Metabolomic Profiling of Antioxidant Compounds in Five Vachellia Species
Abstract
1. Introduction
2. Results
2.1. DPPH Radical Scavenging Activities
2.2. ABTS+ Radical Scavenging Activity
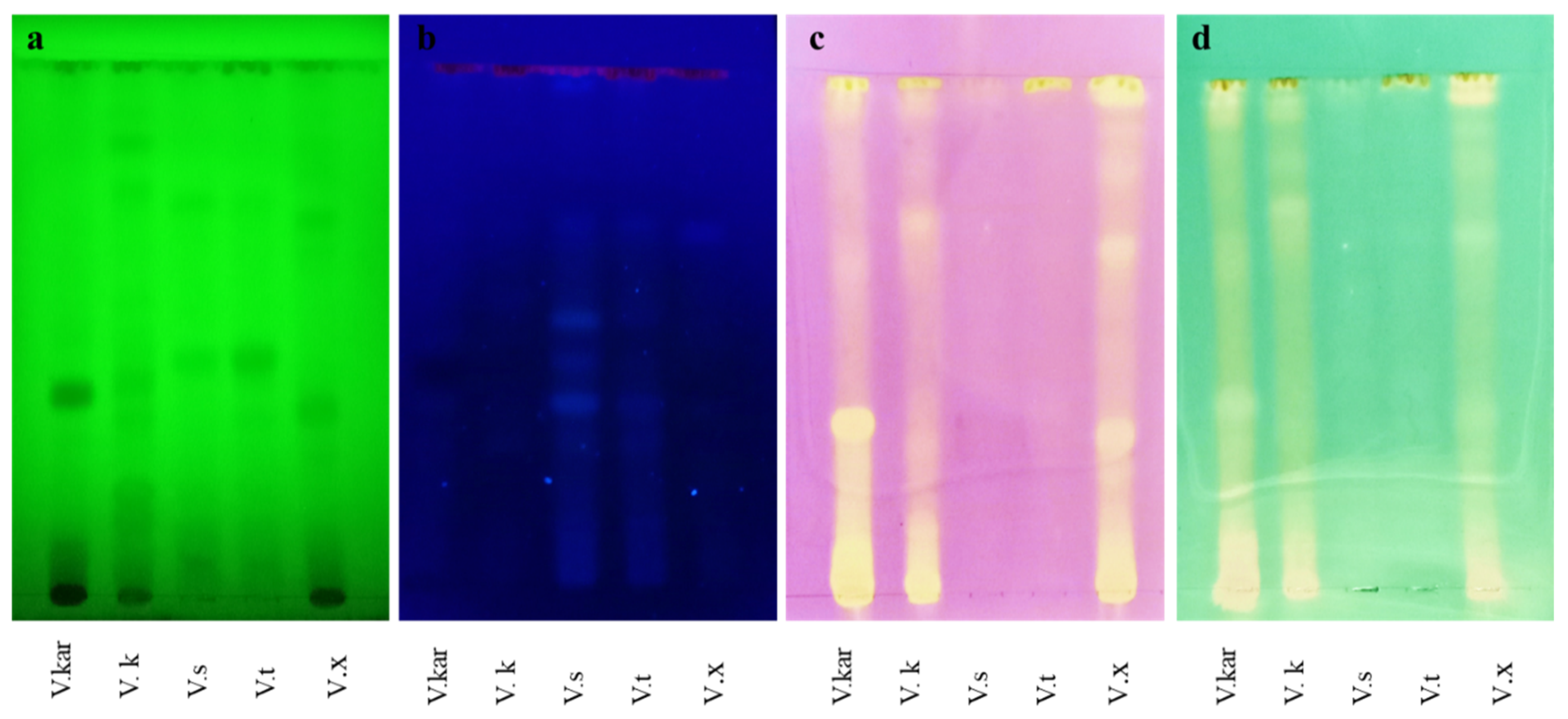
2.3. TLC Bioautographic Radical Scavenging Activities
2.4. Ferric Reduction Antioxidant Power (FRAP) Assay
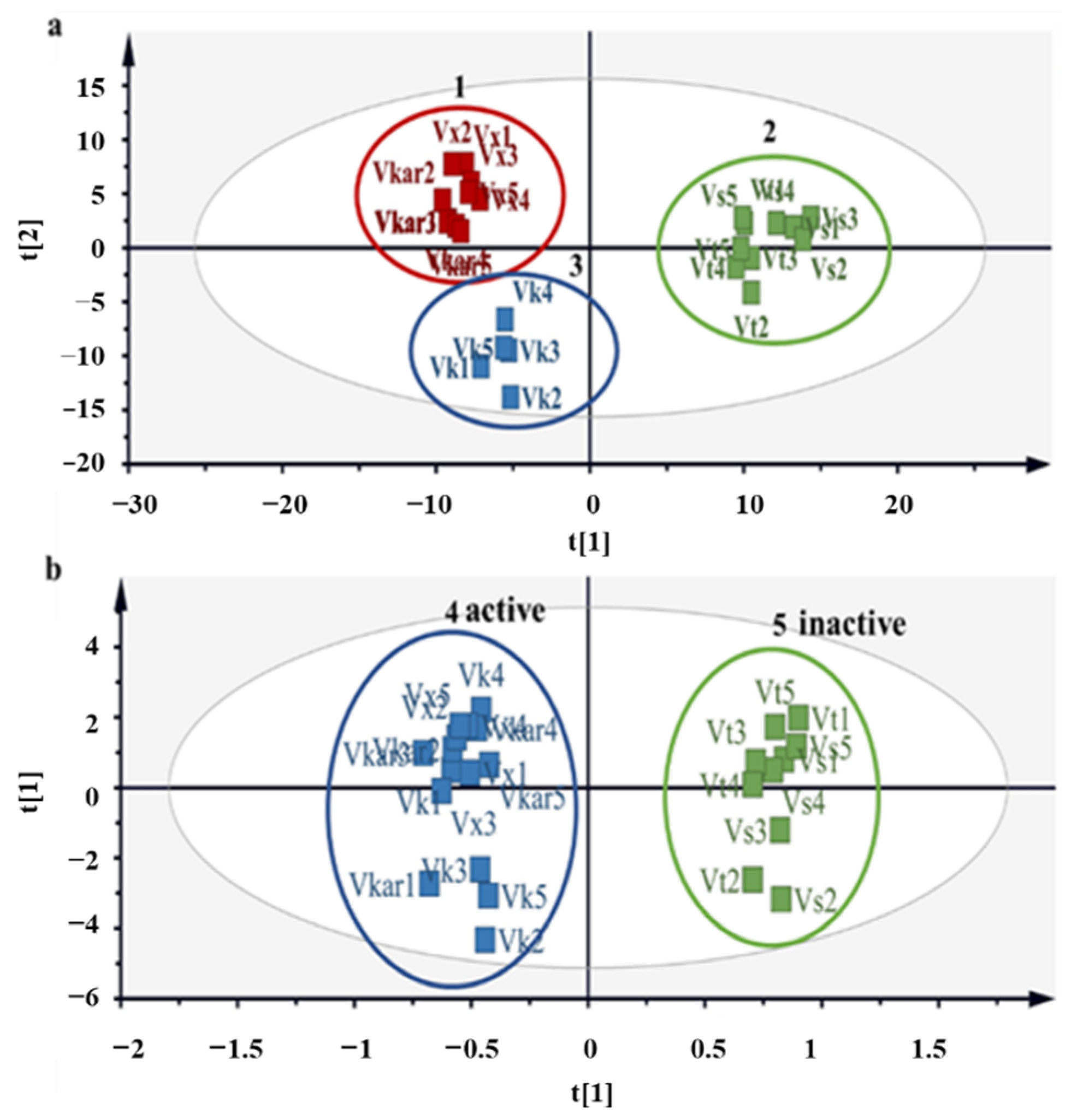
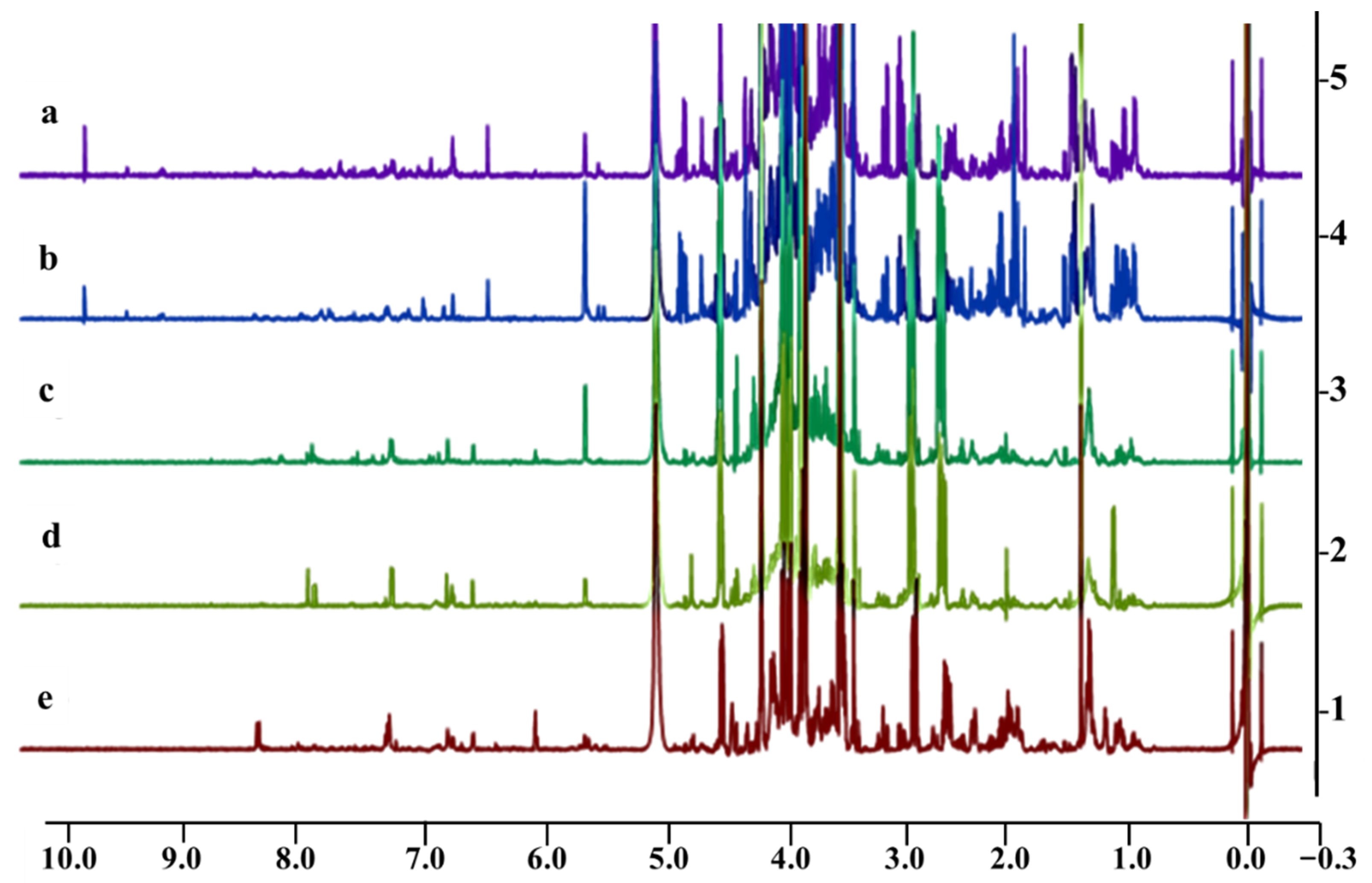
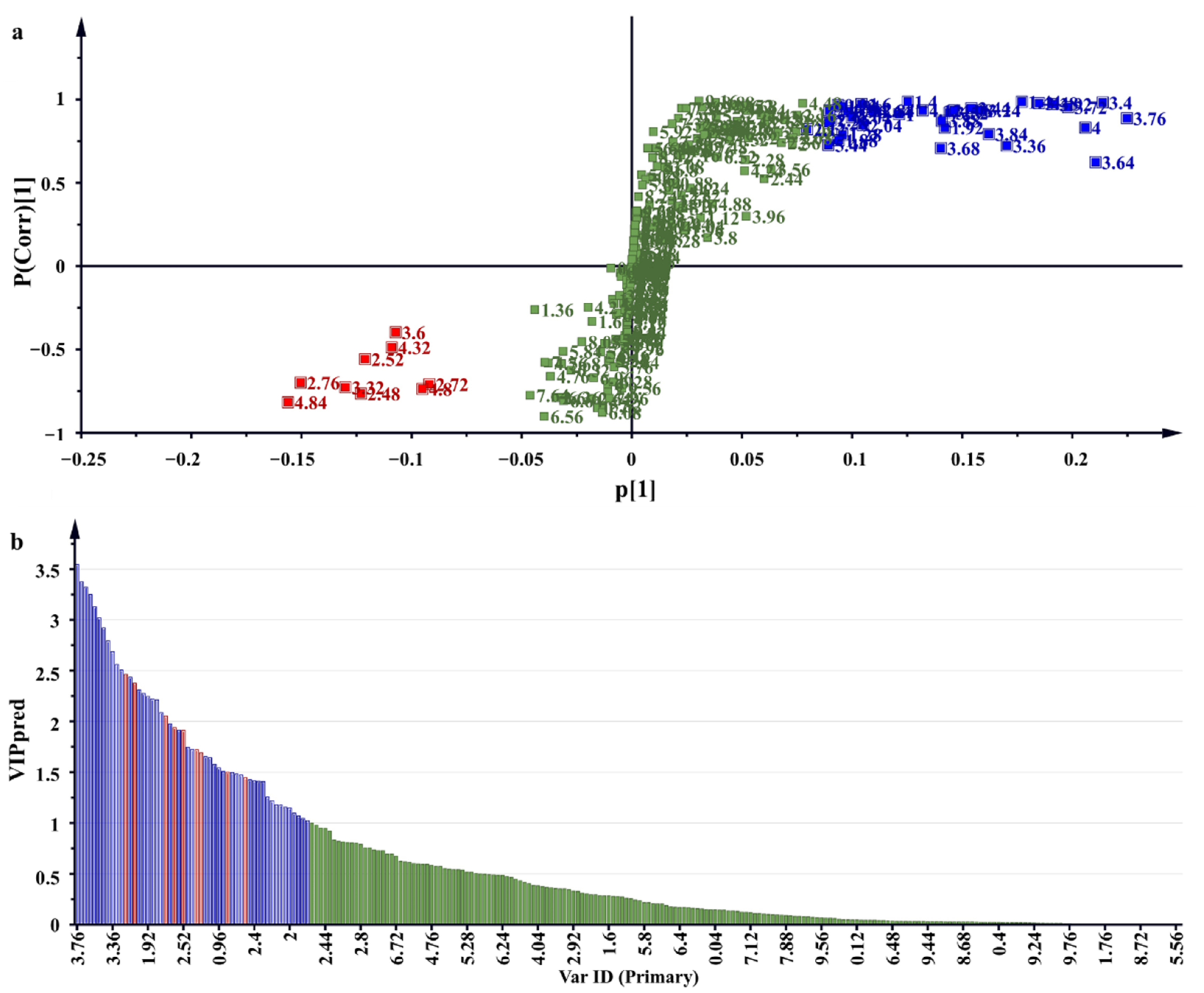
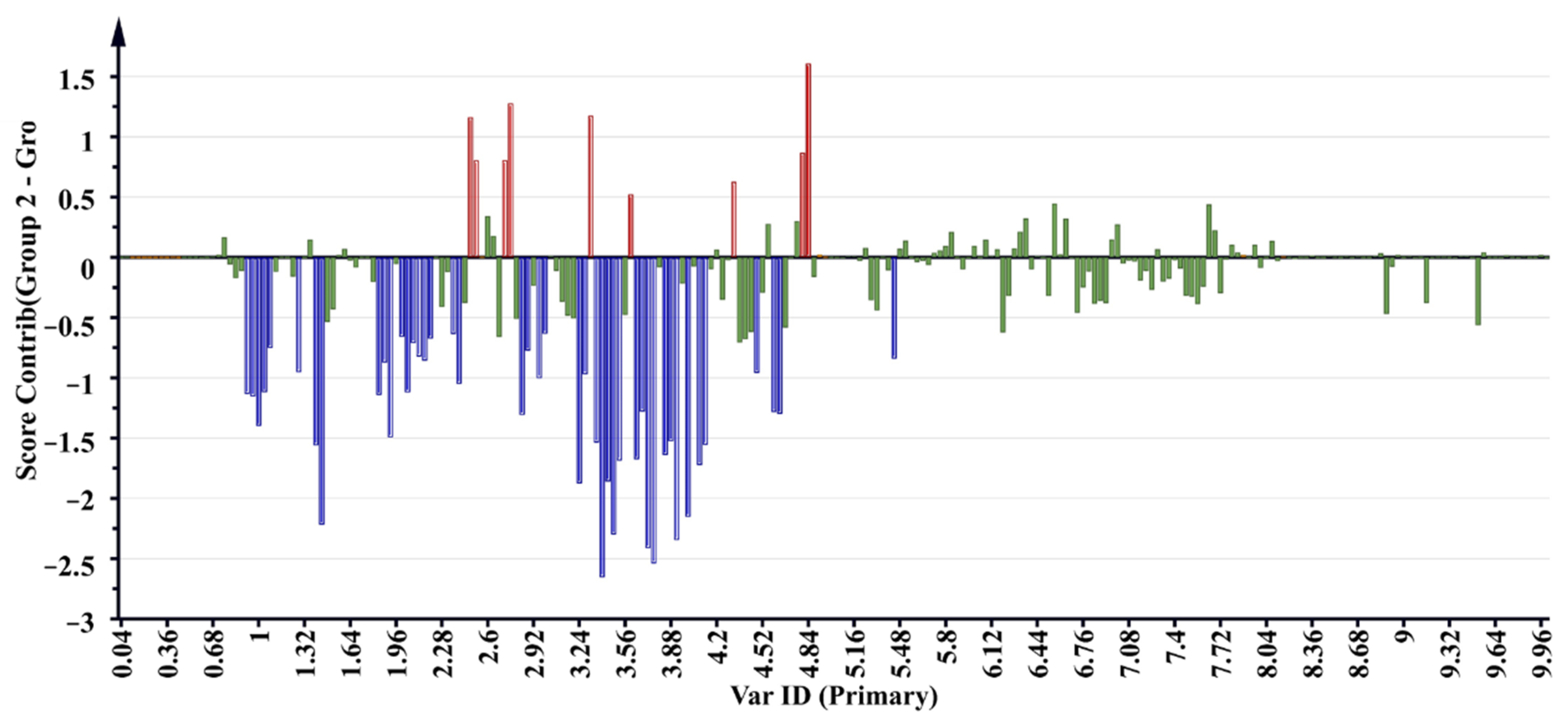
2.5. 1H NMR Metabolomic Profiles and Antioxidant Activity Correlation
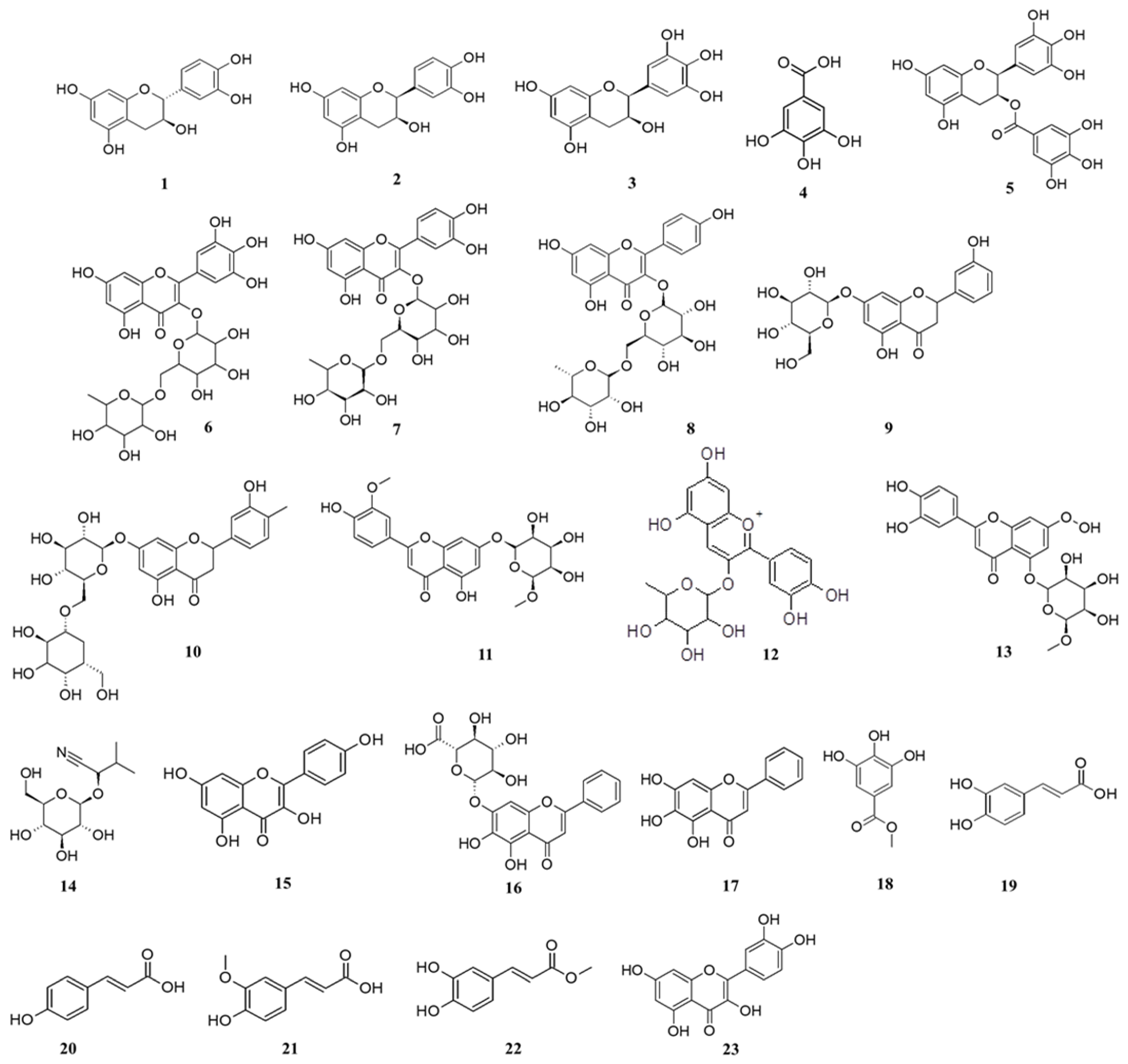
2.6. UHPLC-qTOF-MS Analysis Results
3. Discussion
4. Materials and Methods
4.1. Plant Collection
4.2. Sample Preparation
4.2.1. Plant Extraction
4.2.2. Sample Preparation for 1H NMR Measurement
4.2.3. Sample Preparation UHPLC-qTOF-MS Measurement
4.2.4. 2,2-diphenyl-2-picryl-hydraxyl (DPPH) Free Radical Scavenging Activity Assay
4.2.5. 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic Acid) (ABTS+) Radical Cation Scavenging Activity Assay
4.2.6. Ferric Reducing Antioxidant Power
4.2.7. TLC Bioautographic Radical Scavenging Assay
4.2.8. 1H NMR Measurement and Data Analysis
4.2.9. UHPLC-qTOF-MS Analysis
4.2.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Collin, F. Chemical basis of reactive oxygen species reactivity and involvement in neurodegenerative diseases. Int. J. Mol. Sci. 2019, 20, 2407. [Google Scholar] [CrossRef]
- Clarke, H.D.; Downie, S.R.; Seigler, D.S. Implications of chloroplast DNA restriction site variation for systematics of Acacia (Fabaceae: Mimosoideae). Syst. Bot. 2000, 25, 618–632. [Google Scholar] [CrossRef]
- Dyer, C. New names for the African Acacia species in Vachellia and Senegalia. J. For. Sci. 2014, 76, 1. [Google Scholar] [CrossRef][Green Version]
- Kyalangalilwa, B.; Boatwright, J.S.; Daru, B.H.; Maurin, O.; Van der Bank, M. Phylogenetic position and revised classification of Acacias (Fabaceae: Mimosoideae) in Africa, including new combinations in Vachellia and Senegalia. Bot. J. Linn. Soc. 2013, 172, 500–523. [Google Scholar] [CrossRef]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Katerere, D.R.; Eloff, J.N. Variation in chemical composition, antibacterial and antioxidant activity of fresh and dried Acacia leaf extracts. S. Afr. J. Bot. 2004, 70, 303–305. [Google Scholar] [CrossRef]
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.J.; Li, H.B. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int. J. Mol. Sci. 2017, 5, 96. [Google Scholar] [CrossRef]
- Maroyi, A. Acacia karroo Hayne: Ethnomedicinal uses, phytochemistry and pharmacology of an important medicinal plant in southern Africa. Asian Pac. J. Trop. Med. 2017, 10, 351–360. [Google Scholar] [CrossRef]
- Mbongeni, K. Antioxidant Activity of Vachellia Species, Pork Quality and Fatty Acid Composition from Pigs Supplemented with Graded Levels of Vachellia tortilis Leaf Meal. Ph.D. Thesis, University of Kwazulu-Natal, Pietermaritzburg, South Africa, 2017. Available online: https://researchspace.ukzn.ac.za/xmlui/handle/10413/16725 (accessed on 8 July 2021).
- Nyila, M.A.; Leonard, C.M.; Hussein, A.A.; Lall, N. Activity of South African medicinal plants against Listeria monocytogenes biofilms and isolation of active compounds from Acacia karroo. S. Afr. J. Bot. 2012, 78, 220–227. [Google Scholar] [CrossRef]
- Riedl, J.; Schreiber, R.; Otto, M.; Heilmeier, H.; Altenburger, R.; Schmitt-Jansen, M. Metabolic effect level index links multivariate metabolic fingerprints to ecotoxicological effect assessment. Environ. Sci. Technol. 2015, 49, 8096–8104. [Google Scholar] [CrossRef]
- Verpoorte, R.; Choi, Y.H.; Mustafa, N.R.; Kim, H.K. Metabolomics: Back to basics. Phytochem. Rev. 2008, 7, 525–537. [Google Scholar] [CrossRef]
- Moco, S.I.A.; Bino, R.J.; de Vos, C.H.; Vervoort, J.J.M. Metabolomics technologies and metabolite identification. TrAC Trends Anal. Chem. 2007, 26, 855–866. [Google Scholar] [CrossRef]
- Ursem, R.A.; Tikunov, Y.M.; Bovy, A.G.; van Berloo, R.; van Eeuwijk, F.A. A correlation network approach to metabolic data analysis for tomato fruits. Euphytica 2008, 8, 181–193. [Google Scholar] [CrossRef]
- Lubbe, A.; Pomahačová, B.; Choi, Y.H.; Verpoorte, R. Analysis of metabolic variation and Galanthamine content in narcissus bulbs by HNMR. Phytochem. Anal. 2010, 66, 1157. [Google Scholar] [CrossRef]
- Kim, H.K.; Choi, Y.H.; Verpoorte, R. NMR-based plant metabolomics: Where do we stand, where do we go? Trends Biotechnol. 2011, 29, 267–275. [Google Scholar] [CrossRef]
- Abdel-Farid, I.B.; Sheded, M.G.; Mohamed, E. Metabolomic profiling and antioxidant activity of some Acacia species. Saudi J. Biol. Sci. 2014, 21, 400–408. [Google Scholar] [CrossRef]
- Stafford, G.I.; Jäger, A.K.; Van Staden, J. Activity of traditional South African sedative and potentially CNS-acting plants in the GABA-benzodiazepine receptor assay. J. Ethnopharmacol. 2005, 100, 210–215. [Google Scholar] [CrossRef] [PubMed]
- McGaw, L.J.; Lall, N.; Meyer, J.J.M.; Eloff, J.N. The potential of South African plants against Mycobacterium infections. J. Ethnopharmacol. 2008, 119, 482–500. [Google Scholar] [CrossRef]
- Kubmarawa, D.; Ajoku, G.A.; Enwerem, N.M.; Okorie, D.A. Preliminary phytochemical and antimicrobial screening of 50 medicinal plants from Nigeria. Afr. J. Biotechnol. 2007, 6, 1690–1696. [Google Scholar]
- Cock, I.E.; Van Vuuren, S.F. A review of the traditional use of southern African medicinal plants for the treatment of fungal skin infections. J. Ethnopharmacol. 2020, 241, 112539. [Google Scholar] [CrossRef]
- Johns, T.; Mhoro, E.B.; Sanaya, P.; Kimanani, E.K. Herbal remedies of the Batemi of Ngorongoro District, Tanzania: A quantitative appraisal. Econ. Bot. 1994, 48, 90–95. [Google Scholar] [CrossRef]
- Muthee, J.K.; Gakuya, D.W.; Mbaria, J.M.; Kareru, P.G.; Mulei, C.M.; Njonge, F.K. Ethnobotanical study of anthelmintic and other medicinal plants traditionally used in Loitoktok district of Kenya. J. Ethnopharmacol. 2011, 135, 15–21. [Google Scholar] [CrossRef]
- Corrigan, B.M.; Van Wyk, B.E.; Geldenhuys, C.J.; Jardine, J.M. Ethnobotanical plant uses in the KwaNibela Peninsula, St Lucia, South Africa. S. Afr. J. Bot. 2011, 77, 346–359. [Google Scholar] [CrossRef]
- Mulaudzi, R.B.; Ndhlala, A.R.; Kulkarni, M.G.; Finnie, J.F.; Van Staden, J. Antimicrobial properties and phenolic contents of medicinal plants used by the Venda people for conditions related to venereal diseases. J. Ethnopharmacol. 2011, 135, 330–337. [Google Scholar] [CrossRef]
- van der Merwe, D.; Swan, G.E.; Botha, C.J. Use of ethnoveterinary medicinal plants in cattle by Setswana-speaking people in the Madikwe area of the North West Province of South Africa. J. S. Afr. Vet. Assoc. 2001, 72, 189–196. [Google Scholar] [CrossRef]
- Chigora, P.; Masocha, R.; Mutenheri, F. The role of indigenous medicinal knowledge (IMK) in the treatment of ailments in rural Zimbabwe: The case of Mutirikwi communal Lands. J. Sustain. Dev. Afr. 2007, 9, 26–43. [Google Scholar]
- Al-fatimi, M.; Wurster, M.; Schr, G.; Lindequist, U. Antioxidant, antimicrobial and cytotoxic activities of selected medicinal plants from Yemen. J. Ethnopharmacol. 2007, 111, 657–666. [Google Scholar] [CrossRef]
- Koch, A.; Tamez, P.; Pezzuto, J.; Soejarto, D. Evaluation of plants used for antimalarial treatment by the Maasai of Kenya. J. Ethnopharmacol. 2005, 101, 95–99. [Google Scholar] [CrossRef]
- Worley, B.; Powers, R. Multivariate analysis in metabolomics. Curr. Metabolom. 2013, 1, 92–107. [Google Scholar]
- Mandrekar, J.N. Receiver operating characteristic curve in diagnostic test assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef]
- Tugizimana, F.; Steenkamp, P.A.; Piater, L.A.; Dubery, I.A. A conversation on data mining strategies in LC-MS untargeted metabolomics: Pre-processing and pre-treatment steps. Metabolites 2016, 6, 40. [Google Scholar] [CrossRef] [PubMed]
- Bernatoniene, J.; Kopustinskiene, D.M. The role of catechins in cellular responses to oxidative stress. Molecules 2018, 23, 965. [Google Scholar] [CrossRef]
- Tshikalange, T.E.; Mamba, P.; Adebayo, S.A. Antimicrobial, antioxidant and cytotoxicity studies of medicinal plants used in the treatment of sexually transmitted diseases. Int. J. Pharmacog. Phytochem. Res. 2016, 8, 1891–1895. [Google Scholar]
- Idamokoro, E.M.; Masika, P.J.; Muchenje, V. A Report on the In Vitro Antioxidant Properties of Vachellia karroo Leaf Extract: A Plant Widely Grazed by Goats in the Central Eastern Cape of South Africa. Sustainability 2017, 9, 164. [Google Scholar] [CrossRef]
- Alam, P.; Alajmi, M.F.; Arbab, A.H.; Parvez, M.K.; Siddiqui, N.A.; Alqasoumi, S.I.; Al-Rehaily, A.J.; Al-Dosari, M.S.; Basudan, O.A. Comparative study of antioxidant activity and validated RP-HPTLC analysis of rutin in the leaves of different Acacia species grown in Saudi Arabia. Saudi Pharm. J. 2017, 25, 715–723. [Google Scholar] [CrossRef]
- Kalaivani, T.; Mathew, L. Free radical scavenging activity from leaves of Acacia nilotica (L.) Wild. ex Delile, an Indian medicinal tree. Food Chem. Toxicol. 2010, 48, 298–305. [Google Scholar] [CrossRef]
- Gong, J.; Chu, B.; Gong, L.; Fang, Z.; Zhang, X.; Qiu, S.; Wang, J.; Xiang, Y.; Xiao, G.; Yuan, H.; et al. Comparison of phenolic compounds and the antioxidant activities of fifteen Chrysanthemum morifolium Ramat cv. ‘Hangbaiju’ in China. Antioxidants 2019, 8, 325. [Google Scholar] [CrossRef]
- Wafaa, H.B.; Hassan, W.; Abdelaziz, S.; Al Yousef, H.M. Chemical composition and biological activities of the aqueous fraction of Parkinsonea aculeata L. growing in Saudi Arabia. Arab. J. Chem. 2019, 12, 377–387. [Google Scholar] [CrossRef]
- Kim, E.; Han, S.Y.; Hwang, K.; Kim, D.; Kim, E.M.; Hossain, M.A.; Kim, J.H.; Cho, J.Y. Antioxidant and cytoprotective effects of (−)-epigallocatechin-3-(3″-o-methyl) gallate. Int. J. Mol. Sci. 2019, 20, 3993. [Google Scholar] [CrossRef]
- Abdelhady, M.I.S. A novel polyphenolic compound isolated from Acacia sieberiana. Org. Chem. 2016, 9, 6–8. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Gao, Y.; Granato, D. Effects of epigallocatechin gallate, epigallocatechin and epicatechin gallate on the chemical and cell-based antioxidant activity, sensory properties, and cytotoxicity of a catechin-free model beverage. Food Chem. 2021, 339, 128060. [Google Scholar] [CrossRef] [PubMed]
- Rangkadilok, N.; Sitthimonchai, S.; Worasuttayangkurn, L. Evaluation of free radical scavenging and antityrosinase activities of standardized longan fruit extract. Food Chem. Toxicol. 2007, 45, 328–336. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
| Plant Name | Medicinal Uses | Plant Parts Used | Country of Use/ References |
|---|---|---|---|
| V. sieberiana | Convulsions, sedative (mental illness), dizziness | Bark | Zimbabwe [19] |
| Fever | Leaves, Bark, Roots | South Africa [20] | |
| Sore throat | Root | Nigeria [21] | |
| Diarrhea | Bark | South Africa [22] | |
| V. xanthophloea | Diabetes | Bark | Zimbabwe [19] |
| Emetic/cathartic, sickle cell anemia | Roots | Tanzania [23] | |
| Fatigue, indigestion, skin disorders | Bark, Roots | Kenya [24] | |
| Febrile, fevers, gingivitis, high cholesterol | Leaves, Bark, Roots | South Africa [25] | |
| Malaria, emetic, mouth sores, pharyngitis, tuberculosis symptoms | Bark | South Africa [20] | |
| V. karroo | Diarrhea, dysentery, gastrointestinal, venereal diseases | Leaves, Roots | South Africa [25,26] |
| Fractures, diarrhea | Bark | South Africa [27] | |
| aphrodisiac, sexually transmitted infections, urinary schistosomiasis | Bark, Roots | Zimbabwe [27,28] | |
| V. kosiensis | No literature on the medicinal uses found | ||
| V. tortilis | Cough | Bark | Nigeria [21] |
| Stomach-ache, digestive | Fruits | Yemen [29] | |
| indigestion, malaria, strengthen bones, kidney cleanser | Roots | Kenya [30] | |
| Diarrhea | Branch tips | South Africa [27] | |
| Plant Extracts | DPPH (IC50 µg/mL) | ABTS+ (IC50 µg/mL) | FRAP (IC50 µg/mL) |
|---|---|---|---|
| V. karroo | 4.94 ± 0.44 | 2.23 ± 0.51 | 28.14 ± 0.44 |
| V. kosiensis | 5.13 ± 0.40 | 5.61 ± 0.63 | 11.50 ± 0.34 |
| V. sieberiana | 87.41 ± 0.58 | 52.03 ± 0.59 | 107.09 ± 0.64 |
| V. tortilis | 70.01 ± 0.30 | 45 ± 0.58 | 97.44 ± 0.54 |
| V. xanthophloea | 4.91 ± 0.42 | 3.61 ± 0.45 | 23.20 ± 0.34 |
| Ascorbic acid | 1.40 ± 0.21 | 1.10 ± 0.20 | 15.10 ± 0.39 |
| Compounds | δ1H (ppm) and Multiplicity | Active/Inactive |
|---|---|---|
| Isoleucine | 0.92 (d) | Inactive |
| leucine | 0.96 (d) | Inactive |
| Valine | 1.04 (d) | Inactive |
| Alanine | 1.44 (d) | Inactive |
| Acetate | 1.92 (s) | Inactive |
| Succinate | 2.40 (s) | Active |
| Citric acid | 2.52 (d) | Active |
| Aspartate | 2.70 (m) | Inactive |
| Choline | 3.24 (s) | Inactive |
| Betaine | 3.32 (s) | Inactive |
| Glucose | 4.84 (m) | Inactive |
| Sucrose | 5.44 (d) | Inactive |
| Catechin | 5.92 (s) | Active |
| Ferulate | 6.44 (s) | Active |
| Gallate | 7.10 (s) | Active |
| Trigonelline | 9.15 (s) | Inactive |
| No. | Tentative Metabolites | Empirical Formula | Detected Mass (m/z) | Mass Accuracy (mDa) | MS/MS Fragmentation ions | Data Source | Vachellia karroo | Vachellia kosiensis | Vachellia tortilis | Vachellia sieberiana | Vachellia xanthophloea |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Catechin | C15H14O6 | 289.0705 | −0.7 | Trace | NIST 2014 | ✓ | ✕ | ✕ | ✕ | ✕ |
| 2 | Epicatechin | C15H14O6 | 289.0716 | 0.4 | Trace | NIST 2014 | ✓ | ✕ | ✕ | ✕ | ✕ |
| 3 | Epigallocatechin | C15H14O7 | 305.0659 | −0.2 | Trace | NIST 2014 | ✓ | ✓ | ✕ | ✕ | ✓ |
| 4 | Gallic acid | C7H6O5 | 169.0133 | −0.4 | 125.02 | NIST 2014 | ✕ | ✓ | ✕ | ✕ | ✓ |
| 5 | Epigallocatechin gallate | C22H18O11 | 457.0786 | 1.5 | 169.02 | NIST 2014 | ✕ | ✕ | ✕ | ✕ | # |
| 6 | Myricetin rutinoside | C27H30O17 | 625.1419 | 1.4 | 316.02 | NDP | ✓ | ✓ | ✓ | ✕ | ✓ |
| 7 | Rutin | C27H30O16 | 609.1454 | −0.2 | 300.03 | NIST 2014 | ✓ * | ✓ | ✕ | ✕ | ✕ |
| 8 | Kaempferol rutinoside (Nicotiflorin) | C27H30O15 | 593.1498 | −0.8 | 285.04 | NIST 2014 | ✓ | ✓ | ✓ | ✓ | ✕ |
| 9 | Chrysoeriol rutinoside | C28H32O15 | 607.1663 | −1.6 | 461.10; 299.05 | NDP | ✕ | ✕ | ✓ | ✓ | ✕ |
| 10 | Quercetin rutinoside | C27H30O16 | 609.1467 | 1.1 | 300.03 | NIST 2014 | ✓ * | ✓ | ✓ | ✓ | ✓ |
| 11 | Chrysoeriol glucopyranoside | C22H22O11 | 461.1072 | −1.2 | 299.02 | NDP | ✕ | ✓ | ✓ | ✓ | ✓ |
| 12 | Cyanidin rhamnoside | C21H20O10 | 431.0989 | 1.1 | 285.03 | Metlin DB | ✕ | ✓ | ✓ | ✓ | ✓ |
| 13 | Luteolin glucoside | C21H20O11 | 449.1096 | 1.2 | 287.05 | NIST 2014 | ✕ | ✓ | ✓ | ✓ | ✓ |
| 14 | Dihydroacacipetalin | C11H19NO6 | 262.1281 | 0.2 | Trace | NDP | ✕ | # | ✓ | ✓ | ✓ |
| 15 | Kaempferol | C15H10O6 | 285.0398 | −0.1 | 151.00; 107.01 | NIST 2014 | § | ✓ | ✓ | ✓ | ✓ |
| 16 | Baicalin | C21H18O11 | 445.0748 | −2.3 | Trace | NDP | ✕ | * | ✕ | # | ✕ |
| 17 | Baicalein | C15H10O5 | 269.0443 | −0.7 | Trace | NDP | ✓ | # | ✓ | ✓ | # |
| 18 | Methyl gallate | C8H8O5 | 183.0294 | 0.0 | 183.02; 124.03 | NIST 2014 | ✓ | ✓ | ✕ | ✕ | ✓ |
| 19 | Caffeic acid | C9H8O4 | 179.0319 | −2.5 | Trace | NDP | ✕ | ✕ | ✕ | ✕ | # |
| 20 | p-coumaric acid | C9H8O3 | 163.0394 | −0.1 | 119.04 | NIST 2014 | # | ✕ | § | § | ✕ |
| 21 | Ferulic acid | C10H10O4 | 193.0491 | −1.0 | Trace | NDP | § * | § * | § * | § * | ✕ |
| 22 | Methyl caffeate | C10H10O4 | 193.0501 | −1.9 | Trace | NDP | § * | § * | § * | § * | ✕ |
| 23 | Quercetin | C15H10O7 | 301.0358 | 1.0 | 179.00; 151.00 | NIST 2014 | ✓ | ✓ | ✕ | ✕ | ✓ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
More, G.K.; Meddows-Taylor, S.; Prinsloo, G. Metabolomic Profiling of Antioxidant Compounds in Five Vachellia Species. Molecules 2021, 26, 6214. https://doi.org/10.3390/molecules26206214
More GK, Meddows-Taylor S, Prinsloo G. Metabolomic Profiling of Antioxidant Compounds in Five Vachellia Species. Molecules. 2021; 26(20):6214. https://doi.org/10.3390/molecules26206214
Chicago/Turabian StyleMore, Garland Kgosi, Stephen Meddows-Taylor, and Gerhard Prinsloo. 2021. "Metabolomic Profiling of Antioxidant Compounds in Five Vachellia Species" Molecules 26, no. 20: 6214. https://doi.org/10.3390/molecules26206214
APA StyleMore, G. K., Meddows-Taylor, S., & Prinsloo, G. (2021). Metabolomic Profiling of Antioxidant Compounds in Five Vachellia Species. Molecules, 26(20), 6214. https://doi.org/10.3390/molecules26206214






