Characterization and Optimization of the Tyrosinase Inhibitory Activity of Vitis amurensis Root Using LC-Q-TOF-MS Coupled with a Bioassay and Response Surface Methodology
Abstract
1. Introduction
2. Results and Discussion
2.1. LC-QTOF MS Coupled with a Tyrosinase Inhibitory Assay Using the Root Extract of V. amurensis
2.2. Identification of Tyrosinase Inhibitory Constituents of V. amurensis Root
2.3. Optimization of V. amurensis Root Extraction Using RSM
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. LC-Q-TOF Mass Spectrometry
3.4. LC-Q-TOF-MS Coupled with a Tyrosinase Inhibitory Assay
3.5. Isolation of Tyrosinase Inhibitory Compounds from V. amurensis Root
3.6. Tyrosinase Inhibitory Assay
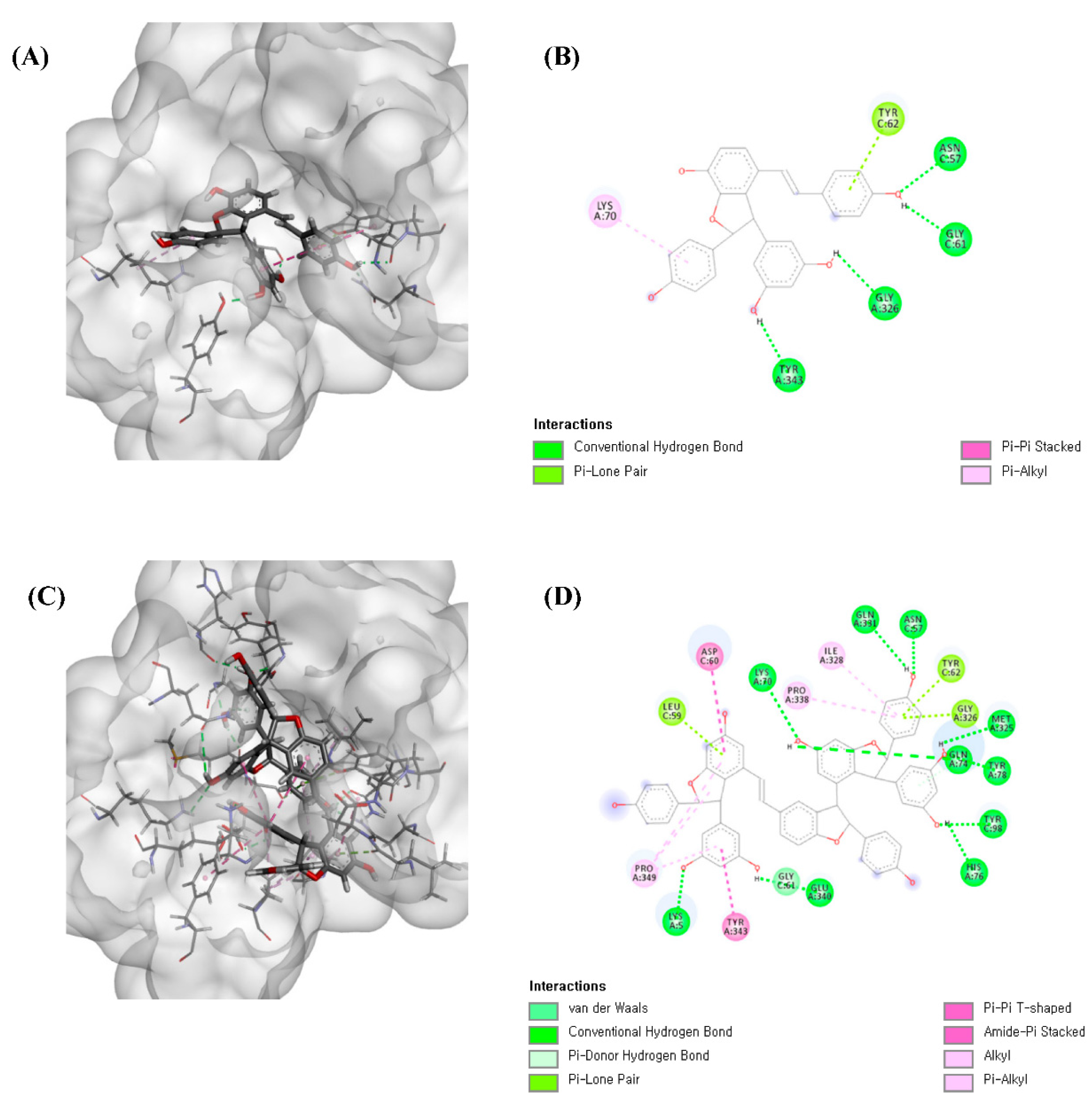
3.7. Molecular Docking Studies
3.8. Experimental Design and Statistical Analysis
3.9. Quantitative Analysis of Tyrosinase-Inhibitory Compounds 1 and 2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ranjbar, S.; Shahvaran, P.S.; Edraki, N.; Khoshneviszadeh, M.; Darroudi, M.; Sarrafi, Y.; Hamzehloueian, M.; Khoshneviszadeh, M. 1, 2, 3-Triazole-linked 5-benzylidene (thio) barbiturates as novel tyrosinase inhibitors and free-radical scavengers. Arch. Pharm. 2020, 353, 2000058. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-S.; Ding, H.-Y.; Lin, H.-C. Identifying 6, 7, 4′-trihydroxyisoflavone as a potent tyrosinase inhibitor. Biosci. Biotechnol. Biochem. 2005, 69, 1999–2001. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, M.; Oshima, T.; Koshio, K.; Itsuzaki, Y.; Anzai, J. Tyrosinase inhibitor from black rice bran. J. Agric. Food Chem. 2003, 51, 6953–6956. [Google Scholar] [CrossRef]
- Chen, Q.; Diao, L.; Song, H.; Zhu, X. Vitis amurensis Rupr: A review of chemistry and pharmacology. Phytomedicine 2018, 49, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.-S.; Oh, Y.N.; Hyun, S.K.; Kwon, H.J.; Kim, B.W. Betulinic acid isolated from Vitis amurensis root inhibits 3-isobutyl-1-methylxanthine induced melanogenesis via the regulation of MEK/ERK and PI3K/Akt pathways in B16F10 cells. Food Chem. Toxicol. 2014, 68, 38–43. [Google Scholar] [CrossRef]
- Kim, H.; Thuong, P.T.; Ngoc, T.M.; Lee, I.; Hung, N.D.; Bae, K. Antioxidant and lipoxygenase inhibitory activity of oligostilbenes from the leaf and stem of Vitis amurensis. J. Ethnopharmacol. 2009, 125, 304–309. [Google Scholar] [CrossRef]
- Jin, K.-S.; Oh, Y.N.; Hyun, S.K.; Kwon, H.J.; Kim, B.W. Vitis amurensis Ruprecht root inhibited α-melanocyte stimulating hormone-induced melanogenesis in B16F10 cells. Nutr. Res. Pract. 2014, 8, 509–515. [Google Scholar] [CrossRef]
- Jang, M.H.; Piao, X.L.; Kim, H.Y.; Cho, E.J.; Baek, S.H.; Kwon, S.W.; Park, J.H. Resveratrol oligomers from Vitis amurensis attenuate β-amyloid-induced oxidative stress in PC12 cells. Biol. Pharm. Bull. 2007, 30, 1130–1134. [Google Scholar] [CrossRef]
- Lee, E.-O.; Lee, H.-J.; Hwang, H.-S.; Ahn, K.-S.; Chae, C.; Kang, K.-S.; Lu, J.; Kim, S.-H. Potent inhibition of Lewis lung cancer growth by heyneanol A from the roots of Vitis amurensis through apoptotic and anti-angiogenic activities. Carcinogen 2006, 27, 2059–2069. [Google Scholar] [CrossRef]
- Bak, M.-J.; Truong, V.L.; Kang, H.-S.; Jun, M.; Jeong, W.-S. Anti-inflammatory effect of procyanidins from wild grape (Vitis amurensis) seeds in LPS-induced RAW 264.7 cells. Oxid. Med. Cell. Longev. 2013, 2013. [Google Scholar] [CrossRef]
- Shin, H.; Chung, H.; Park, B.; Lee, K.Y. Identification of antioxidative constituents from Polygonum aviculare using LC-MS coupled with DPPH assay. Nat. Prod. Sci. 2016, 22, 64–69. [Google Scholar] [CrossRef]
- Park, S.; Shin, H.; Park, Y.; Choi, I.; Park, B.; Lee, K.Y. Characterization of inhibitory constituents of NO production from Catalpa ovata using LC-MS coupled with a cell-based assay. Bioorg. Chem. 2018, 80, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Ingkaninan, K.; De Best, C.; Van Der Heijden, R.; Hofte, A.; Karabatak, B.; Irth, H.; Tjaden, U.; Van der Greef, J.; Verpoorte, R. High-performance liquid chromatography with on-line coupled UV, mass spectrometric and biochemical detection for identification of acetylcholinesterase inhibitors from natural products. J. Chromatogr. A 2000, 872, 61–73. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Witek-Krowiak, A.; Chojnacka, K.; Podstawczyk, D.; Dawiec, A.; Pokomeda, K. Application of response surface methodology and artificial neural network methods in modelling and optimization of biosorption process. Bioresour. Technol. 2014, 160, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Araujo, P.W.; Brereton, R.G. Experimental design I. Screening. Trends Analyt. Chem. 1996, 15, 26–31. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, L.; Zhang, R.; Yang, X.; Sun, Y.; Shi, L.; Xue, P. Optimization of ultrasound-assisted extraction by response surface methodology, antioxidant capacity, and tyrosinase inhibitory activity of anthocyanins from red rice bran. Food Sci. Nutr. 2020, 8, 921–932. [Google Scholar] [CrossRef]
- Weremfo, A.; Adulley, F.; Adarkwah-Yiadom, M. Simultaneous Optimization of Microwave-Assisted Extraction of Phenolic Compounds and Antioxidant Activity of Avocado (Persea americana Mill.) Seeds Using Response Surface Methodology. J. Anal. Methods Chem. 2020, 2020, 7541927. [Google Scholar] [CrossRef]
- Ko, J.; Choi, J.; Bae, S.K.; Kim, J.; Yoon, K.D. Separation of five oligostilbenes from V itis amurensis by flow-rate gradient high-performance counter-current chromatography. J. Sep. Sci. 2013, 36, 3860–3865. [Google Scholar] [CrossRef]
- Wang, K.-T.; Chen, L.-G.; Tseng, S.-H.; Huang, J.-S.; Hsieh, M.-S.; Wang, C.-C. Anti-inflammatory effects of resveratrol and oligostilbenes from Vitis thunbergii var. taiwaniana against lipopolysaccharide-induced arthritis. J. Agric. Food Chem. 2011, 59, 3649–3656. [Google Scholar] [CrossRef]
- Hu, J.; Lin, T.; Xu, J.; Ding, R.; Wang, G.; Shen, R.; Zhang, Y.-W.; Chen, H. Polyphenols isolated from leaves of Vitis thunbergii var. taiwaniana regulate APP related pathway. Bioorg. Med. Chem. Lett. 2016, 26, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Oshima, Y.; Kamijou, A.; Ohizumi, Y.; Niwa, M.; Ito, J.; Hisamichi, K.; Takeshita, M. Novel oligostilbenes from Vitis coignetiae. Tetrahedron 1995, 51, 11979–11986. [Google Scholar] [CrossRef]
- Anna Malinowska, M.; Billet, K.; Drouet, S.; Munsch, T.; Unlubayir, M.; Tungmunnithum, D.; Giglioli-Guivarc’h, N.; Hano, C.; Lanoue, A. Grape Cane Extracts as Multifunctional Rejuvenating Cosmetic Ingredient: Evaluation of Sirtuin Activity, Tyrosinase Inhibition and Bioavailability Potential. Molecules 2020, 25, 2203. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.H.; Oh, K.-E.; Jo, Y.H.; Ahn, J.H.; Liu, Q.; Turk, A.; Jang, J.Y.; Hwang, B.Y.; Lee, K.Y.; Lee, M.K. Characterization of tyrosinase inhibitory constituents from the aerial parts of Humulus japonicus using LC-MS/MS coupled online assay. Bioorg. Med. Chem. 2018, 26, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Kim, C.; Jo, Y.H.; Kim, S.B.; Hwang, B.Y.; Lee, M.K. Synthesis and biological evaluation of resveratrol derivatives as melanogenesis inhibitors. Molecules 2015, 20, 16933–16945. [Google Scholar] [CrossRef] [PubMed]
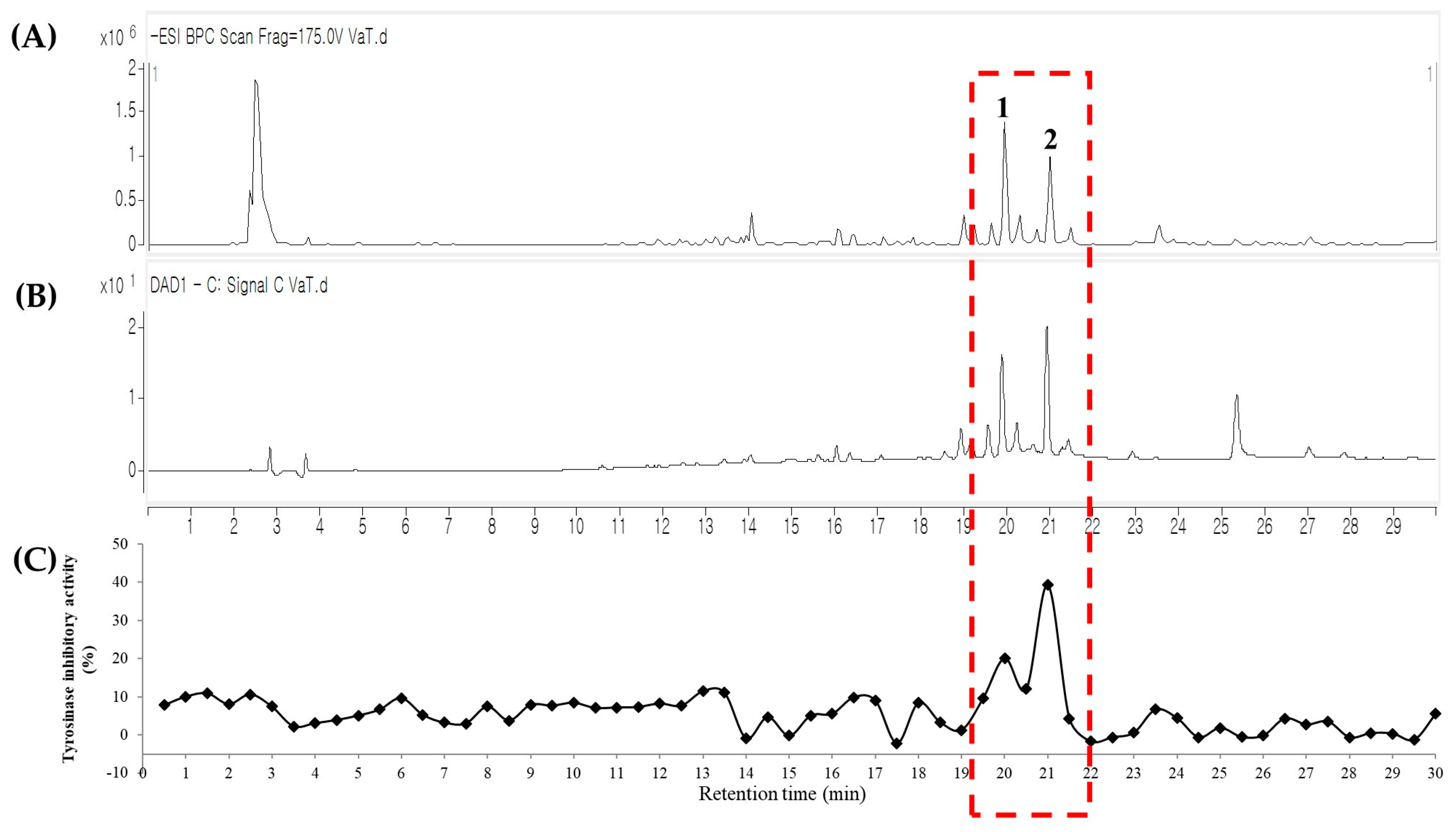
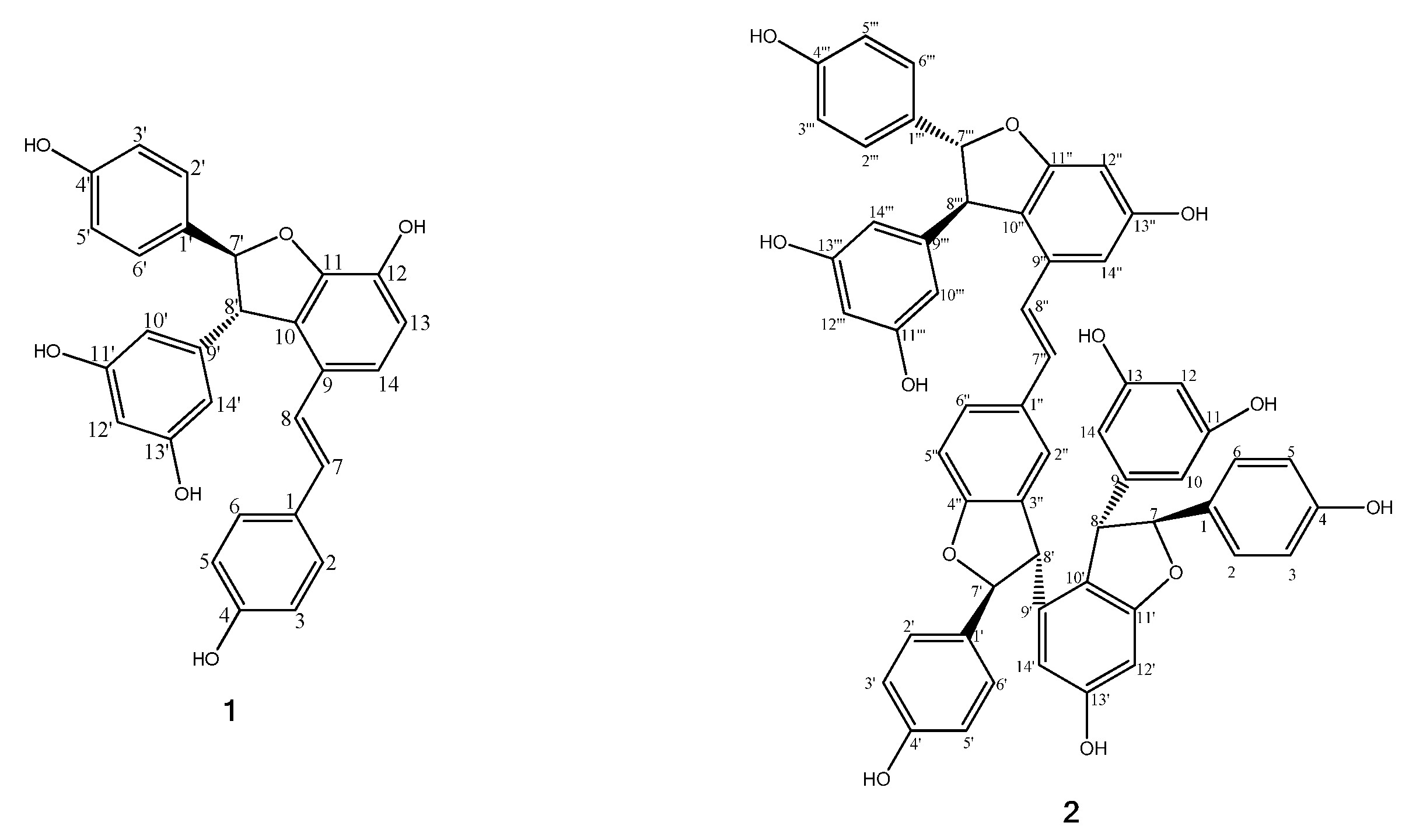
| Peak | Compound Identified | tR (mins) | Observed m/z | Calculated m/z | Molecular Formula [M-H]− | MS/MS Fragments (m/z) | UV (λmax, nm) | IC50 (µM) a | Total Score b |
|---|---|---|---|---|---|---|---|---|---|
| 1 | ε-viniferin | 19.837 | 453.1331 | 453.1344 | C28H21O6 | 359[M-C6H6O-H], 347[M-C7H6O-H], 225[M-C8H7O-C6H5O-H] | 284, 330 | 3.51 ± 0.1 | 8.5024 |
| 2 | vitisin B | 21.328 | 905.2894 | 905.2604 | C56H41O12 | 811[M-C6H6O-H] | 243, 313, 285 | 10.74 ± 1.3 | 9.5492 |
| Run | Extraction Time (min) | MeOH Conc. (%) | Solvent Volume (mL) | Yield (%) | Tyrosinase Inhibitory Activity (%) | Compound 1 (µg/mg) | Compound 2 (µg/mg) | Sum of Compounds 1 and 2 (µg/mg) |
|---|---|---|---|---|---|---|---|---|
| 1 | 40 | 40 | 87.5 | 2.60 | 49.79 | −0.28 | −4.15 | −4.44 |
| 2 | 100 | 40 | 87.5 | 3.47 | 73.34 | 0.69 | 3.59 | 4.28 |
| 3 | 40 | 100 | 87.5 | 2.64 | 88.99 | 18.53 | 64.52 | 83.05 |
| 4 | 100 | 100 | 87.5 | 3.28 | 88.96 | 14.38 | 94.03 | 108.41 |
| 5 | 40 | 70 | 35 | 2.86 | 80.29 | 6.70 | 13.51 | 20.21 |
| 6 | 100 | 70 | 35 | 4.01 | 82.68 | 5.95 | 25.01 | 30.96 |
| 7 | 40 | 70 | 140 | 5.16 | 80.35 | 6.80 | 28.00 | 34.79 |
| 8 | 100 | 70 | 140 | 6.63 | 84.83 | 5.65 | 26.39 | 32.03 |
| 9 | 70 | 40 | 35 | 3.39 | 62.83 | −0.05 | −2.00 | −2.05 |
| 10 | 70 | 100 | 35 | 2.51 | 88.60 | 43.39 | 64.17 | 107.56 |
| 11 | 70 | 40 | 140 | 4.77 | 71.82 | 2.72 | 13.96 | 16.67 |
| 12 | 70 | 100 | 140 | 3.47 | 89.88 | 11.32 | 78.64 | 89.96 |
| 13 | 70 | 70 | 87.5 | 5.06 | 91.08 | 12.82 | 97.69 | 110.51 |
| 14 | 70 | 70 | 87.5 | 4.18 | 88.40 | 12.23 | 82.92 | 95.15 |
| 15 | 70 | 70 | 87.5 | 4.24 | 87.63 | 11.34 | 78.91 | 90.24 |
| Responses | Optimized Conditions | Composite Desirability (D) | Actual Values | Predicted Values | Predictive Capacity (%) | ||
|---|---|---|---|---|---|---|---|
| Extraction Time (X1, min) | MeOH Concentration (X2, %) | Solvent Volume X3, mL) | |||||
| Yield (%) | 100.00 | 64.78 | 140.00 | 0.94 | 6.19 ± 0.36 | 6.21 | 99.73 |
| Tyrosinase inhibitory activity (%) | 65.22 | 100.00 | 140.00 | 0.98 | 91.72 ± 3.48 | 90.37 | 101.50 |
| Compound 1 (µg/mg) | 65.74 | 100.00 | 35.00 | 0.91 | 36.54 ± 1.78 | 37.45 | 97.58 |
| Compound 2 (µg/mg) | 70.00 | 70.00 | 92.24 | 0.99 | 85.94 ± 16.57 | 86.77 | 99.05 |
| Sum of compounds 1 and 2 (µg/mg) | 75.20 | 100.00 | 35.00 | 0.98 | 108.10 ± 19.11 | 107.93 | 100.15 |
| Multiple response optimization | |||||||
| Yield (%) | 100.00 | 66.38 | 140.00 | 0.93 | 5.95 ± 1.13 | 6.20 | 96.03 |
| Tyrosinase inhibitory activity (%) | 0.91 | 85.93 ± 1.57 | 87.25 | 98.48 | |||
| Extraction Time | MeOH Concentration | Solvent Volume | Yield | Tyrosinase Inhibitory Activity | Compound 1 | Compound 2 | Sum of Compounds 1 and 2 | |
|---|---|---|---|---|---|---|---|---|
| Extraction Time | ||||||||
| MeOH concentration | 0.000 | |||||||
| Solvent volume | 0.000 | 0.000 | ||||||
| Yield | 0.338 | −0.191 | 0.594 * | |||||
| Tyrosinase inhibitory activity | 0.245 | 0.794 *** | 0.100 | 0.223 | ||||
| Compound 1 | −0.045 | 0.741 ** | −0.259 | −0.303 | 0.591 * | |||
| Compound 2 | 0.121 | 0.744 *** | 0.119 | 0.040 | 0.817 *** | 0.586 * | ||
| Sum of compounds 1 and 2 | 0.090 | 0.804 *** | 0.036 | −0.041 | 0.828 *** | 0.735 ** | 0.980 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, K.-E.; Shin, H.; Lee, M.K.; Park, B.; Lee, K.Y. Characterization and Optimization of the Tyrosinase Inhibitory Activity of Vitis amurensis Root Using LC-Q-TOF-MS Coupled with a Bioassay and Response Surface Methodology. Molecules 2021, 26, 446. https://doi.org/10.3390/molecules26020446
Oh K-E, Shin H, Lee MK, Park B, Lee KY. Characterization and Optimization of the Tyrosinase Inhibitory Activity of Vitis amurensis Root Using LC-Q-TOF-MS Coupled with a Bioassay and Response Surface Methodology. Molecules. 2021; 26(2):446. https://doi.org/10.3390/molecules26020446
Chicago/Turabian StyleOh, Kyung-Eon, Hyeji Shin, Mi Kyeong Lee, Byoungduck Park, and Ki Yong Lee. 2021. "Characterization and Optimization of the Tyrosinase Inhibitory Activity of Vitis amurensis Root Using LC-Q-TOF-MS Coupled with a Bioassay and Response Surface Methodology" Molecules 26, no. 2: 446. https://doi.org/10.3390/molecules26020446
APA StyleOh, K.-E., Shin, H., Lee, M. K., Park, B., & Lee, K. Y. (2021). Characterization and Optimization of the Tyrosinase Inhibitory Activity of Vitis amurensis Root Using LC-Q-TOF-MS Coupled with a Bioassay and Response Surface Methodology. Molecules, 26(2), 446. https://doi.org/10.3390/molecules26020446






