UPLC–TOF–MS Method for Simultaneous Quantification of Steroid Hormones in Tissue Homogenates of Zebrafish with Solid-Phase Extraction
Abstract
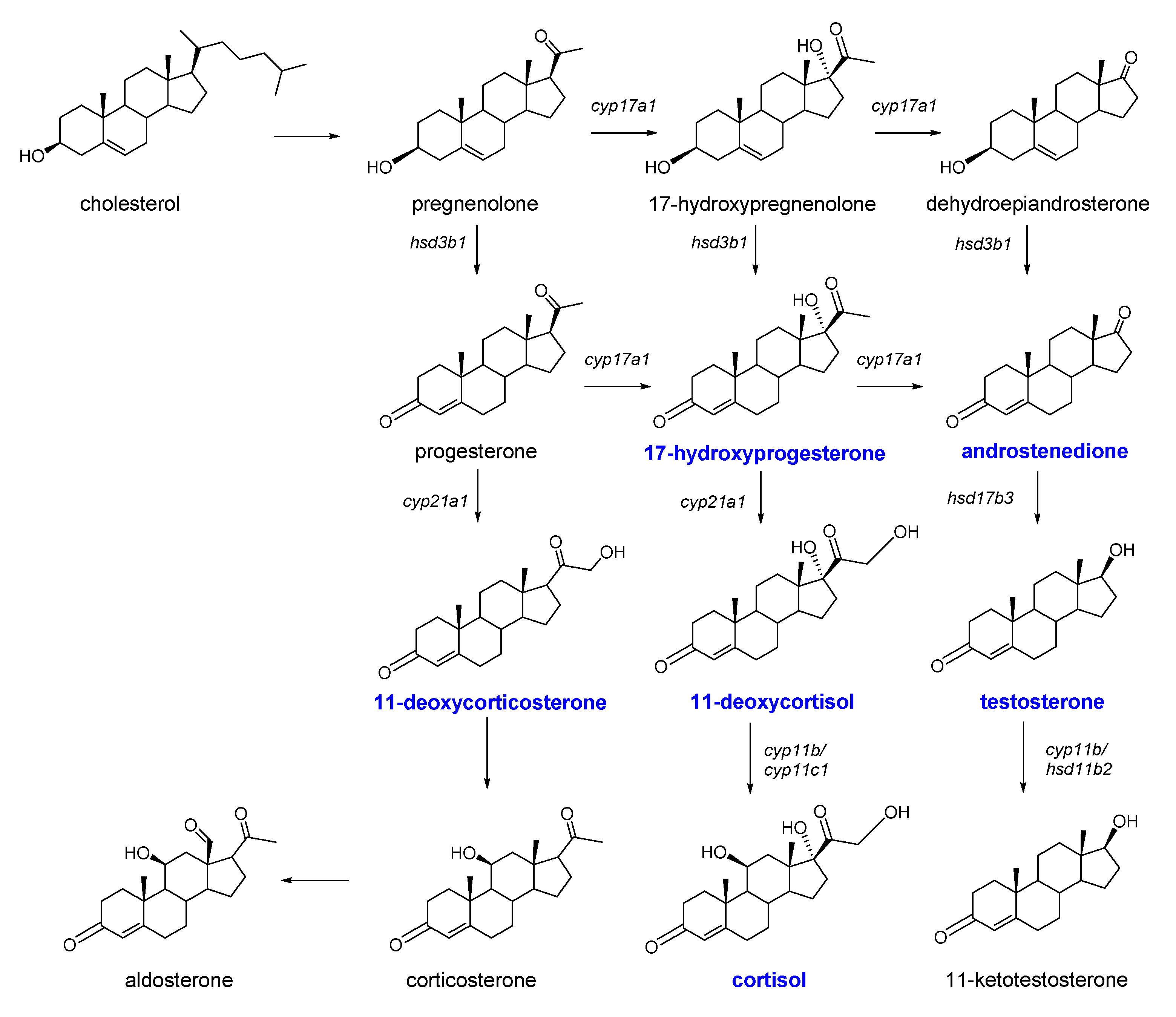
:1. Introduction
2. Results and Discussion
2.1. Method Development
2.2. Calibration and Method Validation
2.3. Recovery and Matrix Effects
2.4. Quantitative Analysis of Steroid Hormones in Zebrafish Homogenates
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Animals and Housing
3.3. Preparation of Calibration Standards, Quality Control, and Internal Standard Solutions
3.4. Tissue Homogenization, Extraction, and Sample Preparation for Quantification
3.5. Quantitative Conditions by UPLC–TOF–MS
3.6. Matrix Effects
3.7. Method Validation
3.8. Acute Exposure Manipulation
3.9. Data Analysis and Statistics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Löhr, H.; Hammerschmidt, M. Zebrafish in endocrine systems: Recent advances and implications for human disease. Annu. Rev. Physiol. 2011, 73, 183–211. [Google Scholar] [CrossRef]
- Lele, Z.; Krone, P. The zebrafish as a model system in developmental, toxicological and transgenic research. Biotechnol. Adv. 1996, 14, 57–72. [Google Scholar] [CrossRef]
- Tokarz, J.; Möller, G.; de Angelis, M.H.; Adamski, J. Zebrafish and steroids: What do we know and what do we need to know? J. Steroid Biochem. Mol. Biol. 2013, 137, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Strüssmann, C.A.; Nakamura, M. Morphology, endocrinology, and environmental modulation of gonadal sex differentiation in teleost fishes. Fish Physiol. Biochem. 2002, 26, 13–29. [Google Scholar] [CrossRef]
- Gorelick, D.A.; Watson, W.; Halpern, M.E. Androgen receptor gene expression in the developing and adult zebrafish brain. Dev. Dynam. 2008, 237, 2987–2995. [Google Scholar] [CrossRef] [PubMed]
- Moraes, A.B.; Giacomini, A.C.; Genario, R.; Marcon, L.; Scolari, N.; Bueno, B.W.; Demin, K.A.; Amstislavskaya, T.G.; Strekalova, T.; Soares, M.C. Pro-social and anxiolytic-like behavior following a single 24-h exposure to 17β-estradiol in adult male zebrafish. Neurosci. Lett. 2021, 747, 135591. [Google Scholar] [CrossRef]
- Fraser, T.W.; Khezri, A.; Lewandowska-Sabat, A.M.; Henry, T.; Ropstad, E. Endocrine disruptors affect larval zebrafish behavior: Testing potential mechanisms and comparisons of behavioral sensitivity to alternative biomarkers. Aquat. Toxicol. 2017, 193, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Egan, R.J.; Bergner, C.L.; Hart, P.C.; Cachat, J.M.; Canavello, P.R.; Elegante, M.F.; Elkhayat, S.I.; Bartels, B.K.; Tien, A.K.; Tien, D.H. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav. Brain Res. 2009, 205, 38–44. [Google Scholar] [CrossRef] [Green Version]
- Ankley, G.T.; Villeneuve, D.L. Temporal changes in biological responses and uncertainty in assessing risks of endocrine-disrupting chemicals: Insights from intensive time-course studies with fish. Toxicol. Sci. 2015, 144, 259–275. [Google Scholar] [CrossRef] [Green Version]
- Ankley, G.T.; Johnson, R.D. Small fish models for identifying and assessing the effects of endocrine-disrupting chemicals. ILAR J. 2004, 45, 469–483. [Google Scholar] [CrossRef] [Green Version]
- Van den Hurk, R.; Lambert, J. Ovarian steroid glucuronides function as sex pheromones for male zebrafish, Brachydanio rerio. Can. J. Zool. 1983, 61, 2381–2387. [Google Scholar] [CrossRef]
- Van den Hurk, R.; Schoonen, W.; Van Zoelen, G.; Lambert, J. The biosynthesis of steroid glucuronides in the testis of the zebrafish, Brachydanio rerio, and their pheromonal function as ovulation inducers. Gen. Comp. Endocr. 1987, 68, 179–188. [Google Scholar] [CrossRef] [Green Version]
- Segner, H.; Caroll, K.; Fenske, M.; Janssen, C.; Maack, G.; Pascoe, D.; Schäfers, C.; Vandenbergh, G.; Watts, M.; Wenzel, A. Identification of endocrine-disrupting effects in aquatic vertebrates and invertebrates: Report from the European IDEA project. Ecotoxicol. Environ. Saf. 2003, 54, 302–314. [Google Scholar] [CrossRef]
- Ramsay, J.M.; Feist, G.W.; Varga, Z.M.; Westerfield, M.; Kent, M.L.; Schreck, C.B. Whole-body cortisol is an indicator of crowding stress in adult zebrafish, Danio rerio. Aquaculture 2006, 258, 565–574. [Google Scholar] [CrossRef]
- Cachat, J.; Stewart, A.; Grossman, L.; Gaikwad, S.; Kadri, F.; Chung, K.M.; Wu, N.; Wong, K.; Roy, S.; Suciu, C. Measuring behavioral and endocrine responses to novelty stress in adult zebrafish. Nat. Protoc. 2010, 5, 1786–1799. [Google Scholar] [CrossRef]
- Thomsson, O.; Ström-Holst, B.; Sjunnesson, Y.; Bergqvist, A.-S. Validation of an enzyme-linked immunosorbent assay developed for measuring cortisol concentration in human saliva and serum for its applicability to analyze cortisol in pig saliva. Acta Vet. Scand. 2014, 56, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, E.; Pearlmutter, P.; Tiangco, M.; Derose, G.; Begdache, L.; Koh, A. Comparison of colorimetric analyses to determine cortisol in human sweat. ACS Omega 2020, 5, 8211–8218. [Google Scholar] [CrossRef]
- Kohen, F.; Lindner, H.; Gilad, S. Development of chemiluminescence monitored immunoassays for steroid hormones. J. Steroid Biochem. 1983, 19, 413–418. [Google Scholar] [CrossRef]
- Wathudura, P.D.; Kavinda, T.; Gunatilake, S.R. Determination of steroidal estrogens in food matrices: Current status and future perspectives. Curr. Opin. Food Sci. 2019, 28, 104–113. [Google Scholar] [CrossRef]
- Handelsman, D.; Wartofsky, L. Requirement for mass spectrometry sex steroid assays in the Journal of Clinical Endocrinology and Metabolism. J. Clin. Endocrinol. Metab. 2013, 98, 3971–3973. [Google Scholar] [CrossRef] [Green Version]
- Taylor, A.E.; Keevil, B.; Huhtaniemi, I.T. Mass spectrometry and immunoassay: How to measure steroid hormones today and tomorrow. Eur. J. Endocrinol. 2015, 173, D1–D12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monaghan, P.J.; Owen, L.J.; Trainer, P.J.; Brabant, G.; Keevil, B.G.; Darby, D. Comparison of serum cortisol measurement by immunoassay and liquid chromatography-tandem mass spectrometry in patients receiving the 11 β-hydroxylase inhibitor metyrapone. Ann. Clin. Biochem. 2011, 48, 441–446. [Google Scholar] [CrossRef] [Green Version]
- Koal, T.; Schmiederer, D.; Pham-Tuan, H.; Röhring, C.; Rauh, M. Standardized LC–MS/MS based steroid hormone profile-analysis. J. Steroid Biochem. 2012, 129, 129–138. [Google Scholar] [CrossRef]
- Rigano, F.; Tranchida, P.Q.; Dugo, P.; Mondello, L. High-performance liquid chromatography combined with electron ionization mass spectrometry: A review. Trac-Trends Anal. Chem. 2019, 118, 112–122. [Google Scholar] [CrossRef]
- Antonelli, G.; Ceccato, F.; Artusi, C.; Marinova, M.; Plebani, M. Salivary cortisol and cortisone by LC–MS/MS: Validation, reference intervals and diagnostic accuracy in Cushing’s syndrome. Clin. Chim. Acta 2015, 451, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.J.; Gaudl, A.; Jaeger, S.; Stadelmann, S.; Hiemisch, A.; Kiess, W.; Willenberg, A.; Schaab, M.; von Klitzing, K.; Thiery, J.; et al. Immunoassay or LC-MS/MS for the measurement of salivary cortisol in children? Clin. Chem. Lab. Med. 2016, 54, 811–822. [Google Scholar] [CrossRef]
- Miller, R.; Plessow, F.; Rauh, M.; Groeschl, M.; Kirschbaum, C. Comparison of salivary cortisol as measured by different immunoassays and tandem mass spectrometry. Psychoneuroendocrinology 2013, 38, 50–57. [Google Scholar] [CrossRef]
- Jensen, M.A.; Hansen, A.M.; Abrahamsson, P.; Norgaard, A.W. Development and evaluation of a liquid chromatography tandem mass spectrometry method for simultaneous determination of salivary melatonin, cortisol and testosterone. J. Chromatogr. B 2011, 879, 2527–2532. [Google Scholar] [CrossRef] [PubMed]
- Montskó, G.; Tarjányi, Z.; Mezősi, E.; Kovács, G.L. A validated method for measurement of serum total, serum free, and salivary cortisol, using high-performance liquid chromatography coupled with high-resolution ESI-TOF mass spectrometry. Anal. Bioanal. Chem. 2014, 406, 2333–2341. [Google Scholar] [CrossRef]
- Nouri, M.Z.; Kroll, K.J.; Webb, M.; Denslow, N.D. Quantification of steroid hormones in low volume plasma and tissue homogenates of fish using LC-MS/MS. Gen. Comp. Endocr. 2020, 296, 113543. [Google Scholar] [CrossRef] [PubMed]
- Büttler, R.M.; Martens, F.; Ackermans, M.T.; Davison, A.S.; van Herwaarden, A.E.; Kortz, L.; Krabbe, J.G.; Lentjes, E.G.W.; Syme, C.; Webster, R.; et al. Comparison of eight routine unpublished LC–MS/MS methods for the simultaneous measurement of testosterone and androstenedione in serum. Clin. Chim. Acta 2016, 454, 112–118. [Google Scholar] [CrossRef]
- Ray, J.A.; Kushnir, M.M.; Yost, R.A.; Rockwood, A.L.; Meikle, A.W. Performance enhancement in the measurement of 5 endogenous steroids by LC–MS/MS combined with differential ion mobility spectrometry. Clin. Chim. Acta 2015, 438, 330–336. [Google Scholar] [CrossRef]
- FDA. Guidance for Industry: Bioanalytical Method Validation. 2001. Available online: https://www.fda.gov/cder/Guidance/4252fnl.pdf (accessed on 1 January 2017).
- Jönsson, B.A.; Malmberg, B.; Amilon, Å.; Garde, A.H.; Ørbæk, P. Determination of cortisol in human saliva using liquid chromatography–electrospray tandem mass spectrometry. J. Chromatogr. B 2003, 784, 63–68. [Google Scholar] [CrossRef]
- Marcos, J.; Renau, N.; Casals, G.; Segura, J.; Ventura, R.; Pozo, O.J. Investigation of endogenous corticosteroids profiles in human urine based on liquid chromatography tandem mass spectrometry. Anal. Chim. Acta 2014, 812, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Ohno, M.; Yamaguchi, I.; Saiki, K.; Yamamoto, I.; Azuma, J. Specific determination of urinary 6β-hydroxycortisol and cortisol by liquid chromatography–atmospheric pressure chemical ionization mass spectrometry. J. Chromatogr. B 2000, 746, 95–101. [Google Scholar] [CrossRef]
- Tomšíková, H.; Aufartová, J.; Solich, P.; Nováková, L.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. High-sensitivity analysis of female-steroid hormones in environmental samples. Trac-Trend. Anal. Chem. 2012, 34, 35–58. [Google Scholar] [CrossRef]
- Gao, W.; Kirschbaum, C.; Grass, J.; Stalder, T. LC–MS based analysis of endogenous steroid hormones in human hair. J. Steroid Biochem. 2016, 162, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Galuska, C.E.; Hartmann, M.F.; Sánchez-Guijo, A.; Bakhaus, K.; Geyer, J.; Schuler, G.; Zimmer, K.-P.; Wudy, S.A. Profiling intact steroid sulfates and unconjugated steroids in biological fluids by liquid chromatography-tandem mass spectrometry (LC-MS-MS). Analyst 2013, 138, 3792–3801. [Google Scholar] [CrossRef] [PubMed]
- Gaudl, A.; Kratzsch, J.; Bae, Y.J.; Kiess, W.; Thiery, J.; Ceglarek, U. Liquid chromatography quadrupole linear ion trap mass spectrometry for quantitative steroid hormone analysis in plasma, urine, saliva and hair. J. Chromatogr. A 2016, 1464, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Rousu, T.; Tolonen, A. Comparison of unit resolution SRM and TOF-MS at 12,000 mass resolution for quantitative bioanalysis of 11 steroids from human plasma. Bioanalysis 2012, 4, 555–563. [Google Scholar] [CrossRef]
- Mutavdžić Pavlović, D.; Babić, S.; Dolar, D.; Ašperger, D.; Košutić, K.; Horvat, A.J.; Kaštelan-Macan, M. Development and optimization of the SPE procedure for determination of pharmaceuticals in water samples by HPLC-diode array detection. J. Sep. Sci. 2010, 33, 258–267. [Google Scholar] [CrossRef]
- Gineys, N.; Giroud, B.; Vulliet, E. Analytical method for the determination of trace levels of steroid hormones and corticosteroids in soil, based on PLE/SPE/LC–MS/MS. Anal. Bioanal. Chem. 2010, 397, 2295–2302. [Google Scholar] [CrossRef]
- Economou, A.; Botitsi, H.; Antoniou, S.; Tsipi, D. Determination of multi-class pesticides in wines by solid-phase extraction and liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2009, 1216, 5856–5867. [Google Scholar] [CrossRef]
- Romero-Martínez, A.; González-Bono, E.; Lila, M.; Moya-Albiol, L. Testosterone/cortisol ratio in response to acute stress: A possible marker of risk for marital violence. Soc. Neurosci. 2013, 8, 240–247. [Google Scholar] [CrossRef]
- Mehta, P.H.; Josephs, R.A. Testosterone and cortisol jointly regulate dominance: Evidence for a dual-hormone hypothesis. Horm. Behav. 2010, 58, 898–906. [Google Scholar] [CrossRef]
- Feist, G.; Schreck, C.B. Ontogeny of the stress response in chinook salmon, Oncorhynchus tshawytscha. Fish Physiol. Biochem. 2001, 25, 31–40. [Google Scholar] [CrossRef]
- Reddy, S.; Iden, C.R.; Brownawell, B.J. Analysis of steroid conjugates in sewage influent and effluent by liquid chromatography—Tandem mass spectrometry. Anal. Chem. 2005, 77, 7032–7038. [Google Scholar] [CrossRef]

| Analyte | QC Level | Recovery (%) | Matrix Effect (%) |
|---|---|---|---|
| Cortisol | LQC | 97.60 | 4.31 |
| MQC | 97.68 | 7.61 | |
| HQC | 95.57 | 9.43 | |
| Testosterone | LQC | 107.47 | −8.19 |
| MQC | 104.91 | −3.12 | |
| HQC | 105.20 | −3.00 | |
| Androstenedione | LQC | 106.93 | 0.99 |
| MQC | 107.92 | −3.37 | |
| HQC | 107.28 | −2.37 | |
| 11-Deoxycortisol | LQC | 92.53 | 8.68 |
| MQC | 93.09 | 6.03 | |
| HQC | 92.07 | 7.50 | |
| 11-Deoxycorticosterone | LQC | 89.73 | 7.17 |
| MQC | 91.12 | 6.89 | |
| HQC | 90.61 | 8.35 | |
| 17-Hydroxyprogesterone | LQC | 96.93 | −1.68 |
| MQC | 95.60 | 0.83 | |
| HQC | 95.17 | 0.79 |
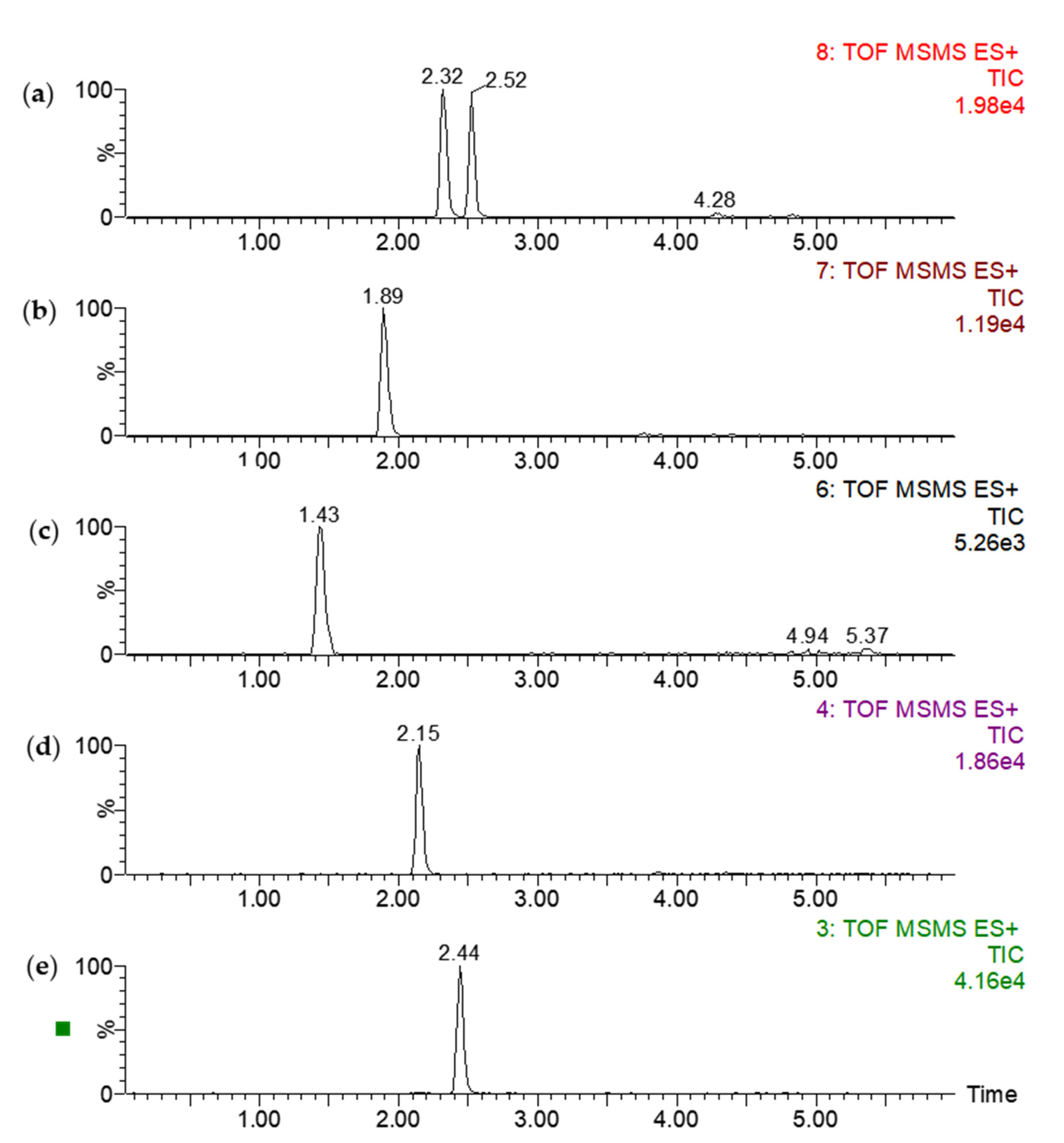
| Analyte | m/z | RT | LR | R2 | LOD | LOQ |
|---|---|---|---|---|---|---|
| Cortisol | 363.2 | 1.43 | 0.3–200 | 0.9999 | 0.1 | 0.3 |
| Testosterone | 289.2 | 2.44 | 0.3–200 | 0.9998 | 0.1 | 0.3 |
| Androstenedione | 287.2 | 2.15 | 0.3–200 | 0.9999 | 0.1 | 0.3 |
| 11-Deoxycortisol | 347.2 | 1.89 | 0.7–200 | 0.9999 | 0.2 | 0.7 |
| 11-Deoxy-Corticosterone | 331.2 | 2.32 | 0.7–200 | 0.9997 | 0.2 | 0.7 |
| 17-Hydroxy-Progesterone | 331.2 | 2.52 | 1.7–200 | 0.9998 | 0.5 | 1.7 |
| Analyte | QC Level | Calculated Concentration (Mean ± SD, ng/mL) | Accuracy (DEV, %) | Precision (RSD, %) | |||
|---|---|---|---|---|---|---|---|
| Intra-Day | Inter-Day | Intra-Day | Inter-Day | Intra-Day | Inter-Day | ||
| Cortisol | LQC | 15.1 ± 1.0 | 14.5 ± 1.3 | 100.8 | 96.7 | 6.6 | 8.6 |
| MQC | 75.4 ± 3.5 | 79.7 ± 3.9 | 100.5 | 106.3 | 4.6 | 4.9 | |
| HQC | 148.9 ± 3.7 | 154.4 ± 4.3 | 99.3 | 102.9 | 2.5 | 2.8 | |
| Testosterone | LQC | 14.2 ± 0.4 | 14.7 ± 0.6 | 94.7 | 97.8 | 2.8 | 4.1 |
| MQC | 74.9 ± 2.3 | 75.1 ± 3.4 | 99.9 | 100.1 | 3.1 | 4.5 | |
| HQC | 143.6 ± 2.8 | 145.3 ± 6.2 | 95.7 | 96.9 | 1.9 | 4.3 | |
| Androstenedione | LQC | 14.3 ± 0.6 | 13.8 ± 0.7 | 95.1 | 92.2 | 4.2 | 5.1 |
| MQC | 74.9 ± 2.3 | 75.9 ± 4.8 | 99.9 | 101.2 | 3.1 | 6.3 | |
| HQC | 146.6 ± 4.5 | 146.4 ± 5.7 | 97.7 | 97.6 | 3.1 | 3.8 | |
| 11-Deoxycortisol | LQC | 14.6 ± 0.8 | 13.9 ± 0.9 | 97.5 | 92.7 | 5.8 | 6.5 |
| MQC | 74.8 ± 2.3 | 76.0 ± 4.4 | 99.7 | 101.3 | 3.1 | 5.8 | |
| HQC | 144.5 ± 5.1 | 137.2 ± 5.5 | 96.4 | 91.5 | 3.5 | 4.0 | |
| 11-Deoxy- Corticosterone | LQC | 15.0 ± 0.6 | 15.1 ± 1.2 | 100.0 | 100.9 | 4.0 | 7.9 |
| MQC | 74.9 ± 2.3 | 78.8 ± 2.7 | 99.8 | 105.0 | 3.1 | 3.5 | |
| HQC | 145.4 ± 5.8 | 144.4 ± 6.1 | 97.0 | 96.2 | 4.0 | 4.2 | |
| 17-Hydroxyprogesterone | LQC | 14.5 ± 0.7 | 14.6 ± 0.8 | 96.6 | 97.3 | 4.8 | 5.5 |
| MQC | 74.8 ± 2.3 | 79.0 ± 3.6 | 99.8 | 105.3 | 3.1 | 4.8 | |
| HQC | 146.7 ± 6.7 | 144.0 ± 7.0 | 97.8 | 96.0 | 4.6 | 4.9 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Yan, Z.; Li, X.; Yin, X.; Li, K. UPLC–TOF–MS Method for Simultaneous Quantification of Steroid Hormones in Tissue Homogenates of Zebrafish with Solid-Phase Extraction. Molecules 2021, 26, 6213. https://doi.org/10.3390/molecules26206213
Li Y, Yan Z, Li X, Yin X, Li K. UPLC–TOF–MS Method for Simultaneous Quantification of Steroid Hormones in Tissue Homogenates of Zebrafish with Solid-Phase Extraction. Molecules. 2021; 26(20):6213. https://doi.org/10.3390/molecules26206213
Chicago/Turabian StyleLi, Yaxi, Zhi Yan, Xiaodong Li, Xiuli Yin, and Ke Li. 2021. "UPLC–TOF–MS Method for Simultaneous Quantification of Steroid Hormones in Tissue Homogenates of Zebrafish with Solid-Phase Extraction" Molecules 26, no. 20: 6213. https://doi.org/10.3390/molecules26206213
APA StyleLi, Y., Yan, Z., Li, X., Yin, X., & Li, K. (2021). UPLC–TOF–MS Method for Simultaneous Quantification of Steroid Hormones in Tissue Homogenates of Zebrafish with Solid-Phase Extraction. Molecules, 26(20), 6213. https://doi.org/10.3390/molecules26206213






