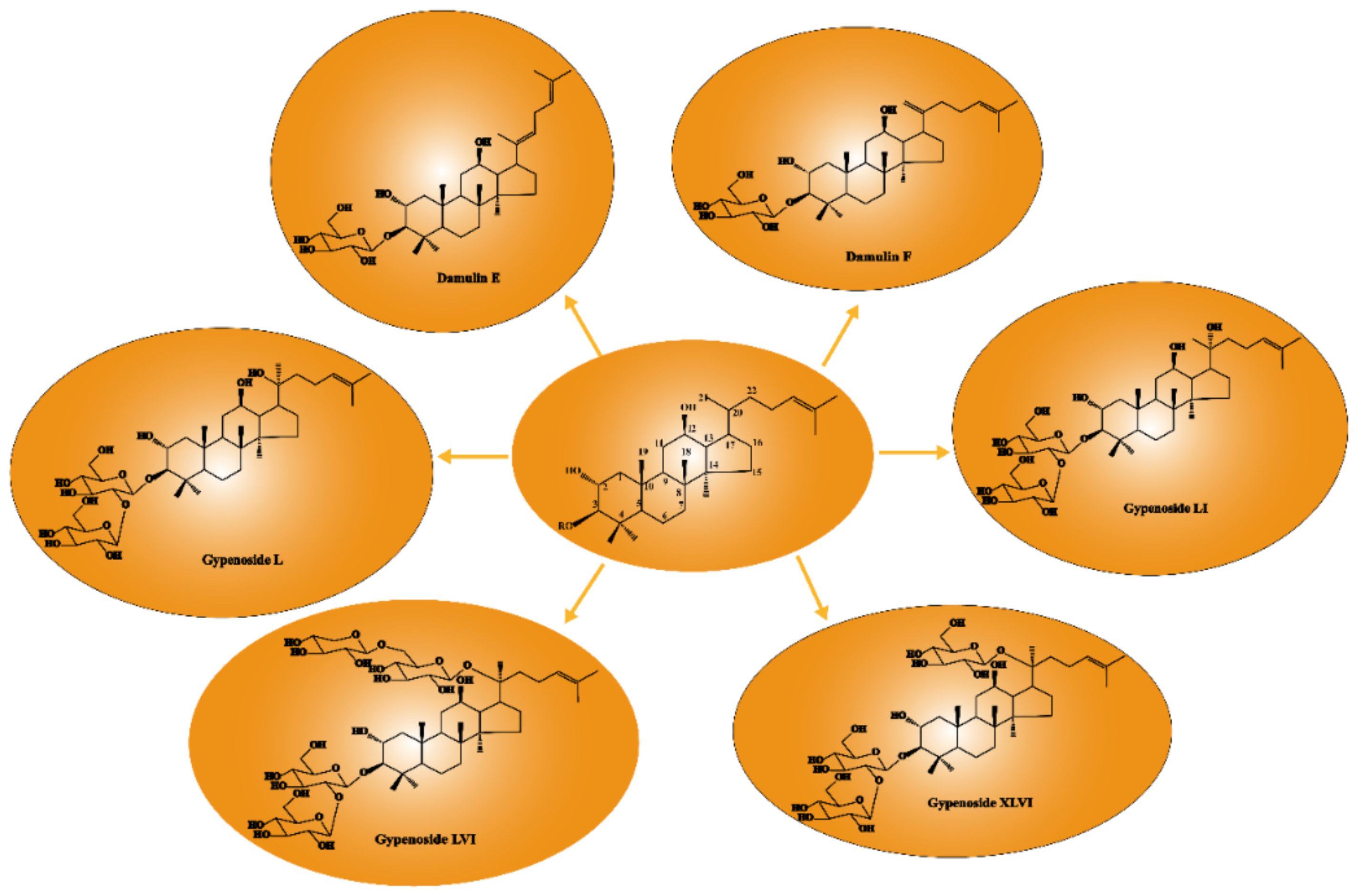
2.1. Gypenosides
2.2. Polysaccharides, Flavonoids, Sterols, Amino Acids, and Inorganic Elements
3. Pharmacological Properties of GP
3.1. Anti-Cancer Effect
3.1.1. Immune Modulation
3.1.2. Induction of Cell Apoptosis
3.1.3. Inhibition of Cell Migration
3.1.4. Regulation of Gut Microbiota
3.1.5. Cell Cycle Arrest
3.1.6. Others
3.1.7. Toxicity on Normal Cells and Cancer Cells
3.1.8. Relationship between Anti-Cancer Activity and Structure of GPS
3.2. Anti-Atherogenic Effect
3.3. Lipid-Regulating Effect
3.3.1. Effect on Hyperlipidemia
3.3.2. Anti-Obesity Effect
3.4. Neuroprotective Effect
3.4.1. Effect on Alzheimer’s Disease
3.4.2. Effect on Vascular Dementia
3.4.3. Anti-Parkinson’s Disease
3.5. Effects on Ischemia–Reperfusion(I/R) Injury
3.6. Hepatoprotective Effects
3.6.1. CCl4-Induced Liver Injury
3.6.2. Exhaustion Exercise-Induced Liver Injury
3.6.3. Alcoholic Liver Injury
3.6.4. High Choline-Induced Liver Injury
3.6.5. Fatty Liver Disease
3.7. Hypoglycemic Effect
3.8. Others
4. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 6-OHDA | 6-hydroxydopamine |
| ABCA1 | ATP-binding cassette transporter A1 |
| ABCG1 | ATP-binding cassette transporter G1 |
| AD | Alzheimer’s disease |
| ADP | Adenosine diphosphate |
| AIF | Apoptosis-inducing factor |
| Akt | Protein kinase B |
| ALT | Alanine aminotransferase |
| AMPK | AMP-activated protein kinase |
| ARE | Antioxidant responsive element |
| AS | Atherosclerosis |
| AST | Aspartate aminotransferase |
| ATF-4 | Activating transcription factor 4 |
| Atg | Autophagy-related gene |
| Aβ | Beta-amyloid protein |
| BDNF | Brain-derived neurotrophic factor |
| BID | BH3-interacting-domain |
| CAT | Catalase |
| CCl4 | Carbon tetrachloride |
| CDC25A | Cell division cycle protein 25A |
| CDK | Cyclin-dependent kinases |
| Chk2 | Checkpoint kinase 2 |
| CHOP | C/EBP-homologous protein |
| COX-2 | Cyclooxygenase-2 |
| Cry | Crytochrome |
| CTL | Cytotoxic T lymphocytes |
| DFF45 | DNA degradation factor 45 KD |
| DRP-1 | Dynamin-related protein 1 |
| E2F1 | E2F transcription factor 1 |
| ECs | Endothelial cells |
| ER | Endoplasm reticulum |
| ERCC6L | Excision repair cross-complementation group 6 like |
| ERK | Extracellular signal-regulated kinase |
| ERα | Estrogen receptor alpha |
| FAK | Focal adhesion kinase |
| FLIP | FLICE inhibitory protein |
| FOXO1 | Forkhead Box O1 |
| GADD153 | Growth arrest and DNA damage-inducible 153 |
| GluT4 | Glucose transporter 4 |
| GluT9 | Glucose transporter 9 |
| GP | Gynostemma pentaphyllum |
| GPE | Gynostemma pentaphyllum extract |
| GPP | Gynostemma pentaphyllum polysaccharides |
| GPS | Gypenosides |
| GRP78 | Glucose regulated protein 78 KD |
| GSH-Px | Glutathione peroxidase |
| GTP | Guanosine triphosphate |
| H5N1 | Highly pathogenic avian influenza virus |
| HDL | High-density lipoprotein |
| HDL-C | High-density lipoprotein cholesterol |
| HGO | Hepatic glucose output |
| HIF-1α | Hypoxia-inducible factor-1α |
| HO-1 | Heme oxygenase 1 |
| HUVECs | Human umbilical vein endothelial cells |
| I/R | Ischemia–reperfusion |
| ICAM-1 | Intercellular cell adhesion molecule-1 |
| IFN | Interferon |
| IL | Interleukin |
| iNOS | Inducible nitric oxide synthase |
| IP3R | Inositol triphosphate receptor |
| IκBα | Inhibitor of NF-κB α |
| JNK | c-Jun N-terminal kinase |
| LC3 | Microtubule-associated protein 1 light chain 3 |
| LDL | Low-density lipoprotein |
| LDL-C | Low-density lipoprotein cholesterol |
| LDLR | Low-density lipoprotein receptor |
| L-DOPA | L-3, 4-Dihydroxyphenylalanine |
| LXR | Liver X receptor |
| MAPK | Mitogen-activated protein kinase |
| MCP-1 | Monocyte chemotactic protein 1 |
| MDA | Malondialdehyde |
| MDCK | Madin-Darby canine kidney |
| MFC | Mouse forestomach carcinoma |
| Mfn2 | Mitofusin 2 |
| MMP-2 | Matrix metalloproteinase-2 |
| MMP-9 | Matrix metalloproteinase-9 |
| MPP+ | 1-Methyl-4-phenylpyridinium ion |
| MPTP | 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| mTOR | Mammalian target of rapamycin |
| MyD88 | Myeloid differentiation factor 88 |
| NAFLD | Nonalcoholic fatty liver disease |
| NFAT | Nuclear factor of activated T cells |
| NF-kB | Nuclear factor-kappa B |
| NK | Natural killer |
| NMDA | N-methyl-D-aspartate |
| NO | Nitric oxide |
| NOS | Nitric oxide synthase |
| NOX4 | Nicotinamide adenine dinucleotide phosphate-oxidase 4 |
| Nrf2 | Nuclear factor-erythroid 2-related factor 2 |
| OAT1 | Organic anion transporter 1 |
| OGD/R | Oxygen-glucose deprivation–reoxygenation |
| OPA1 | Optic atrophy protein 1 |
| Oria1 | Calcium release-activated calcium channel protein 1 |
| Ox-LDL | Oxidized low-density lipoprotein |
| PARP | Poly ADP-ribose polymerase |
| PCNA | Proliferating cell nuclear antigen |
| PCREB | Phosphorylated cAMP response element-binding protein |
| PCSK9 | Proprotein convertase subtilisin/kexin 9 |
| PD | Parkinson’s disease |
| PI3K | Phosphoinositide 3-kinases |
| PPAR | Peroxisome proliferator activated receptor |
| PRRSV | Porcine reproductive and respiratory syndrome virus |
| PTP1B | Protein tyrosine phosphatase 1B |
| RANKL | Receptor Activator of Nuclear Factor-kappa B Ligand |
| RCC | Renal cell carcinoma |
| ROS | Reactive oxygen species |
| SERCA | Sarco/endoplasmic-reticulum-Ca2+-ATPase |
| SIRT1 | Silent information regulator 1 |
| SOC | Store-operated Ca2+ channel |
| SOCS1 | Suppressors of cytokine signaling 1 |
| SOD | Superoxide dismutase |
| SOS | Sevenless homolog |
| SRB1 | Scavenger receptor class B type I |
| STAI | State-Trait Anxiety Inventory |
| STIM1 | Stromal interacting molecule 1 |
| T-AOC | Total antioxidant capacity |
| TC | Total cholesterol |
| TCM | Traditional Chinese medicine |
| TG | Triglycerides |
| TGF-β | Transforming growth factor-β |
| TH | Tyrosine hydrolase |
| TLR4 | Toll-like receptor 4 |
| TNF | Tumor necrosis factor |
| TRAIL | TNF-related apoptosis-inducing ligand |
| T-STAI, | Trait Anxiety Scale of the STAI |
| ULK1 | Unc-51-like kinase 1 |
| uPA | Urokinase-type plasminogen activator |
| URAT1 | Urate transporter 1 |
| VD | Vascular dementia |
| XIAP | X-linked inhibitor of apoptosis protein |
| γH2AX | Phosphorylation of histone H2AX |
References
- Yuan, Z.Y.; Xie, M.Z.; Huang, H.Y. Advances in the chemical constituents and pharmacological studies of gynostemma pentaphyllum. Asia-Pac. Tradit. Med. 2019, 15, 190–197. [Google Scholar]
- Razmovski-Naumovski, V.; Huang, T.H.; Tran, V.H.; Li, G.Q.; Duke, C.C.; Roufogalis, B.D. Chemistry and Pharmacology of Gynostemma pentaphyllum. Phytochem. Rev. 2005, 4, 197–219. [Google Scholar] [CrossRef]
- Wang, J.; Li, C.H.; Farimani, M.M.; Yang, J.L. Dammarane-type saponins from Gynostemma pentaphyllum and their potential anti-AD activity. Phytochem. Lett. 2019, 31, 147–154. [Google Scholar] [CrossRef]
- Chiranthanut, N.; Teekachunhatean, S.; Panthong, A.; Khonsung, P.; Kanjanapothi, D.; Lertprasertsuk, N. Toxicity evaluation of standardized extract of Gynostemma pentaphyllum Makino. J. Ethnopharmacol. 2013, 149, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yuan, D.S. Advances in Studies on Gynostemma Germplasm Resources in China. J. Yunnan Agric. Univ. 2009, 24, 459–461. [Google Scholar]
- Fan, D.D.; Kuang, Y.H.; Xiang, S.X.; Zhang, D.; Zhu, J.J.; Wang, Z.M.; Wang, D.Q.; Li, C.Y. Research progress in chemical constituents and pharmacological activities of gynostemma pentaphyllum. Chin. Pharm. J. 2017, 52, 342–352. [Google Scholar]
- Zhou, W.C. Research on extraction and anti-inflammatory activity of new saponins from Gynostemma pentaphyllum seeds. Yunnan Chem. Technol. 2015, 45, 34–37. [Google Scholar]
- Lou, Y.Y.; Mu, L.; Huang, Y.P.; Zhang, J.; Yin, Z.Q.; Pan, K. A new cucurbitane-type saponin from Gynostemma pentaphyllum. Chin. Tradit. Herb. Drugs 2021, 52, 1872–1876. [Google Scholar]
- Ji, X.L.; Shen, Y.B.; Guo, X.D. Isolation, Structures, and Bioactivities of the Polysaccharides from Gynostemma pentaphyllum (Thunb.) Makino: A Review. Biomed Res. Int. 2018, 2018, 1–14. [Google Scholar]
- Xue, J.J.; Gao, J.D.; Chen, Z.J.; Jing, M.; Qiao, J.; Liu, X. Chemical composition and pharmacological action of Gynostemma pentaphyllum. J. Gansu Univ. Chin. Med. 2018, 35, 86–89. [Google Scholar]
- Ma, L.P.; Zhao, P.R.; Zhang, H.F.; Lou, Z.L. Determination of polysaccharide content in different parts of Gynostemma pentaphyllum. J. Henan Med Univ. 2000, 35, 445–446. [Google Scholar]
- Ling, Y.; Lei, Z.N.; Li, X.Y.; Yan, X.Y.; Wang, S.Y.; Yang, W.L.; Nie, Q.H.; Zhang, Q.; Bao, H.O.; Yu, J.M.; et al. Characterization and identification of the chemical constituents of gynostemma pentaphyllum using high performance liquid Chromatography-Electrospray Ionization-Quadrupole Time-of-Flight tandem mass spectrometry (HPLC-ESI-QTOF-MS/MS). Anal. Lett. 2020, 53, 760–773. [Google Scholar] [CrossRef]
- Shen, Z.L.; Wang, Z.B.; Hou, H.F.; Zhang, L.; Huang, J.J. New progress in research on chemical constituents, pharmacological effects and application of Gynostemma pentaphyllum. Ginseng Res. 2020, 32, 59–64. [Google Scholar]
- Zu, M.L.; Piao, X.L.; Gao, J.M.; Xing, S.F.; Liu, L.H. Monomer gypenoside LI from Gynostemma pentaphyllum inhibits cell proliferation and upregulates expression of miR-128-3p in melanoma cells. J. Biochem. Mol. Toxic. 2020, 34, 1–8. [Google Scholar] [CrossRef]
- Zu, M.L.; Duan, Y.; Xie, J.B.; Qi, Y.S.; Xie, P.; Borjigidai, A.; Piao, X.L. Gypenoside LI arrests the cell cycle of breast cancer in G0/G1 phase by down-regulating E2F1. J. Ethnopharmacol. 2021, 273, 114017. [Google Scholar] [CrossRef]
- Ma, J.X.; Hu, X.P.; Liao, C.H.; Xiao, H.T.; Zhu, Q.C.; Li, Y.; Liu, Z.G.; Tao, A.J.; He, Z.D.; Xu, C.S.; et al. Gypenoside l inhibits proliferation of liver and esophageal cancer cells by inducing senescence. Molecules 2019, 24, 1054. [Google Scholar] [CrossRef] [Green Version]
- Liao, C.H.; Zheng, K.; Li, Y.; Xu, H.; Kang, Q.R.; Fan, L.; Hu, X.P.; Jin, Z.; Zeng, Y.; Kong, X.L.; et al. Gypenoside L inhibits autophagic flux and induces cell death in human esophageal cancer cells through endoplasm reticulum stress-mediated Ca2+ release. Oncotarget 2016, 7, 47387–47402. [Google Scholar] [CrossRef] [Green Version]
- Zheng, K.; Liao, C.H.; Li, Y.; Fan, X.M.; Fan, L.; Xu, H.; Kang, Q.R.; Zeng, Y.; Wu, X.L.; Wu, H.Q.; et al. Gypenoside L, Isolated from Gynostemma pentaphyllum, Induces Cytoplasmic Vacuolation Death in Hepatocellular Carcinoma Cells through Reactive-Oxygen-Species-Mediated Unfolded Protein Response. J. Agric. Food Chem. 2016, 64, 1702–1711. [Google Scholar] [CrossRef]
- Xing, S.F.; Liu, L.H.; Zu, M.L.; Ding, X.F.; Cui, W.Y.; Chang, T.; Piao, X.L. The inhibitory effect of gypenoside stereoisomers, gypenoside L and gypenoside LI, isolated from Gynostemma pentaphyllum on the growth of human lung cancer A549 cells. J. Ethnopharmacol. 2018, 219, 161–172. [Google Scholar] [CrossRef]
- Lu, H.F.; Chen, Y.S.; Yang, J.S.; Chen, J.C.; Lu, K.W.; Chiu, T.H.; Liu, K.C.; Yeh, C.C.; Chen, G.W.; Lin, H.J.; et al. Gypenosides induced G0/G1 arrest via inhibition of cyclin E and induction of apoptosis via activation of caspases-3 and -9 in human lung cancer A-549 cells. In Vivo 2008, 22, 215–221. [Google Scholar]
- Chen, J.C.; Lu, K.W.; Lee, J.H.; Yeh, C.C.; Chung, J.G. Gypenosides induced apoptosis in human colon cancer cells through the mitochondria-dependent pathways and activation of caspase-3. Anticancer Res. 2006, 26, 4313–4326. [Google Scholar]
- Chen, J.C.; Lu, K.W.; Tsai, M.L.; Hsu, S.C.; Kuo, C.L.; Yang, J.S.; Hsia, T.C.; Yu, C.S.; Chou, S.T.; Kao, M.C.; et al. Gypenosides induced G0/G1 arrest via CHk2 and apoptosis through endoplasmic reticulum stress and mitochondria-dependent pathways in human tongue cancer SCC-4 cells. Oral Oncol. 2009, 45, 273–283. [Google Scholar] [CrossRef]
- Liu, J.S.; Chiang, T.H.; Wang, J.S.; Lin, L.J.; Chao, W.C.; Inbaraj, B.S.; Lu, J.F.; Chen, B.H. Induction of p53-independent growth inhibition in lung carcinoma cell A549 by gypenosides. J. Cell. Mol. Med. 2015, 19, 1697–1709. [Google Scholar] [CrossRef]
- Lu, K.W.; Chen, J.C.; Lai, T.Y.; Yang, J.S.; Weng, S.W.; Ma, Y.S.; Lu, P.J.; Weng, J.R.; Chueh, F.S.; Wood, W.G.; et al. Gypenosides inhibits migration and invasion of human oral cancer SAS cells through the inhibition of matrix metalloproteinase-2 -9 and urokinase-plasminogen by ERK1/2 and NF-kappa B signaling pathways. Hum. Exp. Toxicol. 2011, 30, 406–415. [Google Scholar] [CrossRef]
- Lu, K.W.; Chen, J.C.; Lai, T.Y.; Yang, J.S.; Weng, S.W.; Ma, Y.S.; Lin, H.Y.; Wu, R.S.; Wu, K.C.; Wood, W.G.; et al. Gypenosides suppress growth of human oral cancer SAS cells in vitro and in a murine xenograft model. Integr. Cancer Ther. 2012, 11, 129–140. [Google Scholar] [CrossRef]
- Lu, K.W.; Ma, Y.S.; Yu, F.S.; Huang, Y.P.; Chu, Y.L.; Wu, R.S.; Liao, C.L.; Chueh, F.S.; Chung, J.G. Gypenosides induce cell death and alter gene expression in human oral cancer HSC-3 cells. Exp. Ther. Med. 2017, 14, 2469–2476. [Google Scholar] [CrossRef]
- Shi, L.; Pi, Y.Z.; Luo, C.; Zhang, C.H.; Tan, D.H.; Meng, X.J. In vitro inhibitory activities of six gypenosides on human liver cancer cell line HepG2 and possible role of HIF-1α pathway in them. Chem.-Biol. Interact. 2015, 238, 48–54. [Google Scholar] [CrossRef]
- Sun, D.P.; Li, X.X.; Liu, X.L.; Zhao, D.; Qiu, F.Q.; Li, Y.; Ma, P. Gypenosides induce apoptosis by ca2+ overload mediated by Endoplasmic-Reticulum and Store-Operated ca2+ channels in human hepatoma cells. Cancer Biother. Radio. 2013, 28, 320–326. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.; Wang, X.; Wang, Y.; Wang, P.; Xiao, Y. Antiproliferation and anti-migration induced by gypenosides in human colon cancer SW620 and esophageal cancer Eca-109 cells. Hum. Exp. Toxicol. 2014, 33, 522–533. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Wang, X.B.; Niu, J.F.; Wang, Y.Q.; Wang, P.; Liu, Q.H. Anti-cancer effect and the underlying mechanisms of gypenosides on human colorectal cancer SW-480 cells. PLoS ONE 2014, 9, e95609. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.C.; Lu, J.F.; Wang, J.S.; Lin, L.J.; Kuo, H.I.; Chen, B.H. Antiproliferation Effect and Apoptosis Mechanism of Prostate Cancer Cell PC-3 by Flavonoids and Saponins Prepared from Gynostemma pentaphyllum. J. Agric. Food Chem. 2011, 59, 11319–11329. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, L.H.; Ren, Y.G.; Gao, Y.L.; Kang, L.; Qiao, Q. Anticancer and immunoregulatory activity of Gynostemma pentaphyllum polysaccharides in H22 tumor-bearing mice. Int. J. Biol. Macromol. 2014, 69, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Wang, Z.H.; Zhao, Y.X.; Luo, S.J.; Zhang, D.W.; Xiao, S.X.; Peng, Z.H. Isolation and antitumor activities of acidic polysaccharide from Gynostemma pentaphyllum Makino. Carbohyd. Polym. 2012, 89, 942–947. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.T.; Huang, J.J.; Lin, W.J.; Yuan, Z.W.; Feng, S.L.; Xie, Y.; Ma, W.Z. In Vitro Anticancer Activity of a Nonpolar Fraction from Gynostemma pentaphyllum (Thunb.) Makino. Evid.-Based Compl. Alt. 2016, 2016, 1–11. [Google Scholar]
- Xing, S.F.; Liu, L.H.; Zu, M.L.; Lin, M.; Zhai, X.F.; Piao, X.L. Inhibitory Effect of Damulin B from Gynostemma pentaphyllum on Human Lung Cancer Cells. Planta Med. 2019, 85, 394–405. [Google Scholar] [CrossRef]
- Zhang, X.S.; Shi, G.H.; Wu, X.J.; Zhao, Y. Gypensapogenin H from hydrolyzate of total Gynostemma pentaphyllum saponins induces apoptosis in human breast carcinoma cells. Nat. Prod. Res. 2020, 34, 1642–1646. [Google Scholar] [CrossRef]
- Zhang, X.S.; Zhao, C.; Tang, W.Z.; Wu, X.J.; Zhao, Y.Q. Gypensapogenin H, a novel dammarane-type triterpene induces cell cycle arrest and apoptosis on prostate cancer cells. Steroids 2015, 104, 276–283. [Google Scholar] [CrossRef]
- Liu, Y.J.; Liu, J.C.; Wang, Y.F. Effects of Gynostemma pentaphyllum polysaccharides on tumor growth inhibition and immune regulation in MFC gastric cancer-bearing mice. Chin. Tradit. Pat. Med. 2019, 41, 2876–2881. [Google Scholar]
- Liu, X.; Wang, P.J.; Xu, F.X. Study on Gypenosides inhibiting neoplasm growth and elevating immunological function in Lewis lung cancer of mice. J. Anhui Tradit. Chin. Med Coll. 2001, 20, 43–44. [Google Scholar]
- Tan, X.S.; Li, K.N.; Miao, Y.J. Gypenosides inhibits immune escape factors of non-small lung cancer cells by facilitating sTim-3/Tim-3 ratio. Immunol. J. 2021, 37, 520–527. [Google Scholar]
- Liu, H.; Li, X.M.; Duan, Y.; Xie, J.B.; Piao, X.L. Mechanism of gypenosides of Gynostemma pentaphyllum inducing apoptosis of renal cell carcinoma by PI3K/AKT/mTOR pathway. J. Ethnopharmacol. 2021, 271, 113907. [Google Scholar] [CrossRef]
- Khan, I.; Huang, G.X.; Li, X.A.; Liao, W.L.; Leong, W.K.; Xia, W.R.; Bian, X.Q.; Wu, J.L.; Hsiao, W.L.W. Mushroom polysaccharides and jiaogulan saponins exert cancer preventive effects by shaping the gut microbiota and microenvironment in Apc mice. Pharmacol. Res. 2019, 148, 104448. [Google Scholar] [CrossRef]
- Tsui, K.C.; Chiang, T.H.; Wang, J.S.; Lin, L.J.; Chao, W.C.; Chen, B.H.; Lu, J.F. Flavonoids from Gynostemma pentaphyllum Exhibit Differential Induction of Cell Cycle Arrest in H460 and A549 Cancer Cells. Molecules 2014, 19, 17663–17681. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Tan, D.H.; Yan, T.C.; Jiang, D.H.; Hou, M.X. Cytotoxic triterpenes from the acid hydrolyzate of Gynostemma pentaphyllum saponins. J. Asian Nat. Prod. Res. 2018, 20, 182–187. [Google Scholar] [CrossRef]
- Cui, W.Y.; Jin, Y.L.; Liu, H.; Zu, M.L.; Zhai, X.F.; Yang, C.; Gu, Y.L.; Cheng, Y.; Piao, X.L. Dammarane-type saponins from Gynostemma pentaphyllum and their cytotoxicities. Nat. Prod. Res. 2020, 1–9. [Google Scholar] [CrossRef]
- Shi, L.; Meng, X.J.; Cao, J.Q.; Zhao, Y.Q. A new triterpenoid saponin from Gynostemma pentaphyllum. Nat. Prod. Res. Former. Nat. Prod. Lett. 2012, 26, 1419–1422. [Google Scholar] [CrossRef]
- Yin, F.; Zhang, Y.N.; Yang, Z.Y.; Hu, L.H. Nine new dammarane saponins from Gynostemma pentaphyllum. Chem. Biodivers. 2006, 3, 771–782. [Google Scholar] [CrossRef]
- Shi, L.; Cao, J.Q.; Shi, S.M.; Zhao, Y.Q. Triterpenoid saponins fromGynostemma pentaphyllum. J. Asian Nat. Prod. Res. 2011, 13, 168–177. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, H.; Luo, Y.; Zhang, J.; Wang, M.; Liao, P.; Cao, L.; Guo, P.; Sun, G.; Sun, X. Gypenoside XVII prevents atherosclerosis by attenuating endothelial apoptosis and oxidative stress: Insight into the ERα-Mediated PI3K/Akt pathway. Int. J. Mol. Sci. 2017, 18, 77. [Google Scholar] [CrossRef] [Green Version]
- Shou, D.F.; Lu, D.Z.; Wang, P. The mechanism of gypenosides regulate cholesterol homeostasis in foam cells. J. Zhejiang Chin. Med. Univ. 2011, 35, 297–300. [Google Scholar]
- Ge, Q.Q.; Wang, X.F.; Zong, L.; Bu, J.S.; Lu, D.Z. Effects of gypenosides on PPAR-γ/LXR-α/ABCA1 signaling pathway in vascular of ApoE-/- as mice. Zhejiang Med. Educ. 2019, 18, 47–50. [Google Scholar]
- Shen, C.Y.; Jiang, J.G.; Huang, C.L.; Zhu, W. Gypenoside LVI attenuates foam cell formation by promoting cholesterol export and inhibiting inflammation response. J. Funct. Foods 2018, 50, 71–77. [Google Scholar] [CrossRef]
- Song, N.; Yang, F.; Cao, H.M.; Zhang, N.; Jia, L.Q.; Chen, W.N.; Yang, G.L. Gypenoside influences the progression of atherosclerosis in the ApoE-/- mouse through mTOR/ULK1 pathway. Chin J Arter. 2018, 26, 127–132. [Google Scholar]
- Quan, Y.; Qian, M.Z. Effect and Mechanism of Gypenosides on the Inflammatory Molecular Expression in High fat Induced Atherosclerosis Rat. Chin. J. Integr. Tradit. West. 2010, 30, 403–406. [Google Scholar]
- Song, N.; Jia, L.Q.; Cao, H.M.; Ma, Y.X.; Chen, N.; Chen, S.; Lv, X.M.; Yang, G.L. Gypenoside inhibits endothelial cell apoptosis in atherosclerosis by modulating mitochondria through PI3K/Akt/Bad pathway. Biomed Res. Int. 2020, 2020, 1–12. [Google Scholar] [CrossRef]
- Quan, Y.; Yang, Y.; Wang, H.; Shu, B.; Gong, Q.H.; Qian, M. Gypenosides attenuate cholesterol-induced DNA damage by inhibiting the production of reactive oxygen species in human umbilical vein endothelial cells. Mol. Med. Rep. 2015, 11, 2845–2851. [Google Scholar] [CrossRef]
- Bo, H.; Hou, X.W.; Liu, R.H.; Liu, X.H.; Hu, Z.H. Gypenoside inhibits ox-LDL uptake and foam cell formation through enhancing Sirt1-FOXO1 mediated autophagy flux restoration. Life Sci. 2018, 264, 118721. [Google Scholar]
- Song, N.; Chen, N.; Cao, H.M.; Chen, S.; Wang, Y.; Qu, N.N.; Jia, L.Q.; Wang, L. Effects of gynostemma pentaphyllum saponins on atherosclerosis in ApoE-/- mice by regulating biological rhythm related proteins. Chin. Arch. Tradit. Chin. Med. 2021, 39, 248–251. [Google Scholar]
- Sun, X.N.; Song, N.; Yang, X.; Chen, Y.R.; Chen, N.; Cao, H.M. Mechanism of gynostemma saponin a improving ox-LDL-induced EA.hy926 cell injury by regulating mitochondrial function. Chin. Arch. Tradit. Chin. Med. 2020, 38, 77–80. [Google Scholar]
- Zhang, N.; Song, N.; Cao, H.M.; Jia, L.Q.; Chen, W.N.; Leng, X. Effect of gypenosides on the prevention and treatment of atherosclerosis by autophagosome. Nat. Prod. Res. Dev. 2017, 29, 2112–2116. [Google Scholar]
- Yang, J.X.; Ma, F.H.; Zhang, J.T.; Shou, Z.X.; Tang, N.; Tang, C.; Xie, Z.X.; Sun, J.F.; Wu, N. Gypenosides suppress the inflammatory response to atherosclerosis in rats and interfere the expression of TLR4/MyD88/NF-κB signaling pathway. Genom. Appl. Biol. 2021. [Google Scholar]
- Wu, D.; Jiao, X.; Yuan, Z.L.; He, Y.Q. Gypenosides attenuated LPS-induced endothelial cell inflammatory injury. Chin. J. New Drugs 2020, 29, 323–328. [Google Scholar]
- Huang, T.H.; Razmovski-Naumovski, V.; Salam, N.K.; Duke, R.K.; Tran, V.H.; Duke, C.C.; Roufogalis, B.D. A novel LXR-α activator identified from the natural product Gynostemma pentaphyllum. Biochem. Pharmacol. 2005, 70, 1298–1308. [Google Scholar] [CrossRef] [PubMed]
- Wang, W. Gypenosides Regulates Lipid and Carbohydrate Metabolism via Bile Acids Related Pathway. Master’s Thesis, Zunyi Medical University, Zunyi, China, 2017. [Google Scholar]
- Mao, Y. The Effect of Gypenosides Relieving Fat Accumulation in HepG2 Cells. Master’s Thesis, Shenyang Agricultural University, Shenyang, China, 2017. [Google Scholar]
- Zhang, Y.D.; Jiang, X.Y.; Cao, L.J.; Wang, J.; Jiang, C.H.; Zhao, M.G.; Zhang, J.; Yin, Z.Q. Gypenoside granules improved lipid metabolism in C57BL/6J mice with hyperlipidemia. J. China Pharm. Univ. 2019, 50, 713–720. [Google Scholar]
- Wu, L.S.; Qian, M.Z. Effects of gypenosides on PCSK9 gene expression and blood lipids lowered by simvastatin. Chin. J. Pathophysiol. 2017, 33, 79–85. [Google Scholar]
- Wang, Y.S.; Wang, J.; Wang, X.N.; Jiang, C.H.; Zheng, X.; Zhang, J.; Yin, Z.Q. Therapeutic effects of gypenosides on hypercholesterolemia and it protective effect on liver injury. J. China Pharm. Univ. 2021, 52, 84–91. [Google Scholar]
- Yin, M.J.; Zhang, J.J.; Wang, L.Z.; Li, F.Y.; Li, Z.F.; Xiang, W.; Bie, S.T.; Wang, C.H.; Li, Z. Ten New Dammarane-Type Saponins with Hypolipidemia Activity from a Functional Herbal Tea-Gynostemma pentaphyllum. Molecules 2020, 25, 3737. [Google Scholar] [CrossRef]
- Lee, H.S.; Lim, S.; Jung, J.I.; Kim, S.M.; Lee, J.K.; Kim, Y.H.; Cha, K.M.; Oh, T.K.; Moon, J.M.; Kim, T.Y.; et al. Gynostemma pentaphyllum extract ameliorates High-Fat Diet-Induced obesity in C57BL/6N mice by upregulating SIRT1. Nutrients 2019, 11, 2475. [Google Scholar] [CrossRef] [Green Version]
- Gauhar, R.; Hwang, S.; Jeong, S.; Kim, J.; Song, H.; Park, D.C.; Song, K.; Kim, T.Y.; Oh, W.K.; Huh, T. Heat-processed Gynostemma pentaphyllum extract improves obesity in ob/ob mice by activating AMP-activated protein kinase. Biotechnol. Lett. 2012, 34, 1607–1616. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.F.; Yang, P.Y.; Wan, J.C.; Chang, Q.M.; Wang, T.T.Y.; Lu, W.Y.; Zhang, Y.Q.; Wang, Q.; Yu, L.L. Gypenosides reduced the risk of overweight and insulin resistance in C57BL/6J mice through modulating adipose thermogenesis and gut microbiota. J. Agric. Food Chem. 2017, 65, 9237–9246. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Yang, P.Y.; Shi, H.M.; Sun, X.J.; Lee, S.; Yu, L.L. A novel Gynostemma pentaphyllum saponin and its adipogenesis inhibitory effect through modulating Wnt/β-catenin pathway and cell cycle in mitotic clonal expansion. J. Funct. Foods 2015, 17, 552–562. [Google Scholar] [CrossRef]
- Rao, A.; Clayton, P.; Briskey, D. The effect of an orally-dosed Gynostemma pentaphyllum extract (ActivAMP®) on body composition in overweight, adult men and women: A double-blind, randomised, placebo-controlled study. J. Hum. Nutr. Diet. 2021. [Google Scholar] [CrossRef]
- Wang, J.Z.; Hu, G.; Zai, W.H. Degrading blood-fat effect of saponin from gynostemma pentaphyllum in mice with hyperlipidemia. Chin. J. Ethnomedicine Ethnopharmacy 2007, 41–43. [Google Scholar]
- Song, B.Y. Research progress in the pathogenesis of alzheimer’s disease. J. Xi’an Univ. 2020, 23, 77–79. [Google Scholar]
- Yao, B.C.; Ao, Y.; Zhang, G.; Liu, M.X.; Yao, S.Y.; Li, D.M. Effect and mechanism of Gynostemma pentaphyllum Makino on learning and memory ability in dementia mice. Chin J Public Health 2017, 33, 1708–1711. [Google Scholar]
- Cai, H.; Liang, Q.L.; Ge, G.Q. Gypenoside Attenuates β Amyloid-Induced Inflammation in N9 Microglial Cells via SOCS1 Signaling. Neural Plast. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.; Shim, I.; Lee, H. Gypenosides attenuate Lipopolysaccharide-Induced neuroinflammation and memory impairment in rats. Evid.-Based Compl. Alt. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.L.; Sun, T.M.; Wang, M.; Li, R.H.; Yao, B.C.; Tan, H.B. The influence of steven leafs on the cell’s model of AD inducted by aβ1-40. Chin. J. Integr. Med. Cardio-Cerebrovasc. Dis. 2012, 10, 201–204. [Google Scholar]
- Jia, D.; Rao, C.G.; Xue, S.X.; Lei, J.L. Purification, characterization and neuroprotective effects of a polysaccharide from Gynostemma pentaphyllum. Carbohyd. Polym. 2015, 122, 93–100. [Google Scholar] [CrossRef]
- Meng, X.B.; Luo, Y.; Liang, T.; Wang, M.X.; Zhao, J.Y.; Sun, G.B.; Sua, X.B. Gypenoside XVII enhances lysosome biogenesis and autophagy flux and accelerates autophagic. J. Alzheimer’s Dis. 2016, 1135–1150. [Google Scholar] [CrossRef]
- Meng, X.B.; Wang, M.; Sun, G.B.; Ye, J.X.; Zhou, Y.H.; Dong, X.; Wang, T.T.; Lu, S.; Sun, X.B. Attenuation of Aβ25–35-induced parallel autophagic and apoptotic cell death by gypenoside XVII through the estrogen receptor-dependent activation of Nrf2/ARE pathways. Toxicol. Appl. Pharm. 2014, 279, 63–75. [Google Scholar] [CrossRef]
- Hong, S.; Yang, J.; Joh, E.; Kim, H.J.; Kim, D. Gypenoside TN-2 ameliorates scopolamine-induced learning deficit in mice. J. Ethnopharmacol. 2011, 134, 1010–1013. [Google Scholar] [CrossRef]
- Joh, E.; Yang, J.; Kim, D. Gypenoside LXXIV ameliorates Scopolamine-Induced learning deficit in mice. Planta Med. 2010, 76, 793–795. [Google Scholar] [CrossRef]
- Yang, X.; Tang, X.; Su, Y.; Cai, Z.; Zeng, D.; Xu, L. Protective effect and mechanism of gypenosides on hippocampus neurons in rats with chronic cerebral ischemia. Chin J. Mod. Appl. Pharm. 2019, 36, 1487–1491. [Google Scholar]
- Qi, M.F.; Luo, C.L.; Qin, W.; Tian, H.; Chen, Y.S. Gynostemma improves vascular dementia-induced cognitive dysfunction in mice. Acta Acad. Med. Zunyi 2012, 35, 371–374. [Google Scholar]
- Luo, C.L.; Tian, H.; Qin, W.; Chen, Y.S. Effect of Gypenosides on the neurons injury and expression of phosphorylated CREB in the hippocampus tissue of mouse with vascular dementia. J. Zunyi Med. Univ. 2014, 37, 320–323. [Google Scholar]
- Zhang, G.L.; Deng, J.P.; Wang, B.H.; Zhao, Z.W.; Li, J.; Gao, L.; Liu, B.L.; Xong, J.R.; Guo, X.D.; Yan, Z.Q.; et al. Gypenosides improve cognitive impairment induced by chronic cerebral hypoperfusion in rats by suppressing oxidative stress and astrocytic activation. Behav. Pharmacol. 2011, 22, 633–644. [Google Scholar] [CrossRef]
- Wang, P.; Niu, L.; Gao, L.; Li, W.X.; Jia, D.; Wang, X.L.; Gao, G.D. Neuroprotective effect of gypenosides against oxidative injury in the substantia nigra of a mouse model of parkinson’s disease. J. Int. Med. Res. 2010, 38, 1084–1092. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Niu, L.; Guo, X.D.; Gao, L.; Li, W.X.; Jia, D.; Wang, X.L.; Ma, L.T.; Gao, G.D. Gypenosides protects dopaminergic neurons in primary culture against MPP+-induced oxidative injury. Brain Res. Bull. 2010, 83, 266–271. [Google Scholar] [CrossRef]
- Shin, K.S.; Zhao, T.T.; Park, K.H.; Park, H.J.; Hwang, B.Y.; Lee, C.K.; Lee, M.K. Gypenosides attenuate the development of L-DOPA-induced dyskinesia in 6-hydroxydopamine-lesioned rat model of Parkinson’s disease. BMC Neurosci. 2015, 16, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Zhao, T.T.; Kim, K.S.; Shin, K.S.; Park, H.J.; Kim, H.J.; Lee, K.E.; Lee, M.K. Gypenosides ameliorate memory deficits in MPTP-lesioned mouse model of Parkinson’s disease treated with L-DOPA. BMC Complem. Altern. Med. 2017, 17, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, K.S.; Zhao, T.T.; Choi, H.S.; Hwang, B.Y.; Lee, C.K.; Lee, M.K. Effects of gypenosides on anxiety disorders in MPTP-lesioned mouse model of Parkinson’s disease. Brain Res. 2014, 1567, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Park, M.S.; Kim, S.H.; Hwang, B.Y.; Lee, C.K.; Lee, M.K. Neuroprotective Effects of Herbal Ethanol Extracts from Gynostemma pentaphyllum in the 6-Hydroxydopamine-Lesioned Rat Model of Parkinson’s Disease. Molecules 2010, 15, 2814–2824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, F.Y.; Chen, Z.F.; Wu, H.M.; Guo, P.X. Research Progress on the Pathogenesis of Parkinson’s Disease. Chin. J. Ethnomed. Ethnopharmacy 2020, 29, 60–64. [Google Scholar]
- Yu, H.J.; Shi, L.Y.; Qi, G.X.; Zhao, S.J.; Gao, Y.; Li, Y.Z. Gypenoside protects cardiomyocytes against Ischemia-Reperfusion injury via the inhibition of Mitogen-Activated protein kinase mediated nuclear factor kappa b pathway in vitro and in vivo. Front. Pharmacol. 2016, 7, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.J.; Guan, Q.G.; Guo, L.; Zhang, H.S.; Pang, X.F.; Cheng, Y.; Zhang, X.G.; Sun, Y.X. Gypenosides alleviate myocardial ischemia-reperfusion injury via attenuation of oxidative stress and preservation of mitochondrial function in rat heart. Cell Stress Chaperones 2016, 21, 429–437. [Google Scholar] [CrossRef] [Green Version]
- Chang, L.P.; Shi, R.; Wang, X.J.; Bao, Y. Gypenoside a protects ischemia/reperfusion injuries by suppressing miR-143-3p level via the activation of AMPK/Foxo1 pathway. Biofactors 2020, 46, 432–440. [Google Scholar] [CrossRef]
- Yu, H.J.; Zhang, H.S.; Zhao, W.H.; Guo, L.; Li, X.Y.; Li, Y.; Zhang, X.G.; Sun, Y.X. Gypenoside protects against myocardial Ischemia-Reperfusion injury by inhibiting cardiomyocytes apoptosis via inhibition of CHOP pathway and activation of PI3K/Akt pathway in vivo and in vitro. Cell. Physiol. Biochem. 2016, 39, 123–136. [Google Scholar] [CrossRef]
- Ye, Q.F.; Zhu, Y.; Ye, S.J.; Liu, H.; She, X.G.; Niu, Y.; Ming, Y.Z. Gypenoside attenuates renal ischemia/reperfusion injury in mice by inhibition of ERK signaling. Exp. Ther. Med. 2016, 11, 1499–1505. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Ming, Y.Z.; Wan, Q.Q.; Ye, S.J.; Xie, S.; Zhu, Y.; Wang, Y.F.; Zhong, Z.B.; Li, L.; Ye, Q.F. Gypenoside attenuates hepatic ischemia/reperfusion injury in mice via anti-oxidative and anti-apoptotic bioactivities. Exp. Ther. Med. 2014, 7, 1388–1392. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.M. Protective Effect of Gynostemma pentaphyllum Polysaccharide on Liver Injure Induced by Carbon Tetrachloride in Rats. Chin. J. Exp. Tradit. Med. Formulae 2013, 19, 244–247. [Google Scholar]
- Chen, J.; Tsai, C.; Chen, L.; Chen, H.; Wang, W. Therapeutic effect of gypenoside on chronic liver injury and fibrosis induced by CCl4 in rats. Am. J. Chin. Med. 2000, 28, 175–185. [Google Scholar] [CrossRef]
- Chen, J.X.; Zhang, J.G.; Zhang, L.; Liu, J.B. Study on Liver-protective Effect of Total Saponins of Gynostemma Pentaphyllum. China Pharm. 2007, 16, 7–8. [Google Scholar]
- Chen, J.M.; Li, X.W.; Hu, Y.H.; Liu, W.; Zhou, Q.; Zhang, H.; Mu, Y.P.; Liu, P. Gypenosides ameliorate carbon Tetrachloride-Induced liver fibrosis by inhibiting the differentiation of hepatic progenitor cells into myofibroblasts. Am. J. Chin. Med. 2017, 45, 1061–1074. [Google Scholar] [CrossRef]
- Xu, Y.F.; Qi, B. Protective Effects of Polysaccharides from Gynostemma pentaphyllum Makino on the Exhaustive Exercise-induced Hepatocyte Apoptosis. Food Res. Dev. 2016, 37, 158–162. [Google Scholar]
- Nan, Y.; Zhang, W.; Chang, J.R.; Cao, J.; Zhu, Y.X. Gypenosides protect acute alcoholic liver injury through Nrf2 /NF-κB signaling pathway in mice. Chin. Pharmacol. Bull. 2019, 35, 40–45. [Google Scholar]
- Yang, C.C.; Zhao, Y.; Ren, D.Y.; Yang, X.B. Protective Effect of Saponins-Enriched Fraction of Gynostemma pentaphyllum against High Choline-Induced Vascular Endothelial Dysfunction and Hepatic Damage in Mice. Biol. Pharm. Bull. 2020, 43, 463–473. [Google Scholar] [CrossRef] [Green Version]
- Qin, R.N.; Zhang, J.Y.; Li, C.Y.; Zhang, X.Q.; Xiong, A.H.; Huang, F.; Yin, Z.; Li, K.Y.; Qin, W.Y.; Chen, M.Z.; et al. Protective effects of gypenosides against fatty liver disease induced by high fat and cholesterol diet and alcohol in rats. Arch. Pharm. Res. 2012, 35, 1241–1250. [Google Scholar] [CrossRef]
- Bae, U.J.; Park, E.O.; Park, J.; Jung, S.J.; Ham, H.; Yu, K.W.; Park, Y.J.; Chae, S.W.; Park, B.H. Gypenoside UL4-Rich Gynostemma pentaphyllum Extract Exerts a Hepatoprotective Effect on Diet-Induced Nonalcoholic Fatty Liver Disease. Am. J. Chin. Med. 2018, 46, 1315–1332. [Google Scholar] [CrossRef]
- Shen, S.H.; Zhong, T.Y.; Peng, C.; Fang, J.; Lv, B. Structural modulation of gut microbiota during alleviation of non-alcoholic fatty liver disease with Gynostemma pentaphyllum in rats. BMC Complement. Med. Ther. 2020, 20, 1–12. [Google Scholar] [CrossRef]
- Ruan, D.; Lei, J. Beneficial effect of stevenleaf on the imbalance between th17 and treg in nonalcoholic fatty liver disease model rats. Chin J. Mod. Appl. Pharm. 2017, 34, 1683–1688. [Google Scholar]
- Stein, S.A.; Lamos, E.M.; Davis, S.N. A review of the efficacy and safety of oral antidiabetic drugs. Expert Opin. Drug Saf. 2013, 12, 153–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, K.N.; Shou, Q.Y.; Tian, Y.S.; Gu, G.Z.; Hu, Q.J.; Yang, H.X.; Chen, X.P.; Wang, X.C. Research progress on bioactive component groups and mechanisms of Gynostemma pentaphyllum for treatment of diabetes. Chin. Tradit. Herb. Drugs 2020, 51, 2248–2257. [Google Scholar]
- Lin, Z.Z.; Chen, T. Study on hypoglycemic effect of gypenosides on experimental diabetic mice. J. Longyan Univ. 2011, 29, 51–53. [Google Scholar]
- Nguyen, P.H.; Gauhar, R.; Hwang, S.L.; Dao, T.T.; Park, D.C.; Kim, J.E.; Song, H.; Huh, T.L.; Oh, W.K. New dammarane-type glucosides as potential activators of AMP-activated protein kinase (AMPK) from Gynostemma pentaphyllum. Bioorgan. Med. Chem. 2011, 19, 6254–6260. [Google Scholar] [CrossRef]
- Wei, S.R.; Xue, C.K.; He, X.B.; Shen, K.; Yuan, B.; Jiang, P.; Zhu, J.; Li, Y.; Zeng, L. Experimental study on hypoglycemic effect of Gynostemma pentaphyllum polysaccharide. Chin. J. Gerontol. 2005, 25, 418–420. [Google Scholar]
- Du, X.Y.; Hou, Y.; Tan, H.; Han, Y.; Zhang, Y. Hypoglycemic Activity of Polysaccharide from Gynostemma pentaphyllum on Type 2 Diabetic Rats and its Mechanism. Sci. Technol. Eng. 2011, 11, 5754–5758. [Google Scholar]
- Ou, S.Z.; Mo, Y.N.; Chen, S.M.; Gao, L.F.; Ji, L.M.; Wang, D.M.; He, T.; Yu, D.R. Study on the hypoglycemic effect of the ethanol extract of Hainan gynostemma. China Trop. Med. 2014, 14, 38–40. [Google Scholar]
- Yassin, K.; Huyen, V.T.; Hoa, K.N.; Ostenson, C.G. Herbal extract of gynostemma pentaphyllum decreases hepatic glucose output in type 2 diabetic goto-kakizaki rats. Int. J.Biomed. Sci. 2011, 7, 131–136. [Google Scholar]
- Norberg, Å.; Hoa, N.K.; Liepinsh, E.; Van Phan, D.; Thuan, N.D.; Jörnvall, H.; Sillard, R.; östenson, C. A Novel Insulin-releasing Substance, Phanoside, from the Plant Gynostemma pentaphyllum. J. Biol. Chem. 2004, 279, 41361–41367. [Google Scholar] [CrossRef] [Green Version]
- Hoa, N.K.; Norberg, A.; Sillard, R.; Van Phan, D.; Thuan, N.D.; Dzung, D.T.N.; Jörnvall, H.; Östenson, C. The possible mechanisms by which phanoside stimulates insulin secretion from rat islets. J. Endocrinol. 2007, 192, 389–394. [Google Scholar] [CrossRef] [Green Version]
- Lundqvist, L.C.E.; Rattigan, D.; Ehtesham, E.; Demmou, C.; östenson, C.; Sandström, C. Profiling and activity screening of Dammarane-type triterpen saponins from Gynostemma pentaphyllum with glucose-dependent insulin secretory activity. Sci. Rep.-UK 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Megalli, S.; Davies, N.M.; Roufogalis, B.D. Anti-hyperlipidemic and hypoglycemic effects of Gynostemma pentaphyllum in the Zucker fatty rat. J. Pharm. Pharm. Sci. 2006, 9, 281–291. [Google Scholar]
- Wang, Z.; Yin, Y.; Bian, Y.Y.; Tian, J.L.; Shi, L. Triterpenoids from Gynostemma pentaphyllum and their inhibition activity to α-glycosidase and protein tyrosine phosphatase 1B. Chin. Tradit. Herb. Drugs 2020, 51, 6142–6150. [Google Scholar]
- Zhang, X.S.; Bi, X.L.; Xiao, W.; Cao, J.Q.; Xia, X.C.; Diao, Y.P.; Zhao, Y.Q. Protein tyrosine phosphatase 1B inhibitory effect by dammarane-type triterpenes from hydrolyzate of total Gynostemma pentaphyllum saponins. Bioorg. Med. Chem. Lett. 2013, 23, 297–300. [Google Scholar] [CrossRef]
- Zhu, K.N.; Tian, S.S.; Wang, H.; Tian, Y.S.; Gu, G.Z.; Qiu, Y.Y.; Zhang, L.; Yang, H.X. Study on effect of gypenosides on insulin sensitivity in rats with diabetes mellitus via regulating NF-κB signaling pathway. China J. Chin. Mater. Med. 2021, 46, 4488–4496. [Google Scholar]
- Huang, X.F.; Song, Y.; Song, C.W.; Wang, M.Y.; Liu, H.X.; Yu, S.G.; Fang, N.B. Study on hypoglycemic activity of different components of Gynostemma pentaphyllum. Hubei J. TCM 2013, 35, 67–69. [Google Scholar]
- Huyen, V.T.T.; Phan, D.V.; Thang, P.; Hoa, N.K.; östenson, C.G. Gynostemma pentaphyllum Tea Improves Insulin Sensitivity in Type 2 Diabetic Patients. J. Nutr. Metab. 2013, 2013, 765383. [Google Scholar] [CrossRef] [Green Version]
- Huyen, V.T.; Phan, D.V.; Thang, P.; Hoa, N.K.; Ostenson, C.G. Antidiabetic effect of Gynostemma pentaphyllum tea in randomly assigned type 2 diabetic patients. Horm. Metab. Res. 2010, 42, 353–357. [Google Scholar] [CrossRef] [Green Version]
- Lokman, E.F.; Gu, H.F.; Wan Mohamud, W.N.; östenson, C. Evaluation of antidiabetic effects of the traditional medicinal plant gynostemma pentaphyllum and the possible mechanisms of insulin release. Evid.-Based Compl. Alt. 2015, 2015, 120572. [Google Scholar] [CrossRef] [Green Version]
- Su, Q.X.; Cong, J.; Gong, Q. Study on the effect of gypenosides on antioxidative injury in aged mice skin. Chin. J. Aesthetic Med. 2015, 24, 34–36. [Google Scholar]
- Yao, D.D.; Huang, S.H.; Liu, G.H. Effects of gynostemma pentaphyllum makino on hypothalamus in aging rats and its mechanisms. J. Med. Pr. 2007, 20, 1119–1121. [Google Scholar]
- Zhang, R.X. Study about the Effect of Gynostemma Pentaphyllum Polysaccharide on Anti-Oxidation Capability of Aging Mice Induced by D-Galactose. Master’s Thesis, Guangxi University, Guangxi, China, 2020. [Google Scholar]
- Zhao, T.T.; Shin, K.S.; Choi, H.S.; Lee, M.K. Ameliorating effects of gypenosides on chronic stress-induced anxiety disorders in mice. BMC Complementary Altern. Med. 2015, 15, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Choi, E.; Won, Y.H.; Kim, S.; Noh, S.; Park, S.; Jung, S.; Lee, C.K.; Hwang, B.Y.; Lee, M.K.; Ha, K.; et al. Supplementation with extract of Gynostemma pentaphyllum leaves reduces anxiety in healthy subjects with chronic psychological stress: A randomized, double-blind, placebo-controlled clinical trial. Phytomedicine 2018, 52, 198–205. [Google Scholar] [CrossRef]
- Mu, R.H.; Fang, X.Y.; Wang, S.S.; Li, C.F.; Chen, S.M.; Chen, X.M.; Liu, Q.; Li, Y.C.; Yi, L.T. Antidepressant-like effects of standardized gypenosides: Involvement of brain-derived neurotrophic factor signaling in hippocampus. Psychopharmacology 2016, 233, 3211–3221. [Google Scholar] [CrossRef]
- Shang, X.Y.; Chao, Y.; Zhang, Y.; Lu, C.Y.; Xu, C.L.; Niu, W.N. Immunomodulatory and Antioxidant Effects of Polysaccharides from Gynostemma pentaphyllum Makino in Immunosuppressed Mice. Molecules 2016, 21, 1085. [Google Scholar] [CrossRef] [Green Version]
- Rujjanawate, C.; Kanjanapothi, D.; Amornlerdpison, D. The anti-gastric ulcer effect of Gynostemma pentaphyllum Makino. Phytomedicine 2004, 11, 431–435. [Google Scholar] [CrossRef]
- Shan, L.N.; Shi, Y.X. Effects of polysaccharides from Gynostemma pentaphyllum (Thunb.) Makino on physical fatigue. Afr. J. Tradit. Complement Altern. Med. 2014, 11, 112–117. [Google Scholar]
- Kim, Y.H.; Jung, J.I.; Jeon, Y.E.; Kim, S.M.; Oh, T.K.; Lee, J.; Moon, J.M.; Kim, T.Y.; Kim, E.J. Gynostemma pentaphyllum extract and Gypenoside L enhance skeletal muscle differentiation and mitochondrial metabolism by activating the PGC-1α pathway in C2C12 myotubes. Nutr. Res. Pract. 2021, 15, e45. [Google Scholar]
- Yin, X.M. Effects of combination of aspirin and gypenosides on platelet aggregation. Chin. J. Hosp. Pharm. 2001, 21, 468–470. [Google Scholar]
- Jiang, Y.; Chen, X.L. Effects of Gypenosides on sedative hypnosis and neurotransmitters in rats. Zhejiang J. Tradit. Chin. Med. 2016, 51, 459–460. [Google Scholar]
- Srichana, D.; Taengtip, R.; Kondo, S. Antimicrobial activity of Gynostemma pentaphyllum extracts against fungi producing aflatoxin and fumonisin and bacteria causing diarrheal disease. Southeast Asian J. Trop. Med. Public Health 2011, 42, 704–710. [Google Scholar] [PubMed]
- Wang, T.; Guo, Y.S.; Jiang, Y.L.; Li, M.D.; Wu, L.; Liu, H.Q.; Su, P. Study of the immune enhancement effect of Epimedium polysaccharide and Gynostemma polysaccharide on PRRSV attenuated vaccine. Heilongjiang Anim. Sci. Vet. Med. 2021, 122–126. [Google Scholar]
- Sornpet, B.; Potha, T.; Tragoolpua, Y.; Pringproa, K. Antiviral activity of five Asian medicinal pant crude extracts against highly pathogenic H5N1 avian influenza virus. Asian Pac. J. Trop. Med. 2017, 10, 871–876. [Google Scholar] [CrossRef]
- Pang, M.X.; Fang, Y.Y.; Chen, S.H.; Zhu, X.X.; Shan, C.W.; Su, J.; Yu, J.J.; Li, B.; Yang, Y.; Chen, B.; et al. Gypenosides inhibits xanthine oxidoreductase and ameliorates urate excretion in hyperuricemic rats induced by high cholesterol and high fat food (Lipid emulsion). Med. Sci. Monit. 2017, 23, 1129–1140. [Google Scholar] [CrossRef] [Green Version]
- Han, J.K.; Gao, W.; Su, D.Y.; Liu, Y. Gypenoside inhibits RANKL-induced osteoclastogenesis by regulating NF-κB, AKT, and MAPK signaling pathways. J. Cell. Biochem. 2018, 119, 7310–7318. [Google Scholar] [CrossRef]
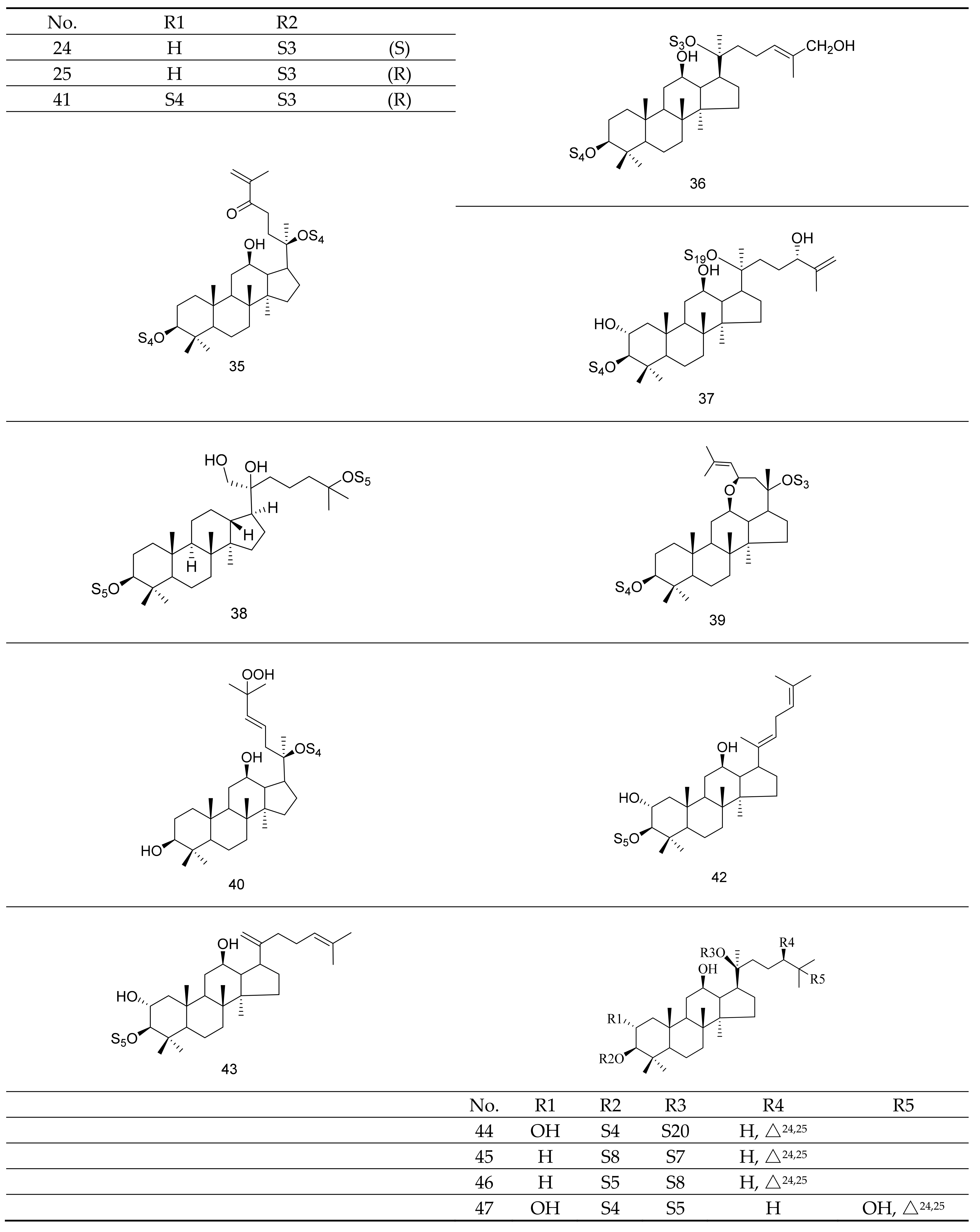
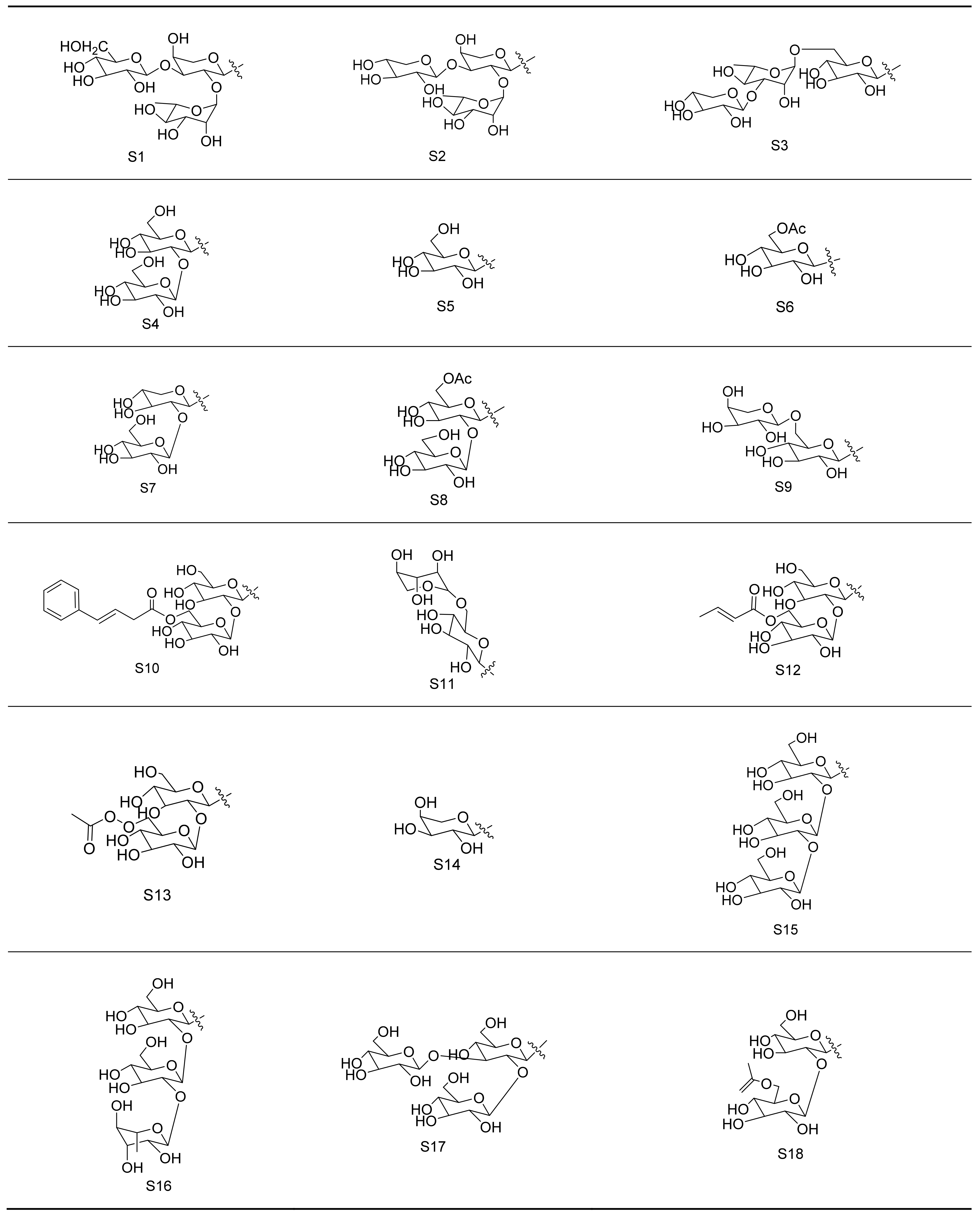
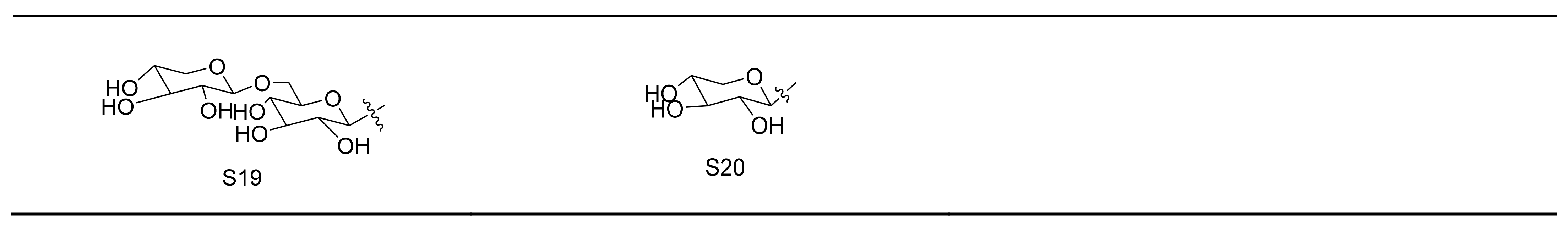
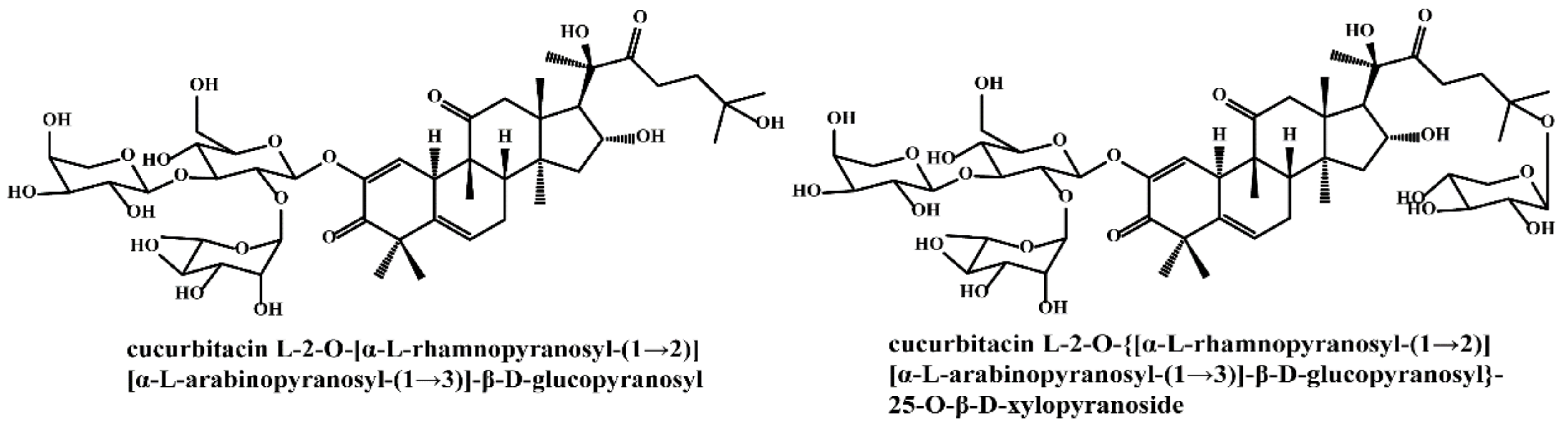


| Active Ingredient | Model | Mechanism | Ref. |
|---|---|---|---|
| Gypenoside LI | Melanoma cells (A375 and SK-MEL-28) | Induced intrinsic apoptosis along with S phase arrest, decreased the levels of CDK2, P-CDK2, Bcl-2, and FLIP, increased the levels of caspase 9, PARP cleavage, and BID death agonist, and increased the level of tumor suppressor miR-128-3p. | [14] |
| Gypenoside LI | Human breast cancer cells (MDA-MB-231 and MCF-7) | Inhibited the proliferation by decreasing the expression of ERCC6L, down-regulated the expression of MMP2 and MMP9, suppressed the migration ability of breast cancer cells, induced cell apoptosis, increased cytochrome c release from mitochondria into the cytoplasm, increased levels of Bax, decreased levels of PARP-1 and Bcl-2, induced cell cycle arrest in G0/G1 phase via down-regulating E2F1 and decreasing CDK2, CDK4, and cyclin D1. | [15] |
| Gypenoside L | Human hepatic cancer HepG2 cells and esophagus cancer ECA-109 cells | Caused cell cycle arrest at S phase, reduced the expression of CDK2, CDK4, CDK6, and cyclin D1, activated senescence-related cell cycle inhibitor proteins (p21 and p27) and their upstream regulators, activated p38 and ERK MAPK pathways and NF-κB pathway to induce senescence. | [16] |
| Gypenoside L | Human esophageal cancer cells (ECA-109 and TE-1) | Induced cell death, which was associated with lysosomal swelling and autophagic flux inhibition, increased the levels of ROS, triggered protein ubiquitination and ER stress response, leading to Ca2+ release from ER IP3R-operated stores and finally cell death. | [17] |
| Gypenoside L | Human hepatocellular carcinoma cells | Increased the intracellular ROS levels, which triggered protein ubiquitination and unfolded protein response, resulting in Ca2+ release from ER IP3R-operated stores and finally cytoplasmic vacuolation and cell death. | [18] |
| Gypenoside L and Gypenoside LI | Human lung cancer A549 cells | Inhibited A549 cell migration from the upper to the lower chamber through the membrane, decreased the expression of MMP2 and MMP9, gypenoside L down-regulated the expression of CDK2 and CDK4 proteins rather than CDK1 protein, while gypenoside LI suppressed the expression of CDK1 protein rather than CDK2 and CDK4 proteins. Gypenoside L induced G0/G1 arrest and gypenoside LI induced G2/M arrest in A549 cells; induced A549 cell apoptosis through extrinsic pathway and intrinsic pathway, boosted the production of ROS, led to more cytochrome c release from mitochondria into cytoplasm and less expression of procaspase 8. | [19] |
| GPS | Human lung cancer A549 cells | Increased p16, p21, p27, and p53 proteins and induced G0/G1 arrest; induced apoptosis by up-regulating Bax, caspase-3, and caspase-9 and down-regulating Bcl-2 levels. | [20] |
| GPS | Human colon cancer 205 cells | Induced DNA fragmentation and cell apoptosis, induced ROS and Ca2+ production, decreased the expression of Bcl-2 and Bcl-xl but increased the expression of Bax, increased the levels of p53 and promoted the release of cytochrome c and the activation of caspase-3, induced G0/G1 arrest, decreased the levels of cyclin D3, cyclin E, CDK4, CDK6 and CDK2, increased the levels of p15, p16, p21, p27, and p53. | [21] |
| GPS | Human tongue cancer SCC-4 cells | Induced ER stress and the production of reactive oxygen species and Ca2+, changed the ratio of Bcl-2 and Bax, followed by the dysfunction of mitochondria, caused cytochrome c release, activation of caspase-3; decreased the levels of cyclin D2 and cyclin E, increased the levels of Chk2, p53, p16, and p21, which led to G0/G1 arrest. | [22] |
| GPS | Human lung carcinoma A549 cells | Induced an arrest at both S phase and G2/M phase, increased the expression of cyclin E and PCNA, decreased the expression of cyclin A and B; induced apoptosis, decreased the expression of Bcl-2 and increased the expression of Bax, activated caspase-3 and the downstream substrates, DFF45 and PARP-1. | [23] |
| GPS | Human oral squamous carcinoma SAS cells | Inhibited the metastatic and invasive capacity of oral cancer cells, decreased the abundance of several proteins, including NF-κB, COX-2, ERK1/2, MMP-9, MMP-2, SOS, Ras, uPA, FAK, and Akt, decreased mRNA levels of MMP-2, MMP-7, and MMP-9. | [24] |
| GPS | Human oral cancer SAS cells | Reduced the levels of CDC25A, cyclin E, cyclin A, and CDK2, increased the levels of p53 and p21, caused G0/G1 phase arrest; triggered apoptotic cell death, increased levels of Bax but inhibited the levels of Bcl-2 and Bcl-xl, stimulated the release of cytochrome c, AIF, and Endo G, increased the translocation of AIF, Endo G, and GADD153, increased protein levels of puma, CAD, caspase-9, and caspase-3, TRAIL, Fas, FasL, ATF-4, and GRP78 but reduced levels of XIAP. | [25] |
| GPS | Human oral cancer HSC-3 cells | Decreased the depolarization of mitochondrial membrane potential, induced cell death, G2/M phase arrest, and apoptosis, altered gene expression such as the expression of GTP binding protein. | [26] |
| GPS | Human hepatocellular carcinoma HepG2 cells | Inhibited HIF-1α mRNA expression, as well as disturbing HepG2 migration and invasion. | [27] |
| GPS | Human hepatocellular carcinoma HepG2 cells | Up-regulated IP3R and SOC proteins (STIM1 and Orai1) and down-regulated SERCA protein, which led to increased extracellular Ca2+ influx and ER Ca2+ release and decreased ER Ca2+ uptake. This process perturbed intracellular calcium homeostasis and finally triggered Ca2+-dependent apoptosis. | [28] |
| GPS | Human colon cancer SW620 cells and Human esophageal cancer Eca-109 cells | Caused cell membrane integrity damage, promoted ROS production, decreased ∆φm level, induced apoptotic morphology such as cell shrinkage and chromatin condensation, initiated apoptotic response, inhibited cell migration. | [29] |
| GPS | Human colon cancer SW-480 cells | Increased the plasma membrane permeability of SW-480 cells, decreased the ∆φm level significantly, increased the level of intracellular ROS level, produced DNA fragmentation and induced apoptosis, caused serious microfilament network collapse as well as the significant decrease in the number of microvilli. | [30] |
| GPS and flavonoids | Prostate cancer PC-3 cells | Induced both S and G2/M phases arrest and cell apoptosis, increased the expression of Bad, Bax, and caspase 3, decreased the level of Bcl-2 and Bcl-xl, modulated the expression of G2 and M checkpoint regulators, cyclin A and B. | [31] |
| A neutral polysaccharide fraction (CGPP) | H22 tumor-bearing mice | Reduced the tumor weight, promoted splenocytes proliferation, promoted cytokine secretion (IL-2, TNF-α, IFN-γ), enhanced the NK cell cytotoxicity and CTL response. | [32] |
| Acidic polysaccharides (GP-B1) | B16 tumor-bearing mice | Inhibited the tumor growth and improved the immune organ status, increased the relative weight of spleen and increased splenocytes proliferation, increased serum TNF-α, IFN-γ, and IL-12 level, reduced IL-10 level. | [33] |
| A nonpolar fraction (EA1.3A) | Human breast adenocarcinoma MDA-MB-453 cells | Inhibited the cell growth and decreased colony formation ability, induced G0/G1 arrest and apoptosis. | [34] |
| Damulin B | Human lung carcinoma cells (A549 and H1299) | Decreased the level of Bcl-2, up-regulated the pro-apoptotic proteins Bax, Bid, and tBid, led to the release of cytochrome c in the cytoplasm, activated the extrinsic protein procaspase-8, cleaved caspase-8 emerged, and reduced the intrinsic protein procaspase-9, initiated cell apoptosis; up-regulated the expression of p53, suppressed the expressions of CDK4, CDK6, and cyclin D1, thus blocking the cell cycle at early G0/G1 phase; inhibited the expression of MMP-2 and MMP-9, up-regulated the expression of IL-24 protein, inhibited cell migration. | [35] |
| Gypensapogenin H | Human breast cancer MDAMB-231 cells | Decreased the expression of cyclin D1, E2F1, CDK4, and CDK2, increased p21 expression, blocked the cell cycle; down-regulated the anti-apoptotic protein Bcl-2, increased the levels of Bax, cytochrome c, cleaved caspase-3, cleaved caspase-9, and P-PARP, initiated cell apoptosis. | [36] |
| Human prostate cancer cells (DU145 and 22RV-1) | Decreased the expression of cyclin D1 and CDK4, increased the expression of p21 to induce cell cycle arrest; decreased anti-apoptotic Bcl-2 protein while Bax, cleaved caspase-3 and -9 increased. | [37] |
| Active Composition | Models | Dosage | Activity/Mechanism | Ref. |
|---|---|---|---|---|
| Gypenoside XVII (GP-17) | A high-fat diet-induced AS model in ApoE-/- mice | 50 mg/kg for 10 weeks, i.g. | Decreased blood lipid levels, increased the expression of antioxidant enzymes (SOD, GSH-Px, CAT) and decreased the MDA level in serum, decreased atherosclerotic lesion size. | [49] |
| Ox-LDL-induced HUVECs injury model | 6.25, 12, 25, 50, 100 µg/mL | Reduced the ROS generation, protected HUVECs against Ox-LDL-induced apoptosis and oxidative stress, increased the expression of ERα, alleviated atherosclerosis via the ERα-mediated PI3K/Akt pathway. | ||
| GPS | Ox-LDL-induced foam cell formation in THP-1 macrophage | 40–200 µg/mL | Down-regulated the expression of the receptor of CD36 in macrophages, up-regulated the expression of the receptor of ABCA1, LXR-α, PPAR-α in macrophages, then reduced the intake of Ox-LDL in macrophages, andpromoted the efflux of Intracellular cholesterol. | [50] |
| GPS | A high-fat diet-induced AS model in ApoE-/- mice | 200 mg/kg and 400 mg/kg for 8 weeks, i.g. | Lowered the level of TC, TG and LDL-C, inhibited the formation of vascular wall hyperplasia, increased the expression of PPAR-γ, LXR-α and ABCA1. It might promote cholesterol efflux through activation of PPAR-γ/LXR-α/ABCA1 signaling pathway. | [51] |
| Gypenoside LVI (GPLVI) | Ox-LDL-induced foam cell formation in RAW264.7 cells | 25, 50 and 100 µg/mL | Inhibited Ox-LDL-induced foam cell formation, promoted cholesterol efflux, inhibited inflammatory response, induced ABCG1, SRB1 and LXRα expression, blocked phosphorylation of ERK and JNK. | [52] |
| GPS | A high-fat diet-induced AS model in ApoE-/- mice | 2.973 g/kg for 4 weeks, i.g. | Lowered the levels of TG, TC and LDL-C, elevated HDL-C level, reduced atheromatous plaques in aortic canal, increased the expression of ULK1, Beclin1 and LC3, decreased p-mTOR expression, relieved the formation of atherosclerotic plaque and prevented atherosclerosis possibly through regulating the autophagy. | [53] |
| GPS | A high-fat diet-induced AS model in SD mice | 40, 80, 160 mg/kg for 7 weeks, i.g. | Inhibited the expression of ICAM-1, MCP-1 and NF-κB P56, delayed the process of atherosclerosis. | [54] |
| GPS | A high-fat diet-induced AS model in ApoE-/- mice | 2.973 mg/kg for 7 weeks, p.o. | Enhanced PI3K and p-Akt expression and down-regulated expression of p-Bad, cytochrome c, cleaved caspase 3, cleaved caspase 9, down-regulated DRP1 and Mfn2 protein levels, inhibited the formation of atherosclerosis via the PI3K/Akt/Bad pathway. | [55] |
| GPS | Cholesterol-induced DNA damage in HUVECs | 1, 10 and 100 µg/mL | Decreased the expression of NOX4 and inhibited ROS production, increased the activity of NOS, decreased the γH2AX expression, alleviated DNA damage. | [56] |
| GPS | Ox-LDL-induced foam cell formation in THP-1 macrophage | 5, 10 and 25 µM | Increased the LC3-II levels, decreased p62 expression, increased the autophagosome puncta numbers, increased SIRT1 and FOXO1 levels, rescued the impaired autophagy flux, reduced the accumulation of Ox-LDL and foam cell formation, prompted lysosome biogenesis and restored its function. | [57] |
| GPS | A high-fat diet-induced AS model in ApoE-/- mice | 2.973 g/kg for 4 weeks, i.g. | Increased the expression of Clock, Bmal1, Period 1, Period 2, Cry1 and Cry 2 proteins, alleviated the formation of atherosclerotic plaque. | [58] |
| Gypenoside A | Ox-LDL-induced injury in ECs (EA.hy926) | 100 µg/mL | Up-regulated the expression of OPA1 and down-regulated the expression of Fission1, increased ATP level, increased the activity of respiratory chain enzyme complexes I, II, III, IV and V, protected ECs by affecting mitochondrial energy metabolism and fusion lysis. | [59] |
| GPS | A high-fat diet-induced AS model in ApoE-/- mice | 2.973 g/kg for 4 weeks, i.g. | Increased the expression of autophagosome related proteins Atg3, Atg4c, Atg5, Atg12, promoted the formation of autophagosome, reduced the serum lipid level. | [60] |
| GPS | AS model established by a high-fat diet (HFD) and vitamin D3 injection in SD mice | 40, 80, 160 mg/kg for 10 weeks, i.g. | Inhibited the expression of TLR4, MyD88, NF-κB, TNF-α, alleviated the inflammatory response. | [61] |
| GPS | Lipopolysaccharide-induced inflammatory injury in HUVECs | 10, 50, 100 and 200 µg/mL | Inhibited the activation of NF-κB via ROS scavenging and reduced the production of inflammatory cytokines. | [62] |
| Models | Medicinal Composition | Dosage | Activity/Mechanism | Ref. |
|---|---|---|---|---|
| MPTP-induced rat model of PD | GPS | 100, 200, and 400 mg/kg, i.p. | Attenuated the motor deficits and striatal dopamine loss in a dose-dependent manner, increased the number of tyrosine hydrolase (TH)-immunopositive neurons, increased glutathione content, and enhanced SOD activity in the substantia nigra. | [90] |
| MPP+-induced oxidative injury of dopaminergic neurons in primary nigral culture | GPS | 50, 100, 200, 400 µg/mL | Increased glutathione content, enhanced activity of GSH-Px and SOD, attenuated MPP+-induced oxidative damage, reduction of dopamine uptake, loss of TH-immunopositive neurons and degeneration of TH-immunopositive neurites, and 200 µg/mL of GPS had the maximum protective effect. | [91] |
| 6-OHDA-induced rat model of PD | GPS and GP-EX | GPS (25 and 50 mg/kg) or GP-EX (50 mg/kg) for 22 days, p.o. | Attenuated the development of L-DOPA-induced dyskinesia without compromising the anti-parkinsonian effects of L-DOPA by modulating the biomarker activities of ΔFosB expression and ERK1/2 phosphorylation. | [92] |
| MPTP-induced rat model of PD | GPS | 50 mg/kg for 21 days, p.o. | Improved the levels of TH-immunopositive cells and dopamine in the substantia nigra and striatum, suppressed NMDA receptor expression, and increased the phosphorylation of ERK1/2 and CREB in the |hippocampus, ameliorated deficits in habit learning and spatial memory by increasing the activation of the dopaminergic neuronal system and modulating NMDA receptor-mediated signaling systems. | [93] |
| MPTP-induced rat model of PD | GPS and GP-EX | GPS (50 mg/kg), GP-EX (50 mg/kg) for 21 days, p.o. | Improved the symptom of anxiety disorders by modulating the brain levels of dopamine and serotonin, increased the number of TH-immunopositive neuronal cells, and increased the density of dopamine neurons in the substantia nigra. | [94] |
| 6-OHDA-induced rat model of PD | GP-EX | 10 mg/kg and 30 mg/kg for 28 days, p.o. | Ameliorated the reduction of TH-immunopositive neurons, recovered the levels of dopamine, 3,4-dihydroxyphenylacetic acid, homovanillic acid, and norepinephrine in the striatum. | [95] |
| Active Composition | Models | Dosage | Activity/Mechanism | Ref. |
|---|---|---|---|---|
| GPS | Oxygen-glucose deprivation– reoxygenation (OGD/R) H9c2 cell model | 5, 10, 20 µM, pretreatment prior to ischemia | Enhanced the cell viability, inhibited the translocation of NF-κB subunit p65 into nuclei, inhibited NF-κB activation by suppressing the MAPK signaling transduction pathway. | [97] |
| Myocardial I/R rat model | 50, 100, 200 mg/kg, pretreatment prior to ischemia, p.o. | Alleviated the impairments on the cardiac function and structure, suppressed the activation of NF-κB via inhibition of phosphorylation of inhibitor of NF-κB α (IκBα), ERK, JNK, and p38. | ||
| GPS | OGD/R H9c2 cell model | 5, 10, 20 µM, pretreatment prior to ischemia | Protected cell viability, reduced ROS production and oxidative stress, and preserved mitochondrial function. | [98] |
| Myocardial I/R rat model | 50, 100, 200 mg/kg, pretreatment prior to ischemia, p.o. | Attenuated infarct size, alleviated I/R-induced pathological changes in the myocardium, and preserved left ventricular function; reduced oxidative stress, preserved mitochondrial function in the cardiomyocytes, maintained mitochondrial membrane integrity, and inhibited the release of cytochrome c from the mitochondria to the cytosol. | ||
| Gypenoside A | OGD/R H9c2 cell model | 10, 20 µM, pretreatment prior to ischemia | Increases cell viability and inhibits apoptosis, exerted its protective effect on H9c2 cells by inhibiting the expression of miR-143-3p via the activation of AMPK. | [99] |
| GPS | OGD/R H9c2 cell model | 10, 20 µM, pretreatment prior to ischemia | Exerted protective effect by inhibiting the ER stress-induced apoptosis via inhibition of CHOP pathway and activation of PI3K/Akt pathway. | [100] |
| Myocardial I/R rat model | 50, 100, 200 mg/kg, pretreatment prior to ischemia, p.o. | |||
| Gypenoside | Renal I/R rat model | 50 mg/kg, pretreatment prior to ischemia, i.v. | Inhibited I/R-induced up-regulation of serum creatinine and blood urea nitrogen, inhibited the production of pro-inflammatory cytokines, inhibited cell apoptosis by activating ERK signaling. | [101] |
| Gypenoside | Hepatic I/R rat model | 50 mg/kg, pretreatment prior to ischemia, p.o. | Attenuated the increase in activity of serum aminotransferases in the hepatic tissue, attenuated the increase in hepatic lipid peroxidation and GSH content in hepatic tissue, up-regulated the protein expression of HO-1, attenuated I/R-induced hepatic cell apoptosis by modulating key apoptosis-related proteins. | [102] |
| Models | Medicinal Composition | Dosage | Activity/Mechanism | Ref. |
|---|---|---|---|---|
| CCl4-induced liver injury in rats | GPP | 40 and 80 g/kg for 30 days, i.g. | Decreased the level of serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT), down-regulated the iNOS mRNA expression in hepatic tissue, elevated the level of Bcl-2/Bax in hepatic tissue and alleviated liver injury. | [103] |
| CCl4-induced chronic liver injury in rats | Gypenoside | 100 mg/5 mL/kg for 8 weeks, 4 times per week, p.o. | Reduced the activities of serum glutamic transaminase and serum glutamic pyruvate transaminase, elevated albumin/globulin ratio, prevented CCl4-induced liver damage, reduced the collagen content, suppressed the onset and enhanced the recovery of liver fibrosis induced by CCl4. | [104] |
| CCl4-induced acute liver injury in rats | GPS | 100 mg/kg, i.v. | Decreased the level of serum ALT, ameliorated liver injury. | [105] |
| CCl4-induced liver fibrosis in rats | GPS | 200 mg/kg for three weeks, i.g. | Ameliorated CCl4-induced liver fibrosis via the inhibition of TGF-β signaling, consequently inhibited the differentiation of hepatic progenitor cells into myofibroblasts. | [106] |
| Exhaustive exercise-induced hepatocyte apoptosis in rats | GPP | 100 and 300 mg/kg, i.g. | Increased the expression of Bcl-2 and decreased the expression of Bax, increased SOD, GSH-Px, and CAT activities and decreased MDA contents in liver, exerted protective effect on oxidative stress and apoptosis induced by exhaustive exercise. | [107] |
| Alcohol-induced liver injury in rats | GPS | 50, 100, and 200 mg/kg for 7 days, i.g. | Decreased the level of MDA and increased the activity of SOD, CAT, and glutathione, improved the level of the antioxidant defense system by activating the Nrf2 signaling pathway, reduced the level of inflammatory cytokines TNF-α and IL-6 by inhibiting the nuclear transposition of NF-kB. | [108] |
| High choline-induced liver injury in rats | GPS | 200, 400, and 800 mg/kg for 8 weeks, i.g. | Elevated T-SOD and GSH-Px activity, decreased the MDA concentration, alleviated the high choline-induced oxidative stress injury, lowered the levels of AST and ALT. | [109] |
| High-fat and cholesterol diet and alcohol-induced fatty liver disease in rats | GPS | 60, 30, and 15 mg/kg for 10 weeks, i.g. | Decreased the levels of serum TG, TC, LDL-C, free fatty acid, increased HDL-C level, increased SOD activity, reduced MDA level and ALT and AST activities, prevented liver fatty degeneration through up-regulation of PPAR-α expression in the liver to inhibit lipid peroxidation and hepatocyte apoptosis, ameliorated hepatic steatosis and damage, and improved hepatic function. | [110] |
| Methionine choline deficient diet-induced NAFLD in rats | UL4-rich GPE | 50, 100, and 200 mg/kg for 8 days, i.g. | Reduced hepatic fat accumulation, hepatocellular injury, inflammation, and fibrosis, increased the expression of SIRT6 and phase 2 anti-oxidant enzymes, and then attenuated oxidative stress in the liver. | [111] |
| Models | Medicinal Composition | Dosage | Activity/Mechanism | Ref. |
|---|---|---|---|---|
| Alloxan-induced hyperglycemia in rats | Gypenoside | 150, 250, 350 mg/kg for 4 weeks, i.g. | Exerted hypoglycemic effect by improving the synthesis of liver glycogen and protected the kidney. | [116] |
| L6 myotube cells | Damulin A | 37.5, 75, 150 µM | Stimulated glucose uptake by stimulating the translocation of GluT4 to the cell membrane via AMPK activation. | [117] |
| Damulin B | 1.2, 6, 12 µM | |||
| Alloxan-induced type 1 diabetes in rats | GPP | 200 mg/kg for 5 d, i.g. | Lowered the fasting blood sugar and glucose tolerance, which might be related to the inhibitory effect on α-amylase. | [118] |
| High-sugar, high-fat diet combined with a small dose of streptozotocin-induced type 2 diabetes in rats | GPP | 50, 100, and 200 mg/kg for 2 months, i.g. | Lowered the fasting blood sugar and glucose tolerance, which might be related to the increased level of serum insulin. | [119] |
| Alloxan-induced hyperglycemia in rats | GP-EX | 1, 2, 4 g/kg for 10 days, i.g. | Lowered the level of blood glucose and had no effect on normal mice. | [120] |
| Glucose-induced hyperglycemia in rats | ||||
| Type 2 diabetic Goto-Kakizaki rats | GPE | 1600 mg/kg/day for three weeks, p.o. | Reduced plasma glucose levels, suppressed hepatic glucose output levels significantly, and improved the hepatic insulin sensitivity by suppressing gluconeogenesis. | [121] |
| Other Bioactivities | Component/Dosage | Activity/Mechanism | Ref. |
|---|---|---|---|
| Anti-aging effect | GPS (8 g/kg) for 30 days, i.g. | Increased the activity of SOD, GSH-Px, and CAT in the skin, reduced the MDA content. | [133] |
| GP (100 mg/kg) for 40 days, i.g. | Increased the activities of antioxidant enzyme SOD and GSH-Px, decreased the contents of NOS and NO in hypothalamus, reduced the strong excitatory toxicity of the two on neurons and delayed aging. | [134] | |
| GPP (50, 100, and 200 mg/kg) for 42 days, i.p. | Increased the activity of antioxidant enzymes (SOD, T-AOC, GSH-Px, CAT) in serum, brain and liver, increased the hydroxyproline content in the skin and reduced the damage caused by peroxidation products (MDA), thus delaying aging. | [135] | |
| Anxiolytic effect | GPS (100, 200 mg/kg); GP-WX (50 mg/kg) for 10 days, p.o. | Recovered the number of open arm entries and the time spent on open arms, reduced the number of marbles buried, increased the spontaneous locomotor activities, increased the levels of dopamine and serotonin in the brain, reduced the serum levels of corticosterone, reduced c-Fos expression. | [136] |
| GP-EX (400 mg) for 8 weeks, p.o. | Reduced “anxiety proneness” as shown by a decrease in the score of T-STAI and the tendency for a decrease in the total score of STAI. | [137] | |
| Antidepressant effect | GPS (25, 50, and 100 mg/kg) for 4 weeks, p.o. | Increased the sucrose preference, reduced the immobility time, increased the hippocampal BDNF expression and neuronal proliferation, and exhibited antidepressant-like effects in mice, which might be mediated by activation of the BDNF-ERK/Akt signaling pathway in the hippocampus. | [138] |
| Immunomodulatory effect | GPP (50, 150, and 250 mg/kg) for 15 days, p.o. | Increased the spleen and thymus indices, activated the macrophages and NK cells, elevated CD4+ T lymphocyte counts as well as the CD4+/CD8+ ratio in a dose-dependent manner, and increased IL-2 level in the sera and spleen. | [139] |
| Anti-gastric ulcer effect | GP butanol fraction (200 and 400 mg/kg), p.o. | Preserved the gastric mucus synthesis and secretion but could not significantly decrease gastric volume and increase gastric pH and acidity. | [140] |
| Anti-fatigue effect | GPP (100, 200, 400 mg/kg) for 30 days, p.o. | Extended the exhaustive swimming time of the rats, elevated the exercise tolerance, lowered the levels of blood lactic acid and urea nitrogen, increased the hemoglobin, liver glycogen, and muscle glycogen concentrations, and postponed the appearance of fatigue. | [141] |
| GPE (20 µg/mL) or GL (0.36 µg/mL) | Stimulated lactate metabolism, promoted the differentiation of myoblasts into myotubes, enhanced exercise endurance capacity. | [142] | |
| Anti-platelet aggregation activity | GPS (10, 20, 40, and 80 mg/kg) for 4 days, i.p. | Inhibited ADP and collagen-induced platelet aggregation, inhibited thrombosis. | [143] |
| Sedative and hypnotic effect | GPS (50, 100 and 200 mg/kg) for 7 days, i.g. | Prolonged the sleep time, increased the contents of 5-hydroxytryptamine and IL-1β, decreased the contents of dopamine and noradrenalin, and showed no influence on glutamate and gamma-aminobutyric acid contents in the hippocampus. | [144] |
| Antimicrobial activity | GPE (100, 1000, 5000, and 10,000 ppm) | Showed antimicrobial activity against fungi producing aflatoxin and fumonisin and bacteria causing diarrheal disease. | [145] |
| Antiviral activity | GPP (150 mg/kg) for 3 days, p.o. | Protected MARC-145 cells from invasion by preventing PRRSV from absorbing marC-145 cells and exerted the best anti-infection effect when the concentration of GPP was 62.5 µg/mL. | [146] |
| GP-EX and GP-WX | Inhibited the H5N1 virus replication in the MDCK cells. | [147] | |
| Effect on hyperuricemia | GPS (15 and 60 mg/kg) for 8 weeks, i.g. | Decreased serum uric acid levels, promoted renal excretion of uric acid, increased the kidney index, down-regulated URAT1 and GLUT9 expression and up-regulated OAT1 expression in the kidney, decreased the levels of xanthine oxidase, adenosime deaminase, and xanthine dehydrogenase expression. | [148] |
| Effect on osteoporosis | 12.5, 25 and 50 µM for 4 days | Inhibited osteoclast formation, inhibited osteoclastogenesis-related markers expression, such as MMP-9, c-Src, NFATc1, and cathepsin K, inhibited RANKL-induced NF-κB and MAPK activation and Akt phosphorylation. | [149] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, C.; Li, N.; Ren, R.; Wang, Y.; Su, X.; Lu, F.; Zong, R.; Yang, L.; Ma, X. Progress in the Medicinal Value, Bioactive Compounds, and Pharmacological Activities of Gynostemma pentaphyllum. Molecules 2021, 26, 6249. https://doi.org/10.3390/molecules26206249
Su C, Li N, Ren R, Wang Y, Su X, Lu F, Zong R, Yang L, Ma X. Progress in the Medicinal Value, Bioactive Compounds, and Pharmacological Activities of Gynostemma pentaphyllum. Molecules. 2021; 26(20):6249. https://doi.org/10.3390/molecules26206249
Chicago/Turabian StyleSu, Chao, Nan Li, Ruru Ren, Yingli Wang, Xiaojuan Su, Fangfang Lu, Rong Zong, Lingling Yang, and Xueqin Ma. 2021. "Progress in the Medicinal Value, Bioactive Compounds, and Pharmacological Activities of Gynostemma pentaphyllum" Molecules 26, no. 20: 6249. https://doi.org/10.3390/molecules26206249
APA StyleSu, C., Li, N., Ren, R., Wang, Y., Su, X., Lu, F., Zong, R., Yang, L., & Ma, X. (2021). Progress in the Medicinal Value, Bioactive Compounds, and Pharmacological Activities of Gynostemma pentaphyllum. Molecules, 26(20), 6249. https://doi.org/10.3390/molecules26206249