Phenolic Characterization and Neuroprotective Properties of Grape Pomace Extracts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phytochemical Analysis
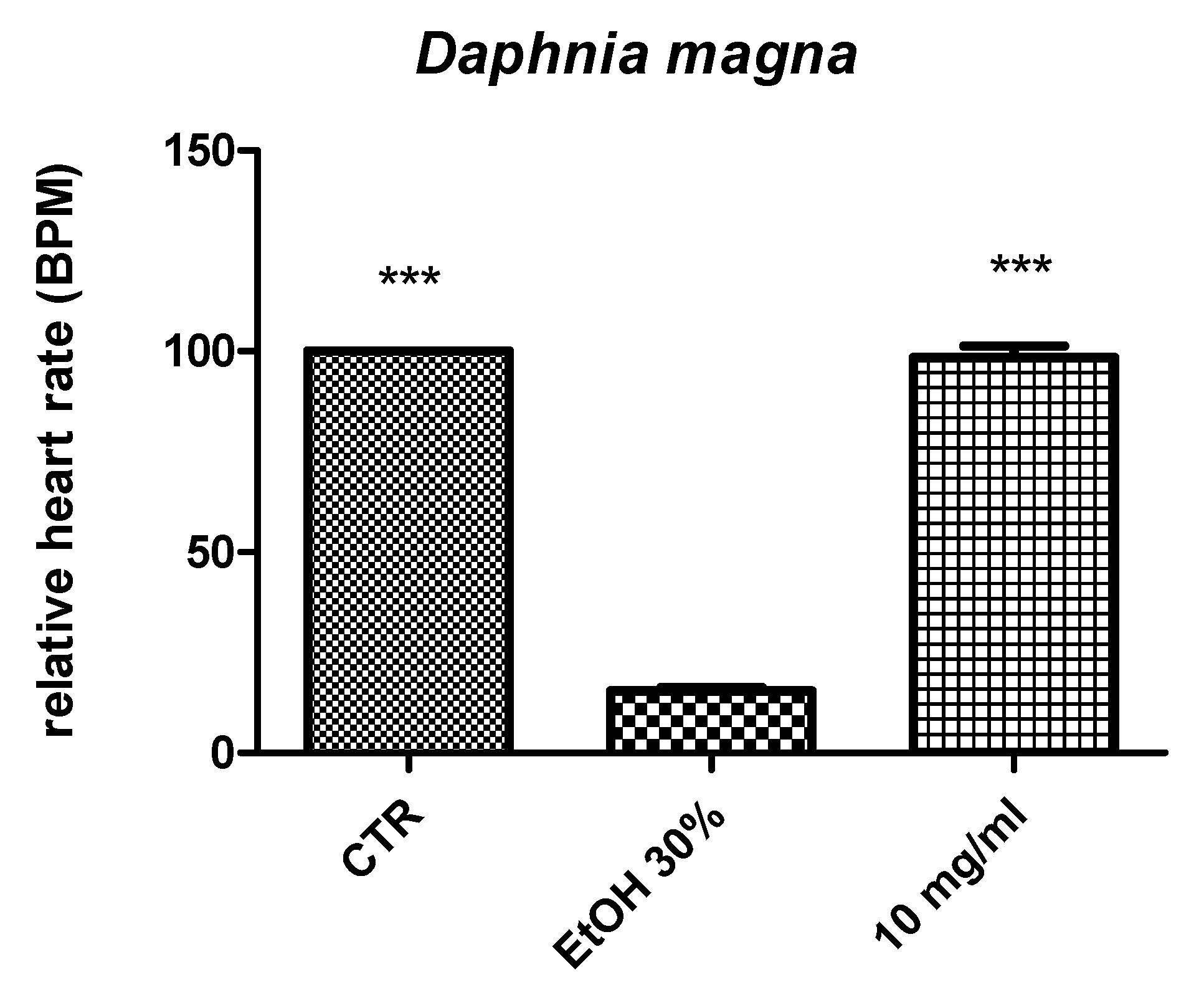
2.2. Eco-Toxicological Assays
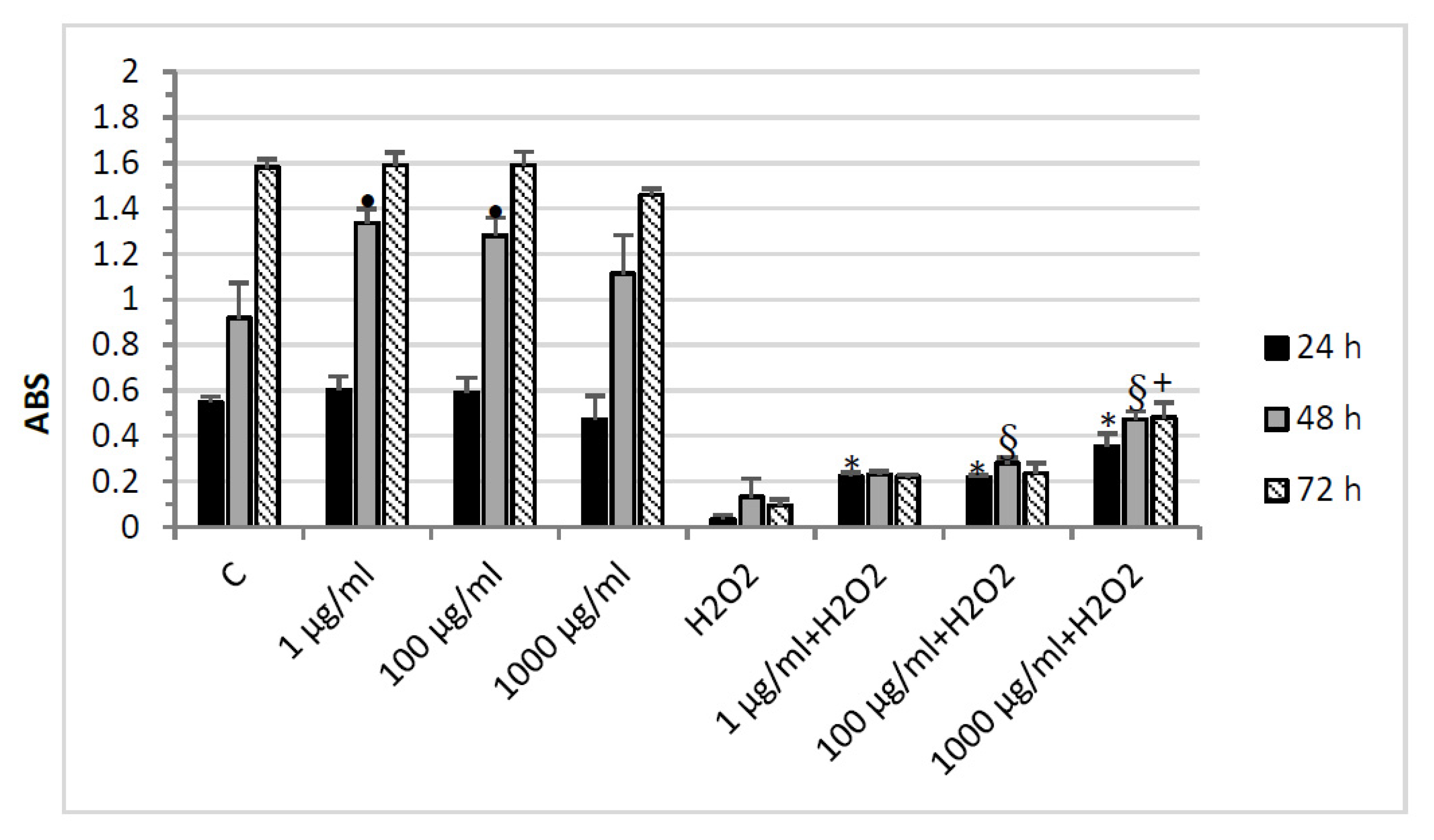
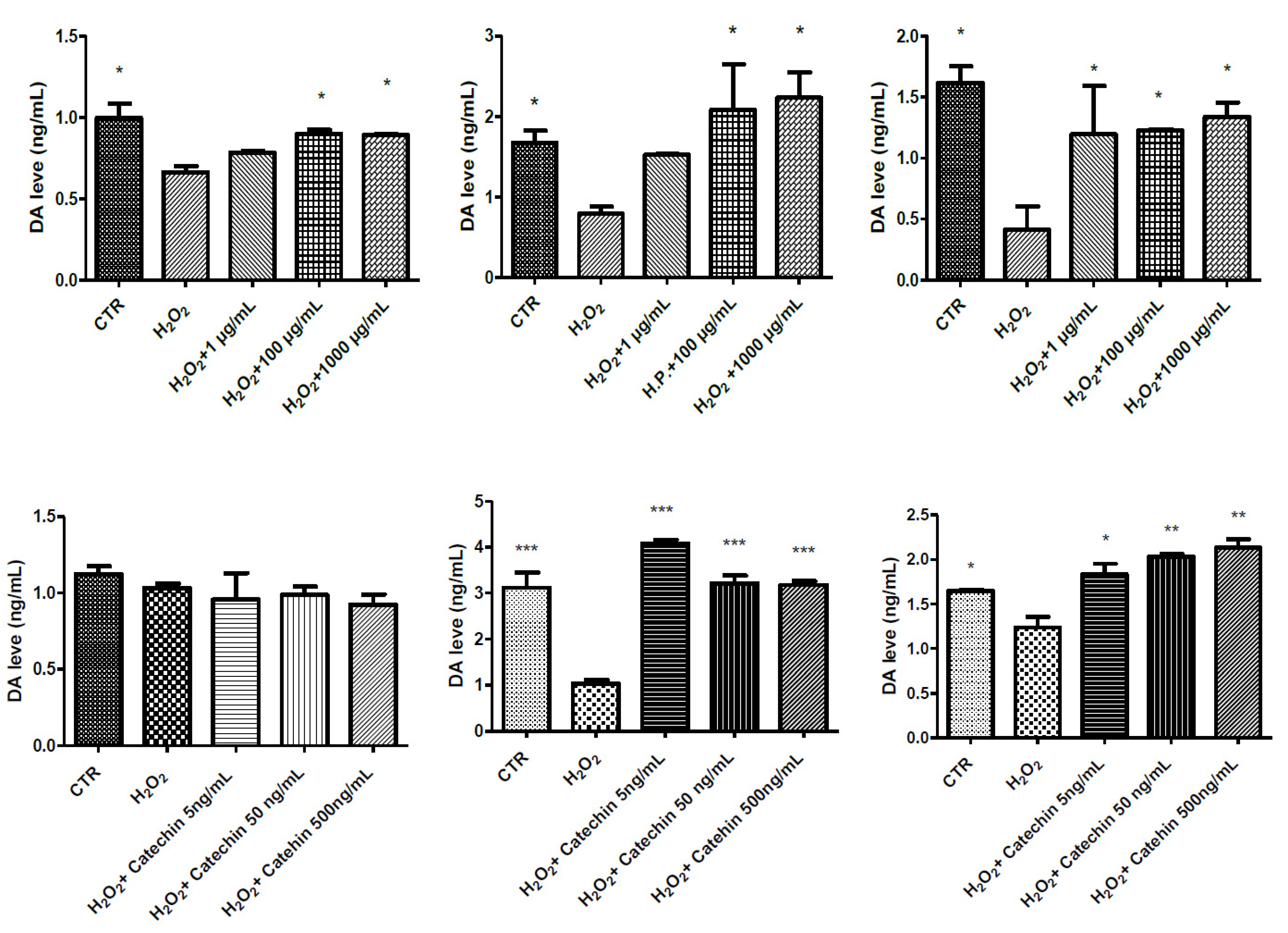
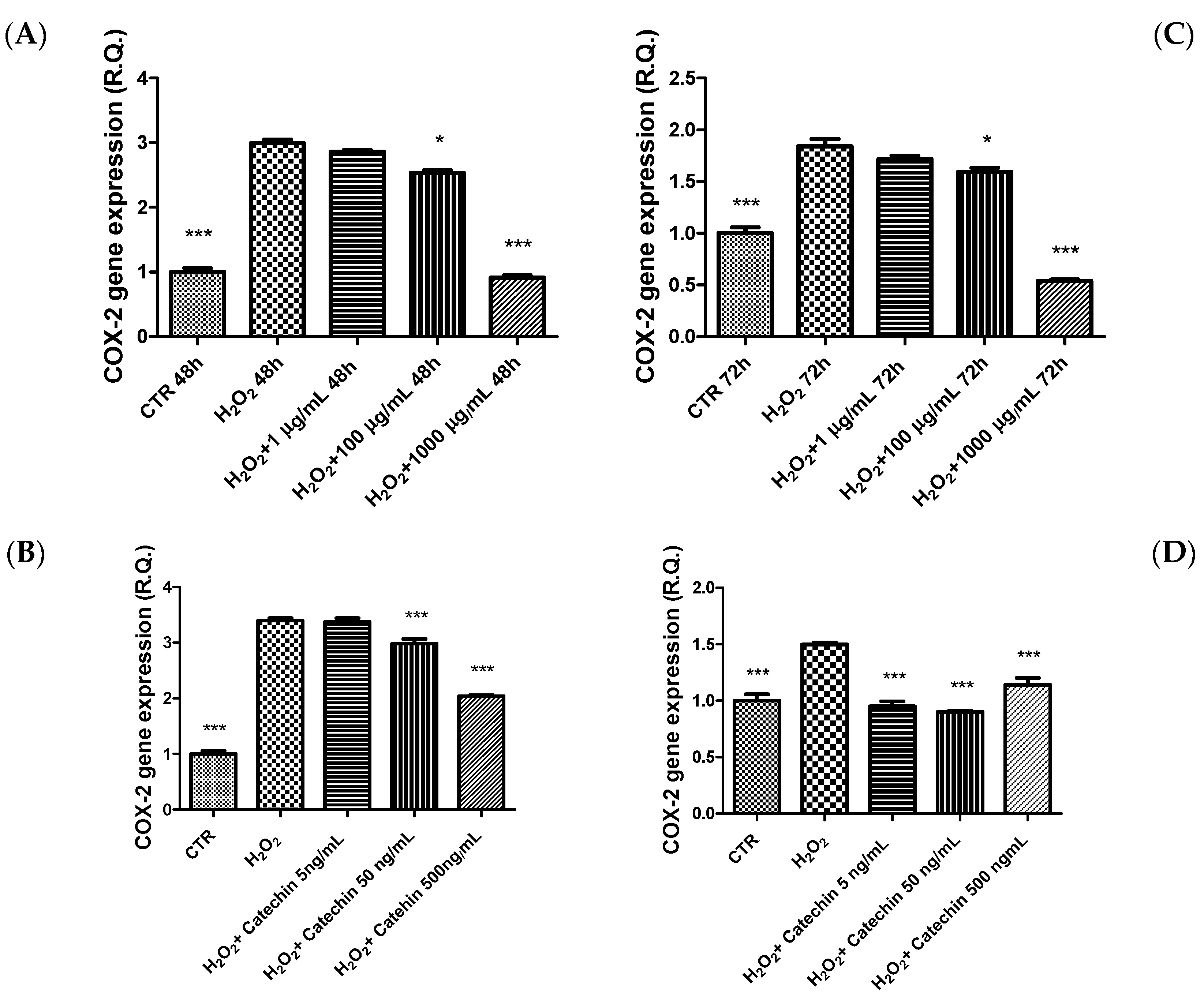
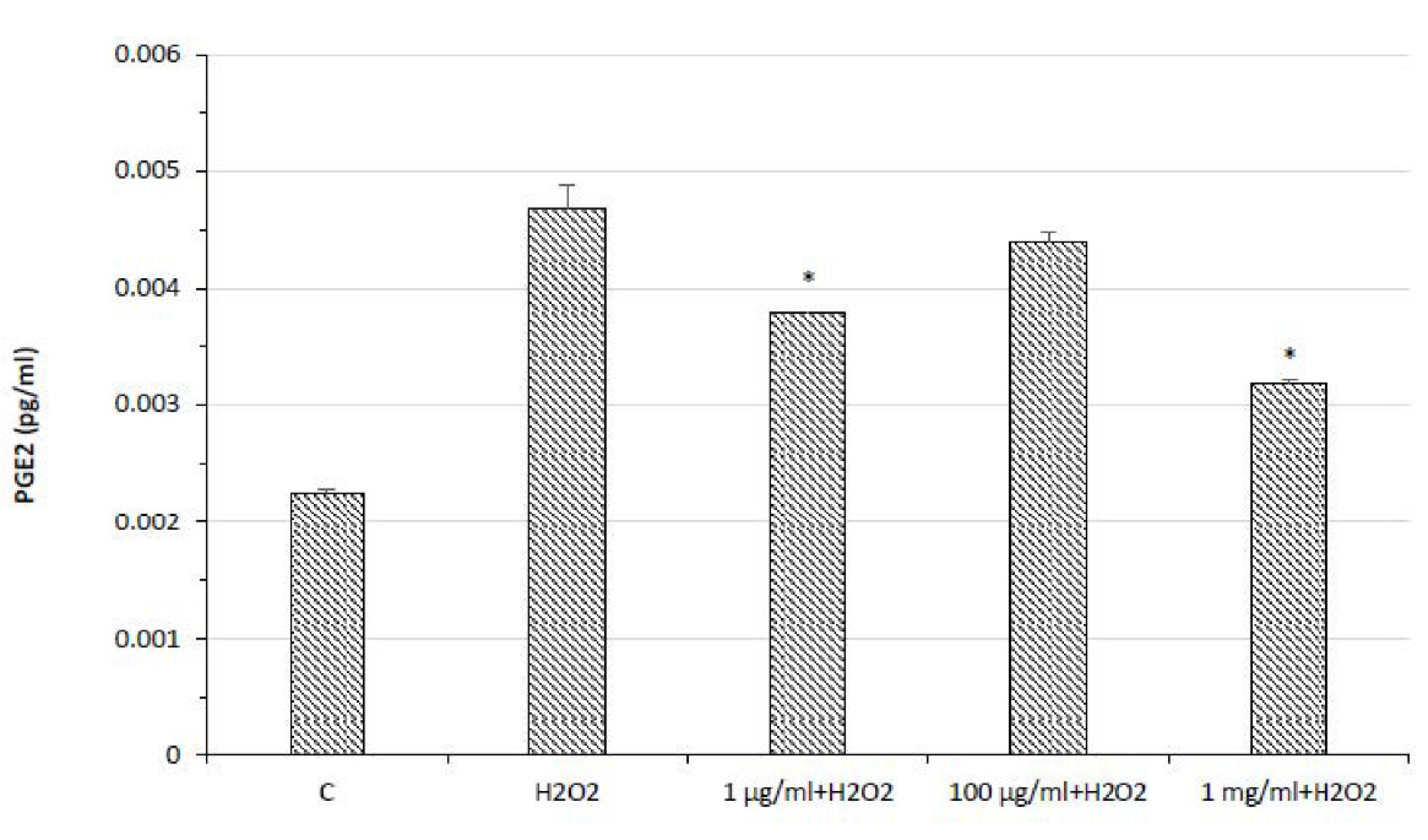
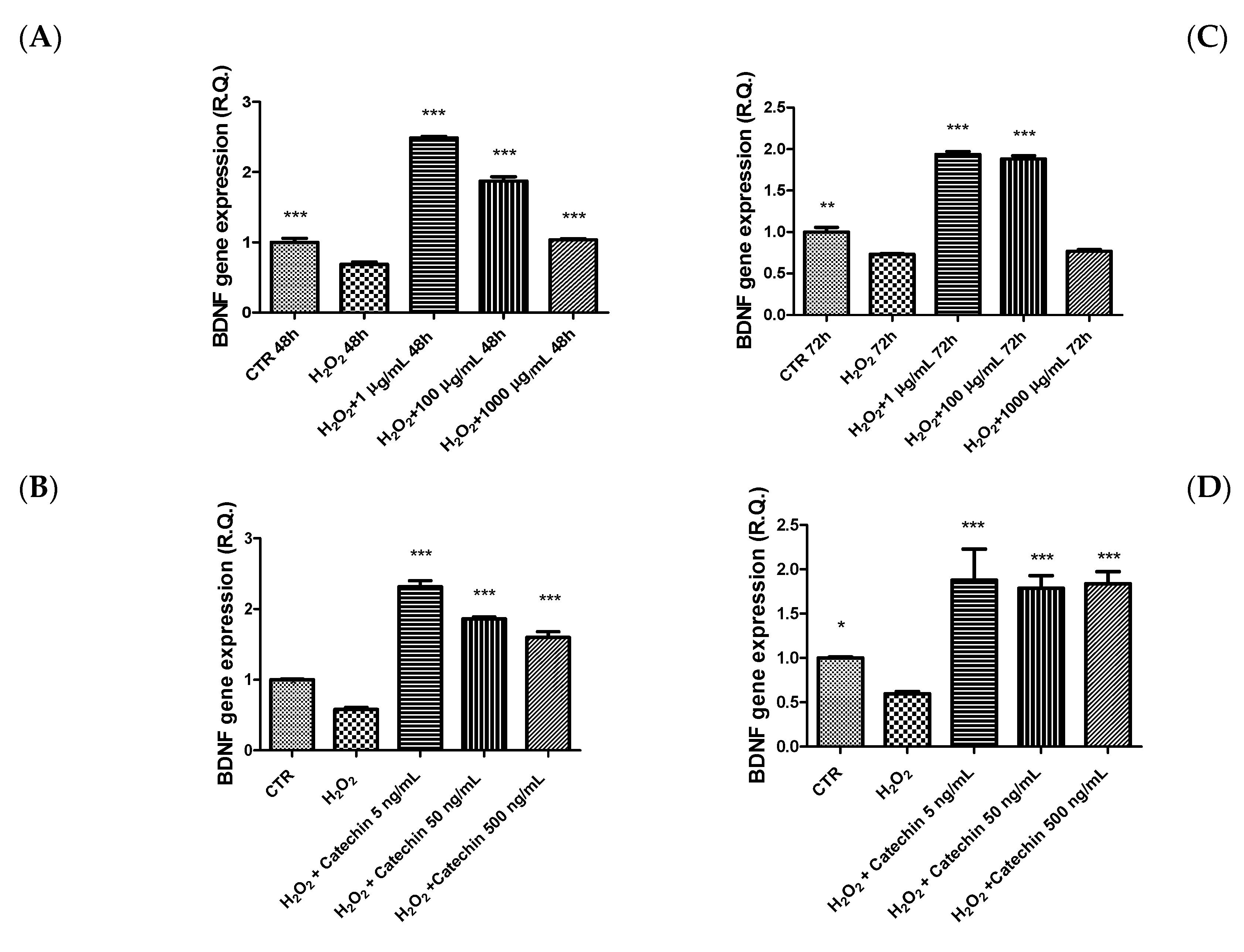
2.3. Protective Effects in HypoE22 Cells
3. Materials and Methods
3.1. Grape Pomace Sample, Reagents, and Solutions
3.2. Grape Pomace Extracts Preparation Solutions
3.3. High Performance Liquid Chromatography (HPLC) Analyses
3.4. HPLC-DAD-MS Determination of Phenolic Compounds
3.5. High Performance Liquid Chromatography (HPLC) Determination of Dopamine (DA)
3.6. Artemia Salina and Daphnia Magna Toxicity Tests
3.7. Cell Culture and Treatment
3.8. MTT Assay
3.9. PGE2 ELISA Assay
3.10. Gene Expression Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Dwyer, K.; Hosseinian, F.; Rod, M.R. The market potential of grape waste alternatives. J. Food Res. 2014, 3, 91. [Google Scholar] [CrossRef]
- Scoma, A.; Rebecchi, S.; Bertin, L.; Fava, F. High impact biowastes from South European agro-industries as feedstock for second-generation biorefineries. Crit. Rev. Biotechnol. 2016, 1, 175–189. [Google Scholar] [CrossRef]
- Antonić, B.; Jančíková, S.; Dordević, D.; Tremlová, B. Grape Pomace Valorization: A Systematic Review and Meta-Analysis. Foods 2020, 9, 1627. [Google Scholar] [CrossRef]
- Bender, A.B.B.; Speroni, C.S.; Moro, K.I.B.; Morisso, F.D.P.; dos Santos, D.R.; da Silva, L.P.; Penna, N.G. Effects of micronization on dietary fiber composition, physicochemical properties, phenolic compounds, and antioxidant capacity of grape pomace and its dietary fiber concentrate. LWT 2020, 117, 108652. [Google Scholar] [CrossRef]
- Beres, C.; Costa, G.N.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.; Cruz, A.P.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.; Freitas, S.P. Towards integral utilization of grape pomace from winemaking process: A review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Escobar, R.; Aliaño-González, M.J.; Cantos-Villar, E. Wine Polyphenol Content and Its Influence on Wine Quality and Properties: A Review. Molecules 2021, 26, 718. [Google Scholar] [CrossRef] [PubMed]
- Sirohi, R.; Tarafdar, A.; Singh, S.; Negi, T.; Gaur, V.K.; Gnansounou, E.; Bharathiraja, B. Green processing and biotechnological potential of grape pomace: Current trends and opportunities for sustainable biorefinery. Bioresour. Technol. 2020, 1314, 23771. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.L.; Lin, K.J.; Wang, P.W.; Chuang, J.H.; Lin, H.Y.; Chen, S.D.; Chuang, Y.C.; Huang, S.T.; Tiao, M.M.; Chen, J.B.; et al. Resveratrol provides neuroprotective effects through modulation of mitochondrial dynamics and ERK1/2 regulated autophagy. Free Radic. Res. 2018, 11–12, 1371–1386. [Google Scholar] [CrossRef] [PubMed]
- Griñán-Ferré, C.; Bellver-Sanchis, A.; Izquierdo, V.; Corpas, R.; Roig-Soriano, J.; Chillón, M.; Andres-Lacueva, C.; Somogyvári, M.; Sőti, C.; Sanfeliu, C.; et al. The pleiotropic neuroprotective effects of resveratrol in cognitive decline and Alzheimer’s disease pathology: From antioxidant to epigenetic therapy. Ageing Res. Rev. 2021, 67, 101271. [Google Scholar] [CrossRef]
- Mirzaei, S.; Gholami, M.H.; Zabolian, A.; Saleki, H.; Farahani, M.V.; Hamzehlou, S.; Far, F.B.; Sharifzadeh, S.O.; Samarghandian, S.; Khan, H.; et al. Caffeic acid and its derivatives as potential modulators of oncogenic molecular pathways: New hope in the fight against cancer. Pharmacol. Res. 2021, 171, 105759. [Google Scholar] [CrossRef]
- Balaha, M.; De Filippis, B.; Cataldi, A.; di Giacomo, V. CAPE and neuroprotection: A review. Biomolecules 2021, 11, 176. [Google Scholar] [CrossRef] [PubMed]
- Fukutomi, R.; Ohishi, T.; Koyama, Y.; Pervin, M.; Nakamura, Y.; Isemura, M. Beneficial Effects of Epigallocatechin-3-O-Gallate, Chlorogenic Acid, Resveratrol, and Curcumin on Neurodegenerative Diseases. Molecules 2021, 26, 415. [Google Scholar] [CrossRef]
- Scapagnini, G.; Vasto, S.; Abraham, N.G.; Caruso, C.; Zella, D.; Fabio, G. Modulation of Nrf2/ARE pathway by food polyphenols: A nutritional neuroprotective strategy for cognitive and neurodegenerative disorders. Mol. Neurobiol. 2011, 44, 192–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nassiri-Asl, M.; Hosseinzadeh, H. Review of the Pharmacological Effects of Vitis vinifera (Grape) and its Bioactive Constituents: An Update. Phytother. Res. 2016, 9, 1392–1403. [Google Scholar] [CrossRef]
- Sapanidou, V.G.; Margaritis, I.; Siahos, N.; Arsenopoulos, K.; Dragatidou, E.; Taitzoglou, I.A.; Zervos, I.A.; Theodoridis, A.; Tsantarliotou, M. Antioxidant effect of a polyphenol-rich grape pomace extract on motility, viability and lipid peroxidation of thawed bovine spermatozoa. J. Biol. Res. 2014, 21, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujishia, K.; Ozawa, T.; Shibata, K.; Tanabe, S.; Sato, Y.; Hisamoto, M.; Okuda, T.; Koizumi, S. Grape seed extract acting on astrocytes reveals neuronal protection against oxidative stress via interleukin-6-mediated mechanisms. Cell Mol. Neurobiol. 2009, 29, 1121–1129. [Google Scholar] [CrossRef]
- Dani, C.; Oliboni, L.S.; Agostini, F.; Funchal, C.; Serafini, L.; Henriques, J.A.; Salvador, M. Phenolic content of grapevine leaves (Vitis labrusca var. Bordo) and its neuroprotective effect against peroxide damage. Toxicol. In Vitro 2010, 24, 148–153. [Google Scholar] [CrossRef]
- Rodrigues, A.D.; Scheffel, T.B.; Scola, G.; Santos, M.T.; de Freitas, S.C.; Dani, C.; Vanderlinde, R.; Henriques, J.A.; Coitinho, A.S.; Salvador, M. Neuroprotective and anticonvulsant effects of organic and conventional purple grape juices on seizures in Wistar rats induced by pentylenetetrazole. Neurochem. Int. 2012, 60, 799–805. [Google Scholar] [CrossRef] [Green Version]
- De la Cerda-Carrasco, A.; López-Solís, R.; Nuñez-Kalasic, H.; Peña-Neira, A.; Obreqeu-Slier, E. Phenolic composition and antioxidant capacity of pomaces from four grape varieties (Vitis vinifera L.). J. Sci. Food Agric. 2015, 95, 1521–1527. [Google Scholar] [CrossRef]
- Fontana, A.R.; Antoniolli, A.; Bottini, R. Grape pomace as a sustainable source of bioactive compounds: Extraction, characterization, and biotechnological applications of phenolics. J. Agric. Food Chem. 2013, 61, 8987–9003. [Google Scholar] [CrossRef]
- Maier, T.; Sanzenbacher, S.; Kammerer, D.R.; Berardini, N.; Conrad, J.; Beifuss, U.; Carle, R.; Schieber, A. Isolation of hydroxycinnamoyltartaric acids from grape pomace by high-speed counter-current chromatography. J. Chromatogr. A 2006, 1128, 61–67. [Google Scholar] [CrossRef]
- Gómez-Mejía, E.; Mikkelsen, L.H.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y.A. combined approach based on matrix solid-phase dispersion extraction assisted by titanium dioxide nanoparticles and liquid chromatography to determine polyphenols from grape residues. J. Cromatogr. A 2021, 1644, 462128. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, L.A.; Lorenzo, J.M.; Trindade, M.A. Fruit and Agro-Industrial Waste Extracts as Potential Antimicrobials in Meat Products: A Brief Review. Foods 2021, 10, 1469. [Google Scholar] [CrossRef] [PubMed]
- Goufo, P.; Singh, R.K.; Cortez, I. A Reference List of Phenolic Compounds (Including Stilbenes) in Grapevine (Vitis vinifera L.) Roots, Woods, Canes, Stems, and Leaves. Antioxidants 2020, 9, 398. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.; Loureiro, J.A.; Pereira, M.C. Green tea extract-biomembrane interaction study: The role of its two major components, (−)-epigallocatechin gallate and (−)-epigallocatechin. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183476. [Google Scholar] [CrossRef]
- Sebastiani, G.; Almeida-Toledano, L.; Serra-Delgado, M.; Navarro-Tapia, E.; Sailer, S.; Valverde, O.; Garcia-Algar, O.; Andreu-Fernández, V. Therapeutic Effects of Catechins in Less Common Neurological and Neurodegenerative Disorders. Nutrients 2021, 13, 2232. [Google Scholar] [CrossRef]
- Gu, Y.; Moroy, G.; Paul, J.-L.; Rebillat, A.-S.; Dierssen, M.; De La Torre, R.; Cieuta-Walti, C.; Dairou, J.; Janel, N. Molecular Rescue of Dyrk1A Overexpression Alterations in Mice with Fontup® Dietary Supplement: Role of Green Tea Catechins. Int. J. Mol. Sci. 2020, 21, 1404. [Google Scholar] [CrossRef] [Green Version]
- Chu, K.; Wang, C.; Chu, C.; Choy, K.W.; Pang, C.; Rogers, M. Uptake and distribution of catechins in fetal organs following in utero exposure in rats. Hum. Reprod. 2007, 22, 280–287. [Google Scholar] [CrossRef] [Green Version]
- Faria, A.; Pestana, D.; Teixeira, D.; Couraud, P.O.; Romero, I.; Weksler, B.; de Freitas, V.; Mateus, N.; Calhau, C. Insights into the putative catechin and epicatechin transport across blood-brain barrier. Food Funct. 2011, 2, 39–44. [Google Scholar] [CrossRef]
- Orlando, G.; Chiavaroli, A.; Adorisio, S.; Delfino, D.V.; Brunetti, L.; Recinella, L.; Leone, S.; Zengin, G.; Acquaviva, A.; Angelini, P.; et al. Unravelling the Phytochemical Composition and the Pharmacological Properties of an Optimized Extract from the Fruit from Prunus mahaleb L.: From Traditional Liqueur Market to the Pharmacy Shelf. Molecules 2021, 26, 4422. [Google Scholar] [CrossRef]
- Cobley, J.N.; Fiorello, M.L.; Bailey, D.M. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018, 15, 490–503. [Google Scholar] [CrossRef]
- Halliwell, B.; Clement, M.V.; Ramalingam, J.; Long, L.H. Hydrogen peroxide. Ubiquitous in cell culture and in vivo? IUBMB Life 2000, 50, 251–257. [Google Scholar] [CrossRef]
- Herrera-Bra, J.; Beltrán-Lissabet, J.F.; Saavedra, K.; Saavedra, N.; Hevia, M.; Alvear, M.; Lanas, F.; Salazar, L.A. Protective effect of Pinot noir pomace extract against the cytotoxicity induced by polycyclic aromatic hydrocarbons on endothelial cells. Food Chem. Toxicol. 2021, 148, 111947. [Google Scholar] [CrossRef]
- Heo, H.J.; Lee, C.Y. Epicatechin and catechin in cocoa inhibit amyloid beta protein induced apoptosis. J. Agric. Food Chem. 2005, 9, 1445–1448. [Google Scholar] [CrossRef]
- Kang, M.K.; Kang, N.J.; Jang, Y.J.; Lee, K.W.; Lee, H.J. Gallic acid induces neuronal cell death through activation of c-Jun N-terminal kinase and downregulation of Bcl-2. Ann. N. Y. Acad. Sci. 2009, 1171, 514–520. [Google Scholar] [CrossRef]
- Sarkaki, A.; Fathimoghaddam, H.; Mansouri, S.M.; Korrani, M.S.; Saki, G.; Farbood, Y. Gallic acid improves cognitive, hippocampal long-term potentiation deficits and brain damage induced by chronic cerebral hypoperfusion in rats. Pak. J. Biol. Sci. 2014, 17, 978–990. [Google Scholar] [CrossRef] [Green Version]
- Orlando, G.; Zengin, G.; Ferrante, C.; Ronci, M.; Recinella, L.; Senkardes, I.; Gevrenova, R.; Zheleva-Dimitrova, D.; Chiavaroli, A.; Leone, S.; et al. Comprehensive Chemical Profiling and Multidirectional Biological Investigation of Two Wild Anthemis Species (Anthemis tinctoria var. Pallida and A. cretica subsp. tenuiloba): Focus on Neuroprotective Effects. Molecules 2019, 24, 2582. [Google Scholar] [CrossRef] [Green Version]
- Orlando, G.; Chiavaroli, A.; Leone, S.; Brunetti, L.; Politi, M.; Menghini, L.; Recinella, L.; Ferrante, C. Inhibitory effects induced by Vicia faba, Uncaria rhyncophylla, and Glycyrrhiza glabra water extracts on oxidative stress biomarkers and dopamine turnover in HypoE22 cells and isolated rat striatum challenged with 6-hydroxydopamine. Antioxidants 2019, 8, 602. [Google Scholar] [CrossRef] [Green Version]
- Koeberle, A.; Werz, O. Inhibitors of the microsomal prostaglandin E(2) synthase-1 as alternative to non steroidal anti-inflammatory drugs (NSAIDs)-a critical review. Curr. Med. Chem. 2009, 16, 4274–4296. [Google Scholar] [CrossRef]
- Ferrante, C.; Chiavaroli, A.; Angelini, P.; Venanzoni, R.; Angeles Flores, G.; Brunetti, L.; Petrucci, M.; Politi, M.; Menghini, L.; Leone, S.; et al. Phenolic Content and Antimicrobial and Anti-Inflammatory Effects of Solidago virga-aurea, Phyllanthus niruri, Epilobium angustifolium, Peumus boldus, and Ononis spinose Extracts. Antibiotics 2020, 9, 783. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.D.; Manadas, B.J.; Melo, C.V.; Gomes, J.R.; Mendes, C.S.; Grãos, M.M.; Carvalho, R.F.; Carvalho, A.P.; Duarte, C.B. Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ. 2005, 12, 1329–1343. [Google Scholar] [CrossRef] [Green Version]
- Annunziata, G.; Capó, X.; Quetglas-Llabrés, M.M.; Monserrat-Mesquida, M.; Tejada, S.; Tur, J.A.; Ciampaglia, R.; Guerra, F.; Maisto, M.; Tenore, G.C.; et al. Ex Vivo Study on the Antioxidant Activity of a Winemaking By-Product Polyphenolic Extract (Taurisolo®) on Human Neutrophils. Antioxidants 2021, 10, 1009. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Bomberg, E.; Billington, C.; Levine, A.; Kotz, C.M. Brain-derived neurotrophic factor in the hypothalamic paraventricular nucleus reduces energy intake. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R1003–R1012. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.J.; Meguid, M.M. LHA dopaminergic activity in obese and lean Zucker rats. Neuroreport 1995, 6, 1191–1194. [Google Scholar] [CrossRef]
- Almeida-Toledano, L.; Andreu-Fernández, V.; Aras-López, R.; García-Algar, Ó.; Martínez, L.; Gómez-Roig, M.D. Epigallocatechin Gallate Ameliorates the Effects of Prenatal Alcohol Exposure in a Fetal Alcohol Spectrum Disorder-Like Mouse Model. Int. J. Mol. Sci. 2021, 22, 715. [Google Scholar] [CrossRef]
- Valassi, E.; Scacchi, M.; Cavagnini, F. Neuroendocrine control of food intake. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 158–168. [Google Scholar] [CrossRef]
- Cho, S.J.; Jung, U.J.; Kim, H.J.; Ryu, R.; Ryoo, J.Y.; Moon, B.S.; Choi, M.S. Effects of the Combined Extracts of Grape Pomace and Omija Fruit on Hyperglycemia and Adiposity in Type 2 Diabetic Mice. Prev. Nutr. Food Sci. 2015, 20, 94–101. [Google Scholar] [CrossRef] [Green Version]
- Recinella, L.; Chiavaroli, A.; di Giacomo, V.; Antolini, M.D.; Acquaviva, A.; Leone, S.; Brunetti, L.; Menghini, L.; Ak, G.; Zengin, G.; et al. Anti-Inflammatory and Neuromodulatory Effects Induced by Tanacetum parthenium Water Extract: Results from In Silico, In Vitro and Ex Vivo Studies. Molecules 2021, 26, 22. [Google Scholar] [CrossRef]
- Orlando, G.; Leone, S.; Ferrante, C.; Chiavaroli, A.; Mollica, A.; Stefanucci, A.; Macedonio, G.; Dimmito, M.P.; Leporini, L.; Menghini, L.; et al. Effects of Kisspeptin-10 on Hypothalamic Neuropeptides and Neurotransmitters Involved in Appetite Control. Molecules 2018, 23, 3071. [Google Scholar] [CrossRef] [Green Version]
- Villegas-Navarro, A.; Rosas-L, E.; Reyes, J.L. The heart of Daphnia magna: Effects of four cardioactive drugs. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2003, 136, 127–134. [Google Scholar] [CrossRef]
- Menghini, L.; Ferrante, C.; Leporini, L.; Recinella, L.; Chiavaroli, A.; Leone, S.; Pintore, G.; Vacca, M.; Orlando, G.; Brunetti, L. An hydroalcoholic chamomile extract modulates inflammatory and immune response in HT29 cells and isolated rat colon. Phytother. Res. 2016, 30, 1513–1518. [Google Scholar] [CrossRef] [PubMed]
- Menghini, L.; Leporini, L.; Vecchiotti, G.; Locatelli, M.; Carradori, S.; Ferrante, C.; Zengin, G.; Recinella, L.; Chiavaroli, A.; Leone, S.; et al. Crocus sativus L. stigmas and byproducts: Qualitative fingerprint, antioxidant potentials and enzyme inhibitory activities. Food Res. Int. 2018, 109, 91–98. [Google Scholar] [CrossRef]
- Chichiriccò, G.; Ferrante, C.; Menghini, L.; Recinella, L.; Leone, S.; Chiavaroli, A.; Brunetti, L.; Di Simone, S.; Ronci, M.; Piccone, P.; et al. Crocus sativus by-products as sources of bioactive extracts: Pharmacological and toxicological focus on anthers. Food Chem. Toxicol. 2019, 126, 7–14. [Google Scholar] [CrossRef] [PubMed]

| Temperature (°C) | Time (min) | Solvent/Plant (v/w) | Gallic Acid | Caftaric Acid | Catechin | Chlorogenic Acid | Epicatechin | Caffeic Acid | Syringic Acid |
|---|---|---|---|---|---|---|---|---|---|
| 20 | 5 | 4 | 3.066 | 3.326 | 1.522 | 1.043 | 1.532 | 0.354 | 0.789 |
| 80 | 5 | 4 | 3.473 | 3.349 | 1.708 | 1.052 | 1.601 | 0.38 | 0.769 |
| 20 | 55 | 4 | 3.452 | 3.368 | 2.096 | 1.0313 | 1.825 | 0.4 | 0.823 |
| 80 | 55 | 4 | 3.728 | 3.354 | 3.003 | 1.234 | 2.097 | 0.414 | 0.969 |
| 20 | 5 | 20 | 1.608 | 3.349 | 2.152 | 1.018 | 1.528 | 0.35 | 0.824 |
| 80 | 5 | 20 | 2.298 | 3.492 | 3.469 | 1.037 | 1.813 | 0.4 | 0.829 |
| 20 | 55 | 20 | 1.952 | 3.434 | 3.437 | 1.029 | 1.602 | 0.387 | 0.789 |
| 80 | 55 | 20 | 2.325 | 3.351 | 1.782 | 1.033 | 1.966 | 0.43 | 0.852 |
| 20 | 30 | 12 | 3.102 | 3.728 | 3.985 | 1.042 | 1.88 | 0.53 | 0.9 |
| 80 | 30 | 12 | 3.665 | 3.846 | 7.865 | 1.048 | 2.473 | 0.513 | 1.01 |
| 50 | 5 | 12 | 2.983 | 3.650 | 6.805 | 1.058 | 1.895 | 0.458 | 0.861 |
| 50 | 55 | 12 | 3.341 | 3.713 | 3.740 | 1.04 | 2.511 | 0.511 | 0.932 |
| 50 | 30 | 4 | 3.181 | 3.323 | 1.957 | 1.035 | 1.523 | 0.399 | 0.772 |
| 50 | 30 | 20 | 2.676 | 3.626 | 6.847 | 1.039 | 1.925 | 0.454 | 0.87 |
| 50 | 30 | 12 | 3.747 | 4.162 | 9.863 | 1.046 | 2.327 | 0.492 | 0.960 |
| Standard | m/z | Wavelengths (nm) | Retention Time (min) |
|---|---|---|---|
| Gallic acid | 169.1 | 254 | 7.937 |
| Caftaric acid | 311.2 | 254 | 10.483 |
| Catechin | 289.3 | 254 | 11.307 |
| Chlorogenic acid | 353.31 | 254 | 12.697 |
| Epicatechin | 289.3 | 254 | 14.653 |
| Caffeic acid | 179.16 | 254 | 16.313 |
| Syringic acid | 197.17 | 254 | 17.510 |
| Coumaric acid | 163.04 | 254 | 23.710 |
| Ferulic acid | 193.1 | 254 | 24.580 |
| Independent Variables | Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Time (min) | 5 | 30 | 55 |
| Temperature (°C) | 20 | 50 | 80 |
| Solvent/plant material (mL/g) | 4 | 12 | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiavaroli, A.; Balaha, M.; Acquaviva, A.; Ferrante, C.; Cataldi, A.; Menghini, L.; Rapino, M.; Orlando, G.; Brunetti, L.; Leone, S.; et al. Phenolic Characterization and Neuroprotective Properties of Grape Pomace Extracts. Molecules 2021, 26, 6216. https://doi.org/10.3390/molecules26206216
Chiavaroli A, Balaha M, Acquaviva A, Ferrante C, Cataldi A, Menghini L, Rapino M, Orlando G, Brunetti L, Leone S, et al. Phenolic Characterization and Neuroprotective Properties of Grape Pomace Extracts. Molecules. 2021; 26(20):6216. https://doi.org/10.3390/molecules26206216
Chicago/Turabian StyleChiavaroli, Annalisa, Marwa Balaha, Alessandra Acquaviva, Claudio Ferrante, Amelia Cataldi, Luigi Menghini, Monica Rapino, Giustino Orlando, Luigi Brunetti, Sheila Leone, and et al. 2021. "Phenolic Characterization and Neuroprotective Properties of Grape Pomace Extracts" Molecules 26, no. 20: 6216. https://doi.org/10.3390/molecules26206216
APA StyleChiavaroli, A., Balaha, M., Acquaviva, A., Ferrante, C., Cataldi, A., Menghini, L., Rapino, M., Orlando, G., Brunetti, L., Leone, S., Recinella, L., & di Giacomo, V. (2021). Phenolic Characterization and Neuroprotective Properties of Grape Pomace Extracts. Molecules, 26(20), 6216. https://doi.org/10.3390/molecules26206216















