Modulating Protein–Protein Interactions by Cyclic and Macrocyclic Peptides. Prominent Strategies and Examples
Abstract
1. Introduction
2. Main Methodologies to Cyclic and Macrocyclic Peptides
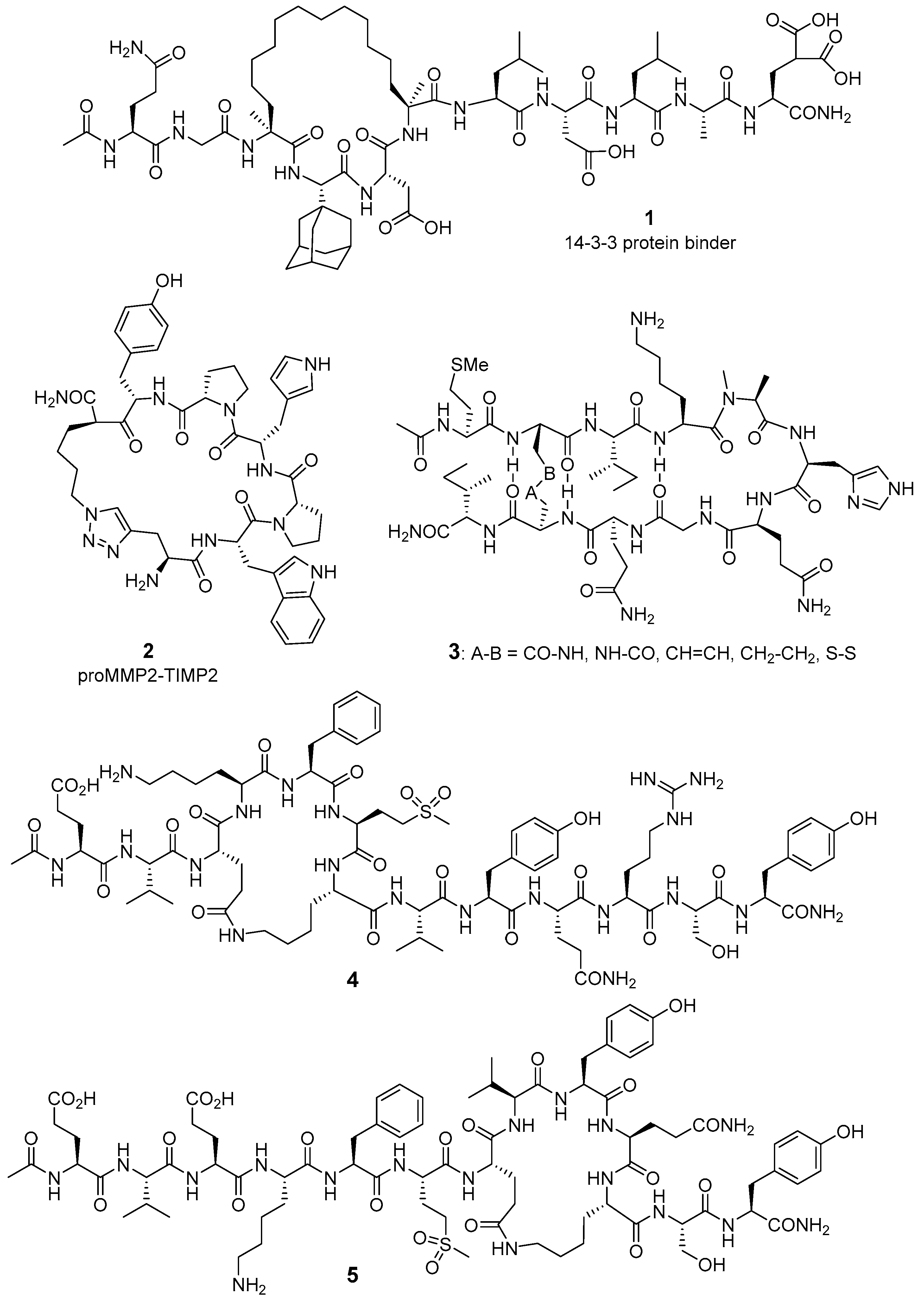
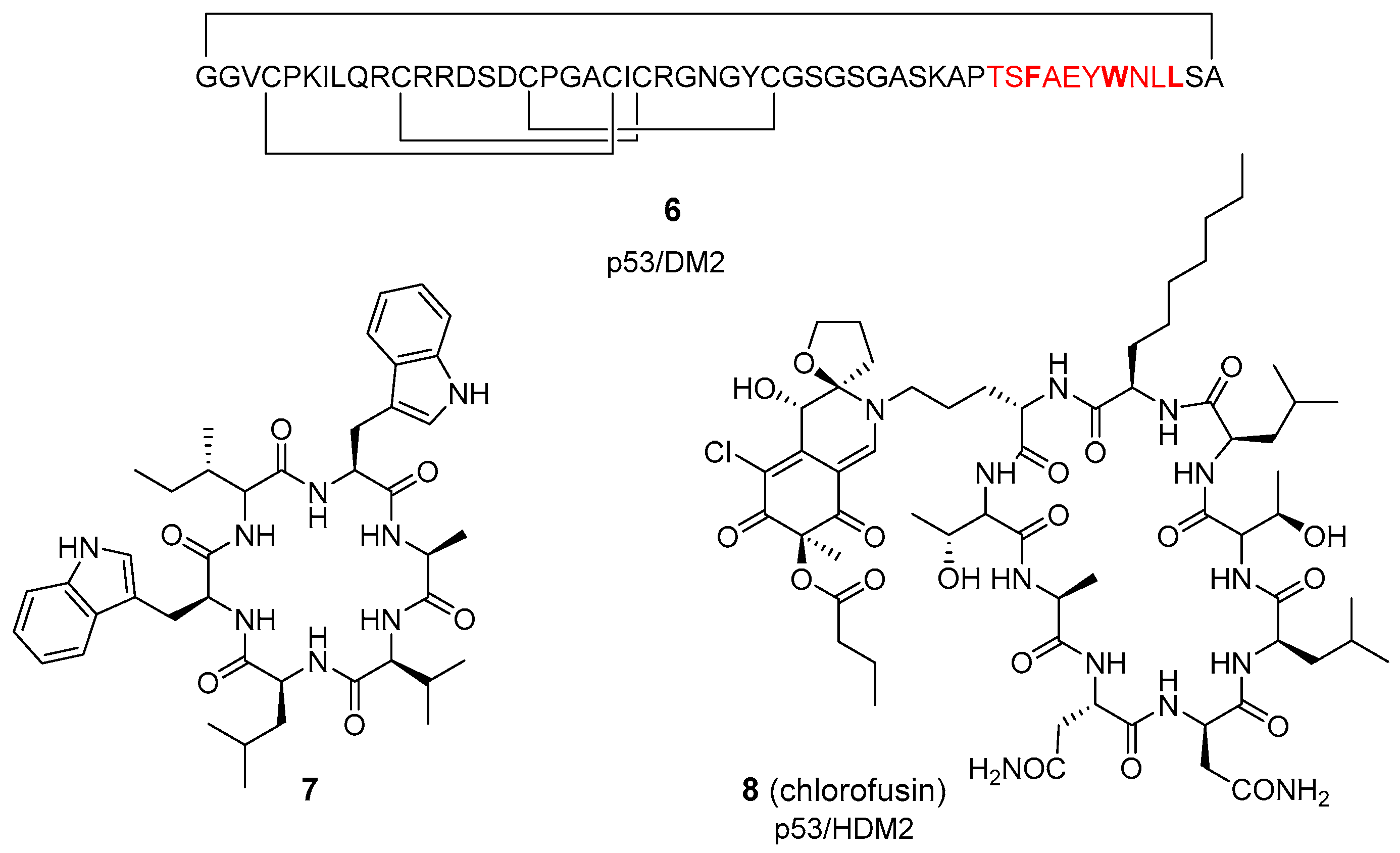
2.1. Computer-Assisted and X-ray-Based Design
2.2. Cyclic Peptides of Natural Origin
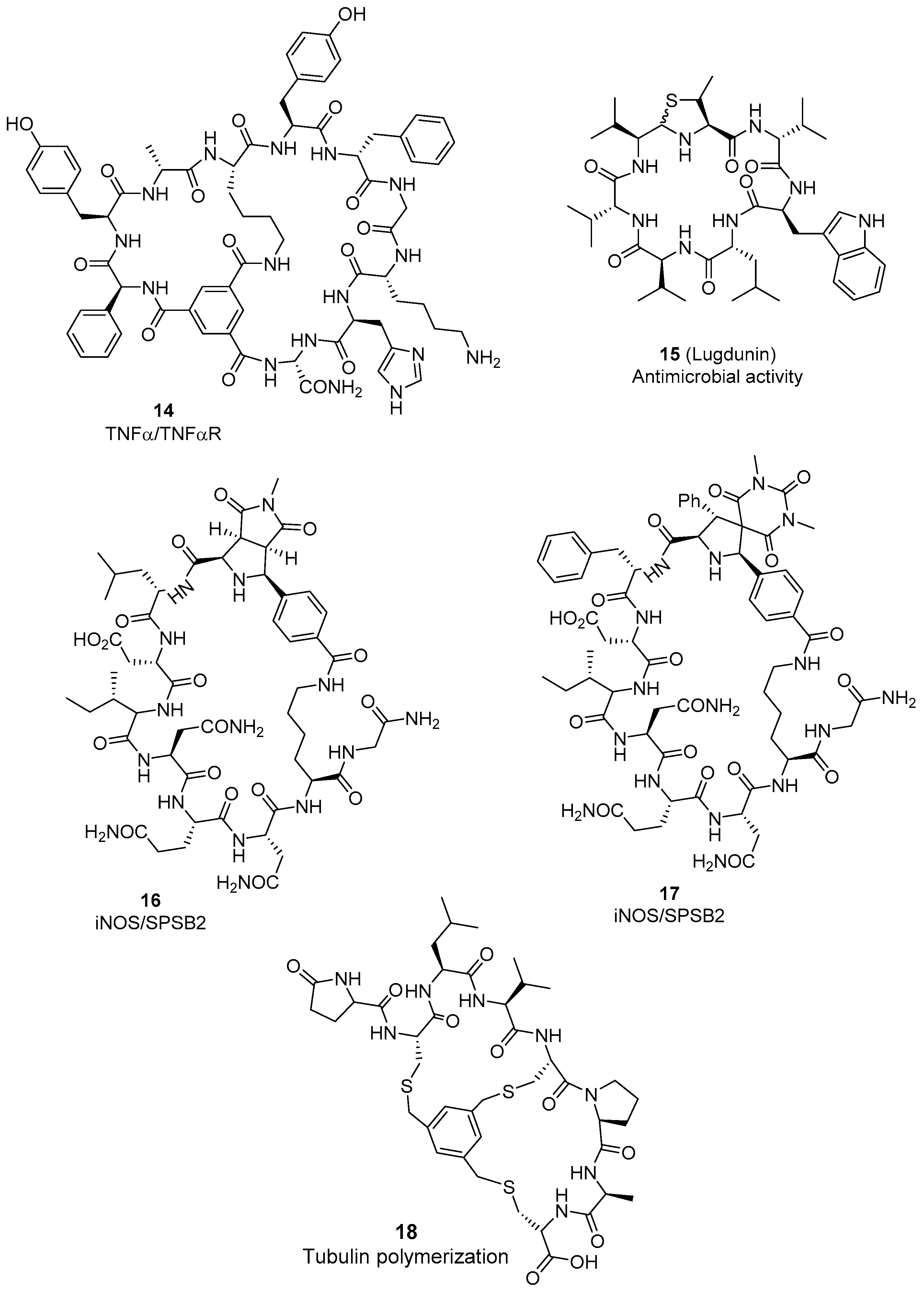
2.3. Biochemical Approaches to Cyclic Peptides
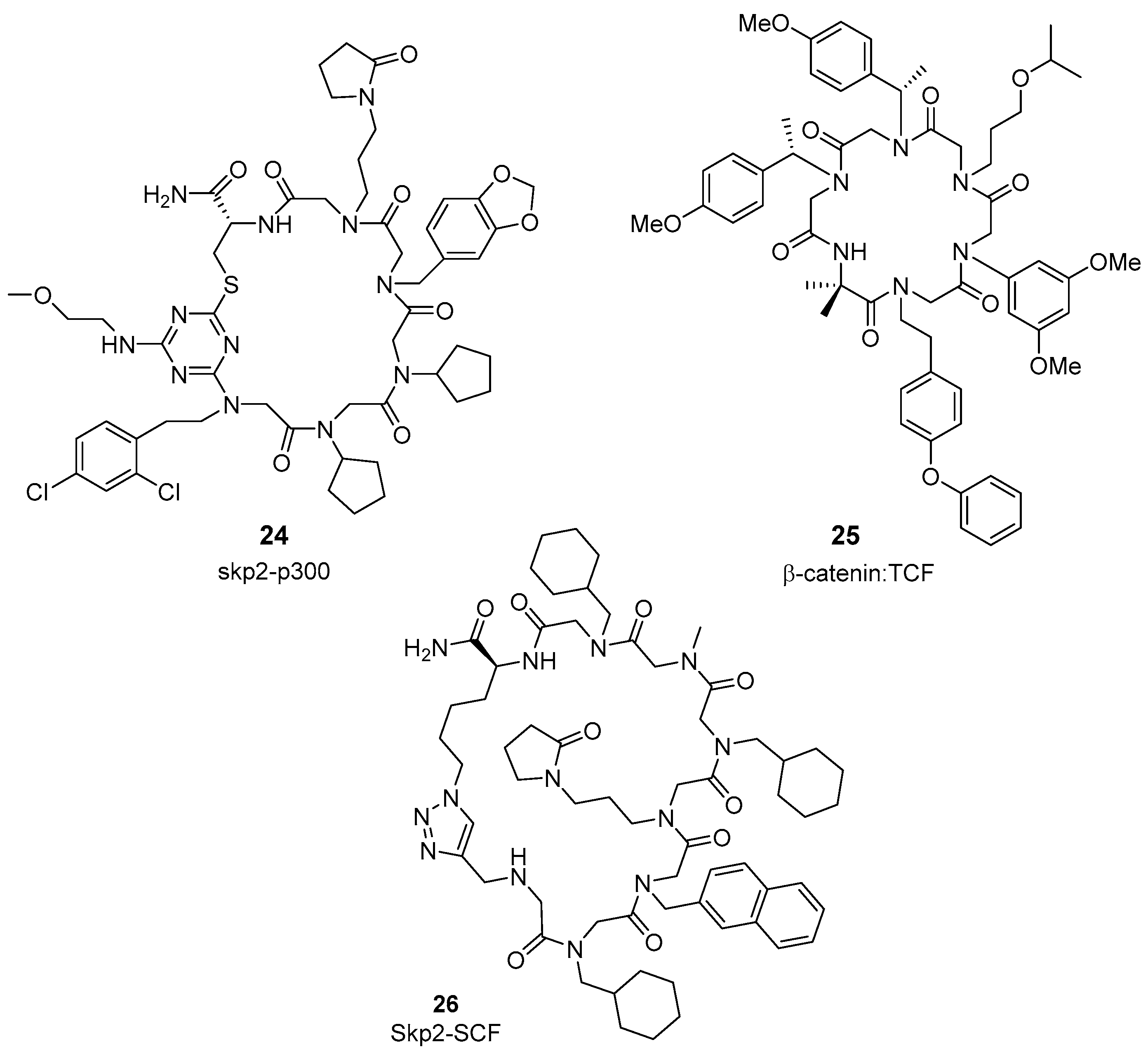
2.4. Synthetic Strategies to Cyclic Peptide Libraries
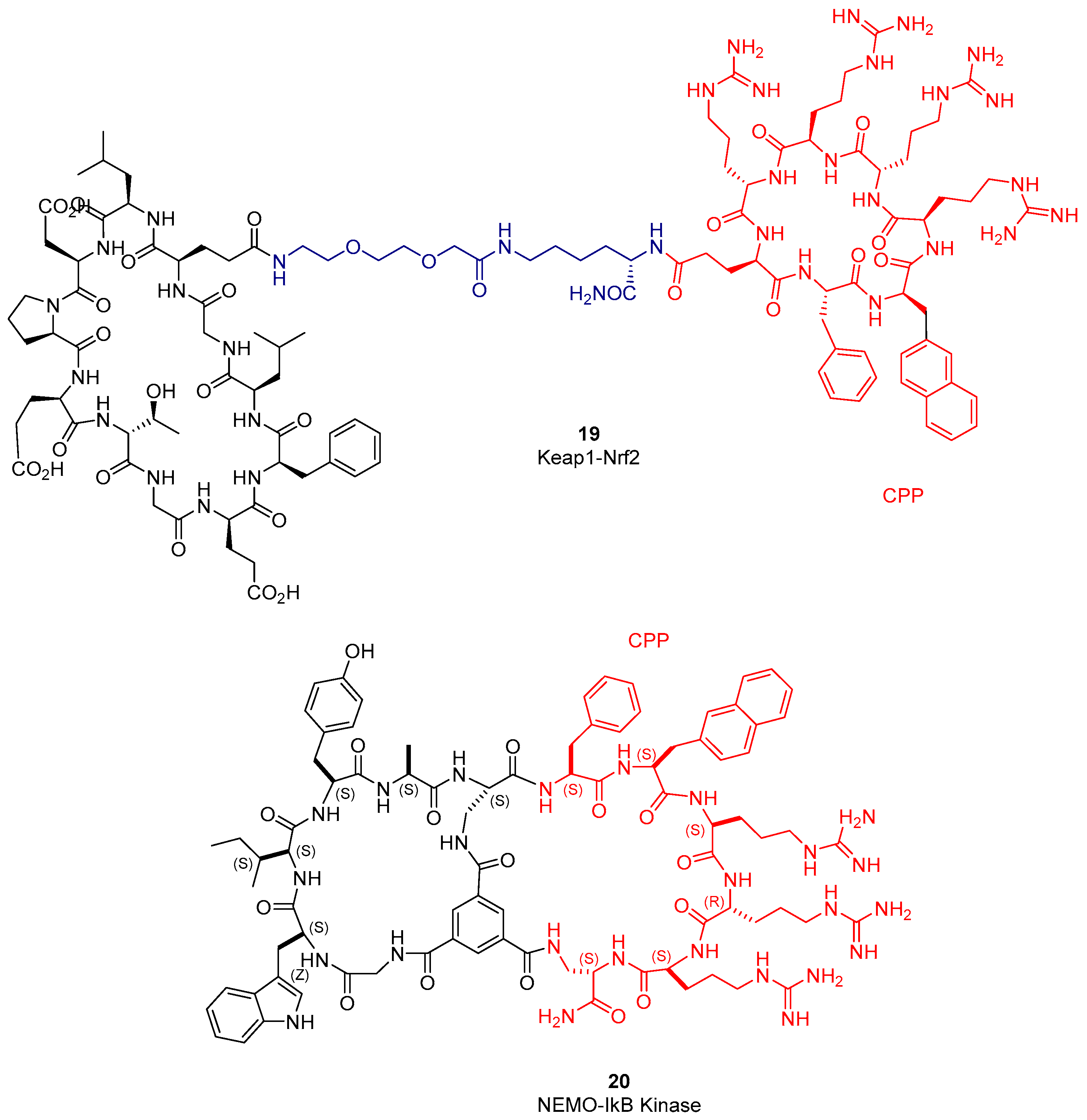
2.5. Cell Penetrating Cyclic Peptides
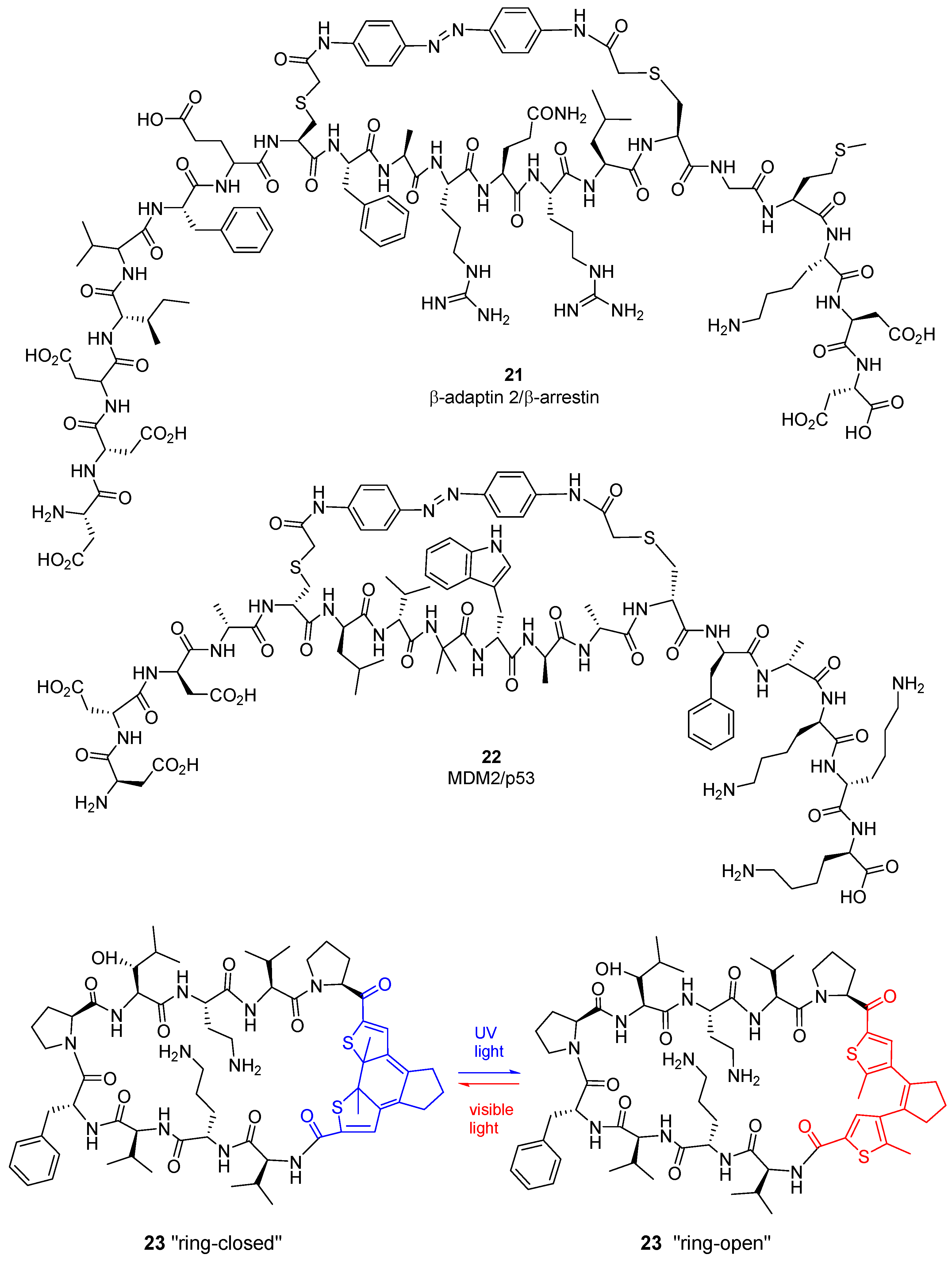
2.6. Photoswitchable Cyclic Peptides
2.7. β-Amino Acid- and Peptoid-Containing Cyclic Peptides
3. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Scott, D.E.; Bayly, A.R.; Abell, C.; Skidmore, J. Small molecules, big targets: Drug discovery faces the protein-protein interaction challenge. Nat. Rev. Drug Discov. 2016, 15, 533–550. [Google Scholar] [CrossRef] [PubMed]
- Skwarczynska, M.; Ottmann, C. Protein-protein interactions as drug targets. Future Med. Chem. 2015, 7, 2195–2219. [Google Scholar] [CrossRef] [PubMed]
- Ghadie, M.A.; Coulombe-Huntington, J.; Xia, Y. Interactome evolution: Insights from genome-wide analyses of protein-protein interactions. Curr. Opin. Struct. Biol. 2018, 50, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, M.P.H.; Thorne, T.; de Silva, E.; Stewart, R.; An, H.J.; Lappe, M.; Wiuf, C. Estimating the size of the human interactome. Proc. Natl. Acad. Sci. USA 2008, 105, 6959–6964. [Google Scholar] [CrossRef] [PubMed]
- Fuller, J.C.; Burgoyne, N.J.; Jackson, R.M. Predicting druggable binding sites at the protein-protein interface. Drug Discov. Today 2009, 14, 155–161. [Google Scholar] [CrossRef]
- Li, H.; Kasam, V.; Tautermann, C.S.; Seeliger, D.; Vaidehi, N. Computational method to identify druggable binding sites that target protein-protein interactions. J. Chem. Inf. Model. 2014, 54, 1391–1400. [Google Scholar] [CrossRef] [PubMed]
- Nevola, L.; Giralt, E. Modulating protein-protein interactions: The potential of peptides. Chem. Commun. 2015, 51, 3302–3315. [Google Scholar] [CrossRef]
- Vinogradov, A.A.; Yin, Y.; Suga, H. Macrocyclic peptides as drug candidates: Recent progress and remaining challenges. J. Am. Chem. Soc. 2019, 141, 4167–4181. [Google Scholar] [CrossRef]
- Cardote, T.A.F.; Ciulli, A. Cyclic and macrocyclic peptides as chemical tools to recognise protein surfaces and probe protein–protein interactions. ChemMedChem 2016, 11, 787–794. [Google Scholar] [CrossRef]
- Dougherty, P.G.; Qian, Z.; Pei, D. Macrocycles as protein-protein interaction inhibitors. Biochem. J. 2017, 474, 1109–1125. [Google Scholar] [CrossRef]
- Bhat, A.; Roberts, L.R.; Dwyer, J.J. Lead discovery and optimization strategies for peptide macrocycles. Eur. J. Med. Chem. 2015, 94, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Doak, B.C.; Zheng, J.; Dobritzsch, D.; Kihlberg, J. How beyond rule of 5 drugs and clinical candidates bind to their targets. J. Med. Chem. 2015, 59, 2312–2327. [Google Scholar] [CrossRef] [PubMed]
- Villar, E.A.; Beglov, D.; Chennamadhavuni, S.; Porco, J.A., Jr.; Kozakov, D.; Vajda, S.; Whitty, A. How proteins bind macrocycles. Nat. Chem. Biol. 2014, 10, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Passioura, T.; Katoh, T.; Goto, Y.; Suga, H. Selection-based discovery of druglike macrocyclic peptides. Annu. Rev. Biochem. 2014, 83, 727–752. [Google Scholar] [CrossRef]
- Pelay-Gimeno, M.; Glas, A.; Koch, O.; Grossmann, T.N. Structure-based design of inhibitors of pProtein-protein interactions: Mimicking peptide binding epitopes. Angew. Chem. Int. Ed. Engl. 2015, 54, 8896–8927. [Google Scholar] [CrossRef]
- Giordanetto, F.; Kihlberg, J. Macrocyclic drugs and clinical candidates: What can medicinal chemists learn from their properties? J. Med. Chem. 2013, 57, 278–295. [Google Scholar] [CrossRef]
- Zorzi, A.; Deyle, K.; Heinis, C. Cyclic peptide therapeutics: Past, present and future. Curr. Opin. Chem. Biol. 2017, 38, 24–29. [Google Scholar] [CrossRef]
- Jebrail, M.J.; Ng, A.H.C.; Rai, V.; Hili, R.; Yudin, A.K.; Wheeler, A.R. Synchronized synthesis of peptide-based macrocycles by digital microfluidics. Angew. Chem. Int. Ed. 2010, 49, 8625–8629. [Google Scholar] [CrossRef]
- Failli, A.; Immer, H.; Götz, M. The synthesis of cyclic peptides by the four component condensation (4 CC). Can. J. Chem. 1979, 57, 3257–3261. [Google Scholar] [CrossRef]
- Itoh, H.; Inoue, M. Full solid-phase total synthesis of macrocyclic natural peptides using four-dimensionally orthogonal protective groups. Org. Biomol. Chem. 2019, 17, 6519–6527. [Google Scholar] [CrossRef]
- Dougherty, P.G.; Sahni, A.; Pei, D. Understanding cell penetration of cyclic peptides. Chem Rev. 2019, 119, 10241–10287. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Atmaj, J.; Van Oosterwijk, N.; Groves, M.R.; Domling, A. Stapled peptides inhibitors: A new window for target drug discovery. Comput. Struct. Biotechnol. J. 2019, 17, 263–281. [Google Scholar] [CrossRef] [PubMed]
- Moiola, M.; Memeo, M.G.; Quadrelli, P. Stapled peptides-a useful improvement for peptide-based drugs. Molecules 2019, 24, 3654. [Google Scholar] [CrossRef] [PubMed]
- Migon, D.; Neubauer, D.; Kamysz, W.; Migon, D. Hydrocarbon stapled antimicrobial peptides. Protein J. 2018, 37, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Iyer, V.V. A Review of Stapled peptides and small molecules to inhibit protein-protein interactions in cancer. Curr. Med. Chem. 2016, 23, 3025–3043. [Google Scholar] [CrossRef]
- Tan, Y.S.; Lane, D.P.; Verma, C.S. Stapled peptide design: Principles and roles of computation. Drug Discov. Today 2016, 21, 1642–1653. [Google Scholar] [CrossRef]
- Cromm, P.M.; Spiegel, J.; Grossmann, T.N. Hydrocarbon stapled peptides as modulators of biological function. ACS Chem. Biol. 2015, 10, 1362–1375. [Google Scholar] [CrossRef]
- Foster, A.D.; Ingram, J.D.; Leitch, E.K.; Lennard, K.R.; Osher, E.L.; Tavassoli, A. Methods for the creation of cyclic Peptide libraries for use in lead discovery. J. Biomol. Screen. 2015, 20, 563–576. [Google Scholar] [CrossRef]
- Gao, M.; Cheng, K.; Yin, H. Targeting protein-protein iInterfaces using macrocyclic peptides. Peptide Sci. 2015, 104, 310–316. [Google Scholar] [CrossRef]
- McHugh, S.M.; Rogers, J.R.; Solomon, S.A.; Yu, H.; Lin, Y.-S. Computational methods to design cyclic peptides. Curr. Opin. Chem. Biol. 2016, 34, 95–102. [Google Scholar] [CrossRef]
- Duffy, F.J.; Devocelle, M.; Shields, D.C. Computational approaches to developing short cyclic peptide modulators of protein-protein interactions. Meth. Mol. Biol. 2015, 1268, 241–271. [Google Scholar] [CrossRef]
- Krüger, D.M.; Glas, A.; Bier, D.; Pospiech, N.; Wallraven, K.; Dietrich, L.; Ottmann, C.; Koch, O.; Hennig, S.; Grossmann, T. Structure-based design of non-natural macrocyclic peptides that inhibit protein–protein interactions. J. Med. Chem. 2017, 60, 8982–8988. [Google Scholar] [CrossRef] [PubMed]
- Nefla, M.; Sudre, L.; Denat, G.; Priam, S.; Andre-Leroux, G.; Berenbaum, F.; Jacques, C. The pro-inflammatory cytokine 14-3-3ε is a ligand of CD13 in cartilage. J. Cell Sci. 2015, 128, 3250–3262. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, P.; Li, Z.; Ren, W.; Wang, S.; Shao, S.; Sun, J.; Ren, X.; Perkins, N.G.; Guo, Z.; Chang, C.-E.A.; et al. Inhibiting matrix metalloproteinase-2 activation by perturbing protein-protein interactions using a cyclic peptide. J. Med. Chem. 2020, 63, 6979–6990. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Cazares, D.; Chavez-Dominguez, R.; Carlos-Reyes, A.; Lopez-Gonzalez, J.S.; Chavez-Dominguez, R.; Lopez-Camarillo, C.; de la Hernadez, C.O.N. Contribution of angiogenesis to inflammation and cCancer. Front. Oncol. 2019, 9, 1399. [Google Scholar] [CrossRef] [PubMed]
- Hartman, G.D.; Fraley, M.E.; Bilodeau, M.T. Kinase insert domain-containing receptor kinase inhibitors as anti-angiogenic agents. Expert Opin. Investig. Drugs 2002, 11, 737–745. [Google Scholar] [CrossRef]
- Garcia-Aranda, M.I.; Marrero, P.; Gautier, B.; Martin-Martinez, M.; Inguimbert, N.; Vidal, M.; Garcia-Lopez, M.T.; Jimenez, M.A.; Gonzalez-Muniz, R.; Perez de Vega, M.J. Parallel solid-phase synthesis of a small library of linear and hydrocarbon-bridged analogues of VEGF81-91: Potential biological tools for studying the VEGF/VEGFR-1 interaction. Bioorg. Med. Chem. 2011, 19, 1978–1986. [Google Scholar] [CrossRef]
- Garcia-Aranda, M.I.; Mirassou, Y.; Gautier, B.; Martin-Martinez, M.; Inguimbert, N.; Vidal, M.; Garcia-Lopez, M.T.; Jimenez, M.A.; Gonzalez-Muniz, R.; Perez de Vega, M.J. Disulfide and amide-bridged cyclic peptide analogues of the VEGF81-91 fragment: Synthesis, conformational analysis and biological evaluation. Bioorg. Med. Chem. 2011, 19, 7526–7533. [Google Scholar] [CrossRef]
- García-Aranda, M.I.; González-López, S.; Santiveri, C.M.; Gagey-Eilstein, N.; Reille-Seroussi, M.; Martín-Martínez, M.; Inguimbert, N.; Vidal, M.; García-López, M.T.; Jiménez, M.A.; et al. Helical peptides from VEGF and Vammin hotspots for modulating the VEGF-VEGFR interaction. Org. Biomol. Chem. 2013, 11, 1896–1905. [Google Scholar] [CrossRef]
- de Veer, S.J.; Weidmann, J.; Craik, D.J. Cyclotides as tools in chemical biology. Acc. Chem. Res. 2017, 50, 1557–1565. [Google Scholar] [CrossRef]
- Chaudhuri, D.; Aboye, T.; Camarero, J.A.; Camarero, J.A.; Camarero, J.A. Using backbone-cyclized Cys-rich polypeptides as molecular scaffolds to target protein-protein interactions. Biochem. J. 2019, 476, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Majumder, S.; Millard, M.; Borra, R.; Bi, T.; Elnagar, A.Y.; Neamati, N.; Shekhtman, A.; Camarero, J.A. In vivo activation of the p53 tumor suppressor pathway by an engineered cyclotide. J. Am. Chem. Soc. 2013, 135, 11623–11633. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.-Y.; Dahiya, R.; Qin, H.-L.; Mourya, R.; Maharaj, S. Natural proline-rich cyclopolypeptides from marine organisms: Chemistry, synthetic methodologies and biological status. Mar. Drugs 2016, 14, 194. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.H.; Attia, E.Z.; Hajjar, D.; Anany, M.A.; Desoukey, S.Y.; Fouad, M.A.; Kamel, M.S.; Id, H.W.; Gulder, T.A.M.; Abdelmohsen, U.R. New cytotoxic cyclic peptide from the marine sponge-associated Nocardiopsis sp. UR67. Mar. Drugs 2018, 16, 290. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.J.; Gruschow, S.; Williams, D.H.; McNicholas, C.; Purewal, R.; Hajek, M.; Gerlitz, M.; Martin, S.; Wrigley, S.K.; Moore, M. Isolation and structure elucidation of Chlorofusin, a novel P53-MDM2 antagonist from a Fusarium sp. J. Am. Chem. Soc. 2001, 123, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.C.; Lee, S.Y.; Searcey, M.; Boger, D.L. The isolation, total synthesis and structure elucidation of chlorofusin, a natural product inhibitor of the p53-MDM2 protein-protein interaction. Nat. Prod. Rep. 2009, 26, 465–477. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cominetti, M.M.D.; Goffin, S.A.; Raffel, E.; Turner, K.D.; Ramoutar, J.C.; O’Connell, M.A.; Howell, L.A.; Searcey, M. Identification of a new p53/MDM2 inhibitor motif inspired by studies of chlorofusin. Bioorg. Med. Chem. Lett. 2015, 25, 4878–4880. [Google Scholar] [CrossRef][Green Version]
- Simonetti, L.; Ivarsson, Y. Genetically encoded cyclic peptide phage display libraries. ACS Cent. Sci. 2020, 6, 336–338. [Google Scholar] [CrossRef]
- Deshayes, K.; Corn, J. Exploring protein-protein interactions using peptide libraries displayed on phage. In Phage Display In Biotechnology and Drug Discovery; Sidhu, S.S., Geyer, C.R., Eds.; CRC Press: Boca Raton, FL, USA, 2015; Volume 14, pp. 181–204. ISBN 978-1-4398-36-50-7. [Google Scholar] [CrossRef]
- Galan, A.; Horvatic, A.; Kules, J.; Guillemin, N.; Mrljak, V.; Bhide, M.; Comor, L. Library-based display technologies: Where do we stand? Int. Mol. BioSyst. 2016, 12, 2342–2358. [Google Scholar] [CrossRef]
- Desimmie, B.A.; Humbert, M.; Lescrinier, E.; Hendrix, J.; Vets, S.; Gijsbers, R.; Ruprecht, R.M.; Dietrich, U.; Debyser, Z.; Christ, F. Phage display-directed discovery of LEDGF/p75 binding cyclic peptide inhibitors of HIV replication. Mol. Ther. 2012, 20, 2064–2075. [Google Scholar] [CrossRef]
- Lipok, M.; Szlachcic, A.; Kindela, K.; Czyrek, A.; Otlewski, J. Identification of a peptide antagonist of the FGF1-FGFR1 signaling axis by phage display selection. FEBS Open Biol. 2019, 9, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.M.; Vitali, F.; Archer, S.A.; Fasan, R. Modular assembly of macrocyclic organo-peptide hHybrids using synthetic and genetically encoded precursors. Angew.Chem. Int. Ed. 2011, 123, 5181–5186. [Google Scholar] [CrossRef]
- Owens, A.E.; Iannuzzelli, J.A.; Gu, Y.; Fasan, R. MOrPH-PhD: An integrated phage display platform for the discovery of functional genetically encoded peptide macrocycle. ACS Cent. Sci. 2020, 6, 368–381. [Google Scholar] [CrossRef] [PubMed]
- Tavassoli, A.; Benkovic, S.J. Split-intein mediated circular ligation used in the synthesis of cyclic peptide libraries in E. coli. Nat. Protoc. 2007, 2, 1126–1133. [Google Scholar] [CrossRef]
- Mistry, I.N.; Tavassoli, A. Reprogramming the transcriptional response to hypoxia with a chromosomally encoded cyclic peptide HIF-1 inhibitor. ACS Synth. Biol. 2017, 6, 518–527. [Google Scholar] [CrossRef]
- Birts, C.N.; Nijjar, S.K.; Mardle, C.A.; Hoakwie, F.; Duriez, P.J.; Blaydes, J.P.; Tavassoli, A. A cyclic peptideinhibitor of C-terminal binding protein dimerization links metabolism with mitotic fidelity in breast cancer cells. Chem. Sci. 2013, 4, 3046–3057. [Google Scholar] [CrossRef]
- Bionda, N.; Cryan, A.L.; Fasan, R. Bioinspired strategy for the ribosomal synthesis of thioether-bridged macrocyclic peptides in bacteria. ACS Chem. Biol. 2014, 9, 2008–2013. [Google Scholar] [CrossRef]
- Bacon, K.; Blain, A.; Burroughs, M.; McArthrur, N.; Rao, B.M.; Menegatti, S. Isolation of chemically cyclized peptide binders using yeast surface display. ACS Comb. Sci. 2020, 22, 519–532. [Google Scholar] [CrossRef]
- Pandya, P.; Sayers, R.O.; Ting, J.P.; Morshedian, S.; Torres, C.; Cudal, J.S.; Zhang, K.; Fitchett, J.R.; Zhang, Q.; Zhang, F.F.; et al. Integration of phage and yeast display platforms: A reliable and cost effective approach for binning of peptides as displayed on-phage. PLoS ONE 2020, 15, e0233961. [Google Scholar] [CrossRef]
- Menegatti, S.; Hussain, M.; Naik, A.D.; Carbonell, R.G.; Rao, B.M. mRNA display selection and solid-phase synthesis of Fc-binding cyclic peptide affinity ligands. Biotechnol. Bioeng. 2013, 110, 857–870. [Google Scholar] [CrossRef]
- Ma, Z.; Hartman, M.C.T. In vitro selection of unnatural cyclic peptide libraries via mRNA display. Methods Mol. Biol. 2012, 805, 367–390. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, G.; Neri, D. DNA-encoded chemical libraries: Foundations and applications in lead discovery. Drug Discov. Today 2016, 21, 1828–1834. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, S.; Sidhu, G.A.W. No TitleDNA-emncoded peptide libraries and drug discovery. Anticancer. Drug Dev. 2002, 237–248. [Google Scholar] [CrossRef]
- Zhu, Z.; Shaginian, A.; Grady, L.C.; O’Keeffe, T.; Shi, X.E.; Davie, C.P.; Simpson, G.L.; Messer, J.A.; Evindar, G.; Bream, R.N.; et al. Design and application of a DNA-encoded macrocyclic peptide library. ACS Chem. Biol. 2018, 13, 53–59. [Google Scholar] [CrossRef]
- Wills, R.; Adebomi, V.; Raj, M. Site-selective peptide macrocyclization. ChemBioChem 2020. Ahead of Print. [Google Scholar] [CrossRef]
- Lian, W.; Upadhyaya, P.; Rhodes, C.A.; Liu, Y.; Pei, D. Screening bicyclic peptide libraries for protein–protein interaction inhibitors: Discovery of a tumor necrosis factor-α antagonist. J. Am. Chem. Soc. 2013, 135, 11990–11995. [Google Scholar] [CrossRef]
- Luo, Q.; Tao, Y.; Sheng, W.; Lu, J.; Wang, H. Dinitroimidazoles as bifunctional bioconjugation reagents for protein functionalization and peptide macrocyclization. Nat. Commun. 2019, 10, 142. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Q.; Wong, C.T.T.; Li, X. Chemoselective Peptide Cyclization and Bicyclization Directly on Unprotected Peptides. J. Am. Chem. Soc. 2019, 141, 12274–12279. [Google Scholar] [CrossRef]
- Reguera, L.; Rivera, D.G. Multicomponent Reaction Toolbox for Peptide Macrocyclization and Stapling. Chem. Rev. 2019, 119, 9836–9860. [Google Scholar] [CrossRef]
- Neochoritis, C.G.; Miraki, M.K.; Abdelraheem, E.M.M.; Surmiak, E.; Zarganes-Tzitzikas, T.; Łabuzek, B.; Holak, T.A.; Domling, A. Design of indole- and MCR-based macrocycles as p53-MDM2 antagonists. Beilstein J. Org. Chem. 2019, 15, 513–520. [Google Scholar] [CrossRef]
- Malins, L.R.; deGruyter, J.N.; Robbins, K.J.; Scola, P.M.; Eastgate, M.D.; Ghadiri, M.R.; Baran, P.S. Peptide macrocyclization inspired by non-ribosomal imine natural products. J. Am. Chem. Soc. 2017, 139, 5233–5241. [Google Scholar] [CrossRef] [PubMed]
- Gueret, S.M.; Thavam, S.; Carbajo, R.J.; Potowski, M.; Larsson, N.; Dahl, G.; Dellsen, A.; Grossmann, T.N.; Plowright, A.T.; Valeur, E.; et al. Macrocyclic modalities combining peptide epitopes and natural product fragments. J. Am. Chem. Soc. 2020, 142, 4904–4915. [Google Scholar] [CrossRef] [PubMed]
- Thombare, V.J.; Holden, J.A.; Reynolds, E.C.; O’Brien-Simpson, N.M.; Hutton, C.A. Celogentin mimetics as inhibitors of tubulin. J. Pep. Sci. 2020, 26, e3239. [Google Scholar] [CrossRef] [PubMed]
- Bernhagen, D.; Jungbluth, V.; Quilis, N.G.; Dostalek, J.; White, P.B.; Jalink, K.; Timmermann, P. High-affinity α,β-integrin-selective bicyclic RGD peptides identified via screening of designed random libraries. ACS Comb. Sci. 2019, 21, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Kubi, G.A.; Dougherty, P.G.; Pei, D. Designing cell-permeable macrocyclic peptides. Methods Mol. Biol. 2019, 2001, 41–59. [Google Scholar] [CrossRef]
- Salim, H.; Song, J.; Sahni, A.; Pei, D. Development of a Cell-Permeable Cyclic Peptidyl Inhibitor against the Keap1–Nrf2 Interaction. J. Org. Chem. 2020, 85, 1416–1424. [Google Scholar] [CrossRef]
- Colarusso, S.; De Simone, D.; Frattarelli, T.; Andreini, M.; Cerretani, M.; Missineo, A.; Moretti, D.; Tambone, S.; Kempf, G.; Augustin, M.; et al. Optimization of linear and cyclic peptide inhibitors of KEAP1-NRF2 protein-protein interaction. Bioorg. Med. Chem. 2020, 28, 115738. [Google Scholar] [CrossRef]
- Dougherty, P.G.; Wen, J.; Pan, X.; Koley, A.; Ren, J.-G.; Sahni, A.; Basu, R.; Salim, H.; Appiah Kubi, G.; Qian, Z.; et al. Enhancing the cell permeability of stapled peptides with a cyclic cell-penetrating peptide. J. Med. Chem. 2019, 62, 10098–10107. [Google Scholar] [CrossRef]
- Rhodes, C.A.; Dougherty, P.G.; Cooper, J.K.; Qian, Z.; Lindert, S.; Wang, Q.-E.; Pei, D. Cell-permeable bicyclic peptidyl inhibitors against NEMO-IκB kinase iInteraction directly from a combinatorial library. J. Am. Chem. Soc. 2018, 140, 12102–12110. [Google Scholar] [CrossRef]
- Fujiwara, D.; Kitada, H.; Oguri, M.; Nishihara, T.; Michigami, M.; Shiraishi, K.; Yuba, E.; Nakase, I.; Im, H.; Cho, S.; et al. A Cyclized helix-loop-helix peptide as a molecular scaffold for the design of inhibitors of intracellular protein-protein interactions by epitope and arginine grafting. Angew. Chem. Int. Ed. 2016, 55, 10612–10615. [Google Scholar] [CrossRef]
- Hemmati, S.; Behzadipour, Y.; Haddad, M. Decoding the proteome of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) for cell-penetrating peptides involved in pathogenesis or applicable as drug delivery vectors. Infect. Genet. Evol. 2020, 85, 104474. [Google Scholar] [CrossRef] [PubMed]
- Albert, L.; Vazquez, O. Photoswitchable peptides for spatiotemporal control of biological functions. Chem. Commun. 2019, 55, 10192–10213. [Google Scholar] [CrossRef] [PubMed]
- Martin-Quiros, A.; Nevola, L.; Eckelt, K.; Madurga, S.; Gorostiza, P.; Giralt, E. Absence of a stable secondary structure is not a limitation for photoswitchable inhibitors of β-arrestin/β-adaptin 2 protein-protein interaction. Chem. Biol. 2015, 22, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Nevola, L.; Varese, M.; Martin-Quiros, A.; Mari, G.; Eckelt, K.; Gorostiza, P.; Giralt, E. Targeted nanoswitchable inhibitors of protein-protein interactions involved in apoptosis. ChemMedChem 2019, 14, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Babii, O.; Afonin, S.; Ishchenko, A.Y.; Schober, T.; Negelia, A.O.; Tolstanova, G.M.; Garmanchuk, L.V.; Ostapchenko, L.I.; Komarov, I.V.; Ulrich, A.S. Structure−activity relationships of photoswitchable diarylethene based β-hairpin peptides as membranolytic antimicrobial and anticancer agents. J. Med. Chem. 2018, 61, 10793–10813. [Google Scholar] [CrossRef] [PubMed]
- Afonin, S.; Babii, O.; Reuter, A.; Middel, V.; Takamiya, M.; Strähle, U.; Komarov, I.V.; Ulrich, A.S. Light-controllable dithienylethene-modified cyclic peptides: Photoswitching the in vivo toxicity in zebrafish embryos. Beilstein J. Org. Chem. 2020, 16, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Frackenpohl, J.; Arvidsson, P.I.; Schreiber, J.V.; Seebach, D. The Outstanding Biological Stability of beta-and-gamma-Peptides toward Proteolytic Enzymes: An In Vitro Investigation with Fifteen Peptidases. ChemBioChem. 2001, 2, 445–455. [Google Scholar] [CrossRef]
- Peterson-Kaufman, K.J.; Haase, H.S.; Boersma, M.D.; Lee, E.F.; Fairlie, W.D.; Gellman, S.H. Residue-Based Preorganization of BH3-Derived α/β-Peptides: Modulating Affinity, Selectivity and Proteolytic Susceptibility in α-Helix Mimics. Chem. Biol. 2015, 10, 1667–1675. [Google Scholar] [CrossRef]
- Fülöp, F.; Martinek, T.A.; Toth, G.K. Application of alicyclic β-amino acids in peptide chemistry. Chem. Soc. Rev. 2006, 35, 323. [Google Scholar] [CrossRef]
- Kritzer, J.A.; Stephens, O.M.; Guarracino, D.A.; Reznika, S.K.; Schepartza, A. β-Peptides as inhibitors of protein–protein interactions. Bioorg. Med. Chem. 2005, 13, 11–16. [Google Scholar] [CrossRef]
- Smith, B.J.; Lee, E.F.; Checco, J.W.; Evangelista, M.; Gellman, S.H.; Fairlie, W.D. Structure-guided rational design of α/β-peptide foldamers with high affinity for BCL-2 family prosurvival proteins. ChemBioChem 2013, 14, 1564–1572. [Google Scholar] [CrossRef] [PubMed]
- Haase, H.S.; Peterson-Kaufman, K.J.; Lan Levengood, S.K.; Checco, J.W.; Murphy, W.L.; Gellman, S.H. Extending Foldamer Design Beyond α-Helix Mimicry: α/β-Peptide inhibitors of vascular endothelial growth factor Signaling. J. Am. Chem. Soc. 2012, 134, 7652–7655. [Google Scholar] [CrossRef]
- Bautista, A.D.; Appelbaum, J.S.; Craig, C.J.; Michel, J.; Schepartz, A. Bridged β3-peptide inhibitors of p53-hDM2 complexation: Correlation between affinity and cell permeability. J. Am. Chem. Soc. 2010, 132, 2904–2906. [Google Scholar] [CrossRef] [PubMed]
- Checco, J.W.; Lee, E.F.; Evangelista, M.; Sleebs, N.J.; Rogers, K.; Pettikiriarachchi, A.; Kershaw, N.J.; Eddinger, G.A.; Belair, D.G.; Wilson, J.L.; et al. α/β-peptide foldamers targeting intracellular protein-protein interactions with activity in living cells. J. Am. Chem. Soc. 2015, 137, 11365–11375. [Google Scholar] [CrossRef] [PubMed]
- Udugamasooriya, D.G.; Dineen, S.P.; Brekken, R.A.; Kodadek, T. A Peptoid “antibody surrogate” that antagonizes VEGF receptor 2 activity. J. Am. Chem. Soc. 2008, 130, 5744–5752. [Google Scholar] [CrossRef] [PubMed]
- Mondragón, L.; Orzáez, M.; Sanclimens, G.; Moure, A.; Armiñán, A.; Sepúlveda, P.; Messeguer, A.; Vicent, M.J.; Pérez-Payá, E. Modulation of cellular apoptosis with apoptotic protease-activating factor 1 (Apaf-1) inhibitors. J. Med. Chem. 2008, 51, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.; Lee, J.H.; Moon, H.; Hyun, Y.J.; Lim, H.S. A Chemical inhibitor of the Skp2/p300 Interaction that promotes p53-mediated apoptosis. Angew. Chem. Int. Ed. 2016, 11, 602–606. [Google Scholar] [CrossRef]
- Drew, K.; Renfrew, P.D.; Craven, T.W.; Butterfoss, G.L.; Chou, F.-C.; Lyskov, S.; Bullock, B.N.; Watkins, A.; Labonte, J.W.; Pacella, M.; et al. Adding diverse noncanonical backbones to rosetta: Enabling peptidomimetic design. PLoS ONE 2013, 8, e67051. [Google Scholar] [CrossRef]
- Schneider, J.A.; Craven, T.W.; Kasper, A.C.; Yun, C.; Haugbro, M.; Briggs, E.M.; Svetlov, V.; Nudler, E.; Knaut, H.; Bonneau, R.; et al. Design of Peptoid-peptide Macrocycles to Inhibit the β-catenin TCF Interaction in Prostate Cancer. Nature 2018, 9, 4396. [Google Scholar] [CrossRef]
- Shin, M.H.; Lee, K.S.; Lim, H.-S. DNA-Encoded combinatorial library of macrocyclic peptoids. Bioconjugate Chem. 2019, 30, 2931–2938. [Google Scholar] [CrossRef]
- Stone, T.A.; Deber, C.M. Therapeutic design of peptide modulators of protein-protein interactions in membranes. Biochim. Biophys. Acta 2017, 1859, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Messina, F.; Giombini, E.; Montaldo, C.; Sharma, A.A.; Piacentini, M.; Zoccoli, A.; Sekaly, R.-P.; Locatelli, F.; Zumla, A.; Maeurer, M.; et al. Looking for pathways related to COVID-19 phenotypes: Confirmation of pathogenic mechanisms by SARS-CoV-2—Host interactome. bioRxiv 2020, 1–23. [Google Scholar] [CrossRef]
- Bojadzic, D.; Alcazar, O.; Chen, J.; Buchwald, P. Small-molecule In Vitro inhibitors of the coronavirus spike—ACE2 protein-protein interaction as blockers of viral attachment and entry for SARS-CoV-2. bioRxiv 2020, 1–60. [Google Scholar] [CrossRef]
- Cantuti-Castelvetri1, L.; Ojha, R.; Pedro, L.D.; Djannatian, M.; Franz, J.; Kuivanen, S.; van der Meer, F.; Kallio, K.; Kaya, T.; Anastasina, M.; et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 2020, 370, 856–860. [Google Scholar] [CrossRef]
- Bello, M.; Martinez-Munoz, A.; Balbuena-Rebolledo, I. Identification of saquinavir as a potent inhibitor of dimeric SARS-CoV2 main protease through MM/GBSA. J. Mol. Model. 2020, 26, 340. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Muñiz, R.; Bonache, M.Á.; Pérez de Vega, M.J. Modulating Protein–Protein Interactions by Cyclic and Macrocyclic Peptides. Prominent Strategies and Examples. Molecules 2021, 26, 445. https://doi.org/10.3390/molecules26020445
González-Muñiz R, Bonache MÁ, Pérez de Vega MJ. Modulating Protein–Protein Interactions by Cyclic and Macrocyclic Peptides. Prominent Strategies and Examples. Molecules. 2021; 26(2):445. https://doi.org/10.3390/molecules26020445
Chicago/Turabian StyleGonzález-Muñiz, Rosario, María Ángeles Bonache, and María Jesús Pérez de Vega. 2021. "Modulating Protein–Protein Interactions by Cyclic and Macrocyclic Peptides. Prominent Strategies and Examples" Molecules 26, no. 2: 445. https://doi.org/10.3390/molecules26020445
APA StyleGonzález-Muñiz, R., Bonache, M. Á., & Pérez de Vega, M. J. (2021). Modulating Protein–Protein Interactions by Cyclic and Macrocyclic Peptides. Prominent Strategies and Examples. Molecules, 26(2), 445. https://doi.org/10.3390/molecules26020445





