Identification of Organic Volatile Markers Associated with Aroma during Maturation of Strawberry Fruits
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Plant Material
3.2. Chemical Reagents
3.3. Preparation of Samples for Volatile Analysis
3.4. Headspace-Solid Phase Microextraction (HS-SPME)
3.5. Gas Chromatography Mass Spectrometry (GC/MS)
3.6. Identification and Quantification of Volatile Organic Compounds Related to Strawberry Aroma
3.7. Quality Assurance and Control
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- El Hadi, M.A.M.; Zhang, F.-J.; Wu, F.-F.; Zhou, C.-H.; Tao, J. Advances in fruit aroma volatile research. Molecules 2013, 18, 8200–8229. [Google Scholar] [CrossRef] [PubMed]
- Teiri, H.; Pourzamani, H.; Hajizadeh, Y. Phytoremediation of VOCs from indoor air by ornamental potted plants: A pilot study using a palm species under the controlled environment. Chemosphere 2018, 197, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Agathokleous, E.; Kitao, M.; Calabrese, E.J. Emission of volatile organic compounds from plants shows a biphasic pattern within an hormetic context. Environ. Pollut. 2018, 239, 318–321. [Google Scholar] [CrossRef]
- Vivaldo, G.; Masi, E.; Taiti, C.; Caldarelli, G.; Mancuso, S. The network of plants volatile organic compounds. Sci. Rep. 2017, 7, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, H.; Wei, Y.; Wang, X.; Xu, C.; Shao, X. The marine yeast Sporidiobolus pararoseus ZMY-1 has antagonistic properties against Botrytis cinerea in vitro and in strawberry fruit. Postharvest Biol. Technol. 2019, 150, 1–8. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, Y.; Tang, X.; Jin, W.; Han, Z. Differences in volatile ester composition between Fragaria× ananassa and F. vesca and implications for strawberry aroma patterns. Sci. Hortic. 2013, 150, 47–53. [Google Scholar] [CrossRef]
- Hakala, M.A.; Lapveteläinen, A.T.; Kallio, H.P. Volatile compounds of selected strawberry varieties analyzed by purge-and-trap headspace GC-MS. J. Agric. Food Chem. 2002, 50, 1133–1142. [Google Scholar] [CrossRef]
- Farneti, B.; Khomenko, I.; Cappellin, L.; Ting, V.; Costa, G.; Biasioli, F.; Costa, F. Dynamic volatile organic compound fingerprinting of apple fruit during processing. LWT-Food Sci. Technol. 2015, 63, 21–28. [Google Scholar] [CrossRef]
- Yan, J.w.; Ban, Z.j.; Lu, H.y.; Li, D.; Poverenov, E.; Luo, Z.s.; Li, L. The aroma volatile repertoire in strawberry fruit: A review. J. Agric. Food Chem. 2018, 98, 4395–4402. [Google Scholar] [CrossRef]
- Ulrich, D.; Kecke, S.; Olbricht, K. What do we know about the chemistry of strawberry aroma? J. Agric. Food Chem. 2018, 66, 3291–3301. [Google Scholar] [CrossRef]
- Ceccarelli, A.; Farneti, B.; Frisina, C.; Allen, D.; Donati, I.; Cellini, A.; Costa, G.; Spinelli, F.; Stefanelli, D. Harvest maturity stage and cold storage length influence on flavour development in peach fruit. Agronomy 2019, 9, 10. [Google Scholar] [CrossRef] [Green Version]
- Maoz, I.; Kaplunov, T.; Beno-Mualem, D.; Lewinsohn, E.; Lichter, A. Variability in Volatile Composition of Crimson Seedless (Vitis vinifera) in Association with Maturity at Harvest. Am. J. Enol. Vitic. 2018, 69, 125–132. [Google Scholar] [CrossRef]
- Bondada, B.; Harbertson, E.; Shrestha, P.M.; Keller, M. Temporal extension of ripening beyond its physiological limits imposes physical and osmotic challenges perturbing metabolism in grape (Vitis vinifera L.) berries. Sci. Hortic. 2017, 219, 135–143. [Google Scholar] [CrossRef]
- Ménager, I.; Jost, M.; Aubert, C. Changes in physicochemical characteristics and volatile constituents of strawberry (Cv. Cigaline) during maturation. J. Agric. Food Chem. 2004, 52, 1248–1254. [Google Scholar] [CrossRef]
- Miszczak, A.; Forney, C.F.; Prange, R.K. Development of aroma volatiles and color during postharvest ripening ofKent’strawberries. J. Am. Soc. Hortic. Sci. 1995, 120, 650–655. [Google Scholar] [CrossRef]
- Parra-Palma, C.; Úbeda, C.; Gil, M.; Ramos, P.; Castro, R.I.; Morales-Quintana, L. Comparative study of the volatile organic compounds of four strawberry cultivars and it relation to alcohol acyltransferase enzymatic activity. Sci. Hortic. 2019, 251, 65–72. [Google Scholar] [CrossRef]
- Lu, H.; Wang, K.; Wang, L.; Li, D.; Yan, J.; Ban, Z.; Luo, Z.; Li, L.; Yang, D. Effect of superatmospheric oxygen exposure on strawberry (Fragaria × ananassa Fuch.) volatiles, sensory and chemical attributes. Postharvest Biol. Technol. 2018, 142, 60–71. [Google Scholar] [CrossRef]
- Camelo, A.L.; Oliveira, F.C.; Correia, F.T.; Barbosa, F.G.; Mafezoli, J.; Diógenes, I.C.; Araújo, A.J.; Costa-Lotufo, L.V.; Longhinotti, E. Discrimination of VOCs of the Plectranthus grandis by hydrodistillation, HS-SPME and cytotoxic activity. Ind. Crop. Pro. 2019, 127, 225–231. [Google Scholar] [CrossRef]
- Mahendran, T.; Brennan, J.G.; Hariharan, G. Aroma volatiles components of ‘Fuerte’Avocado (Persea americana Mill.) stored under different modified atmospheric conditions. J. Essential Oil Res. 2019, 31, 34–42. [Google Scholar] [CrossRef]
- Kirillov, V.; Stikhareva, T.; Atazhanova, G.; Makubayeva, A. Composition of essential oil of leaves and fruits of green strawberry (Fragaria viridis Weston) growing wild in Northern Kazakhstan. J. Appl. Botany Food Qual. 2019, 92, 39. [Google Scholar] [CrossRef]
- Chaudhary, P.R.; Jayaprakasha, G.; Patil, B.S. Identification of volatile profiles of Rio Red grapefruit at various developmental to maturity stages. J. EssEntial Oil rEsEarch 2018, 30, 77–83. [Google Scholar] [CrossRef]
- Kafkas, E.; Kafkas, S. Identification of strawberry (Fragaria × ananassa‘Rubygem’) volatiles using various SPME fibres by GC/MS. In Proceedings of the VIII International Strawberry Symposium 1156, Québec City, QC, Canada, 20 April 2017; pp. 689–694. [Google Scholar]
- Du, X.; Rouseff, R. Aroma active volatiles in four southern highbush blueberry cultivars determined by gas chromatography–olfactometry (GC-O) and gas chromatography–mass spectrometry (GC-MS). J. Agric. Food Chem. 2014, 62, 4537–4543. [Google Scholar] [CrossRef] [PubMed]
- Oz, A.; Baktemur, G.; Kargi, S.P.; Kafkas, E. Volatile compounds of strawberry varieties. Chem. Nat. Compd. 2016, 52, 507–509. [Google Scholar] [CrossRef]
- Vendel, I.; Hertog, M.; Nicolaï, B. Fast analysis of strawberry aroma using SIFT-MS: A new technique in postharvest research. Postharvest Biol. Technol. 2019, 152, 127–138. [Google Scholar] [CrossRef]
- Nan, L.; Guo, M.; Chen, S.; Li, Y.; Cui, C.; Song, Y.; Huang, J.; Chen, G. Effect of peduncle on aroma of cabernet sauvignon dry red wine. In Proceedings of the 2018 International Conference on Biotechnology and Bioengineering (8TH ICBB), Budapest, Hungary, 24–26 October 2018; p. 020008. [Google Scholar]
- Cheng, H.; Chen, J.; Watkins, P.J.; Chen, S.; Wu, D.; Liu, D.; Ye, X. Discrimination of aroma characteristics for cubeb berries by sensomics approach with chemometrics. Molecules 2018, 23, 1627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, W.; Sun, P.; Chen, L.; Gao, S.; Shao, W.; Li, J. Comparative analysis of fruit volatiles and related gene expression between the wild strawberry Fragaria pentaphylla and cultivated Fragaria × ananassa. Eur. Food Res. Technol. 2018, 244, 57–72. [Google Scholar] [CrossRef]
- Severo, J.; De Oliveira, I.R.; Bott, R.; Le Bourvellec, C.; Renard, C.M.; Page, D.; Chaves, F.C.; Rombaldi, C.V. Preharvest UV-C radiation impacts strawberry metabolite content and volatile organic compound production. LWT-Food Sci. Technol. 2017, 85, 390–393. [Google Scholar] [CrossRef]
- Naidoo, S.; Christie, N.; Acosta, J.J.; Mphahlele, M.M.; Payn, K.G.; Myburg, A.A.; Külheim, C. Terpenes associated with resistance against the gall wasp, Leptocybe invasa, in Eucalyptus grandis. Plant Cell Environ. 2018, 41, 1840–1851. [Google Scholar] [CrossRef] [Green Version]
- Ceuppens, B.; Ameye, M.; Van Langenhove, H.; Roldan-Ruiz, I.; Smagghe, G. Characterization of volatiles in strawberry varieties ‘Elsanta’ and ‘Sonata’ and their effect on bumblebee flower visiting. Arthropod-Plant Interact. 2015, 9, 281–287. [Google Scholar] [CrossRef]
- Forney, C.F.; Kalt, W.; Jordan, M.A. The composition of strawberry aroma is influenced by cultivar, maturity, and storage. HortScience 2000, 35, 1022–1025. [Google Scholar] [CrossRef] [Green Version]
- Sangha, B.; Agehara, S. Optimization of growth-stage specific nitrogen fertilization improves strawberry growth and yield. In Proceedings of the 129th Annual Meeting of the Florida State Horticultural Society, Stuart, FL, USA, 12–14 June 2016; pp. 137–139. [Google Scholar]
- Parvez, S.; Wani, I.A. Postharvest biology and technology of strawberry. In Postharvest Biology and Technology of Temperate Fruits; Springer: Cham, Switzerland, 2018; pp. 331–348. [Google Scholar]
- Rahman, M.M.; Moniruzzaman, M.; Ahmad, M.R.; Sarker, B.; Alam, M.K. Maturity stages affect the postharvest quality and shelf-life of fruits of strawberry genotypes growing in subtropical regions. J. Saudi Soc. Agric. Sci. 2016, 15, 28–37. [Google Scholar] [CrossRef] [Green Version]
- Hori, M.; Ohuchi, K.; Matsuda, K. Role of host plant volatile in the host-finding behavior of the strawberry leaf beetle, Galerucella vittaticollis Baly (Coleoptera: Chrysomelidae). Appl. Entomol. Zool. 2006, 41, 357–363. [Google Scholar] [CrossRef] [Green Version]
- Kafkas, E.; Türemiş, N.; Bilgili, B.; Zarifikhosroshahi, M.; Burgut, A.; Kafkas, S. Aroma profiles of organically grown ‘Benicia’ and ‘Albion’strawberries. In Proceedings of the VIII International Strawberry Symposium 1156, Québec City, QC, Canada, 20 April 2017; pp. 703–708. [Google Scholar]
- Lee, J.H.; Jayaprakasha, G.; Avila, C.A.; Crosby, K.M.; Patil, B.S. Metabolomic studies of volatiles from tomatoes grown in net-house and open-field conditions. Food Chem. 2019, 275, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Padilla-Jimenez, S.M.; Angoa-Pérez, M.V.; Mena-Violante, H.G.; Oyoque-Salcedo, G.; Renteria-Ortega, M.; Oregel-Zamudio, E. Changes in the Aroma of Organic Blackberries (Rubus Fruticosus) During Ripeness. Anal. Chem. Lett. 2019, 9, 64–73. [Google Scholar] [CrossRef]
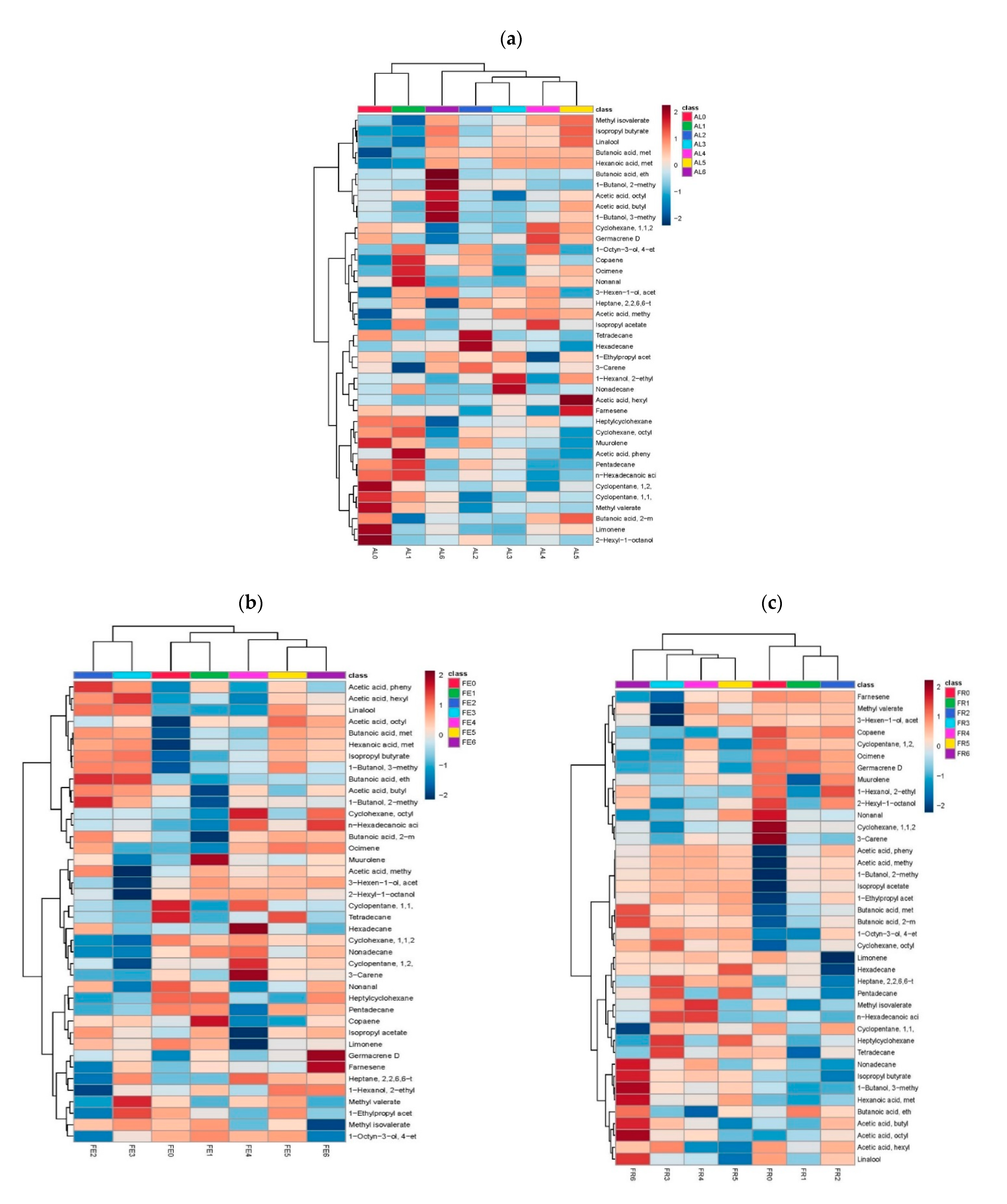
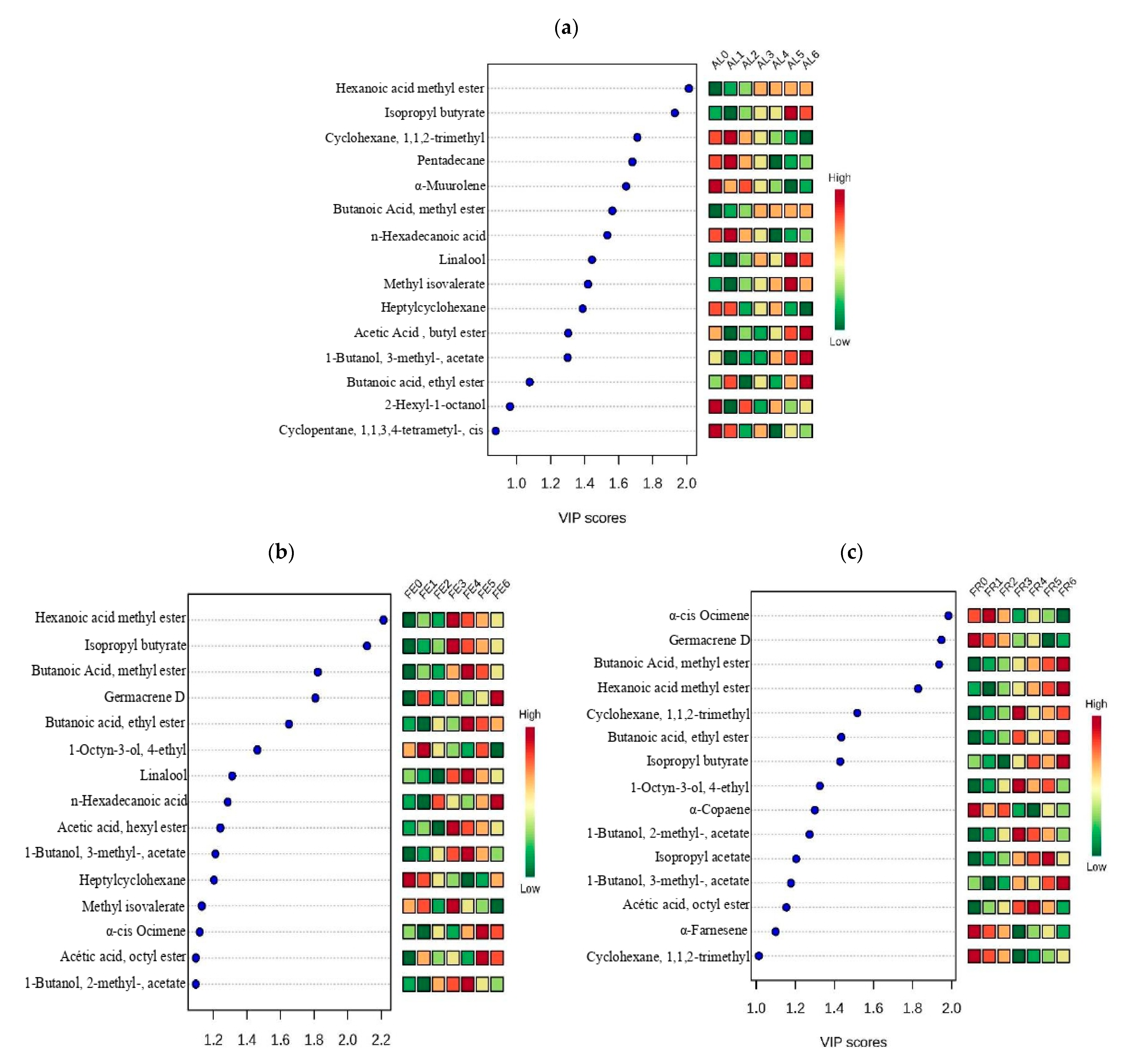
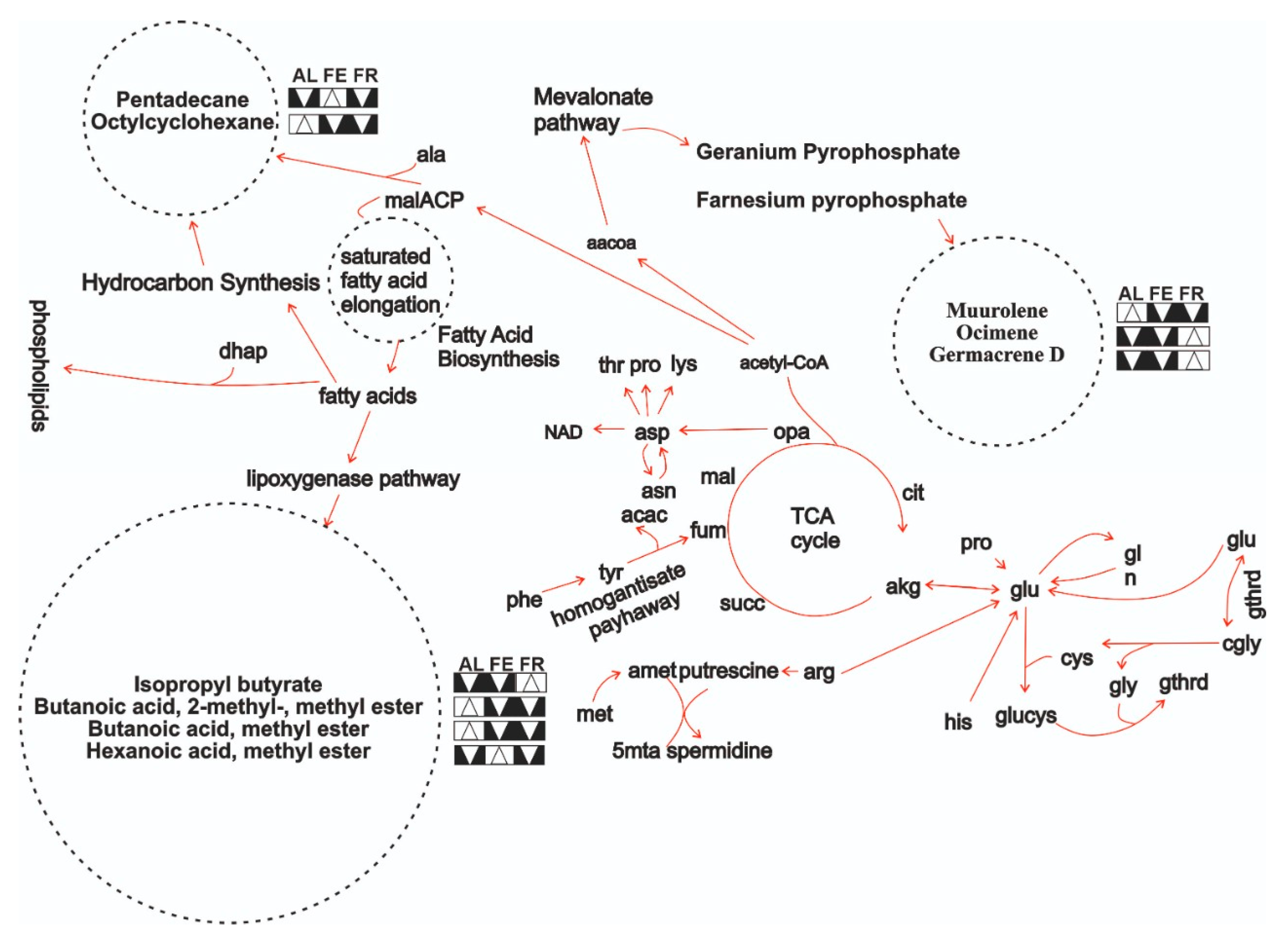
| # | Compound | KI | AL0 | AL1 | AL2 | AL3 | AL4 | AL5 | AL6 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Methyl acetate + | 498 | 0.22 ± 0.01 c | 3.60 ± 0.10 b | 5.68 ± 0.72 a | 5.31 ± 0.73 a | 5.38 ± 0.30 a | 3.94 ± 0.07 b | 5.72 ± 0.18 a |
| 2 | Isopropyl acetate * | 575 | 0.24 ± 0.03 f | 0.87 ± 0.06 e | 1.45 ± 0.21 dc | 1.77 ± 0.09 a | 1.55 ± 0.02 bc | 1.26 ± 0.12 d | 1.68 ± 0.05 ba |
| 3 | Butanoic Acid, methyl ester * | 724 | 0.08 ± 0.01 f | 2.26 ± 0.12 f | 8.85 ± 0.28 e | 26.79 ± 2.01 c | 18.81 ± 0.94 d | 33.58 ± 3.43 b | 42.42 ± 2.29 a |
| 4 | Butanoic Acid, 2-methyl-, methyl ester * | 783 | 0.22 ± 0.01 d | 0.01 ± 0.00 g | 0.09 ± 0.01 f | 0.11 ± 0.01 e | 0.25 ± 0.00 c | 0.39 ± 0.01 a | 0.33 ± 0.00 b |
| 5 | Methyl isovalerate + | 783 | 0.15 ± 0.01 d | 0.01 ± 0.00 d | 0.33 ± 0.02 d | 0.97 ± 0.09 c | 1.19 ± 0.08 cb | 1.49 ± 0.22 b | 3.11 ± 0.40 a |
| 6 | Butanoic acid, ethyl ester * | 810 | 0.13 ± 0.01 b | 0.13 ± 0.02 b | 0.10 ± 0.01 b | 0.26 ± 0.02 b | 0.17 ± 0.00 b | 0.31 ± 0.00 b | 22.10 ± 2.72 a |
| 7 | Cyclopentane, 1,1,3,4-tetrametyl-, cis * | 817 | 0.64 ± 0.01 b | 0.25 ± 0.03 d | 0.02 ± 0.00 f | 0.15 ± 0.02 e | 0.57 ± 0.03 c | 0.27 ± 0.03 d | 0.88 ± 0.04 a |
| 8 | Acetic Acid, butyl ester * | 825 | 0.22 ± 0.01 c | 0.03 ± 0.00 c | 0.25 ± 0.01 c | 0.16 ± 0.01 c | 0.30 ± 0.02 c | 1.34 ± 0.06 b | 13.12 ± 0.62 a |
| 9 | Methyl valerate * | 835 | 0.23 ± 0.01 b | 0.12 ± 0.02 e | 0.05 ± 0.01 f | 0.17 ± 0.02 c | 0.15 ± 0.02 dc | 0.14 ± 0.02 de | 0.44 ± 0.04 a |
| 10 | Isopropyl butyrate + | 854 | 0.09 ± 0.00 c | 0.04 ± 0.00 d | 0.37 ± 0.02 dc | 2.59 ± 0.12 c | 1.89 ± 0.02 dc | 7.78 ± 0.24 b | 20.57 ± 2.39 a |
| 11 | 1-Ethylpropyl acetate * | 858 | 0.19 ± 0.01 d | 0.07 ± 0.00 e | 0.25 ± 0.03 dc | 0.44 ± 0.04 b | 0.01 ± 0.00 e | 0.32 ± 0.02 c | 0.76 ± 0.10 a |
| 12 | Cyclopentane, 1,2,3,4,5-pentamethyl * | 876 | 0.55 ± 0.03 a | 0.13 ± 0.02 d | 0.08 ± 0.01 e | 0.29 ± 0.02 c | 0.01 ± 0.00 f | 0.26 ± 0.02 c | 0.42 ± 0.04 b |
| 13 | 1-Butanol, 3-methyl-, acetate * | 886 | 0.23 ± 0.02 de | 0.14 ± 0.02 e | 0.32 ± 0.04 dc | 0.42 ± 0.00 c | 0.88 ± 0.10 b | 0.77 ± 0.07 b | 4.59 ± 0.13 a |
| 14 | 1-Butanol, 2-methyl-, acetate * | 888 | 0.10 ± 0.01 b | 0.02 ± 0.00 b | 0.21 ± 0.03 b | 0.33 ± 0.05 b | 0.01 ± 0.00 b | 0.02 ± 0.00 b | 6.62 ± 0.85 a |
| 15 | Cyclohexane, 1,1,2-trimethyl * | 889 | 4.37 ± 0.53 a | 1.71 ± 0.12 c | 1.72 ± 0.20 c | 1.29 ± 0.03 c | 4.01 ± 0.38 a | 2.50 ± 0.24 b | 0.26 ± 0.02 d |
| 16 | Hexanoic acid methyl ester + | 933 | 0.04 ± 0.00 d | 0.10 ± 0.00 d | 2.06 ± 0.19 d | 10.06 ± 0.55 c | 7.31 ± 0.29 c | 17.23 ± 1.69 b | 32.79 ± 3.74 a |
| 17 | Acetic acid, hexyl ester * | 987 | 0.21 ± 0.01 dc | 0.10 ± 0.01 d | 0.28 ± 0.03 c | 0.60 ± 0.03 b | 0.51 ± 0.05 b | 1.40 ± 0.16 a | 0.49 ± 0.07 b |
| 18 | Heptane, 2,2,6,6-tetramethyl * | 1003 | 0.08 ± 0.00 c | 0.11 ± 0.01 c | 0.25 ± 0.01 a | 0.19 ± 0.02 b | 0.22 ± 0.03 a | 0.18 ± 0.01 b | 0.02 ± 0.00 d |
| 19 | 3-Hexen-1-ol, acetate, (E)- * | 1013 | 0.01 ± 0.00 e | 0.10 ± 0.01 c | 0.07 ± 0.01 c | 0.20 ± 0.02 b | 0.20 ± 0.01 b | 0.03 ± 0.00 de | 0.61 ± 0.05 a |
| 20 | 3-Carene + | 1034 | 0.15 ± 0.01 d | 0.01 ± 0.00 e | 0.36 ± 0.02 b | 0.30 ± 0.02 c | 0.17 ± 0.03 d | 0.27 ± 0.02 c | 0.73 ± 0.06 a |
| 21 | Limonene + | 1038 | 0.70 ± 0.05 a | 0.08 ± 0.00 b | 0.07 ± 0.01 b | 0.11 ± 0.00 b | 0.74 ± 0.06 a | 0.71 ± 0.09 a | 0.77 ± 0.10 a |
| 22 | 1-Hexanol, 2-ethyl + | 1040 | 0.11 ± 0.01 e | 0.09 ± 0.00 e | 0.17 ± 0.00 c | 0.76 ± 0.02 a | 0.07 ± 0.01 f | 0.35 ± 0.00 b | 0.21 ± 0.00 c |
| 23 | α-cis Ocimene * | 1052 | 0.02 ± 0.00 d | 0.17 ± 0.01 b | 0.14 ± 0.00 c | 0.02 ± 0.00 d | 0.14 ± 0.02 c | 0.22 ± 0.02 a | 0.03 ± 0.00 d |
| 24 | 1-Octyn-3-ol, 4-ethyl * | 1095 | 0.06 ± 0.01 e | 0.12 ± 0.00 d | 0.23 ± 0.02 b | 0.10 ± 0.02 d | 0.26 ± 0.01 a | 0.07 ± 0.00 e | 0.20 ± 0.01 c |
| 25 | Nonanal + | 1105 | 0.09 ± 0.01 d | 0.23 ± 0.01 b | 0.04 ± 0.01 e | 0.06 ± 0.01 e | 0.20 ± 0.01 c | 0.25 ± 0.00 a | 0.02 ± 0.00 f |
| 26 | Linalool + | 1110 | 0.18 ± 0.00 d | 0.02 ± 0.00 d | 0.49 ± 0.05 d | 2.25 ± 0.15 b | 1.21 ± 0.07 c | 2.38 ± 0.10 b | 6.31 ± 0.48 a |
| 27 | 2-Hexyl-1-octanol * | 1110 | 0.47 ± 0.04 a | 0.03 ± 0.00 f | 0.29 ± 0.03 c | 0.11 ± 0.02 e | 0.18 ± 0.01 d | 0.11 ± 0.01 e | 0.39 ± 0.01 b |
| 28 | Acetic acid, phenylmethyl ester * | 1169 | 0.12 ± 0.01 de | 0.18 ± 0.02 c | 0.15 ± 0.00 dc | 0.25 ± 0.04 b | 0.15 ± 0.01 dc | 0.08 ± 0.01 e | 0.57 ± 0.04 c |
| 29 | Acétic acid, octyl ester * | 1213 | 0.16 ± 0.02 cb | 0.14 ± 0.02 c | 0.17 ± 0.02 cb | 0.07 ± 0.01 c | 0.24 ± 0.00 cb | 0.38 ± 0.04 b | 2.25 ± 0.30 a |
| 30 | α-Copaene * | 1357 | 0.04 ± 0.00 d | 0.17 ± 0.01 c | 0.26 ± 0.03 b | 0.08 ± 0.01 d | 0.19 ± 0.02 c | 0.08 ± 0.00 d | 0.39 ± 0.06 a |
| 31 | Heptylcyclohexane * | 1359 | 7.30 ± 0.93 a | 5.62 ± 0.83 b | 4.61 ± 0.30 b | 2.69 ± 0.01 c | 2.18 ± 0.05 c | 2.24 ± 0.20 c | 0.62 ± 0.09 d |
| 32 | Nonadecane + | 1407 | 0.15 ± 0.01 c | 1.61 ± 0.04 b | 0.05 ± 0.00 c | 3.84 ± 0.28 a | 0.02 ± 0.00 c | 0.29 ± 0.02 c | 0.16 ± 0.01 c |
| 33 | Tetradecane + | 1415 | 5.50 ± 0.72 b | 0.10 ± 0.01 c | 8.70 ± 1.02 a | 0.31 ± 0.01 c | 0.95 ± 0.05 c | 0.12 ± 0.02 c | 1.03 ± 0.07 c |
| 34 | Hexadecane * | 1416 | 0.05 ± 0.00 c | 0.12 ± 0.02 c | 1.86 ± 0.28 a | 0.27 ± 0.01 c | 0.15 ± 0.01 c | 0.01 ± 0.00 c | 0.58 ± 0.07 b |
| 35 | Octylcyclohexane * | 1474 | 8.39 ± 0.45 ba | 9.15 ± 1.32 a | 7.31 ± 0.61 b | 2.93 ± 0.09 c | 1.89 ± 0.14 c | 0.18 ± 0.00 c | 0.18 ± 0.03 c |
| 36 | Germacrene D * | 1501 | 0.21 ± 0.03 dc | 0.06 ± 0.01 e | 0.14 ± 0.01 de | 0.25 ± 0.01 c | 0.88 ± 0.08 a | 0.34 ± 0.03 b | 0.12 ± 0.00 e |
| 37 | α-Farnesene + | 1506 | 0.11 ± 0.01 c | 0.05 ± 0.01 d | 0.03 ± 0.00 d | 0.14 ± 0.00 c | 0.01 ± 0.00 d | 0.39 ± 0.04 a | 0.24 ± 0.03 b |
| 38 | α-Muurolene + | 1507 | 12.96 ± 0.30 a | 4.83 ± 0.27 c | 7.99 ± 1.02 b | 2.01 ± 0.14 d | 1.00 ± 0.07 ed | 0.07 ± 0.01 e | 0.64 ± 0.01 e |
| 39 | Pentadecane * | 1605 | 6.23 ± 0.77 a | 6.64 ± 0.53 a | 5.84 ± 0.02 a | 2.22 ± 0.06 b | 0.19 ± 0.02 c | 0.35 ± 0.00 c | 0.87 ± 0.12 c |
| 40 | n-Hexadecanoic acid * | 1825 | 0.32 ± 0.02 d | 0.43 ± 0.01 b | 0.35 ± 0.04 dc | 0.38 ± 0.04 c | 0.12 ± 0.01 f | 0.24 ± 0.03 e | 0.50 ± 0.03 a |
| # | Compound | KI | FE0 | FE1 | FE2 | FE3 | FE4 | FE5 | FE6 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Methyl acetate + | 498 | 0.69 ± 0.08 d | 1.24 ± 0.08 dc | 1.73 ± 0.17 c | 4.77 ± 0.05 b | 5.86 ± 0.52 a | 4.64 ± 0.61 b | 1.81 ± 0.22 c |
| 2 | Isopropyl acetate * | 575 | 0.05 ± 0.00 e | 0.01 ± 0.00 e | 0.27 ± 0.03 d | 0.89 ± 0.07 a | 0.94 ± 0.06 a | 0.52 ± 0.05 c | 0.70 ± 0.03 b |
| 3 | Butanoic Acid, methyl ester * | 724 | 0.08 ± 0.00 d | 0.67 ± 0.05 d | 1.25 ± 0.06 d | 16.25 ± 0.91 c | 21.24 ± 1.19 b | 18.65 ± 0.08 cb | 32.61 ± 3.85 a |
| 4 | Butanoic Acid, 2-methyl-, methyl ester * | 783 | 0.02 ± 0.00 e | 0.10 ± 0.00 d | 0.00 ± 0.00 e | 0.51 ± 0.00 b | 0.82 ± 0.04 a | 0.54 ± 0.04 b | 0.40 ± 0.03 c |
| 5 | Methyl isovalerate + | 783 | 0.06 ± 0.00 ed | 0.01 ± 0.00 e | 0.13 ± 0.02 cd | 0.01 ± 0.00 e | 0.24 ± 0.02 b | 0.18 ± 0.00 cb | 0.74 ± 0.09 a |
| 6 | Butanoic acid, ethyl ester * | 810 | 0.07 ± 0.01 c | 0.11 ± 0.01 c | 0.04 ± 0.00 c | 0.46 ± 0.06 c | 9.85 ± 0.36 b | 0.38 ± 0.02 c | 14.64 ± 2.01 a |
| 7 | Cyclopentane, 1,1,3,4-tetrametyl-, cis * | 817 | 0.38 ± 0.03 a | 0.42 ± 0.05 a | 0.07 ± 0.01 c | 0.42 ± 0.02 a | 0.33 ± 0.05 b | 0.38 ± 0.00 a | 0.42 ± 0.02 a |
| 8 | Acetic Acid, butyl ester * | 825 | 0.07 ± 0.01 d | 0.09 ± 0.01 d | 0.00 ± 0.00 d | 0.31 ± 0.04 c | 0.75 ± 0.11 b | 0.05 ± 0.00 d | 1.06 ± 0.09 a |
| 9 | Methyl valerate * | 835 | 0.06 ± 0.01 dc | 0.09 ± 0.01 dc | 0.03 ± 0.01 d | 0.11 ± 0.01 c | 0.08 ± 0.01 dc | 0.35 ± 0.03 b | 1.27 ± 0.07 a |
| 10 | Isopropyl butyrate + | 854 | 0.00 ± 0.00 d | 0.13 ± 0.01 d | 0.02 ± 0.00 d | 1.67 ± 0.07 c | 4.65 ± 0.49 b | 2.33 ± 0.20 c | 11.95 ± 0.63 a |
| 11 | 1-Ethylpropyl acetate * | 858 | 0.06 ± 0.01 d | 0.03 ± 0.00 d | 0.05 ± 0.01 d | 0.11 ± 0.01 c | 0.05 ± 0.00 d | 0.28 ± 0.03 b | 0.73 ± 0.03 a |
| 12 | Cyclopentane, 1,2,3,4,5-pentamethyl * | 876 | 0.07 ± 0.00 e | 0.32 ± 0.01 b | 0.10 ± 0.01 ed | 0.49 ± 0.05 a | 0.26 ± 0.02 c | 0.36 ± 0.00 b | 0.14 ± 0.01 d |
| 13 | 1-Butanol, 3-methyl-, acetate * | 886 | 0.01 ± 0.00 d | 0.04 ± 0.00 d | 0.03 ± 0.00 d | 0.13 ± 0.01 c | 0.42 ± 0.06 b | 0.39 ± 0.01 b | 0.71 ± 0.02 a |
| 14 | 1-Butanol, 2-methyl-, acetate * | 888 | 0.05 ± 0.00 c | 0.13 ± 0.00 a | 0.00 ± 0.00 cb | 0.35 ± 0.03 cb | 7.74 ± 0.46 cb | 0.39 ± 0.02 cb | 2.30 ± 0.01 cb |
| 15 | Cyclohexane, 1,1,2-trimethyl * | 889 | 2.58 ± 0.10 c | 3.09 ± 0.42 c | 1.75 ± 0.13 d | 5.07 ± 0.73 a | 0.38 ± 0.04 e | 3.85 ± 0.07 b | 0.34 ± 0.03 e |
| 16 | Hexanoic acid methyl ester + | 933 | 0.00 ± 0.00 d | 0.42 ± 0.05 d | 0.83 ± 0.04 d | 12.40 ± 0.55 c | 24.01 ± 2.62 b | 21.24 ± 2.63 b | 66.02 ± 6.34 a |
| 17 | Acetic acid, hexyl ester * | 987 | 0.02 ± 0.00 c | 0.02 ± 0.00 c | 0.09 ± 0.01 c | 0.59 ± 0.05 c | 1.81 ± 0.04 b | 0.78 ± 0.08 cb | 8.96 ± 1.13 a |
| 18 | Heptane, 2,2,6,6-tetramethyl * | 1003 | 0.01 ± 0.00 d | 0.13 ± 0.02 c | 0.02 ± 0.00 d | 0.22 ± 0.03 b | 0.03 ± 0.00 d | 0.22 ± 0.02 b | 0.52 ± 0.05 a |
| 19 | 3-Hexen-1-ol, acetate, (E)- * | 1013 | 0.04 ± 0.00 d | 0.09 ± 0.00 c | 0.14 ± 0.00 c | 0.45 ± 0.02 a | 0.11 ± 0.01 c | 0.31 ± 0.05 b | 0.05 ± 0.00 d |
| 20 | 3-Carene + | 1034 | 0.09 ± 0.01 dc | 1.32 ± 0.02 a | 0.04 ± 0.00 e | 0.35 ± 0.02 b | 0.07 ± 0.00 d | 0.33 ± 0.01 b | 0.11 ± 0.00 c |
| 21 | Limonene + | 1038 | 0.42 ± 0.01 c | 0.00 ± 0.00 e | 0.33 ± 0.04 d | 0.44 ± 0.02 c | 0.88 ± 0.04 a | 0.31 ± 0.03 d | 0.70 ± 0.09 b |
| 22 | 1-Hexanol, 2-ethyl + | 1040 | 0.02 ± 0.00 c | 0.03 ± 0.00 c | 0.07 ± 0.01 c | 0.44 ± 0.06 a | 0.01 ± 0.00 c | 0.39 ± 0.02 a | 0.23 ± 0.01 b |
| 23 | α-cis Ocimene * | 1052 | 0.03 ± 0.00 f | 0.10 ± 0.00 e | 0.03 ± 0.00 f | 0.43 ± 0.02 a | 0.32 ± 0.03 c | 0.38 ± 0.04 b | 0.20 ± 0.01 d |
| 24 | 1-Octyn-3-ol, 4-ethyl * | 1095 | 0.05 ± 0.00 d | 0.07 ± 0.01 d | 0.13 ± 0.02 c | 0.01 ± 0.00 e | 0.01 ± 0.00 e | 0.29 ± 0.03 a | 0.17 ± 0.01 b |
| 25 | Nonanal + | 1105 | 0.09 ± 0.00 ed | 0.05 ± 0.00 e | 0.10 ± 0.01 d | 0.39 ± 0.04 a | 0.32 ± 0.03 b | 0.23 ± 0.00 c | 0.25 ± 0.00 c |
| 26 | Linalool + | 1110 | 0.09 ± 0.00 e | 0.11 ± 0.01 e | 0.12 ± 0.01 e | 1.59 ± 0.23 d | 3.48 ± 0.20 b | 2.65 ± 0.18 c | 5.79 ± 0.06 a |
| 27 | 2-Hexyl-1-octanol * | 1110 | 0.05 ± 0.00 d | 0.14 ± 0.02 c | 0.14 ± 0.01 c | 0.22 ± 0.03 b | 0.14 ± 0.02 c | 0.36 ± 0.04 a | 0.02 ± 0.00 d |
| 28 | Acetic acid, phenylmethyl ester * | 1169 | 0.04 ± 0.01 d | 0.06 ± 0.01 d | 0.13 ± 0.00 d | 0.27 ± 0.01 c | 0.65 ± 0.09 b | 0.35 ± 0.03 c | 0.83 ± 0.10 a |
| 29 | Acétic acid, octyl ester * | 1213 | 0.01 ± 0.00 e | 0.05 ± 0.00 d | 0.05 ± 0.00 d | 0.25 ± 0.00 b | 0.11 ± 0.01 c | 0.27 ± 0.02 a | 0.26 ± 0.02 ba |
| 30 | α-Copaene * | 1357 | 0.02 ± 0.00 d | 0.00 ± 0.00 d | 3.06 ± 0.12 a | 0.24 ± 0.02 c | 0.24 ± 0.03 c | 0.01 ± 0.00 d | 0.57 ± 0.06 b |
| 31 | Heptylcyclohexane * | 1359 | 3.07 ± 0.46 c | 0.08 ± 0.00 d | 4.52 ± 0.22 b | 8.59 ± 0.47 a | 0.05 ± 0.01 d | 0.06 ± 0.01 d | 0.24 ± 0.03 d |
| 32 | Nonadecane + | 1407 | 1.19 ± 0.02 e | 11.27 ± 0.92 b | 8.08 ± 0.10 c | 12.43 ± 0.77 a | 0.25 ± 0.01 e | 2.57 ± 0.26 d | 0.35 ± 0.02 e |
| 33 | Tetradecane + | 1415 | 6.85 ± 0.32 b | 0.14 ± 0.01 c | 0.02 ± 0.00 c | 0.23 ± 0.02 c | 0.24 ± 0.01 c | 14.36 ± 0.52 a | 0.16 ± 0.02 c |
| 34 | Hexadecane * | 1416 | 0.07 ± 0.00 e | 1.76 ± 0.06 a | 0.08 ± 0.01 e | 0.19 ± 0.01 d | 0.98 ± 0.06 b | 0.39 ± 0.03 c | 0.34 ± 0.02 c |
| 35 | Octylcyclohexane * | 1474 | 0.06 ± 0.01 c | 7.10 ± 0.72 b | 0.01 ± 0.00 c | 8.68 ± 0.20 a | 0.35 ± 0.02 c | 0.20 ± 0.01 c | 0.75 ± 0.04 c |
| 36 | Germacrene D * | 1501 | 0.00 ± 0.00 e | 0.02 ± 0.00 de | 0.10 ± 0.01 dc | 9.51 ± 0.09 a | 0.12 ± 0.00 c | 0.18 ± 0.02 c | 0.47 ± 0.00 b |
| 37 | α-Farnesene + | 1506 | 0.01 ± 0.00 c | 0.06 ± 0.01 c | 0.09 ± 0.01 c | 7.10 ± 0.25 a | 0.01 ± 0.00 c | 0.13 ± 0.01 c | 0.69 ± 0.04 b |
| 38 | α-Muurolene + | 1507 | 0.02 ± 0.00 c | 0.07 ± 0.01 c | 2.60 ± 0.18 a | 0.43 ± 0.06 b | 0.34 ± 0.00 b | 0.16 ± 0.01 c | 0.03 ± 0.00 c |
| 39 | Pentadecane * | 1605 | 3.84 ± 0.22 c | 0.01 ± 0.00 e | 4.08 ± 0.12 c | 6.82 ± 0.15 b | 0.12 ± 0.01 e | 8.64 ± 0.12 a | 0.56 ± 0.01 d |
| 40 | n-Hexadecanoic acid * | 1825 | 0.02 ± 0.00 d | 0.47 ± 0.02 b | 0.02 ± 0.00 d | 2.31 ± 0.19 a | 0.21 ± 0.03 c | 0.35 ± 0.03 cb | 0.50 ± 0.05 b |
| # | Compound | KI | FR0 | FR1 | FR2 | FR3 | FR4 | FR5 | FR6 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Methyl acetate + | 498 | 2.13 ± 0.22 c | 81.61 ± 4.00 a | 4.00 ± 0.02 c | 3.81 ± 0.31 c | 4.82 ± 0.03 cb | 5.97 ± 0.76 cb | 8.15 ± 0.25 b |
| 2 | Isopropyl acetate * | 575 | 2.50 ± 0.20 d | 42.07 ± 0.67 a | 1.68 ± 0.02 ef | 1.60 ± 0.18 f | 2.24 ± 0.23 ed | 3.99 ± 0.05 c | 5.90 ± 0.26 b |
| 3 | Butanoic Acid, methyl ester * | 724 | 0.46 ± 0.04 d | 7.37 ± 0.54 b | 0.47 ± 0.02 d | 0.45 ± 0.03 d | 0.64 ± 0.08 d | 2.10 ± 0.23 c | 16.23 ± 1.12 a |
| 4 | Butanoic Acid, 2-methyl-, methyl ester * | 783 | 0.97 ± 0.06 c | 4.82 ± 0.29 a | 0.36 ± 0.04 d | 0.31 ± 0.04 d | 0.28 ± 0.04 d | 0.55 ± 0.03 dc | 3.95 ± 0.52 b |
| 5 | Methyl isovalerate + | 783 | 1.01 ± 0.08 a | 0.27 ± 0.02 b | 0.02 ± 0.00 c | 0.04 ± 0.01 c | 0.09 ± 0.01 c | 0.02 ± 0.00 c | 0.08 ± 0.00 c |
| 6 | Butanoic acid, ethyl ester * | 810 | 0.85 ± 0.11 b | 3.14 ± 0.09 a | 0.06 ± 0.00 d | 0.01 ± 0.00 d | 0.01 ± 0.00 d | 0.07 ± 0.00 d | 0.36 ± 0.01 c |
| 7 | Cyclopentane, 1,1,3,4-tetrametyl-, cis * | 817 | 13.29 ± 0.45 a | 7.68 ± 0.89 b | 0.62 ± 0.01 c | 0.33 ± 0.02 c | 0.36 ± 0.04 c | 0.48 ± 0.05 c | 0.35 ± 0.04 c |
| 8 | Acetic Acid, butyl ester * | 825 | 1.25 ± 0.18 a | 0.97 ± 0.02 b | 0.14 ± 0.01 c | 0.08 ± 0.01 c | 0.06 ± 0.01 c | 0.03 ± 0.00 c | 1.00 ± 0.10 b |
| 9 | Methyl valerate * | 835 | 2.50 ± 0.34 d | 3.06 ± 0.44 d | 0.12 ± 0.01 d | 0.01 ± 0.00 c | 0.10 ± 0.01 b | 0.10 ± 0.01 d | 0.27 ± 0.01 a |
| 10 | Isopropyl butyrate + | 854 | 0.85 ± 0.09 b | 0.85 ± 0.13 b | 0.01 ± 0.00 c | 0.06 ± 0.01 c | 0.10 ± 0.01 c | 0.14 ± 0.01 c | 1.82 ± 0.02 a |
| 11 | 1-Ethylpropyl acetate * | 858 | 0.82 ± 0.03 b | 4.57 ± 0.39 a | 0.31 ± 0.02 cb | 0.24 ± 0.03 d | 0.24 ± 0.02 d | 0.50 ± 0.02 cbd | 0.67 ± 0.04 cb |
| 12 | Cyclopentane, 1,2,3,4,5-pentamethyl * | 876 | 4.61 ± 0.02 a | 2.52 ± 0.24 b | 0.10 ± 0.00 c | 0.01 ± 0.00 c | 0.09 ± 0.00 c | 0.02 ± 0.00 c | 0.13 ± 0.02 c |
| 13 | 1-Butanol, 3-methyl-, acetate * | 886 | 1.74 ± 0.15 a | 1.09 ± 0.16 b | 0.05 ± 0.01 c | 0.07 ± 0.01 c | 0.07 ± 0.00 c | 0.18 ± 0.02 c | 1.61 ± 0.22 a |
| 14 | 1-Butanol, 2-methyl-, acetate * | 888 | 1.28 ± 0.01 c | 5.12 ± 6.56 a | 5.71 ± 0.17 cb | 5.20 ± 0.61 cb | 5.48 ± 0.23 b | 7.89 ± 0.25 cb | 10.68 ± 0.24 b |
| 15 | Cyclohexane, 1,1,2-trimethyl * | 889 | 52.60 ± 6.26 a | 3.37 ± 0.15 b | 0.13 ± 0.02 b | 0.01 ± 0.00 b | 0.06 ± 0.01 b | 0.13 ± 0.01 b | 0.31 ± 0.04 b |
| 16 | Hexanoic acid methyl ester + | 933 | 2.83 ± 0.08 b | 2.25 ± 0.21 b | 0.19 ± 0.01 c | 0.26 ± 0.01 c | 0.35 ± 0.05 c | 1.81 ± 0.19 cb | 21.03 ± 1.58 a |
| 17 | Acetic acid, hexyl ester * | 987 | 2.34 ± 0.11 a | 1.98 ± 0.26 b | 0.10 ± 0.01 c | 0.07 ± 0.01 c | 0.03 ± 0.00 c | 0.05 ± 0.01 c | 0.27 ± 0.01 c |
| 18 | Heptane, 2,2,6,6-tetramethyl * | 1003 | 1.74 ± 0.15 b | 5.01 ± 0.22 a | 0.05 ± 0.01 d | 0.37 ± 0.04 c | 0.22 ± 0.03 dc | 0.42 ± 0.03 c | 0.37 ± 0.04 c |
| 19 | 3-Hexen-1-ol, acetate, (E)- * | 1013 | 8.64 ± 5.25 a | 5.65 ± 4.57 a | 2.39 ± 0.34 b | 0.02 ± 0.00 b | 1.51 ± 0.12 b | 4.42 ± 0.62 b | 2.87 ± 0.36 b |
| 20 | 3-Carene + | 1034 | 12.52 ± 0.90 a | 1.40 ± 0.08 b | 0.04 ± 0.00 c | 0.02 ± 0.00 c | 0.07 ± 0.01 c | 0.09 ± 0.01 c | 0.20 ± 0.01 c |
| 21 | Limonene + | 1038 | 2.56 ± 0.28 a | 2.29 ± 0.32 a | 0.03 ± 0.00 b | 0.06 ± 0.01 b | 0.08 ± 0.01 b | 0.11 ± 0.01 b | 0.27 ± 0.03 b |
| 22 | 1-Hexanol, 2-ethyl + | 1040 | 31.11 ± 3.61 a | 0.35 ± 0.04 b | 2.19 ± 0.13 b | 0.04 ± 0.00 b | 0.05 ± 0.01 b | 0.05 ± 0.01 b | 1.31 ± 0.16 b |
| 23 | α-cis Ocimene * | 1052 | 14.43 ± 1.22 b | 21.56 ± 6.06 a | 3.72 ± 0.43 c | 0.05 ± 0.00 c | 0.85 ± 0.05 c | 0.12 ± 0.00 c | 0.16 ± 0.02 c |
| 24 | 1-Octyn-3-ol, 4-ethyl * | 1095 | 0.21 ± 0.02 b | 0.27 ± 0.04 a | 0.14 ± 0.01 c | 0.20 ± 0.01 b | 0.15 ± 0.01 c | 0.28 ± 0.03 a | 0.26 ± 0.01 a |
| 25 | Nonanal + | 1105 | 20.06 ± 1.71 a | 5.26 ± 0.25 b | 0.18 ± 0.02 c | 0.06 ± 0.00 c | 0.15 ± 0.01 c | 0.54 ± 0.04 c | 0.22 ± 0.03 c |
| 26 | Linalool + | 1110 | 2.86 ± 0.41 a | 0.35 ± 0.01 c | 0.08 ± 0.01 c | 0.02 ± 0.00 c | 0.02 ± 0.00 c | 0.00 ± 0.00 c | 1.27 ± 0.10 b |
| 27 | 2-Hexyl-1-octanol * | 1110 | 3.01 ± 0.23 a | 2.40 ± 0.34 b | 0.13 ± 0.01 c | 0.05 ± 0.00 c | 0.07 ± 0.00 c | 0.13 ± 0.01 c | 0.33 ± 0.03 c |
| 28 | Acetic acid, phenylmethyl ester * | 1169 | 1.98 ± 0.17 b | 13.25 ± 0.50 a | 0.85 ± 0.03 c | 0.53 ± 0.00 c | 0.66 ± 0.05 c | 0.92 ± 0.02 c | 1.56 ± 0.01 b |
| 29 | Acétic acid, octyl ester * | 1213 | 1.16 ± 0.00 b | 2.33 ± 0.25 a | 0.13 ± 0.01 c | 0.08 ± 0.01 c | 0.10 ± 0.01 c | 0.08 ± 0.00 c | 1.32 ± 0.18 b |
| 30 | α-Copaene * | 1357 | 12.80 ± 1.83 a | 8.89 ± 0.36 b | 0.42 ± 0.01 c | 0.04 ± 0.00 c | 0.04 ± 0.00 c | 0.12 ± 0.01 c | 0.21 ± 0.01 c |
| 31 | Heptylcyclohexane * | 1359 | 5.42 ± 0.79 a | 2.64 ± 0.25 b | 0.21 ± 0.00 c | 0.55 ± 0.03 c | 0.10 ± 0.01 c | 0.88 ± 0.13 c | 0.26 ± 0.01 c |
| 32 | Nonadecane + | 1407 | 1.10 ± 0.11 a | 0.74 ± 0.02 b | 0.02 ± 0.00 d | 0.03 ± 0.00 d | 0.05 ± 0.00 d | 0.03 ± 0.00 d | 0.38 ± 0.03 c |
| 33 | Tetradecane + | 1415 | 23.21 ± 1.44 a | 2.71 ± 0.33 b | 0.68 ± 0.07 c | 1.75 ± 0.18 cb | 0.40 ± 0.00 c | 1.36 ± 0.03 cb | 0.89 ± 0.01 c |
| 34 | Hexadecane * | 1416 | 2.41 ± 0.14 a | 2.68 ± 0.32 a | 0.01 ± 0.00 d | 0.07 ± 0.00 cd | 0.09 ± 0.01 cd | 0.55 ± 0.02 b | 0.38 ± 0.01 cb |
| 35 | Octylcyclohexane * | 1474 | 0.06 ± 0.00 fe | 0.50 ± 0.01 a | 0.07 ± 0.00 e | 0.33 ± 0.01 c | 0.05 ± 0.00 f | 0.16 ± 0.01 d | 0.49 ± 0.01 b |
| 36 | Germacrene D * | 1501 | 17.53 ± 3.71 a | 18.78 ± 6.03 a | 3.89 ± 0.37 b | 0.08 ± 0.01 b | 1.03 ± 0.11 b | 0.10 ± 0.01 b | 0.29 ± 0.00 b |
| 37 | α-Farnesene + | 1506 | 45.02 ± 5.49 a | 51.11 ± 3.73 a | 1.36 ± 0.20 b | 0.03 ± 0.00 b | 0.53 ± 0.00 b | 1.08 ± 0.05 b | 0.31 ± 0.03 b |
| 38 | α-Muurolene + | 1507 | 14.61 ± 0.66 a | 0.12 ± 0.01 b | 0.50 ± 0.07 b | 0.02 ± 0.00 b | 0.14 ± 0.01 b | 0.13 ± 0.01 b | 0.25 ± 0.03 b |
| 39 | Pentadecane * | 1605 | 2.44 ± 0.14 b | 3.65 ± 0.08 a | 0.03 ± 0.00 e | 0.72 ± 0.08 d | 0.05 ± 0.00 e | 1.25 ± 0.11 c | 0.62 ± 0.08 d |
| 40 | n-Hexadecanoic acid * | 1825 | 2.16 ± 0.03 b | 3.79 ± 0.55 a | 0.08 ± 0.01 c | 0.39 ± 0.02 c | 0.49 ± 0.07 c | 0.11 ± 0.01 c | 0.42 ± 0.03 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padilla-Jiménez, S.M.; Angoa-Pérez, M.V.; Mena-Violante, H.G.; Oyoque-Salcedo, G.; Montañez-Soto, J.L.; Oregel-Zamudio, E. Identification of Organic Volatile Markers Associated with Aroma during Maturation of Strawberry Fruits. Molecules 2021, 26, 504. https://doi.org/10.3390/molecules26020504
Padilla-Jiménez SM, Angoa-Pérez MV, Mena-Violante HG, Oyoque-Salcedo G, Montañez-Soto JL, Oregel-Zamudio E. Identification of Organic Volatile Markers Associated with Aroma during Maturation of Strawberry Fruits. Molecules. 2021; 26(2):504. https://doi.org/10.3390/molecules26020504
Chicago/Turabian StylePadilla-Jiménez, Samuel Macario, María Valentina Angoa-Pérez, Hortencia Gabriela Mena-Violante, Guadalupe Oyoque-Salcedo, José Luis Montañez-Soto, and Ernesto Oregel-Zamudio. 2021. "Identification of Organic Volatile Markers Associated with Aroma during Maturation of Strawberry Fruits" Molecules 26, no. 2: 504. https://doi.org/10.3390/molecules26020504
APA StylePadilla-Jiménez, S. M., Angoa-Pérez, M. V., Mena-Violante, H. G., Oyoque-Salcedo, G., Montañez-Soto, J. L., & Oregel-Zamudio, E. (2021). Identification of Organic Volatile Markers Associated with Aroma during Maturation of Strawberry Fruits. Molecules, 26(2), 504. https://doi.org/10.3390/molecules26020504







