Cytokinin Plant Hormones Have Neuroprotective Activity in In Vitro Models of Parkinson’s Disease
Abstract
1. Introduction
2. Results and Discussion
2.1. Cytokinins’ Oxygen Radical Absorbance Capacity (ORAC)
2.2. Differentiation of SH-SY5Y Cells
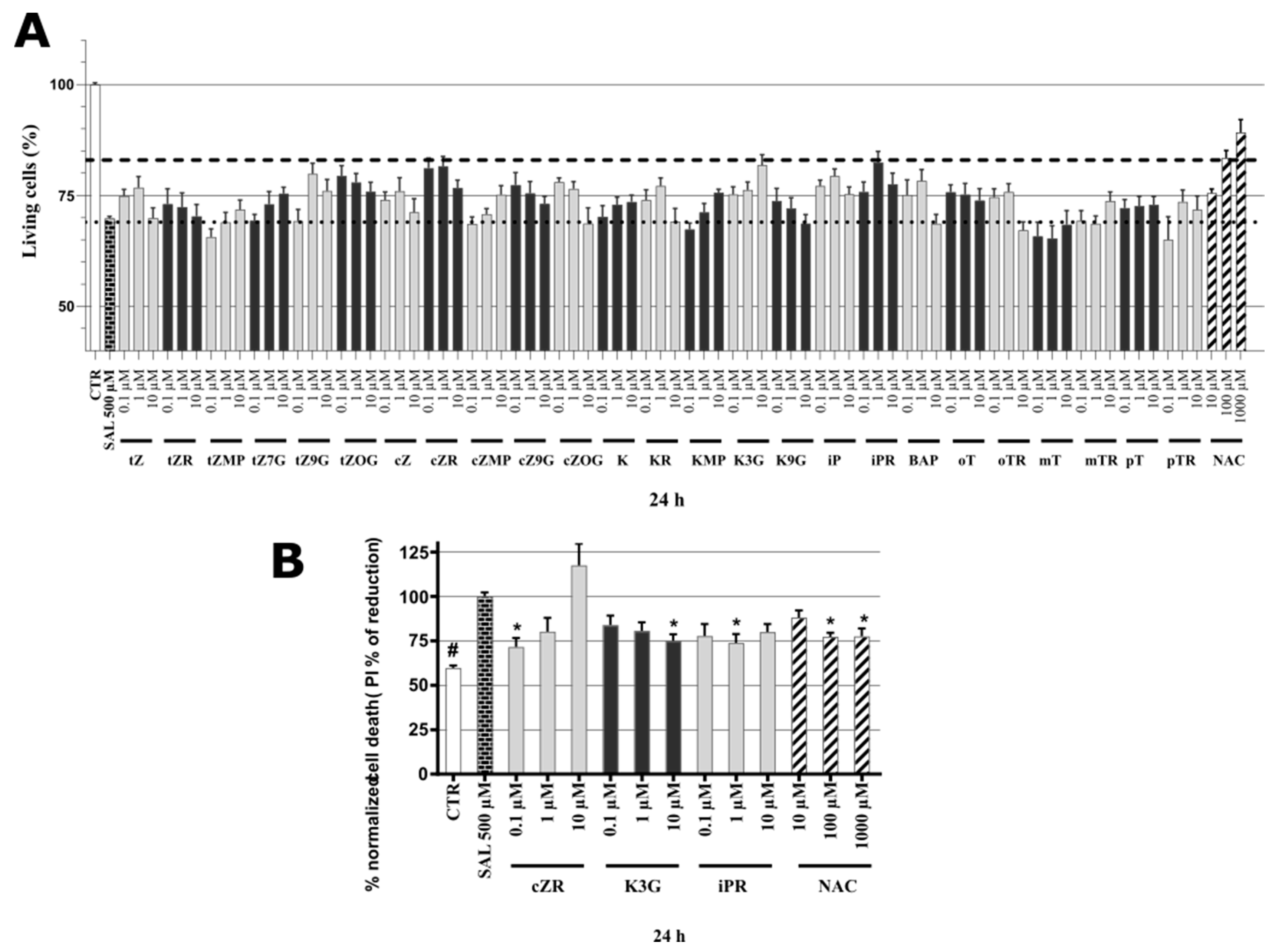
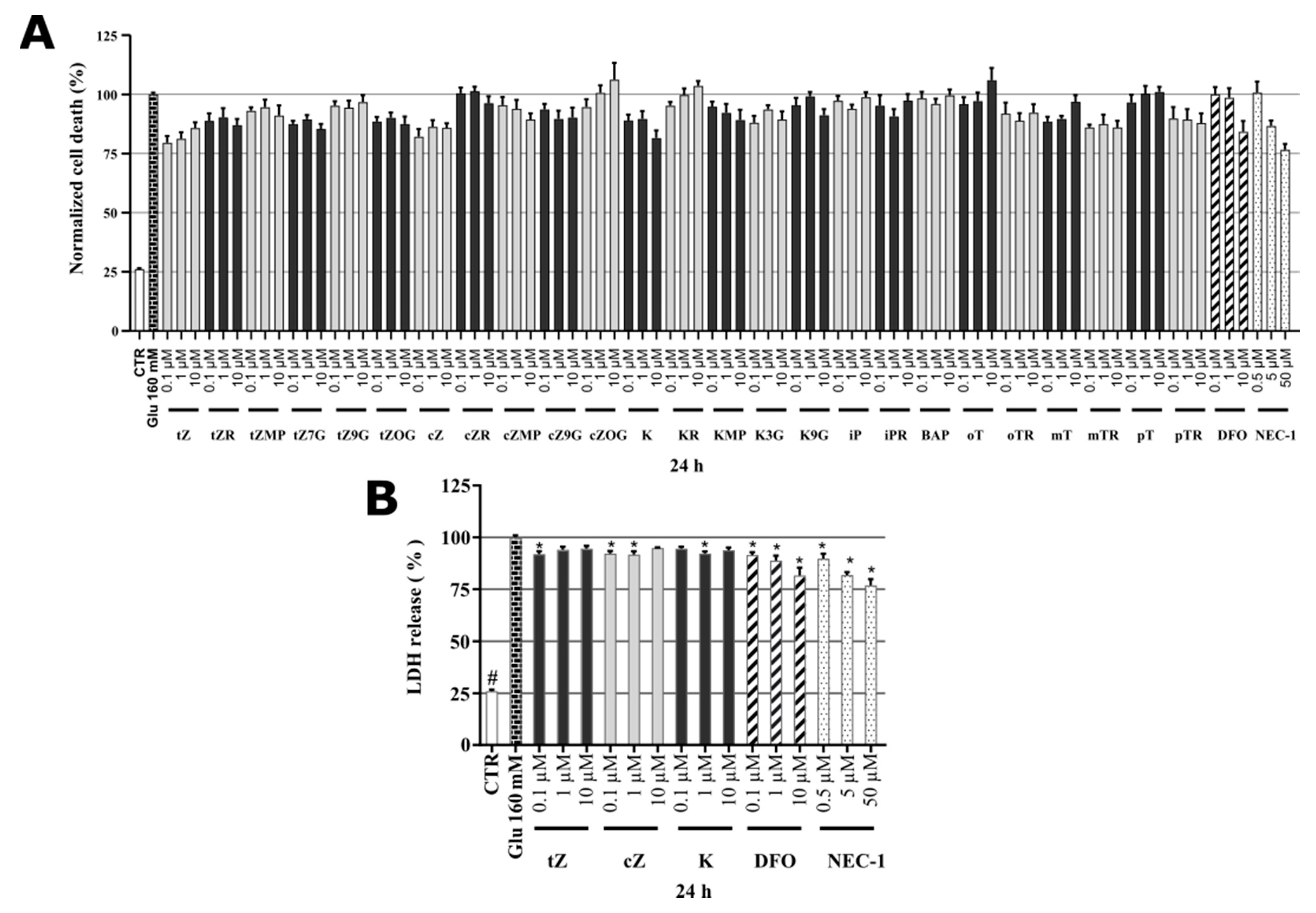
2.3. Cytotoxicity of Cytokinins towards Neuron-like SH-SY5Y Cells
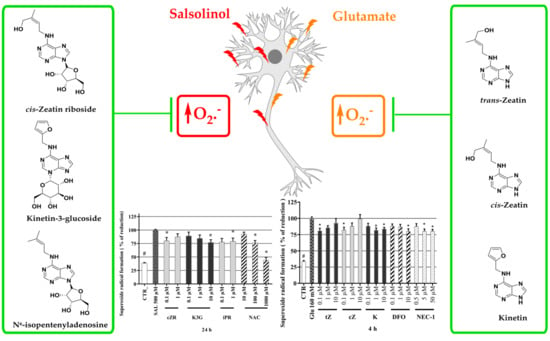
2.4. Identification of Neuroprotective Cytokinins in the SAL-induced Model of PD
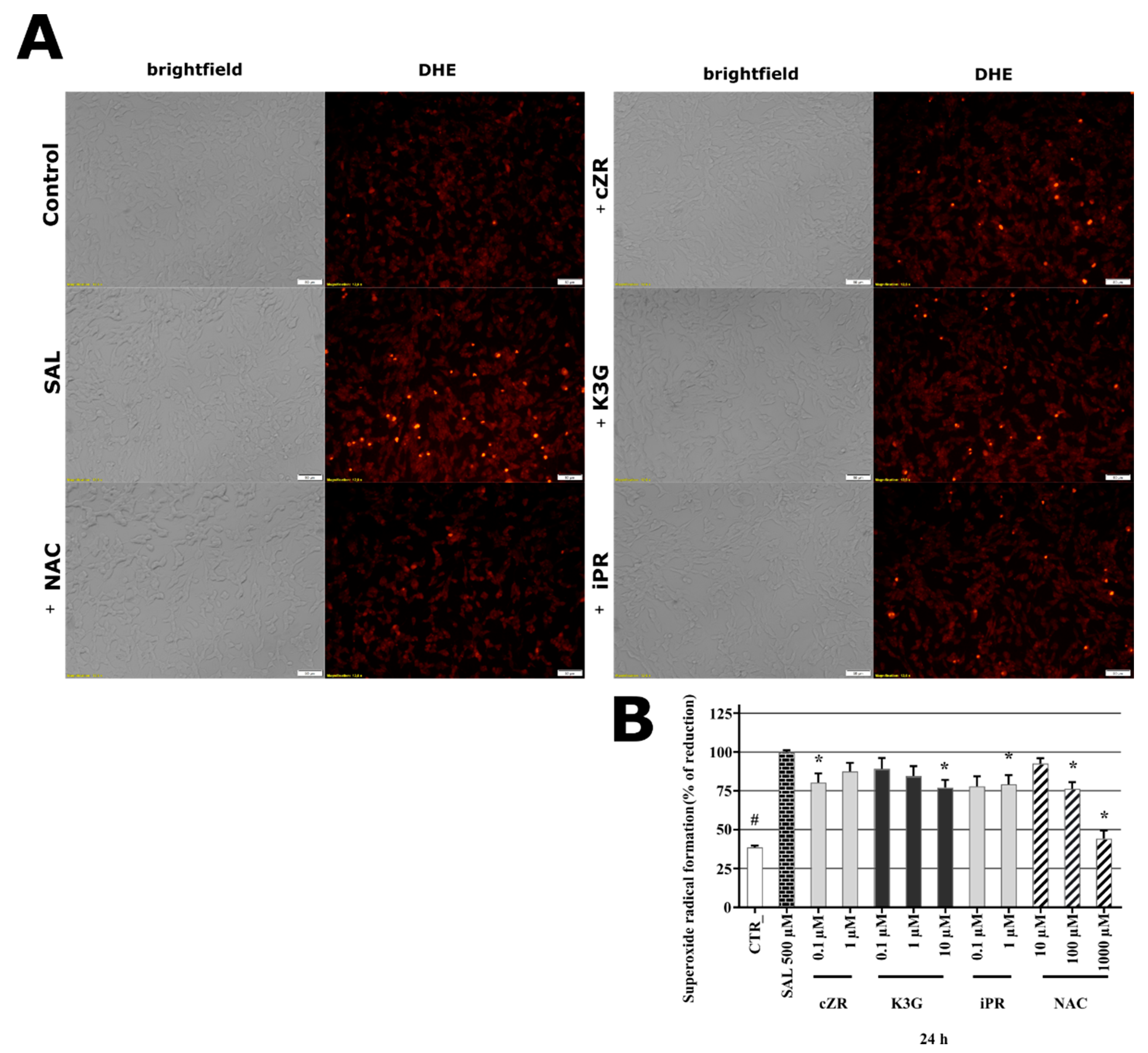
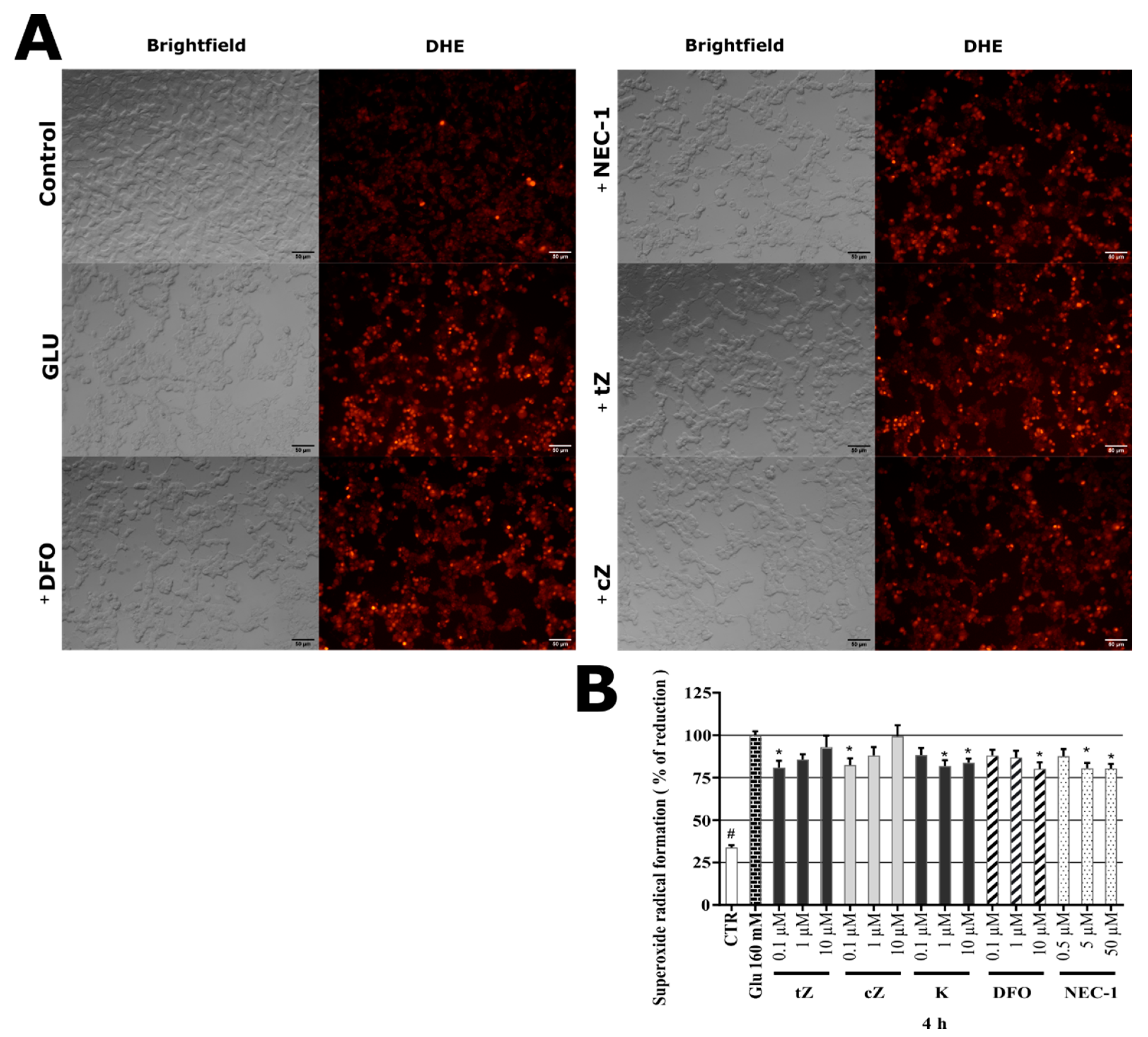
2.5. Cytokinins Decrease SAL-induced Superoxide Radical Formation
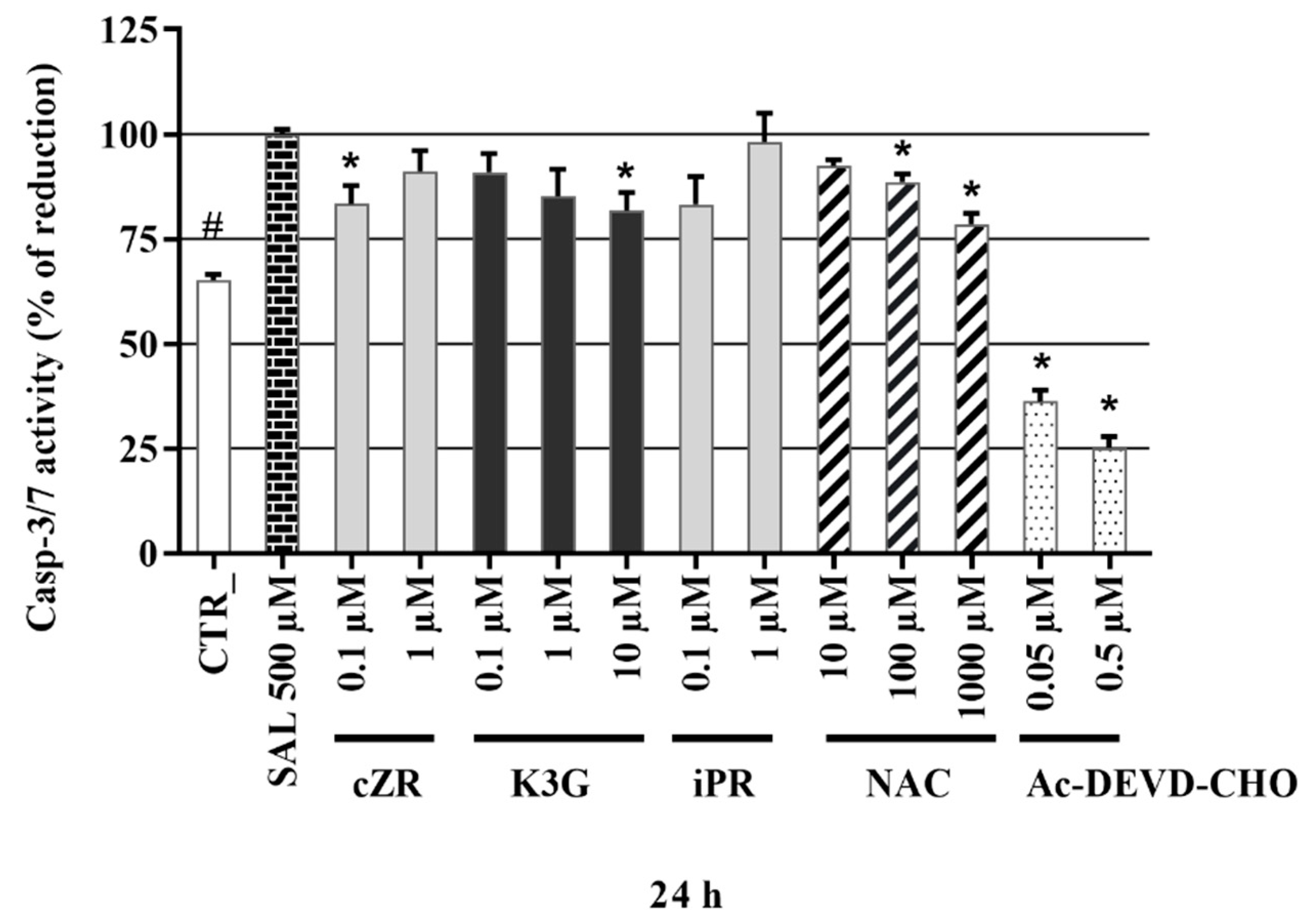
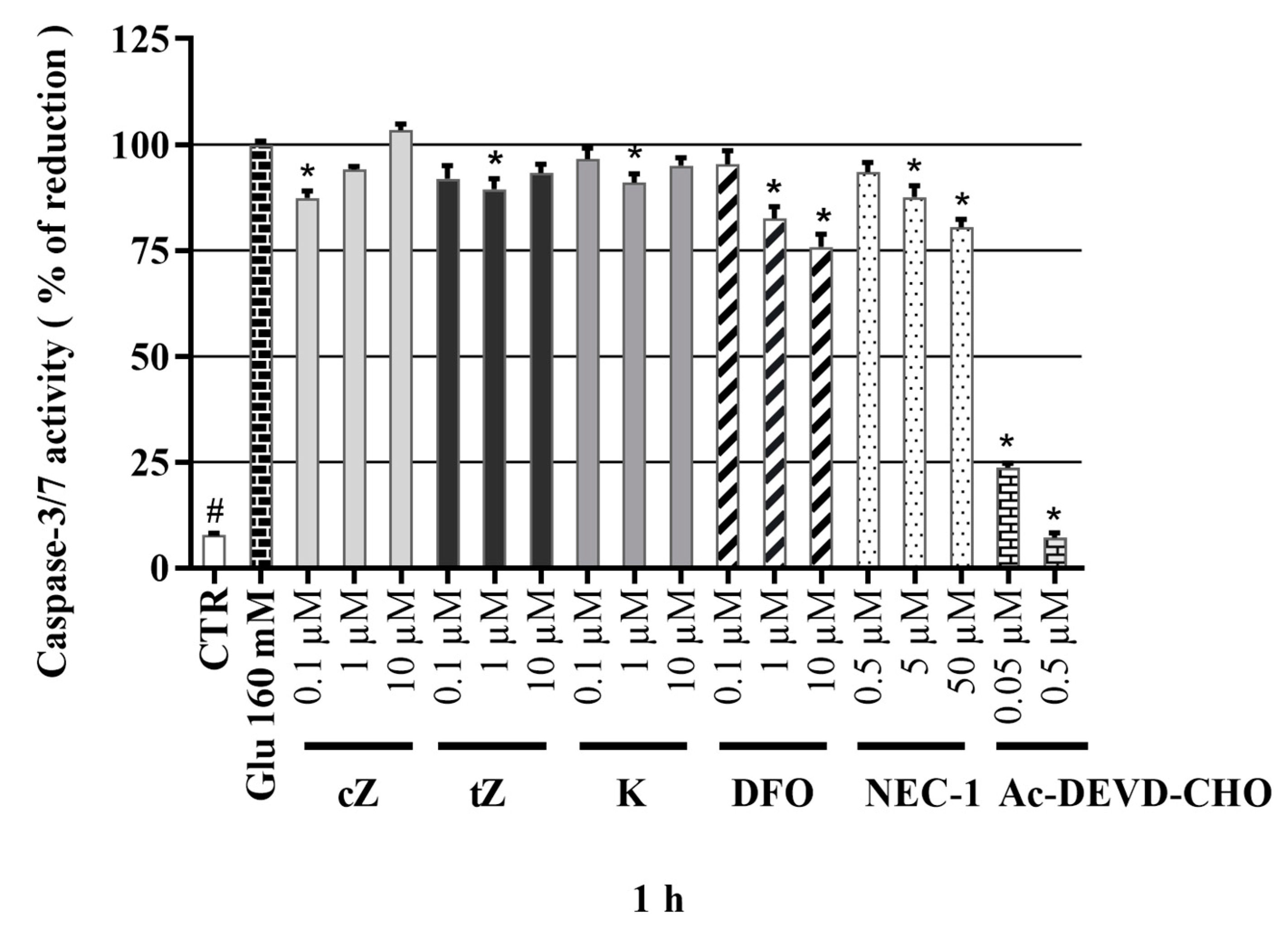
2.6. Anti-Apoptotic Effects of Cytokinins Determined by Caspase-3/7 Activity Measurements
2.7. Identification of Cytokinin Neuroprotective Activity in the Glutamate-induced Cell Death Model
2.8. Effects of Cytokinins on Glu-induced Oxidative Stress in SH-SY5Y cells
2.9. Effects of Cytokinins on Caspase-3/7 Activity in the Glu-induced Cell Death Model
3. Conclusions
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. ORAC Radical Scavenging Activity Assays
4.3. SH-SY5Y Cell Culture
4.4. Microscopy
4.5. Cell Membrane Staining (Neurite Outgrowth kit, Invitrogen™)
4.6. Cell Treatment
4.7. Cell Viability and Cell Death
4.8. Measurement of Oxidative Stress by the Dihydroethidium (DHE) assay
4.9. Measurement of Caspase-3/7 Activity
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Dorsey, E.R.; Sherer, T.; Okun, M.S.; Bloem, B.R. The Emerging Evidence of the Parkinson Pandemic. J. Parkinsons Dis. 2018, 8, S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Rizek, P.; Kumar, N.; Jog, M.S. An update on the diagnosis and treatment of Parkinson disease. CMAJ Can. Med Assoc. J. 2016, 188, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J. Progression of Parkinson disease: Are we making progress in charting the course? Arch. Neurol. 2005, 62, 351–352. [Google Scholar] [CrossRef] [PubMed]
- Sian, J.; Dexter, D.T.; Lees, A.J.; Daniel, S.; Agid, Y.; Javoy-Agid, F.; Jenner, P.; Marsden, C.D. Alterations in glutathione levels in Parkinson’s disease and other neurodegenerative disorders affecting basal ganglia. Ann. Neurol. 1994, 36, 348–355. [Google Scholar] [CrossRef]
- Alam, Z.I.; Daniel, S.E.; Lees, A.J.; Marsden, D.C.; Jenner, P.; Halliwell, B. A generalised increase in protein carbonyls in the brain in Parkinson’s but not incidental Lewy body disease. J. Neurochem. 1997, 69, 1326–1329. [Google Scholar] [CrossRef]
- Alam, Z.I.; Jenner, A.; Daniel, S.E.; Lees, A.J.; Cairns, N.; Marsden, C.D.; Jenner, P.; Halliwell, B. Oxidative DNA damage in the parkinsonian brain: An apparent selective increase in 8-hydroxyguanine levels in substantia nigra. J. Neurochem. 1997, 69, 1196–1203. [Google Scholar] [CrossRef]
- Park, J.-S.; Davis, R.L.; Sue, C.M. Mitochondrial Dysfunction in Parkinson’s Disease: New Mechanistic Insights and Therapeutic Perspectives. Curr. Neurol. Neurosci. Rep. 2018, 18, 21. [Google Scholar] [CrossRef]
- Ambrosi, G.; Cerri, S.; Blandini, F. A further update on the role of excitotoxicity in the pathogenesis of Parkinson’s disease. J. Neural Transm. 2014, 121, 849–859. [Google Scholar] [CrossRef]
- Dantuma, N.P.; Bott, L.C. The ubiquitin-proteasome system in neurodegenerative diseases: Precipitating factor, yet part of the solution. Front. Mol. Neurosci. 2014, 7, 70. [Google Scholar] [CrossRef]
- Cookson, M.R.; Bandmann, O. Parkinson’s disease: Insights from pathways. Hum. Mol. Genet. 2010, 19, R21–R27. [Google Scholar] [CrossRef]
- Rinne, U.K. Problems associated with long-term levodopa treatment of Parkinson’s disease. Acta Neurol. Scand. Suppl. 1983, 95, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Corona, J.C. Natural Compounds for the Management of Parkinson’s Disease and Attention-Deficit/Hyperactivity Disorder. BioMed Res. Int. 2018, 2018, 4067597. [Google Scholar] [CrossRef] [PubMed]
- Kieber, J.J. Tribute to Folke Skoog: Recent Advances in our Understanding of Cytokinin Biology. J. Plant Growth Regul. 2002, 21, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Voller, J.; Maková, B.; Kadlecová, A.; Gonzalez, G.; Strnad, M. Plant Hormone Cytokinins for Modulating Human Aging and Age-Related Diseases. In Hormones in Ageing and Longevity; Rattan, S., Sharma, R., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 311–335. [Google Scholar]
- Rattan, S.I.; Sodagam, L. Gerontomodulatory and youth-preserving effects of zeatin on human skin fibroblasts undergoing aging in vitro. Rejuvenation Res 2005, 8, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Z.; Yang, X. Kinetin protects against lipid peroxidation and improves antioxidant status in cultured astrocytes and mouse brain exposed to D-galactose. Afr. J. Biotechnol. 2011, 10, 11721–11727. [Google Scholar]
- Hertz, N.T.; Berthet, A.; Sos, M.L.; Thorn, K.S.; Burlingame, A.L.; Nakamura, K.; Shokat, K.M. A neo-substrate that amplifies catalytic activity of parkinson’s-disease-related kinase PINK1. Cell 2013, 154, 737–747. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, D.; Zheng, Y.; Hao, C.; Li, H.; Ouyang, W. Neuroprotective Effects of Kinetin Against Glutamate-Induced Oxidative Cytotoxicity in HT22 Cells: Involvement of Nrf2 and Heme Oxygenase-1. Neurotox. Res. 2018, 33, 725–737. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Yang, Y.-C.; Huang, C.-L.; Kuo, T.-Y.; Lin, J.-H.; Yang, D.-M.; Huang, N.-K. When Cytokinin, a Plant Hormone, Meets the Adenosine A2A Receptor: A Novel Neuroprotectant and Lead for Treating Neurodegenerative Disorders? PLoS ONE 2012, 7, e38865. [Google Scholar] [CrossRef]
- Brizzolari, A.; Marinello, C.; Carini, M.; Santaniello, E.; Biondi, P.A. Evaluation of the antioxidant activity and capacity of some natural N6-substituted adenine derivatives (cytokinins) by fluorimetric and spectrophotometric assays. J. Chromatogr. B 2016, 1019, 164–168. [Google Scholar] [CrossRef]
- McDaniel, D.H.; Neudecker, B.A.; DiNardo, J.C.; Lewis Ii, J.A.; Maibach, H.I. Idebenone: A new antioxidant—Part I. Relative assessment of oxidative stress protection capacity compared to commonly known antioxidants. J. Cosmet. Dermatol. 2005, 4, 10–17. [Google Scholar] [CrossRef]
- Dassano, A.; Mancuso, M.; Giardullo, P.; De Cecco, L.; Ciuffreda, P.; Santaniello, E.; Saran, A.; Dragani, T.A.; Colombo, F. N(6)-isopentenyladenosine and analogs activate the NRF2-mediated antioxidant response. Redox Biol. 2014, 2, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Forster, J.I.; Köglsberger, S.; Trefois, C.; Boyd, O.; Baumuratov, A.S.; Buck, L.; Balling, R.; Antony, P.M. Characterization of Differentiated SH-SY5Y as Neuronal Screening Model Reveals Increased Oxidative Vulnerability. J. Biomol. Screen. 2016, 21, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Dwane, S.; Durack, E.; Kiely, P.A. Optimising parameters for the differentiation of SH-SY5Y cells to study cell adhesion and cell migration. BMC Res. Notes 2013, 6, 366. [Google Scholar] [CrossRef] [PubMed]
- Xicoy, H.; Wieringa, B.; Martens, G.J.M. The SH-SY5Y cell line in Parkinson’s disease research: A systematic review. Mol. Neurodegener. 2017, 12, 10. [Google Scholar] [CrossRef]
- Kurnik-Łucka, M.; Panula, P.; Bugajski, A.; Gil, K. Salsolinol: An Unintelligible and Double-Faced Molecule—Lessons Learned from In Vivo and In Vitro Experiments. Neurotox. Res. 2018, 33, 485–514. [Google Scholar] [CrossRef]
- Li, C.; Chai, S.; Ju, Y.; Hou, L.; Zhao, H.; Ma, W.; Li, T.; Sheng, J.; Shi, W. Pu-erh Tea Protects the Nervous System by Inhibiting the Expression of Metabotropic Glutamate Receptor 5. Mol. Neurobiol. 2017, 54, 5286–5299. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- McBean, G.J.; López, M.G.; Wallner, F.K. Redox-based therapeutics in neurodegenerative disease. Br. J. Pharmacol. 2017, 174, 1750–1770. [Google Scholar] [CrossRef]
- Cheung, Y.-T.; Lau, W.K.-W.; Yu, M.-S.; Lai, C.S.-W.; Yeung, S.-C.; So, K.-F.; Chang, R.C.-C. Effects of all-trans-retinoic acid on human SH-SY5Y neuroblastoma as in vitro model in neurotoxicity research. Neurotoxicology 2009, 30, 127–135. [Google Scholar] [CrossRef]
- Rárová, L.; Steigerová, J.; Kvasnica, M.; Bartůněk, P.; Křížová, K.; Chodounská, H.; Kolář, Z.; Sedlák, D.; Oklestkova, J.; Strnad, M. Structure activity relationship studies on cytotoxicity and the effects on steroid receptors of AB-functionalized cholestanes. J. Steroid Biochem. Mol. Biol. 2016, 159, 154–169. [Google Scholar] [CrossRef]
- Voller, J.; Zatloukal, M.; Lenobel, R.; Dolezal, K.; Béres, T.; Krystof, V.; Spíchal, L.; Niemann, P.; Dzubák, P.; Hajdúch, M.; et al. Anticancer activity of natural cytokinins: A structure-activity relationship study. Phytochemistry 2010, 71, 1350–1359. [Google Scholar] [CrossRef] [PubMed]
- Texel, S.J.; Zhang, J.; Camandola, S.; Unger, E.L.; Taub, D.D.; Koehler, R.C.; Harris, Z.L.; Mattson, M.P. Ceruloplasmin Deficiency Reduces Levels of Iron and BDNF in the Cortex and Striatum of Young Mice and Increases Their Vulnerability to Stroke. PLoS ONE 2011, 6, e25077. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Yang, H.; Yang, M.; Yanagisawa, D.; Bellier, J.-P.; Mori, M.; Takahata, S.; Nonaka, T.; Zhao, S.; Tooyama, I. Mitochondrial ferritin protects SH-SY5Y cells against H2O2-induced oxidative stress and modulates α-synuclein expression. Exp. Neurol. 2017, 291, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, N.; Aki, T.; Funakoshi, T.; Noritake, K.; Unuma, K.; Uemura, K. Necrosis in human neuronal cells exposed to paraquat. J. Toxicol. Sci. 2018, 43, 193–202. [Google Scholar] [CrossRef]
- Ito, K.; Eguchi, Y.; Imagawa, Y.; Akai, S.; Mochizuki, H.; Tsujimoto, Y. MPP+ induces necrostatin-1- and ferrostatin-1-sensitive necrotic death of neuronal SH-SY5Y cells. Cell Death Discov. 2017, 3, 17013. [Google Scholar] [CrossRef]
- Wanpen, S.; Govitrapong, P.; Shavali, S.; Sangchot, P.; Ebadi, M. Salsolinol, a dopamine-derived tetrahydroisoquinoline, induces cell death by causing oxidative stress in dopaminergic SH-SY5Y cells, and the said effect is attenuated by metallothionein. Brain Res. 2004, 1005, 67–76. [Google Scholar] [CrossRef]
- Dengler, W.A.; Schulte, J.; Berger, D.P.; Mertelsmann, R.; Fiebig, H.H. Development of a propidium iodide fluorescence assay for proliferation and cytotoxicity assays. Anti-Cancer Drugs 1995, 6, 522–532. [Google Scholar] [CrossRef]
- Othman, E.M.; Naseem, M.; Awad, E.; Dandekar, T.; Stopper, H. The Plant Hormone Cytokinin Confers Protection against Oxidative Stress in Mammalian Cells. PLoS ONE 2016, 11, e0168386. [Google Scholar] [CrossRef]
- Yap, Y.; Omasanggar, R.; Koh, Y.L.; Yew, M.Y.; Lai, H.T.; Ling, A.P.K.; Chye, S.M.; Ng, K.Y.; Koh, R.Y. Neurotoxic effect of salsolinol through oxidative stress induction and Nrf2-Keap1 signalling regulation. J. Chem. Pharm. Res. 2016, 8, 30–38. [Google Scholar]
- Bindokas, V.P.; Jordan, J.; Lee, C.C.; Miller, R.J. Superoxide production in rat hippocampal neurons: Selective imaging with hydroethidine. J. Neurosci. 1996, 16, 1324–1336. [Google Scholar] [CrossRef]
- Carter, W.O.; Narayanan, P.K.; Robinson, J.P. Intracellular hydrogen peroxide and superoxide anion detection in endothelial cells. J. Leukoc. Biol. 1994, 55, 253–258. [Google Scholar] [PubMed]
- Wanpen, S.; Kooncumchoo, P.; Shavali, S.; Govitrapong, P.; Ebadi, M. Salsolinol, an endogenous neurotoxin, activates JNK and NF-kappaB signaling pathways in human neuroblastoma cells. Neurochem. Res. 2007, 32, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska-Trypuć, A.; Matejczyk, M.; Czerpak, R. N6-benzyladenine and kinetin influence antioxidative stress parameters in human skin fibroblasts. Mol. Cell. Biochem. 2016, 413, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.; Czapski, G. SOD-like activity studies of cytokinin-copper(II) complexes. Free Radic. Res. Commun. 1991, 12, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Štarha, P.; Trávníček, Z.; Herchel, R.; Popa, I.; Suchý, P.; Vančo, J. Dinuclear copper(II) complexes containing 6-(benzylamino)purines as bridging ligands: Synthesis, characterization, and in vitro and in vivo antioxidant activities. J. Inorg. Biochem. 2009, 103, 432–440. [Google Scholar] [CrossRef] [PubMed]
- De Lazzari, F.; Bubacco, L.; Whitworth, A.J.; Bisaglia, M. Superoxide Radical Dismutation as New Therapeutic Strategy in Parkinson’s Disease. Aging Dis. 2018, 9, 716–728. [Google Scholar] [CrossRef]
- Lalkovičová, M.; Danielisová, V. Neuroprotection and antioxidants. Neural Regen. Res. 2016, 11, 865–874. [Google Scholar] [CrossRef]
- Surendran, S.; Raja Sankar, S. Parkinson’s disease: Oxidative stress and therapeutic approaches. Neurol. Sci. 2010, 31, 531–540. [Google Scholar] [CrossRef]
- Lee, K.H.; Cha, M.; Lee, B.H. Neuroprotective Effect of Antioxidants in the Brain. Int. J. Mol. Sci. 2020, 21, 7152. [Google Scholar] [CrossRef]
- Bollimuntha, S.; Ebadi, M.; Singh, B.B. TRPC1 protects human SH-SY5Y cells against salsolinol-induced cytotoxicity by inhibiting apoptosis. Brain Res. 2006, 1099, 141–149. [Google Scholar] [CrossRef]
- Walsh, J.G.; Cullen, S.P.; Sheridan, C.; Lüthi, A.U.; Gerner, C.; Martin, S.J. Executioner caspase-3 and caspase-7 are functionally distinct proteases. Proc. Natl. Acad. Sci. USA 2008, 105, 12815. [Google Scholar] [CrossRef] [PubMed]
- Jantas, D.; Piotrowski, M.; Lason, W. An Involvement of PI3-K/Akt Activation and Inhibition of AIF Translocation in Neuroprotective Effects of Undecylenic Acid (UDA) Against Pro-Apoptotic Factors-Induced Cell Death in Human Neuroblastoma SH-SY5Y Cells. J. Cell. Biochem. 2015, 116, 2882–2895. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.; Tamas, A.; Reglödi, D.; Tizabi, Y. PACAP Protects Against Salsolinol-Induced Toxicity in Dopaminergic SH-SY5Y Cells: Implication for Parkinson’s Disease. J. Mol. Neurosci. 2013, 50, 600–607. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cheng, B.; Anand, P.; Kuang, A.; Akhtar, F.; Scofield, V.L. N-Acetylcysteine in Combination with IGF-1 Enhances Neuroprotection against Proteasome Dysfunction-Induced Neurotoxicity in SH-SY5Y Cells. Parkinsons Dis. 2016, 2016, 6564212. [Google Scholar] [CrossRef]
- Li, J.; Meng, Z.; Zhang, G.; Xing, Y.; Feng, L.; Fan, S.; Fan, F.; Buren, B.; Liu, Q. N-acetylcysteine relieves oxidative stress and protects hippocampus of rat from radiation-induced apoptosis by inhibiting caspase-3. Biomed. Pharmacother. 2015, 70, 1–6. [Google Scholar] [CrossRef]
- Rakshit, J.; Mallick, A.; Roy, S.; Sarbajna, A.; Dutta, M.; Bandyopadhyay, J. Iron-Induced Apoptotic Cell Death and Autophagy Dysfunction in Human Neuroblastoma Cell Line SH-SY5Y. Biol. Trace Elem. Res. 2019, 193, 138–151. [Google Scholar] [CrossRef]
- Rakshit, J.; Priyam, A.; Gowrishetty, K.K.; Mishra, S.; Bandyopadhyay, J. Iron chelator Deferoxamine protects human neuroblastoma cell line SH-SY5Y from 6-Hydroxydopamine-induced apoptosis and autophagy dysfunction. J. Trace Elem. Med. Biol. 2020, 57, 126406. [Google Scholar] [CrossRef]
- Ma, X.W.; Guo, R.Y. Dose-dependent effect of Curcuma longa for the treatment of Parkinson’s disease. Exp. Ther. Med. 2017, 13, 1799–1805. [Google Scholar] [CrossRef]
- Naoi, M.; Maruyama, W.; Takahashi, T.; Akao, Y.; Nakagawa, Y. Involvement of endogenous N-methyl(R)salsolinol in Parkinson’s disease: Induction of apoptosis and protection by (-)deprenyl. In Advances in Research on Neurodegeneration; Mizuno, Y., Calne, D.B., Horowski, R., Poewe, W., Riederer, P., Youdim, M.B.H., Eds.; Springer: Vienna, Austria, 2000; pp. 111–121. [Google Scholar]
- Kulikov, A.V.; Rzhaninova, A.A.; Goldshtein, D.V.; Boldyrev, A.A. Expression of NMDA receptors in multipotent stromal cells of human adipose tissue under conditions of retinoic acid-induced differentiation. Bull. Exp. Biol. Med. 2007, 144, 626–629. [Google Scholar] [CrossRef]
- Kritis, A.A.; Stamoula, E.G.; Paniskaki, K.A.; Vavilis, T.D. Researching glutamate—Induced cytotoxicity in different cell lines: A comparative/collective analysis/study. Front. Cell. Neurosci. 2015, 9, 91. [Google Scholar] [CrossRef]
- Sun, X.; Shi, X.; Lu, L.; Jiang, Y.; Liu, B. Stimulus-dependent neuronal cell responses in SH-SY5Y neuroblastoma cells. Mol. Med. Rep. 2016, 13, 2215–2220. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.P.; Lieberknecht, V.; Ramos-Hryb, A.B.; Olescowicz, G.; Ludka, F.K.; Tasca, C.I.; Gabilan, N.H.; Rodrigues, A.L.S. Creatine affords protection against glutamate-induced nitrosative and oxidative stress. Neurochem. Int. 2016, 95, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Mou, Y.; Wang, J.; Wu, J.; He, D.; Zhang, C.; Duan, C.; Li, B. Ferroptosis, a new form of cell death: Opportunities and challenges in cancer. J. Hematol. Oncol. 2019, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Zille, M.; Kumar, A.; Kundu, N.; Bourassa, M.W.; Wong, V.S.C.; Willis, D.; Karuppagounder, S.S.; Ratan, R.R. Ferroptosis in Neurons and Cancer Cells Is Similar But Differentially Regulated by Histone Deacetylase Inhibitors. eNeuro 2019, 6. [Google Scholar] [CrossRef]
- Jantas, D.; Chwastek, J.; Grygier, B.; Lasoń, W. Neuroprotective Effects of Necrostatin-1 Against Oxidative Stress–Induced Cell Damage: An Involvement of Cathepsin D Inhibition. Neurotox. Res. 2020, 37, 525–542. [Google Scholar] [CrossRef]
- Sun, Z.W.; Zhang, L.; Zhu, S.J.; Chen, W.C.; Mei, B. Excitotoxicity effects of glutamate on human neuroblastoma SH-SY5Y cells via oxidative damage. Neurosci. Bull. 2010, 26, 8–16. [Google Scholar] [CrossRef]
- Chu, J.; Liu, C.-X.; Song, R.; Li, Q.-L. Ferrostatin-1 protects HT-22 cells from oxidative toxicity. Neural Regen. Res. 2020, 15, 528–536. [Google Scholar]
- Xu, X.; Chua, C.C.; Kong, J.; Kostrzewa, R.M.; Kumaraguru, U.; Hamdy, R.C.; Chua, B.H. Necrostatin-1 protects against glutamate-induced glutathione depletion and caspase-independent cell death in HT-22 cells. J. Neurochem. 2007, 103, 2004–2014. [Google Scholar] [CrossRef]
- Shirlee, T.; David, S.; Pamela, M. Oxytosis: A Novel Form of Programmed Cell Death. Curr. Top. Med. Chem. 2001, 1, 497–506. [Google Scholar] [CrossRef]
- Nikolova, S.; Lee, Y.S.; Lee, Y.-S.; Kim, J.-A. Rac1-NADPH oxidase-regulated generation of reactive oxygen species mediates glutamate-induced apoptosis in SH-SY5Y human neuroblastoma cells. Free Radic. Res. 2005, 39, 1295–1304. [Google Scholar] [CrossRef]
- Jelinek, A.; Heyder, L.; Daude, M.; Plessner, M.; Krippner, S.; Grosse, R.; Diederich, W.; Culmsee, C. Mitochondrial rescue prevents glutathione peroxidase-dependent ferroptosis. Free Radic. Biol. Med. 2018, 117, 45–57. [Google Scholar] [CrossRef]
- Olsen, A.; Siboska, G.E.; Clark, B.F.C.; Rattan, S.I.S. N6-Furfuryladenine, Kinetin, Protects against Fenton Reaction-Mediated Oxidative Damage to DNA. Biochem. Biophys. Res. Commun. 1999, 265, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.P.; Kaur, J.; Rattan, S.I.S. Increased longevity of kinetin-fed Zaprionus fruitflies is accompanied by their reduced fecundity and enhanced catalase activity. IUBMB Life 1997, 41, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Jeong, C.H.; Choi, S.G.; Chun, J.Y.; Kim, Y.J.; Lee, J.; Shin, D.H.; Heo, H.J. Zeatin prevents amyloid beta-induced neurotoxicity and scopolamine-induced cognitive deficits. J. Med. Food 2009, 12, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-J.; Han, A.R.; Kim, E.-A.; Yang, J.W.; Ahn, J.-Y.; Na, J.-M.; Cho, S.-W. KHG21834 attenuates glutamate-induced mitochondrial damage, apoptosis, and NLRP3 inflammasome activation in SH-SY5Y human neuroblastoma cells. Eur. J. Pharmacol. 2019, 856, 172412. [Google Scholar] [CrossRef] [PubMed]
- Yuksel, T.N.; Yayla, M.; Halici, Z.; Cadirci, E.; Polat, B.; Kose, D. Protective effect of 5-HT7 receptor activation against glutamate-induced neurotoxicity in human neuroblastoma SH-SY5Y cells via antioxidative and antiapoptotic pathways. Neurotoxicol. Teratol. 2019, 72, 22–28. [Google Scholar] [CrossRef]
- Lee, H.J.; Spandidos, D.A.; Tsatsakis, A.; Margina, D.; Izotov, B.N.; Yang, S.H. Neuroprotective effects of Scrophularia buergeriana extract against glutamate-induced toxicity in SH-SY5Y cells. Int. J. Mol. Med. 2019, 43, 2144–2152. [Google Scholar] [CrossRef]
- Hu, Y.; Li, J.; Liu, P.; Chen, X.; Guo, D.-H.; Li, Q.-S.; Rahman, K. Protection of SH-SY5Y Neuronal Cells from Glutamate-Induced Apoptosis by 3,6′-Disinapoyl Sucrose, a Bioactive Compound Isolated from Radix Polygala. J. Biomed. Biotechnol. 2012, 2012, 728342. [Google Scholar] [CrossRef]
- Geng, N.; Shi, B.J.; Li, S.L.; Zhong, Z.Y.; Li, Y.C.; Xua, W.L.; Zhou, H.; Cai, J.H. Knockdown of ferroportin accelerates erastin-induced ferroptosis in neuroblastoma cells. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 3826–3836. [Google Scholar]
- Krishnamurthy, P.; Mays, J.; Bijur, G.; Johnson, G. Transient oxidative stress in SH-SY5Y human neuroblastoma cells results in caspase dependent and independent cell death and tau proteolysis. J. Neurosci. Res. 2000, 61, 515–523. [Google Scholar] [CrossRef]
- Stone, W.L.; Qui, M.; Smith, M. Lipopolysaccharide enhances the cytotoxicity of 2-chloroethyl ethyl sulfide. BMC Cell Biol. 2003, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, R.A.; Stamm, N.B.; Patel, B.K.R. One-step cellular caspase-3/7 assay. BioTechniques 2003, 34, 1064–1067. [Google Scholar] [CrossRef] [PubMed]
- Hammer, O.; Harper, D.; Ryan, P. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]

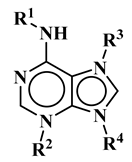
| R1 | R2 | R3 | R4 | Trivial Name | Abbreviation |
|---|---|---|---|---|---|
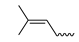 | - | - | H | N6-isopentenyladenine | iP |
| - | - | ribosyl | N6-isopentenyladenosine | iPR | |
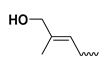 | - | - | H | trans-zeatin | tZ |
| - | - | ribosyl | t-zeatin riboside | tZR | |
| - | glycosyl | - | t-zeatin-7-glucoside | tZ7G | |
| - | - | glycosyl | t-zeatin-9-glucoside | tZ9G | |
| - | - | ribonucleotide | tZR-5‘-monophosphate | tZMP | |
 | - | - | - | t-zeatin-O-glucoside | tZOG |
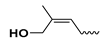 | - | - | H | cis-zeatin | cZ |
| - | - | ribosyl | c-zeatin riboside | cZR | |
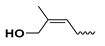 | - | - | glycosyl | c-zeatin-9-glucoside | cZ9G |
| - | - | ribonucleotide | cZR-5‘-monophosphate | cZMP | |
 | - | - | - | c-zeatin-O-glucoside | cZOG |
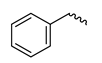 | - | - | H | 6-benzylaminopurine | BAP |
 | - | - | H | meta-topolin | mT |
| - | - | ribosyl | meta-topolin riboside | mTR | |
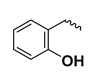 | - | - | H | ortho-topolin | oT |
| - | - | ribosyl | ortho-topolin riboside | oTR | |
 | - | - | H | para-topolin | pT |
| - | - | ribosyl | para-topolin riboside | pTR | |
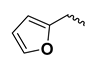 | - | - | H | kinetin | K |
| - | - | ribosyl | kinetin riboside | KR | |
| - | - | ribotide | KR-5‘-monophosphate | KMP | |
| glycosyl | - | - | kinetin-3-glucoside | K3G | |
| - | - | glycosyl | kinetin-9-glucoside | K9G | |
 | |||||
| Deferoxamine | DFO | ||||
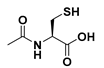 | N-acetylcysteine | NAC | |||
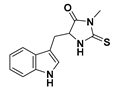 | Necrostatin 1 | NEC-1 | |||
| Average (TE) | SD (n = 3) | Average (TE) | SD (n = 3) | ||
|---|---|---|---|---|---|
| tZ | 0.073 | 0.012 | K | 2.082 | 0.256 |
| tZR | 0.13 | 0.012 | KR | 1.673 | 0.294 |
| tZMP | 0.326 | 0.017 | KMP | 0.726 | 0.026 |
| tZ7G | 0.082 | 0.006 | K3G | 0.53 | 0.035 |
| tZ9G | 0.063 | 0.004 | K9G | 0.906 | 0.025 |
| tZOG | 0.042 | 0.006 | BAP | N/A * | N/A * |
| cZ | 0.151 | 0.009 | oT | 7.026 | 1.179 |
| cZR | 0.178 | 0.005 | oTR | 3.241 | 0.140 |
| cZMP | 0.173 | 0.036 | mT | 4.509 | 0.687 |
| cZ9G | 0.085 | 0.009 | mTR | 3.171 | 0.239 |
| cZOG | 3.301 | 0.036 | pT | 16.799 | 0.829 |
| iP | 0.384 | 0.005 | pTR | 4.147 | 0.238 |
| iPR | 0.224 | 0.024 |
| Compound | Viability a | Compound | Viability a |
|---|---|---|---|
| (%, 10 µM) | (%, 10 µM) | ||
| tZ | 102.3 ± 2.20 | K | 97.9 ± 1.81 |
| tZR | 98.8 ± 1.82 | KR | 88.1 ± 3.09 |
| tZMP | 101.5 ± 4.59 | KMP | 93.5 ± 3.57 |
| tZ7G | 101.4 ± 2.44 | K3G | 99.8 ± 1.13 |
| tZ9G | 97.6 ± 1.59 | K9G | 98.3 ± 1.11 |
| tZOG | 94.3 ± 1.67 | BAP | 98.8 ± 1.40 |
| cZ | 104.0 ± 1.79 | oT | 95.5 ± 4.02 |
| cZR | 100.0 ± 1.23 | oTR | 90.8 ± 3.81 |
| cZMP | 93.8 ± 2.13 | mT | 90.4 ± 6.03 |
| cZ9G | 101.7 ± 2.08 | mTR | 99.5 ± 3.69 |
| cZOG | 98.18 ± 1.59 | pT | 90.6 ± 3.25 |
| iP | 104.5 ± 0.97 | pTR | 89.5 ± 3.89 |
| iPR | 96.9 ± 3.25 | ||
| NAC (1 mM) | 90.2 ± 5.06 | DFO (100µM) | 102.1 ± 4.22 |
| NEC-1 (50 µM) | 95,8 ± 3.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez, G.; Grúz, J.; D’Acunto, C.W.; Kaňovský, P.; Strnad, M. Cytokinin Plant Hormones Have Neuroprotective Activity in In Vitro Models of Parkinson’s Disease. Molecules 2021, 26, 361. https://doi.org/10.3390/molecules26020361
Gonzalez G, Grúz J, D’Acunto CW, Kaňovský P, Strnad M. Cytokinin Plant Hormones Have Neuroprotective Activity in In Vitro Models of Parkinson’s Disease. Molecules. 2021; 26(2):361. https://doi.org/10.3390/molecules26020361
Chicago/Turabian StyleGonzalez, Gabriel, Jiří Grúz, Cosimo Walter D’Acunto, Petr Kaňovský, and Miroslav Strnad. 2021. "Cytokinin Plant Hormones Have Neuroprotective Activity in In Vitro Models of Parkinson’s Disease" Molecules 26, no. 2: 361. https://doi.org/10.3390/molecules26020361
APA StyleGonzalez, G., Grúz, J., D’Acunto, C. W., Kaňovský, P., & Strnad, M. (2021). Cytokinin Plant Hormones Have Neuroprotective Activity in In Vitro Models of Parkinson’s Disease. Molecules, 26(2), 361. https://doi.org/10.3390/molecules26020361