Physicochemical Characterization, Antioxidant Capacity, and Sensory Properties of Murici (Byrsonima crassifolia (L.) Kunth) and Taperebá (Spondias mombin L.) Beverages
Abstract
1. Introduction
2. Results and Discussion
2.1. Physicochemical Analysis of Taperebá and Murici Pulp
2.2. Total Phenolic and Flavonoids Content of Taperebá and Murici Pulp
2.3. Antioxidant Activity of Murici and Taperebá Pulp
2.4. Physicochemical Analysis of Beverages
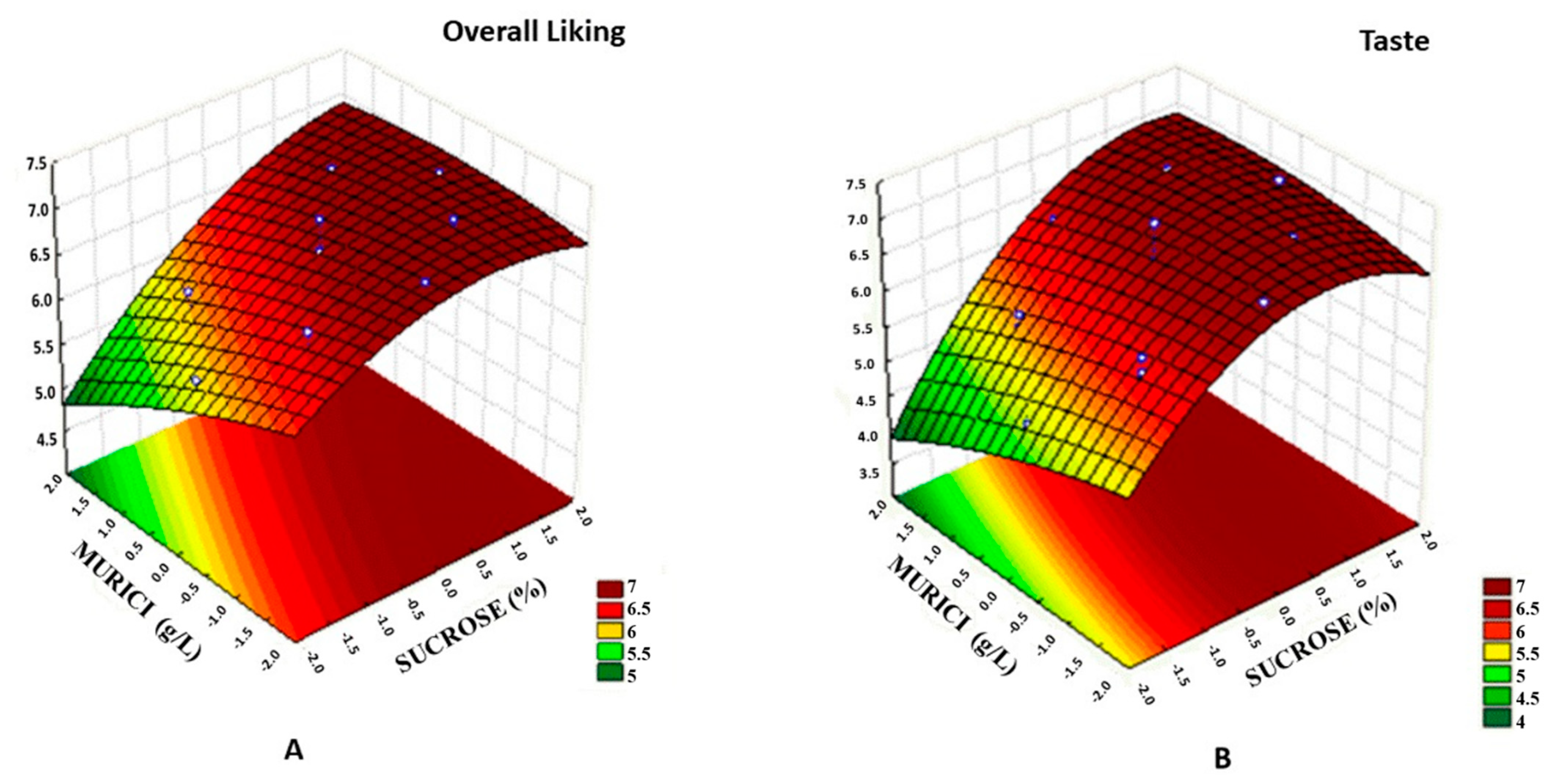
2.5. Sensory Analysis
2.6. Beverage Optimization
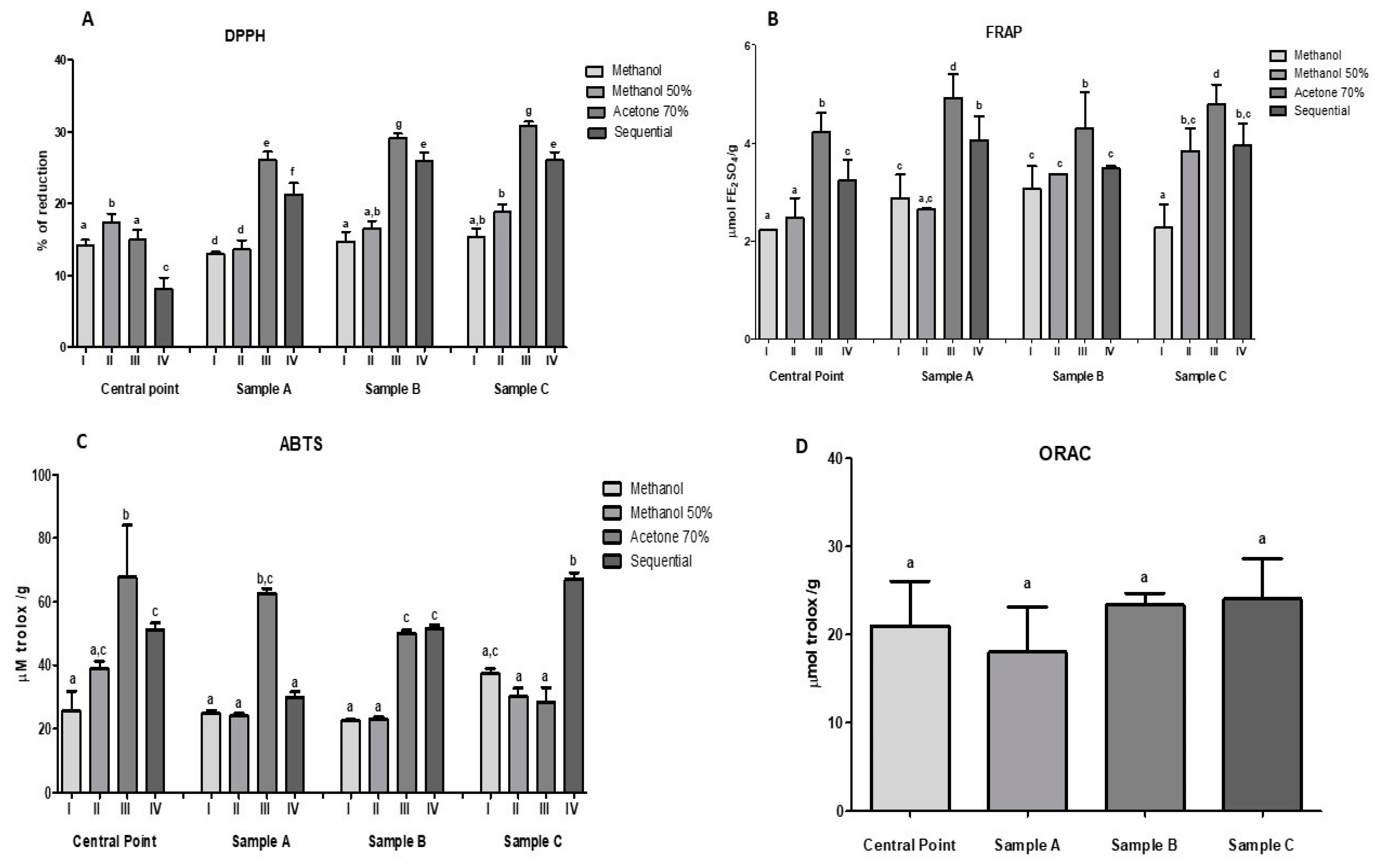
2.7. Antioxidant Capacity of Optimized Beverages
3. Materials and Methods
3.1. Material and Samples
3.2. Physicochemical Characterization
3.3. Extraction
3.4. Determination of the Total Phenolic Content
3.5. Determination of the Flavonoids Content
3.6. TEAC Assay
3.7. DPPH (Free Radical-Scavenging) Assay
3.8. FRAP Assay
3.9. ORAC (Oxygen Radical Absorbance Capacity) Assay
3.10. Experimental Design
3.11. Beverage Preparation
3.12. Sensory Analysis
3.13. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Neves, L.C.; Benedette, R.M.; Chagas, E.A. Characterization of the Antioxidant Capacity of Natives Fruits From the Brazilian Amazon Region 1. Rev. Bras. Frutic. 2012, 34, 1165–1173. [Google Scholar] [CrossRef]
- Canuto, G.B.; Xavier, A.A.O.; Neves, L.C.; Benassi, M.D.E.T. Physical and Chemical Characterization of Fruit Pulps from Amazonia and Their Correlation To Free Radical Scavenger Activity. Rev. Bras. Frutic. 2010, 32, 1196–1205. [Google Scholar] [CrossRef]
- do Rufino, M.S.M.; Alves, R.E.; de Brito, E.S.; Pérez-Jiménez, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem. 2010, 121, 996–1002. [Google Scholar] [CrossRef]
- Malta, L.G.; Tessaro, E.P.; Eberlin, M.; Pastore, G.M.; Liu, R.H. Assessment of antioxidant and antiproliferative activities and the identification of phenolic compounds of exotic Brazilian fruits. Food Res. Int. 2013, 53, 417–425. [Google Scholar] [CrossRef]
- Morais, L.; Maia, G.A.; Figueiredo, R.W. De Desenvolvimento De Néctares Mistos À Base De Manga E Cajá Enriquecidos Com Frutooligossacarídeos Ou Inulina. Aliment. Nutr. 2011, 22, 149–154. [Google Scholar]
- Alberto, P.S.; Silva, F.G.; Cabral, J.S.R.; De Fátima Sales, J.; Pereira, F.D. Methods to overcome of the dormancy in murici (Byrsonima verbascifolia Rich) seeds. Semin. Agrar. 2011, 32, 1015–1020. [Google Scholar] [CrossRef]
- Mariutti, L.R.B.; Rodrigues, E.; Chisté, R.C.; Fernandes, E.; Mercadante, A.Z. The Amazonian fruit Byrsonima crassifolia effectively scavenges reactive oxygen and nitrogen species and protects human erythrocytes against oxidative damage. Food Res. Int. 2014, 64, 618–625. [Google Scholar] [CrossRef]
- Gordon, A.; Jungfer, E.; Da Silva, B.A.; Maia, J.G.S.; Marx, F. Phenolic constituents and antioxidant capacity of four underutilized fruits from the amazon region. J. Agric. Food Chem. 2011, 59, 7688–7699. [Google Scholar] [CrossRef]
- De Souza, V.R.; Pereira, P.A.P.; Queiroz, F.; Borges, S.V.; De Carneiro, J.D.S. Determination of bioactive compounds, antioxidant activity and chemical composition of Cerrado Brazilian fruits. Food Chem. 2012, 134, 381–386. [Google Scholar] [CrossRef]
- Mariutti, L.R.B.; Rodrigues, E.; Mercadante, A.Z. Carotenoids from Byrsonima crassifolia: Identification, quantification and in vitro scavenging capacity against peroxyl radicals. J. Food Compos. Anal. 2013, 31, 155–160. [Google Scholar] [CrossRef]
- Murillo, E.; Meléndez-Martínez, A.J.; Portugal, F. Screening of vegetables and fruits from Panama for rich sources of lutein and zeaxanthin. Food Chem. 2010, 122, 167–172. [Google Scholar] [CrossRef]
- Maldonado-Astudillo, Y.I.; Alia-Tejacal, I.; Núñez-Colín, C.A.; Jiménez-Hernández, J.; Pelayo-Zaldívar, C.; López-Martínez, V.; Andrade-Rodríguez, M.; Bautista-Baños, S.; Valle-Guadarrama, S. Postharvest physiology and technology of Spondias purpurea L. and S. mombin L. Sci. Hortic. 2014, 174, 193–206. [Google Scholar] [CrossRef]
- Tiburski, J.H.; Rosenthal, A.; Deliza, R.; de Godoy, R.L.O.; Pacheco, S. Nutritional properties of yellow mombin (Spondias mombin L.) pulp. Food Res. Int. 2011, 44, 2326–2331. [Google Scholar] [CrossRef]
- Prati, P.; Moretti, R.H.; Cardello, H.M.A.B. Elaboration of beverage composed by blends of clarified-stabilized sugar cane and juice’s acid fruits. Cienc. Tecnol. Aliment. 2005, 25, 147–152. [Google Scholar] [CrossRef][Green Version]
- Oludemi, F.O.; Akanbi, C.T. Chemical, antioxidant and sensory properties of tomato-watermelon-pineapple blends, and changes in their total antioxidant capacity during storage. Int. J. Food Sci. Technol. 2013, 48, 1416–1425. [Google Scholar] [CrossRef]
- Neves, L.C.; Benedette, R.M.; Tosin, J.M.; Chagas, E.A.; Da Silva, V.X.; Prill, M.A.D.S.; Roberto, S.R. Production of Blends based on tropical and native fruits from brazilian Amazon. Rev. Bras. Frutic. 2011, 33, 187–197. [Google Scholar] [CrossRef][Green Version]
- Rebouças, M.C.; Rodrigues, M.D.C.P.; Afonso, M.R.A. Optimization of the Acceptance of Prebiotic Beverage Made from Cashew Nut Kernels and Passion Fruit Juice. J. Food Sci. 2014, 79, S1393–S1398. [Google Scholar] [CrossRef]
- Lawless, L.J.R.; Threlfall, R.T.; Meullenet, J.F.; Howard, L.R. Consumer-based optimization of blackberry, blueberry and concord juice blends. J. Sens. Stud. 2012, 27, 439–450. [Google Scholar] [CrossRef]
- Kumar, S.B.; Ravi, R.; Saraswathi, G. Optimization of fruit punch using mixture design. J. Food Sci. 2010, 75, S1–S7. [Google Scholar] [CrossRef]
- Hamacek, F.R.; Martino, H.S.D.; Pinheiro-Sant’Ana, H.M. Murici, fruit from the Cerrado of Minas Gerais, Brazil: Physical and physicochemical characteristics, and occurrence and concentration of carotenoids and vitamins. Fruits 2014, 69, 459–472. [Google Scholar] [CrossRef][Green Version]
- de Mattietto, R.A.; Lopes, A.S.; de Menezes, H.C.M. Physical and physicochemical characterization of caja fruit (Spondias mombin L.) ans its pulp, obtained using two types of extractor. Braz. J. Food Technol. 2010, 13, 156–164. [Google Scholar]
- Curi, P.N.; Tavares, B.S.; Almeida, A.B.; Pio, R.; Pasqual, M.; Peche, P.M.; de Souza, V.R. Characterization and influence of subtropical persimmon cultivars on juice and jelly characteristics. An. Acad. Bras. Cienc. 2017, 89, 1205–1220. [Google Scholar] [CrossRef] [PubMed]
- Dias, F.; Abadio, B.; Galv, I.; Botelho, R. Physicochemical characteristics and antioxidant activity of three native fruits from brazilian savannah (cerrado) *. Aliment. Nutr. 2012, 23, 1–6. [Google Scholar]
- Rongtong, B.; Suwonsichon, T.; Ritthiruangdej, P.; Kasemsumran, S. Determination of water activity, total soluble solids and moisture, sucrose, glucose and fructose contents in osmotically dehydrated papaya using near-infrared spectroscopy. Agric. Nat. Resour. 2018, 52, 557–564. [Google Scholar] [CrossRef]
- Alves, A.M.; Dias, T.; Hassimotto, N.M.A.; Naves, M.M.V. Ascorbic acid and phenolic contents, antioxidant capacity and flavonoids composition of Brazilian savannah native fruits. Food Sci. Technol. 2017, 37, 564–569. [Google Scholar] [CrossRef]
- Almeida, M.M.B.; de Sousa, P.H.M.; Arriaga, Â.M.C.; do Prado, G.M.; Magalhães, C.E.D.C.; Maia, G.A.; de Lemos, T.L.G. Bioactive compounds and antioxidant activity of fresh exotic fruits from northeastern Brazil. Food Res. Int. 2011, 44, 2155–2159. [Google Scholar] [CrossRef]
- Zielinski, A.A.F.; Ávila, S.; Ito, V.; Nogueira, A.; Wosiacki, G.; Haminiuk, C.W.I. The Association between Chromaticity, Phenolics, Carotenoids, and In Vitro Antioxidant Activity of Frozen Fruit Pulp in Brazil: An Application of Chemometrics. J. Food Sci. 2014, 79, 510–516. [Google Scholar] [CrossRef]
- de Souza, V.R.; Aniceto, A.; Abreu, J.P.; Montenegro, J.; Boquimpani, B.; de Jesuz, V.A.; de Campos, M.B.E.; Marcellini, P.S.; Freitas-Silva, O.; Cadena, R.; et al. Fruit-based drink sensory, physicochemical, and antioxidant properties in the Amazon region: Murici (Byrsonima crassifolia (L.) Kunth and verbascifolia (L.) DC) and tapereba (Spondia mombin). Food Sci. Nutr. 2020, 8, 2341–2347. [Google Scholar] [CrossRef]
- Brunelli, L.T.; Venturini Filho, W.G. Caracterização Química E Sensorial De Bebida Mista De Soja E Uva *. Aliment. Nutr. 2012, 23, 467–473. [Google Scholar]
- De Carvalho-Silva, L.B.; Dionísio, A.P.; Pereira, A.C.D.S.; Wurlitzer, N.J.; De Brito, E.S.; Bataglion, G.A.; Brasil, I.M.; Eberlin, M.N.; Liu, R.H. Antiproliferative, antimutagenic and antioxidant activities of a Brazilian tropical fruit juice. LWT Food Sci. Technol. 2014, 59, 1319–1324. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by meas of Folin-Ciocalteu reagent. Methods Enzym. 1999, 299, 152–178. [Google Scholar]
- Pękal, A.; Pyrzynska, K. Evaluation of Aluminium Complexation Reaction for Flavonoid Content Assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Brand-Wiliams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Abreu, J.; Quintino, I.; Pascoal, G.; Postingher, B.; Cadena, R.; Teodoro, A. Antioxidant capacity, phenolic compound content and sensory properties of cookies produced from organic grape peel (Vitis labrusca) flour. Int. J. Food Sci. Technol. 2019, 54, 1215–1224. [Google Scholar] [CrossRef]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORACFL)) of plasma and other biological and food samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef]
- Freitas, D.; Mattietto, R. Ideal sweetness of mixed juices from Amazon fruits. Cienc. Tecnol. Aliment. 2013, 33, 440. [Google Scholar] [CrossRef][Green Version]
| Parameter | Murici | Taperebá |
|---|---|---|
| Acidity (g citric acid · 100 g−1) | 0.75 ± 0.03 a | 1.74 ± 0.05 b |
| Vitamin C (mg · 100 g−1) | 58.88 ± 1.63 a | 25.93 ± 1.65 b |
| Total Soluble Solids (°Brix) | 4.20 ± 0.01 a | 9.80 ± 0.10 b |
| pH | 3.36 ± 0.01 a | 2.60 ± 0.01 b |
| Total Phenolics Compounds (mg GAE. 100 g−1) | 307.52 ± 19.73 a | 1340.15 ± 19.14 a |
| Flavonoids (µg QE/g) | 174.87 ± 1.76 a | 129.46 ± 10.68 b |
| DPPH (% of reduction) | 52.94 ± 2.41 a | 74.14 ± 1.34 b |
| FRAP (µmol FE2SO4/g) | 7.38 ± 0.98 a | 14.36 ± 3.47 b |
| ABTS (μmol trolox/g) | 79.49 ± 3.35 a | 188.24 ± 65.46 b |
| ORAC (µmol trolox/g) | 312.54 ± 82.95 a | 332.46 ± 86.82 a |
| Physicochemical Atributes (*) | ||||
|---|---|---|---|---|
| Samples | pH | Acidity | Soluble Solids | Vitamin C |
| (g·100 g−1) | (°Brix) | (mg·100 g−1) | ||
| 1 | 3.05 ± 0.01 bc | 0.18 ± 0.01 g | 8.80 ± 0.00 m | 17.57 ± 2.77 abc |
| 2 | 2.87 ± 0.01 h | 0.26 ± 0.01 de | 9.73 ± 0.23 j | 14.40 ± 4.16 abc |
| 3 | 3.00 ± 0.05 cde | 0.24 ± 0.00 e | 9.40 ± 0.00 l | 18.77 ± 0.69 ab |
| 4 | 3.20 ± 0.03 a | 0.19 ± 0.01 fg | 11.53 ± 0.12 h | 14.18 ± 2.36 abc |
| 5 | 2.94 ± 0.03fg | 0.35 ± 0.01 a | 13.00 ± 0.00f | 13.30 ± 2.95 bc |
| 6 | 3.00 ± 0.01 cde | 0.27 ± 0.00 de | 12.13 ± 0.12 g | 15.97 ± 0.69 abc |
| 7 | 3.02 ± 0.01 bcd | 0.31 ± 0.04 b | 10.20 ± 0.00 i | 17.77 ± 1.18 abc |
| 8 | 3.07 ± 0.01 b | 0.18 ± 0.00 fg | 14.00 ± 0.00 e | 13.75 ± 0.70 abc |
| 9 | 3.03 ± 0.01 bcd | 0.27 ± 0.00 de | 14.60 ± 0.00 c | 15.25 ± 0.70 abc |
| 10 | 2.99 ± 0.01 def | 0.21 ± 0.00 f | 12.00 ± 0.00 g | 19.10 ± 1.86 ab |
| 11 | 3.00 ± 0.01 cde | 0.31 ± 0.01 bc | 13.00 ± 0.00 f | 16.13 ± 1.85 abc |
| 12 | 3.00 ± 0.01 cde | 0.27 ± 0.01 cde | 12.03 ± 0.06 g | 16.77 ± 1.20 abc |
| 13 | 3.15 ± 0.01 a | 0.26 ± 0.01 de | 14.27 ± 0.12 d | 12.96 ± 1.40 c |
| 14 | 2.96 ± 0.01 ef | 0.36 ± 0.01 a | 15.47 ± 0.12 b | 19.25 ± 1.20 a |
| 15 | 3.02 ± 0.01 bcd | 0.27 ± 0.00 de | 8.00 ± 0.00 n | 14.63 ± 0.68 abc |
| 16 | 2.90 ± 0.02 gh | 0.27 ± 0.01 de | 16.47 ± 0.12 a | 13.69 ± 2.51 abc |
| 17 | 2.98 ± 0.03 def | 0.28 ± 0.01 bcd | 12.00 ± 0.00 g | 15.17 ± 0.69 abc |
| Sensory Atributes (*) | |||||
|---|---|---|---|---|---|
| Samples | Aroma | Taste | Appearance | Texture | Overall Liking |
| 1 | 6.09 abc | 6.06 bcd | 6.54 bc | 5.99 bcde | 6.30 bcdef |
| 2 | 6.31 abc | 6.26 abcd | 6.77 abc | 6.40 abcde | 6.58 abcde |
| 3 | 5.66 bc | 5.57 de | 6.36 c | 5.55 e | 5.78 f |
| 4 | 5.87 bc | 6.35 abcd | 6.34 c | 6.16 abcde | 6.38 abcdef |
| 5 | 6.21 abc | 6.35 abcd | 6.85 abc | 6.60 abcd | 6.59 abcde |
| 6 | 6.18 abc | 6.59 abc | 6.92 abc | 6.56 abcd | 6.68 abcd |
| 7 | 6.16 abc | 5.77 cde | 6.97 abc | 6.08 abcde | 6.17 def |
| 8 | 6.38 abc | 6.79 ab | 7.00 abc | 6.78 abc | 6.79 abcd |
| 9 | 6.64 ab | 6.99 a | 7.32 a | 6.94 a | 7.10 a |
| 10 | 6.73 a | 6.92 ab | 7.28 ab | 6.85 ab | 7.04 ab |
| 11 | 6.28 abc | 6.29 abcd | 6.64 abc | 5.84 de | 6.24 cdef |
| 12 | 6.38 abc | 6.57 abc | 7.05 abc | 6.20 abcde | 6.54 abcdef |
| 13 | 6.71 a | 6.94 ab | 6.69 abc | 6.56 abcd | 6.84 abcd |
| 14 | 6.83 a | 6.76 ab | 6.78 abc | 6.55 abcd | 6.86 abcd |
| 15 | 6.25 abc | 5.09 e | 6.82 abc | 5.93 cde | 5.87 ef |
| 16 | 6.77 a | 7.01 a | 6.97 abc | 6.79 abc | 6.98 abc |
| 17 | 6.78 a | 7.13 a | 6.96 abc | 6.70 abcd | 7.03 ab |
| p Values | |||||
|---|---|---|---|---|---|
| Constituents | Aroma | Taste | Appearance | Texture | Overall Liking |
| Taperebá (1) | 0.0752 | 0.5944 | 0.0028 | 0.0290 | 0.0675 |
| Taperebá quadratic | 0.0616 | 0.0740 | 0.0089 | 0.5122 | 0.1461 |
| Murici (2) | 0.3420 | 0.0260 | 0.0048 | 0.0025 | 0.0056 |
| Murici quadratic | 0.7539 | 0.5594 | 0.8678 | 0.3881 | 0.6006 |
| Sucrose (3) | 0.0051 | 0.0000 | 0.0202 | 0.0004 | 0.0001 |
| Sucrose quadratic | 0.7342 | 0.0063 | 0.4605 | 0.4384 | 0.0789 |
| 1 and 2 | 0.8259 | 0.5309 | 0.6887 | 0.9249 | 0.7183 |
| 1 and 3 | 0.5964 | 0.5309 | 0.2702 | 0.1665 | 0.5010 |
| 2 and 3 | 0.1158 | 0.1625 | 0.0459 | 0.7779 | 0.1665 |
| Samples | Taperebá | Murici | Sucrose |
|---|---|---|---|
| (g/L) | (g/L) | (%) | |
| A | 150 (0) | 105 (−1) | 12.5 (+1) |
| B | 150 (0) | 150 (0) | 14.2 (+1.68) |
| C | 150 (0) | 195 (+1) | 12.5 (+1) |
| Central Point | 150 (0) | 150 (0) | 10 (0) |
| Uncoded And Coded Values | |||
|---|---|---|---|
| Samples | Taperebá | Murici | Sucrose |
| (g/L) | (g/L) | (%) | |
| 1 | 105 (−1) | 105 (−1) | 7.5 (−1) |
| 2 | 195 (+1) | 105 (−1) | 7.5 (−1) |
| 3 | 105 (−1) | 195 (+1) | 7.5 (−1) |
| 4 | 74.4 (−1.68) | 150 (0) | 10 (0) |
| 5 | 225.6 (+1.68) | 150 (0) | 10 (0) |
| 6 | 150 (0) | 150 (0) | 10 (0) |
| 7 | 195 (+1) | 195 (+1) | 7.5 (−1) |
| 8 | 105 (−1) | 105 (−1) | 12.5 (+1) |
| 9 | 195 (+1) | 105 (−1) | 12.5 (+1) |
| 10 | 150 (0) | 74.4 (−1.68) | 10 (0) |
| 11 | 150 (0) | 225.6 (+1.68) | 10 (0) |
| 12 | 150 (0) | 150 (0) | 10 (0) |
| 13 | 105 (−1) | 195 (+1) | 12.5 (+1) |
| 14 | 195 (+1) | 195 (+1) | 12.5 (+1) |
| 15 | 150 (0) | 150 (0) | 5.8 (−1.68) |
| 16 | 150 (0) | 150 (0) | 14.2 (+1.68) |
| 17 | 150 (0) | 150 (0) | 10 (0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aniceto, A.; Montenegro, J.; Cadena, R.d.S.; Teodoro, A.J. Physicochemical Characterization, Antioxidant Capacity, and Sensory Properties of Murici (Byrsonima crassifolia (L.) Kunth) and Taperebá (Spondias mombin L.) Beverages. Molecules 2021, 26, 332. https://doi.org/10.3390/molecules26020332
Aniceto A, Montenegro J, Cadena RdS, Teodoro AJ. Physicochemical Characterization, Antioxidant Capacity, and Sensory Properties of Murici (Byrsonima crassifolia (L.) Kunth) and Taperebá (Spondias mombin L.) Beverages. Molecules. 2021; 26(2):332. https://doi.org/10.3390/molecules26020332
Chicago/Turabian StyleAniceto, Adriana, Julia Montenegro, Rafael da Silva Cadena, and Anderson Junger Teodoro. 2021. "Physicochemical Characterization, Antioxidant Capacity, and Sensory Properties of Murici (Byrsonima crassifolia (L.) Kunth) and Taperebá (Spondias mombin L.) Beverages" Molecules 26, no. 2: 332. https://doi.org/10.3390/molecules26020332
APA StyleAniceto, A., Montenegro, J., Cadena, R. d. S., & Teodoro, A. J. (2021). Physicochemical Characterization, Antioxidant Capacity, and Sensory Properties of Murici (Byrsonima crassifolia (L.) Kunth) and Taperebá (Spondias mombin L.) Beverages. Molecules, 26(2), 332. https://doi.org/10.3390/molecules26020332