Abstract
Single-walled carbon nanotubes (SWCNT) have recently been attracting the attention of plant biologists as a prospective tool for modulation of photosynthesis in higher plants. However, the exact mode of action of SWCNT on the photosynthetic electron transport chain remains unknown. In this work, we examined the effect of foliar application of polymer-grafted SWCNT on the donor side of photosystem II, the intersystem electron transfer chain and the acceptor side of photosystem I. Analysis of the induction curves of chlorophyll fluorescence via JIP test and construction of differential curves revealed that SWCNT concentrations up to 100 mg/L did not affect the photosynthetic electron transport chain. SWCNT concentration of 300 mg/L had no effect on the photosystem II donor side but provoked inactivation of photosystem II reaction centres and slowed down the reduction of the plastoquinone pool and the photosystem I end acceptors. Changes in the modulated reflection at 820 nm, too, indicated slower re-reduction of photosystem I reaction centres in SWCNT-treated leaves. We conclude that SWCNT are likely to be able to divert electrons from the photosynthetic electron transport chain at the level of photosystem I end acceptors and plastoquinone pool in vivo. Further research is needed to unequivocally prove if the observed effects are due to specific interaction between SWCNT and the photosynthetic apparatus.
1. Introduction
In the recent decade, carbon-based nanomaterials and single-walled carbon nanotubes (SWCNT) in particular have been involved in the development of state-of-the-art approaches in agronomy and plant biotechnology. SWCNT were applied in the newly evolving field of plant-based nanobionics turning living plants into monitoring systems for nitroaromatic compounds [1]. Chitosan-complexed SWCNT were demonstrated to serve as a DNA carrier in a novel technique for genetic transformation of the chloroplast genome in a number of plant species [2]. The spectroscopic and electronic properties of SWCNT [3] intuitively make them highly appropriate candidate to be utilized in development of novel techniques for augmentation of photosynthesis and modulation of stress responses in photosynthetic organisms. Many of the predicted applications of SWCNT in plant biotechnologies require knowledge of their modes of action on the photosynthetic machinery. SWCNT were assumed to endow chloroplasts with wider photosynthetic action spectrum due to their absorbance in the ultraviolet, visible and near-infrared regions [4]. Giraldo et al. [4] also argued that composite nanoparticles consisting of semiconducting SWCNT and nanoceria are able to passively enter isolated higher plant chloroplasts and enhance photosystem II (PSII) activity and photosynthetic electron transport possibly by transferring excitons to the photosynthetic apparatus which might be employed as a beneficial strategy for increasing plant survival in shade conditions. In vitro studies of the interaction between photosystem I (PSI) and SWCNT point to electron transfer from PSI electron carriers FA/B to SWCNT [5,6]. It is to be noted, however, that this effect was only observable at particular orientation between SWCNT and PSI complexes. Furthermore, Dorogi et al. [7] proved that SWCNT stabilize the charge separation in isolated purple bacterial reaction centres, thus providing another evidence for the possibility for direct interaction between SWCNT and components of the photosynthetic apparatus.
SWCNT can penetrate the plant cell wall and cytoplasmic membrane and exert effects ranging from stimulation of cell growth (at low dose) to reactive oxygen species formation and necrosis (at high dose) in Arabidopsis mesophyll protoplasts [8]. Shen et al. [9] also, report on adverse effects of protoplast viability after treatment with SWCNT. SWCNT translocated to the chloroplasts are thought to cause significant changes in the thylakoid membrane’s architecture [8]. Indeed, the expression of genes related to chloroplast development was enhanced in rice seedlings treated with SWCNT [10]. Besides, the information gained from in vitro studies, relatively little is known on how SWCNT affect higher plants photosynthesis in vivo. While the chlorophyll (Chl) a and b content of rice seedlings grown from SWCNT-treated seeds did not change, the photosynthesis rate of these plants significantly increased [10]. Arabidopsis thaliana leaves infiltrated with SWCNT showed increased photosynthetic electron transport rate [4]. In addition to augmentation of photosynthesis, the action of SWCNT in intact plants was related to increased expression of antioxidant enzymes [10] which was found to alleviate the drought stress effects [11]. Our recent findings demonstrated that high doses of polymer-grafted SWCNT exert negative effects on the rate and efficiency of photosynthesis, when applied via foliar spraying [12]. We hypothesized that SWCNT interact with the components of electron transport chain. Extensive and detailed study of the Chl fluorescence of SWCNT-treated plants can help to answer the question of whether transfer of excitons and/or electrons from SWCNT towards photosynthetic complexes occurs in vivo and how it affects the efficiency of the photosynthetic process. JIP test of the prompt Chl a fluorescence related to the electron transport in PSII and analysis of the modulated reflection at 820 nm (MR) associated with the electron transport at the level of PSI are widely applied approaches in numerous works aimed at revealing not only the stress responses of the photosynthetic apparatus but also the general physiological condition of plants subjected to various types of stress [13,14,15,16,17,18].
In the current work we aimed to shed light on the putative interaction between SWCNT and the photosynthetic complexes in intact pea plants sprayed with aqueous solution of SWCNT grafted with ‘Pluronic’ P85 triblock co-polymer [19]. We found that, besides partial inactivation of PSII reaction centres, SWCNT also interfere with the photosynthetic electron transport chain at the level of intersystem electron carriers and the PSI acceptor side.
2. Results
2.1. Prompt Chlorophyll Fluorescence Induction Curves
The prompt Chl fluorescence transients of the control, as well as all treated plants, exhibited the characteristic points O, J, I and P (Figure 1). The fluorescence rise from O to J is related to the reduction state of QA, with equilibration between the rates of QA reduction and re-oxidation being reached at J point [14,20]. Increase of fluorescence in the J-I phase is ascribed to gradual reduction of the PQ pool [21]; the fluorescence rise in the I point slows down due to reaching equilibrium between PQ reduction and re-oxidation; the P point is related to full reduction of the pool of PSI end electron acceptors [22]. Treatment with 10 mg/L nanotubes (SWCNT10) had little effect on both the shape and intensity of the prompt fluorescence curve. In the samples treated with 100 mg/L SWCNT (SWCNT100) we observed decrease of the fluorescence intensity after the J point and in leaves sprayed with 300 mg/L SWCNT (SWCNT300) an overall lowering of the prompt fluorescence intensity was visible (Figure 1). Notably, the latter effect was the most strongly expressed for P (maximal fluorescence, FM)—ca. 25% decrease in SWCNT300 relative to the control, while the fluorescence intensity at O was decreased by ca. 15%. The prompt fluorescence transients of pea plants treated only with the co-polymer in the corresponding concentrations, i.e., P8510, P85100 or P85300, were indistinguishable from the ones that are characteristic of the control plants (Figure S1A).
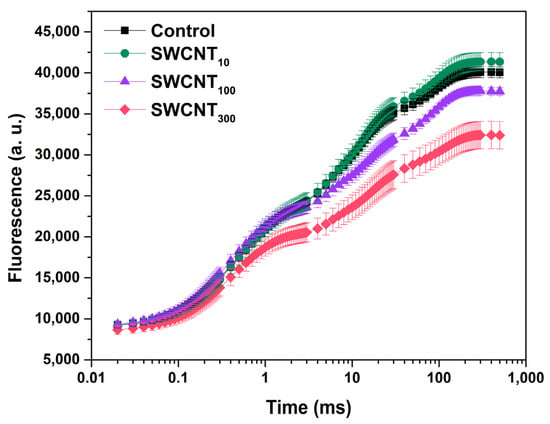
Figure 1.
Prompt Chl fluorescence induction curves (±SEM) of intact pea leaves sprayed with distilled water (control), 10, 100 or 300 mg/L of polymer-grafted SWCNT.
2.2. Differential Curves—Variable Chlorophyll Fluorescence Differences during J-I and I-P Induction Phases
Detailed examination of the shape of the prompt Chl fluorescence curves was done by construction of differential curves. Treatments with the P85 polymer only, in any of the selected concentrations, did not induce significant alterations in the shape of the fluorescence induction curves (Figure S1). SWCNT10 did not strongly affect the shape of the fluorescence transients as seen from the resulting differential curves with values approximating 0. The differential curves constructed in the phase between the O and J points (ΔWOJ), known to bear information about the oxygen-evolving complex functionality [23], did not show significant deviation from the control for any of the tested SWCNT concentrations (data not shown). However, well reproducible negative bands in the differential curves in the J-I (ΔWJI, Figure 2A) and I-P (ΔWIP, Figure 2B) phases were found for the leaves treated with SWCNT100 and SWCNT300. These bands revealed significant changes in the shape of prompt Chl fluorescence transients in both variants relative to the control due to slower increase of the fluorescence intensity in the respective phases. This effect was concentration-dependent since it was manifested to a higher extent for the SWCNT300 concentration.
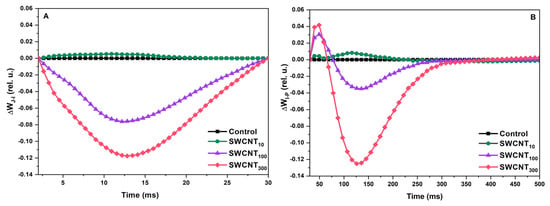
Figure 2.
Differential curves constructed for the J-I (A) and I-P (B) phases of the prompt Chl fluorescence transients of pea plants sprayed with distilled water (control), 10, 100 and 300 mg/L SWCNT. The differential curves were obtained by subtraction of the control J-I and I-P phases from the respective ones of treated plants following normalization between the J and I or I and P points, respectively: ΔWJI = WJItreated–WJIcontrol and ΔWIP = WIPtreated–WIPcontrol.
2.3. JIP Test
To further substantiate our observations on the photosynthetic electron transport in SWCNT-treated plants we analysed the variable Chl fluorescence transients according to the mathematical expressions contained in the JIP test (Table S1) [14,17,20,24]. The fluxes of light energy which is absorbed (ABS/RC), trapped (TR0/RC, data not shown) and consequently utilized for electron transport (ET0/RC, data not shown) were not changed upon SWCNT10 (Table 1) and P85 (data not shown) application. None of the applied SWCNT concentrations affected the initial rate of active PSII reaction centres closure, M0 (Table 1). However, the turnover number of QA reduction/re-oxidation (N) increased along with SWCNT concentration reaching values higher by 32% in the SWCNT300 than in the control. The enhancement of N was correlated with similar increase in the Sm parameter. Sm corresponds to the area above the JIP curve complementary to FM, and reflects the capacity of the intersystem electron carriers and the PSI end acceptors pools to take electrons until full reduction of QA, i.e., closure of all active PSII reaction centres.

Table 1.
Mean values of selected JIP test parameters for intact pea leaves treated with 10, 100 or 300 mg/L polymer-grafted SWCNT.
Analysis of the electron transfer quantum yields and probabilities revealed that the electron transfer beyond QA- to the intersystem electron carriers was not significantly affected by the applied treatment (parameters φEo and ψEo, Table 1, Figure 3).
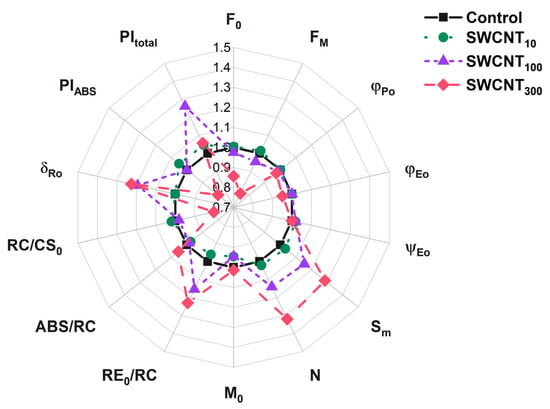
Figure 3.
Selected JIP parameters for intact pea leaves treated with distilled water (control), 10, 100 or 300 mg/L of polymer-grafted SWCNT. The JIP parameters values were normalized to those of the control.
The maximum quantum yield of PSII, φPo, was slightly decreased (by 3% on average) only in the variant treated with SWCNT300. However, the probability of transfer of an electron from the intersystem electron carriers to the terminal PSI acceptors, δRo, and the electron flux towards the PSI end acceptors, RE0/RC, both were enhanced significantly by about 20% in SWCNT100 and SWCNT300 (Figure 3).
Although the maximum quantum yield of PSII primary photochemical reaction was only slightly decreased, the PIABS parameter, reflecting the performance of PSII absorbed energy conservation as reduced intersystem electron carriers, was lower by about 15% in SWCNT300 relative to the control. Interestingly, the overall performance of PSI, PSII and the intersystem electron transport, PItotal, was not significantly affected. Although the RC/CS0 parameter evidenced for 20% decrease in the number of active PSII reaction centres, this change was not paralleled by increase in the absorption per active reaction centre (ABS/RC Table 1, Figure 3).
2.4. PSI Activity
As the JIP test performed on SWCNT100 and SWCNT300 samples indicated higher electron flux towards the PSI acceptor side, we further analysed the modulated reflection at 820 nm for more detailed inspection of the function of PSI. Adopting approach similar to the one applied by Guo et al. [25] we examined the relative MR transients (Figure 4) by calculating the amplitude of MR signal changes (Figure 5A) and the rate of P700 oxidation (Vox) and re-reduction (Vred, Figure 5B).
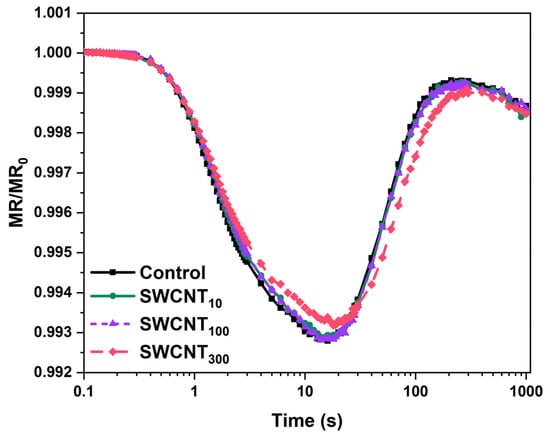
Figure 4.
Curves of the modulated reflection (MR) at 820 nm (relative to MR0 and presented on a semi-logarithmic scale) recorded for intact pea leaves sprayed with distilled water (control), 10, 100 or 300 mg/L SWCNT.
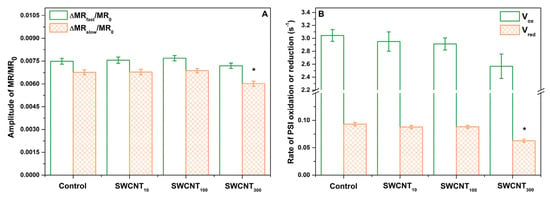
Figure 5.
Relative amplitudes of initial decrease (ΔMRfast) and subsequent rise (ΔMRslow) of the MR signal (A) and rates of P700 oxidation and re-reduction (B). Asterisks indicate statistically significant differences to the respective control revealed with ANOVA and Holm-Sidak ad hoc test at α = 0.05.
The MR transients recorded during illumination with actinic light showed characteristic shape including initial fast decrease of the MR signal (ΔMRfast) from MR0 to a minimal level reached at about 10 ms, that was followed by slower increase in MR intensity (ΔMRslow) reaching a plateau at 100 ms (Figure 4). The decrease of the MR at 820 nm (and respectively–increase in absorption at 820 nm) is accompanied by lowering of the absorption at 700 nm i.e., photobleaching at 700 nm, which in turn correlates with enhancement of primary P700 and PC oxidation [26]. Thus, this MR phase is governed by the primary photochemical activity of PSI. The following increase of MR reflects the gradual re-reduction of P700+ and PC+ by electrons donated by the intersystem electron carriers, leaving this phase strongly dependent on the PSII function [26]. Therefore, at the point of the minimal MR the rates of P700 and PC oxidation and re-reduction are equal.
In all the tested variants, the amplitude of ΔMRfast was higher than the amplitude of ΔMRslow indicating that PSI does not reach full re-reduction after the act of its primary oxidation. The SWCNT treated plants did not show any variation from the control in that respect.
Moreover, neither the amplitudes of MR, nor the rates of oxidation and re-reduction of P700 and PC were influenced by the treatment with P85 only (Figure S1B).
While, the amplitude of ΔMRfast decrease did not change significantly upon SWCNT treatment in any of the tested concentrations, we found significant decrease in the extent of re-reduction of P700+ and PC+ as indicated by the smaller ΔMRslow rise in SWCNT300 (Figure 5A). Similarly, while Vox remained unchanged in the SWCN-treated plants, the rate of re-reduction, Vred (being highly dependent on PSII activity and the redox poise of the intersystem electron carriers), decreased by about 30% in SWCNT300 (Figure 5B). The amplitudes of MR signal variation and the rates of P700 (and PC) oxidation and re-reduction were not significantly changed by SWCNT10 and SWCNT100 (Figure 5).
3. Discussion
The complementary pieces of information obtained through analyses of both Chl fluorescence induction curves and the modulated reflection at 820 nm allowed for comprehensive examination of the operation of the donor side of PSII, the acceptor side of PSI and the intersystem electron transport in pea plants treated with different concentrations of polymer-modified SWCNT and polymer only.
The polymer and the lowest tested concentration of SWCNT–10 mg/L did not appear to cause any changes in the functionality of both PSI and PSII based on the analyses of the fluorescence induction curves intensity and shape (Figure 1 and Figure 2, Figure S1), JIP test (Table 1, Figure 3) and the reflection at 820 nm (Figure 4 and Figure 5). In all of the applied analyses, SWCNT100 exhibited intermediary values between SWCNT10 and SWCNT300 following the same tendency as the highest tested concentration and strongly suggesting concentration-dependent effect.
Foliar spraying with carbon-based nanomaterials, especially when in high concentrations, may leave dark spots on leaf surface as illustrated by IMAGING-PAM, (Figure S2). SWCNT on the leaf lamina might decrease the intensity of measuring light reaching the photosynthetic apparatus or re-absorb part of the Chl fluorescence thus affecting the objectivity of Chl fluorescence analyses. In an attempt to assess the extent to which these effects bias our results, we used IMAGING-PAM to compare the mean values of selected fluorescence parameters in the whole leaf area with those in dark spot-free regions in the SWCNT300 variant (Figure S3). Indeed, F0 and FM in dark spot-free leaf regions were higher than in whole leaf area by less than 10%, and no difference in FV/FM ratio was observed (Figure S3). Thus, the unspecific effects of the SWCNT presence on the leaf surface (i.e., re-absorption and/or shading) might be accounted for about 10% of the detected changes in Chl fluorescence intensity. Importantly, data provided by OJIP curves, recorded on the leaf area consisting of both dark spots and spot-free regions, demonstrate more substantial differences in F0 and FM when control and SWCNT300 plants are compared, F0 and FM for SWCNT300 being lower by 15% and 23%, respectively (Table 1). Hence, these changes are not only due to the altered optical leaf properties.
We found that in SWCNT300 samples the functionality of the donor side of PSII was unaffected with no significant changes within the initial 2 ms of the fluorescence induction curve, i.e., the function of the oxygen evolving complex was preserved [23]. Also, according to the JIP test parameters M0, φEo and ψEo (Table 1), no changes were found in the rate of PSII reaction centres closure and the efficiency of electron transfer to intersystem electron carriers. The functionality of PSII as judged by φPo was slightly but significantly inhibited by the SWCNT treatment (Table 1). Next, the concentration-dependent slowdown of fluorescence rises in the J-I phase (Figure 2A) indicated retarded reduction of the PQ pool [21]. The negative bands at the I-P phase differential curves (Figure 2B) reflect impeded reduction of the terminal electron acceptors of PSI. When considering the negative bands at the J-I and I-P phases it should be taken into account that fluorescence rise is affected both by the donor and the acceptor sides, i.e., by decrease of the number of active PSII reaction centres and/or altered activity of PSI. Indeed, we observed decrease in the density of active PSII centres (RC/CS0, Table 1) which means a smaller electron flux towards the PQ pool leading to slower rise of fluorescence. This effect might be due to increase in the reactive oxygen species formation as reported previously for SWCNT-treated plants [8,10]. The increased flux of electrons towards PSI end electron acceptors (RE0/RC) and the higher probability for transfer of an electron from the intersystem electron carriers to PSI acceptor side (δRo, Table 1) indicate that the fluorescence rise slowdown at the J-I and I-P phases might be also caused by enhanced PSI activity. Counterintuitively, neither the initial drop in the amplitude of the MR signal (ΔMRfast, Figure 5A), nor the rate of initial P700 oxidation (Vox, Figure 5B), which are defined by the PSI functionality solely, were affected. However, the ΔMRslow and Vred were significantly lower in SWCNT300 relative to the control (Figure 5). Schansker et al. [22] argued that the recovery of I-P phase in the process of light to dark adaptation occurs simultaneously with recovery of the MR slow rise. Similarly, Strasser et al. [17] ascribed the gradual decrease of the amplitude of MR rise, which was accompanied by disappearance of the I phase in desiccated plants, to inhibition of the intersystem electron transport from PQH2 to PC. A rough parallel can be drawn between the effects of SWCNT300 concentration and the data presented by Guo et al. and Schansker et al. [25,27] regarding the action of dibromothymoquinone (DBMIB, acting as a Cyt b6f inhibitor preventing electron transfer from PQH2 to PC) and methylviologen (MV, pulling electrons from P700 ahead of ferredoxin-NADP reductase). Both DBMIB and MV caused appearance of negative I-P bands and decrease in both Vox and Vred which was more strongly expressed for Vred [25]. The action of MV was related to strongly lowered or no increase of MR signal reflecting P700 (and PC) re-reduction which was explained by the fact that MV pulls electrons from P700 thus not allowing electrons to accumulate in the PQ pool and to re-reduce P700 [25,27]. Hence, by complementing prompt Chl fluorescence with MR data, it can be hypothesized that in the SWCNT300-treated plants the slower fluorescence rise in the I-P and J-I phases is at least partially due to increased electron flow respectively (i) at the acceptor side of PSI and (ii) at the level of PQH2, and/or at the level of Cyt b6f. The latter hypothesis should be taken with caution due to the significant deactivation of QA reducing PSII reaction centres (Table 1, Figure 3). The significant increase of Sm and N parameters of the JIP test (Table 1, Figure 3) corroborates with those hypotheses as they indicate for larger pool of electron carriers, which have to be reduced before full reduction of all QA in all the active RC. Interestingly, the increase of Sm and N is not accompanied by changes in the probability of electron transport from QA- towards PQ (parameter ψEo, Table 1). This effect might be due to decreased number and photochemical activity of the PSII reaction centres (RC/CS0, φPo, Table 1) or possibly due to donation of electrons to SWCNT only at the level of PQ and beyond (reflected after the J point of the OJIP curves). The retarded fluorescence rise in the J-I and I-P phases might be due to downregulation of electron transport through Cyt b6f known to be a subject of ‘photosynthetic control’ by thylakoid lumen acidity [28]. Our recent study demonstrates that the proton motive force in SWCNT300 treated plants was slightly lower than in control plants [12] indicating that the slower increase of Chl fluorescence in the J-P phase is not due to Cyt b6f control. It is still to be cleared out if SWCNT are capable of pulling electrons at the PSI or Cyt b6f level or the observed phenomenon represents indirect effect of the SWCNT treatment.
In concert with our results, it was earlier suggested that PSI is capable of electron donation to SWCNT when the two are physically interacting [5,6]. Thus, it appears highly possible that the agent extracting electrons from the photosynthetic electron transport chain is SWCNT. Moreover, SWCNT were found to be capable of reaching and entering the chloroplasts in Arabidopsis [4,8]. It should be noted that in these experiments SWCNT were either applied on a leaf surface with compromised epidermis or infiltrated into the leaves, thus omitting the barrier properties of cuticular waxes. The results of our recent work, however, suggest penetration of SWCNT into chloroplasts upon non-invasive foliar application of polymer-grafted SWCNT [12] which was also applied in this work.
The contribution of CEF to the observed SWCNT-induced effect should be taken into account as well. Short-term heat stress leads to substantial enhancement of the NAD(P)H dehydrogenase-dependent CEF and it was argued that this response improves heat stress resistance of some rice lines [29]. Zhou et al. [30] reported that maize plants resistant to drought stress differ from the susceptible ones by their capability to retain the operation of CEF. Since SWCNT can be viewed as a novel and unexplored abiotic stress factor, it cannot be ruled out that the lower Vred and ΔMRslow in SWCNT300 reflects activation of CEF. Our recent work illustrates that foliar application of SWCNT alters CO2 assimilation [12], which might lead to over-reduction of the photosynthetic electron transport chain and activation of CEF as a safety valve.
Recent investigation of the photosynthetic activity of Arabidopsis and maize plants treated with SWCNT evidenced for increased photosynthetic electron transport [4,10]. Giraldo et al. [4] ascribed these results to increased light absorption properties of the photosynthetic apparatus due to the wider absorption spectrum of SWCNT, which were argued to transfer excitons to the photosynthetic apparatus. Contrary to these observations, in our experimental conditions we did not find significantly enhanced overall photosynthetic activity (as judged by the PItotal parameter, Table 1), probably due to differences in the type, surface modification and method of application of SWCNT utilized. It should be noted that the instrumentation utilized in the current work employs excitation light with wavelength strongly limited to 650 nm. Thus, conclusions regarding the question of complementation of the absorption spectra of the photosynthetic complexes with SWCNT are not relevant.
4. Materials and Methods
4.1. SWCNT Preparation
SWCNT were purchased from Sigma-Aldrich (>77% carbon as SWCNT; diameter: 0.7–1.1 nm, length: 300–2300 nm). With the aim of achieving stable dispersion of the water suspensions of SWCNT, the nanotubes were grafted with poly(ethylene oxide)26-block-poly(propylene oxide)40-block-poly(ethylene oxide)26 triblock copolymer (“Pluronic” P85, from BASF) as in a procedure described in [19,31]. In brief, deionized water solution of the triblock P85 co-polymer and SWCNT were mixed during sonication and further diluted with deionized water to 10, 100 and 300 mg SWCNT/L (denoted as SWCNT10, SWCNT100, SWCNT300). Immediately prior to treatment SWCNT suspensions were sonicated for 30 min.
4.2. Plant Material
7-day-old pea plantlets (Pisum sativum cv. RAN1) with two fully developed leaf pairs, grown in the same conditions as in [32] were sprayed with around 3 mL/plant of SWCNT at concentrations of 10, 100 or 300 mg/L. Chl fluorescence and modulated reflection at 820 nm measurements of intact pea leaves were performed 7 days after the SWCNT-treatment. Control plants were sprayed with distilled water. In addition, to check for specific effects due to P85 co-polymer, we examined pea plants sprayed with P85 solutions with concentrations corresponding to those used for SWCNT dispersion preparations.
4.3. Photosynthetic Performance
Fast kinetics of prompt Chl a fluorescence and modulated reflection at 820 nm of dark adapted (for 30 min) plants were recorded simultaneously within 0.5 s with M-PEA fluorimeter (Hansatech Instruments Ltd., King’s Lynn, UK).
4.4. Analysis of Chl Fluorescence Induction Curves
Prompt Chl fluorescence induction curves were obtained upon application of high-intensity 3000 µmol photons m−2 s−1 light pulse at 650 ± 10 nm wavelength. JIP analysis of the fluorescence induction curves was done according to Strasser et al. [20] and Goltsev et al. [14]. The formulae used for calculation of selected JIP parameters examined in the current work are presented in Table S1. Variable Chl fluorescence curves were obtained by double normalization according to the formula:
where Ft is the fluorescence intensity at time t after starting the measuring protocol, F0 is the minimal fluorescence recorded at 20 µs and FP is the maximal fluorescence intensity (at around 300 ms, also known as FM). Differential curves of three complementary parts of the Chl fluorescence transients were constructed by subtraction of the respective double normalized curves, recorded for the control, from the curves of each SWCNT- or P85-treated sample:
where:
FJ being fluorescence intensity at 2 ms, and FI—fluorescence intensity at 30 ms.
4.5. Analysis of Modulated Reflection at 820 nm
For modulated reflection (MR) measurements, a LED light at 820 ± 25 nm and 100% intensity was applied. MR values were presented relative to the initial reflection at 820 nm (MR0): MR/MR0. The amplitude of the fast drop of MR signal was defined as:
and the amplitude of the slow rise of MR signal was calculated by the formula:
where MR0 is the average reflection registered between 0.02 and 0.07 ms after beginning of measurement, MRmin is the lowest MR value reached after initial fast decrease of MR signal and MRterminal is the averaged MR signal within 270 to 400 ms of the measurement protocol. The slopes of the initial decrease and subsequent increase of the MR signal, representing the apparent rates of P700 oxidation and re-reduction, were calculated by linear regression analysis in the time ranges 0.6–1.1 ms and 30–70 ms, respectively.
4.6. Statistical Analysis
One-way ANOVA with α = 0.05 and Holm-Sidak ad hoc test was performed in OriginLab 2018. The summarized results (averaged values with standard errors) represent data obtained in 3 independent experiments each involving 10 measurements per variant.
5. Conclusions
The specific physical properties of carbon-based nanotubes offer various possibilities for their application in agriculture and biotechnology. This requires in-depth knowledge of possible interactions with the photosynthetic apparatus. Here we have focused on the possible interactions between SWCNT and the components of electron transport chain. Although SWCNT did not affect the donor side of PSII, they caused decrease in the number of active PSII centres. Our data suggest that foliar application of polymer-grafted SWCNT affects the higher plant photosynthetic apparatus at several levels. SWCNT slowed down the electron transfer by the intersystem electron carriers and lowered the extent and rate of reduction of the end acceptors of PSI. These observations provide the first in vivo indication for possible electron transfer between the photosynthetic electron-transport chain (the end acceptors of PSI and PQH2) and SWCNT, thus, supporting our hypothesis on interaction between the SWCNT and photosynthetic electron transport chain (Figure 6). However, further research is needed to unequivocally prove if the observed effects are due to specific interaction between SWCNT and the photosynthetic apparatus.
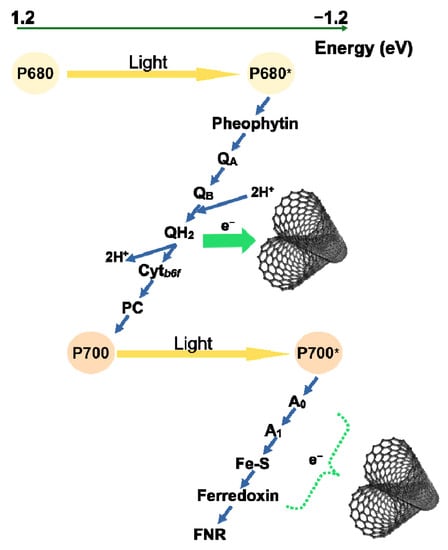
Figure 6.
Proposed mechanism of interaction between the photosynthetic electron transport chain of higher plants and P85 polymer-modified SWCNT. SWCNT are capable to divert electrons from the photosynthetic electron transport chain at the level of plastoquinone pool and/or photosystem I end acceptors in vivo.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/molecules26195958/s1. Figure S1: Prompt fluorescence induction curves (A) and modulated reflection at 820 nm (B) of intact pea leaves sprayed with distilled water or polymer P85 only at concentrations of 10, 100 and 300 mg/L. Figure S2. Representative chlorophyll fluorescence images of ΦPSII in pea plants sprayed with H2O, polymer (10 mg/L, P–10; 100 mg/L, P–100; and 300 mg/L, P–300) and SWCNT (10 mg/L, SWCNT–10; 100 mg/L, SWCNT–100; and 300 mg/L, SWCNT–300). Figure S3. Selected Chl fluorescence parameters measured in pea leaves sprayed with 300 mg/L SWCNT. Analysis was performed on whole leaf area as well as on selected leaf area free of dark spots. Table S1. Definition of selected JIP test parameters.
Author Contributions
Conceptualization, N.P., S.K. and V.V.; methodology, M.P., V.G., P.P.; formal analysis, N.P.; investigation, N.P.; data curation, N.P. and M.P.; writing—original draft preparation, N.P. and M.P.; writing—review and editing, S.K., V.G. and V.V.; visualization, N.P.; supervision, S.K.; project administration, N.P.; funding acquisition, N.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the manuscript and the Supplementary Materials.
Acknowledgments
N.P. is grateful for the support from the National Programme ‘Young Scientists and Post-doctoral fellows’ (DCM #577/17.08.2018, N.P.) by the Bulgarian Ministry of Education and Science. V.V., P.P and S.K. were supported by the National Science Fund, Bulgaria (grant number KP-06-H36/8/13.12.2019).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Wong, M.H.; Giraldo, J.P.; Kwak, S.-Y.; Koman, V.B.; Sinclair, R.; Lew, T.T.S.; Bisker, G.; Liu, P.; Strano, M.S. Nitroaromatic detection and infrared communication from wild-type plants using plant nanobionics. Nat. Mater. 2017, 16, 264–272. [Google Scholar] [CrossRef]
- Kwak, S.-Y.; Lew, T.T.S.; Sweeney, C.J.; Koman, V.B.; Wong, M.H.; Bohmert-Tatarev, K.; Snell, K.D.; Seo, J.S.; Chua, N.-H.; Strano, M.S. Chloroplast-selective gene delivery and expression in planta using chitosan-complexed single-walled carbon nanotube carriers. Nat. Nanotechnol. 2019, 14, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Odom, T.W.; Huang, J.-L.; Kim, P.; Lieber, C.M. Atomic structure and electronic properties of single-walled carbon nanotubes. Nature 1998, 391, 62–64. [Google Scholar] [CrossRef]
- Giraldo, J.P.; Landry, M.P.; Faltermeier, S.M.; McNicholas, T.P.; Iverson, N.M.; Boghossian, A.A.; Reuel, N.F.; Hilmer, A.J.; Sen, F.; Brew, J.A. Plant nanobionics approach to augment photosynthesis and biochemical sensing. Nat. Mater. 2014, 13, 400–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaniber, S.M.; Brandstetter, M.; Simmel, F.C.; Carmeli, I.; Holleitner, A.W. On-chip functionalization of carbon nanotubes with photosystem I. J. Am. Chem. Soc. 2010, 132, 2872–2873. [Google Scholar] [CrossRef]
- Nii, D.; Miyachi, M.; Shimada, Y.; Nozawa, Y.; Ito, M.; Homma, Y.; Ikehira, S.; Yamanoi, Y.; Nishihara, H.; Tomo, T. Conjugates between photosystem I and a carbon nanotube for a photoresponse device. Photosynth. Res. 2017, 133, 155–162. [Google Scholar] [CrossRef]
- Dorogi, M.; Bálint, Z.; Mikó, C.; Vileno, B.; Milas, M.; Hernádi, K.; Forró, L.; Váró, G.; Nagy, L. Stabilization effect of single-walled carbon nanotubes on the functioning of photosynthetic reaction centers. J. Phys. Chem. B 2006, 110, 21473–21479. [Google Scholar] [CrossRef]
- Yuan, H.; Hu, S.; Huang, P.; Song, H.; Wang, K.; Ruan, J.; He, R.; Cui, D. Single walled carbon nanotubes exhibit dual-phase regulation to exposed Arabidopsis mesophyll cell. Nanoscale Res. Lett. 2011, 6, 44. [Google Scholar] [CrossRef] [Green Version]
- Shen, C.X.; Zhang, Q.F.; Li, J.; Bi, F.C.; Yao, N. Induction of programmed cell death in Arabidopsis and rice by single-wall carbon nanotubes. Am. J. Bot. 2010, 97, 1602–1609. [Google Scholar] [CrossRef]
- Zhang, H.; Yue, M.; Zheng, X.; Xie, C.; Zhou, H.; Li, L. Physiological effects of single-and multi-walled carbon nanotubes on rice seedlings. IEEE Trans. NanoBioscience 2017, 16, 563–570. [Google Scholar] [CrossRef]
- Hatami, M.; Hadian, J.; Ghorbanpour, M. Mechanisms underlying toxicity and stimulatory role of single-walled carbon nanotubes in Hyoscyamus niger during drought stress simulated by polyethylene glycol. J. Hazard. Mater. 2017, 324, 306–320. [Google Scholar] [CrossRef]
- Velikova, V.; Petrova, N.; Kovács, L.; Petrova, A.; Koleva, D.; Tsonev, T.; Taneva, S.; Petrov, P.; Krumova, S. Single-walled carbon nanotubes modify leaf micromorphology, chloroplast ultrastructure and photosynthetic activity of pea plants. Int. J. Mol. Sci. 2021, 22, 4878. [Google Scholar] [CrossRef]
- Dąbrowski, P.; Kalaji, H.; Baczewska, A.; Pawluśkiewicz, B.; Mastalerczuk, G.; Borawska-Jarmułowicz, B.; Paunov, M.; Goltsev, V. Delayed chlorophyll a fluorescence, MR 820, and gas exchange changes in perennial ryegrass under salt stress. J. Lumin. 2017, 183, 322–333. [Google Scholar] [CrossRef]
- Goltsev, V.; Kalaji, H.; Paunov, M.; Bąba, W.; Horaczek, T.; Mojski, J.; Kociel, H.; Allakhverdiev, S. Variable chlorophyll fluorescence and its use for assessing physiological condition of plant photosynthetic apparatus. Russ. J. Plant Physiol. 2016, 63, 869–893. [Google Scholar] [CrossRef]
- Kalaji, H.; Rastogi, A.; Živčák, M.; Brestic, M.; Daszkowska-Golec, A.; Sitko, K.; Alsharafa, K.; Lotfi, R.; Stypiński, P.; Samborska, I. Prompt chlorophyll fluorescence as a tool for crop phenotyping: An example of barley landraces exposed to various abiotic stress factors. Photosynthetica 2018, 56, 953–961. [Google Scholar] [CrossRef] [Green Version]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef] [Green Version]
- Strasser, R.J.; Tsimilli-Michael, M.; Qiang, S.; Goltsev, V. Simultaneous in vivo recording of prompt and delayed fluorescence and 820-nm reflection changes during drying and after rehydration of the resurrection plant Haberlea rhodopensis. Biochim. Biophys. Acta Bioenerg. 2010, 1797, 1313–1326. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Li, P.; Ma, F.; Goltsev, V. Photosynthetic performance during leaf expansion in Malus micromalus probed by chlorophyll a fluorescence and modulated 820 nm reflection. J. Photochem. Photobiol. B Biol. 2014, 137, 144–150. [Google Scholar] [CrossRef]
- Petrov, P.D.; Georgiev, G.L. Fabrication of super-macroporous nanocomposites by deposition of carbon nanotubes onto polymer cryogels. Eur. Polym. J. 2012, 48, 1366–1373. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the Chlorophyll a Fluorescence Transient. In Chlorophyll Fluorescence; Papageorgiou, G.C., Ed.; Springer: Dorbrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar]
- Tóth, S.Z.; Schansker, G.; Strasser, R.J. A non-invasive assay of the plastoquinone pool redox state based on the OJIP-transient. Photosynth. Res. 2007, 93, 193–203. [Google Scholar] [CrossRef] [Green Version]
- Schansker, G.; Tóth, S.Z.; Strasser, R.J. Dark recovery of the Chl a fluorescence transient (OJIP) after light adaptation: The qT-component of non-photochemical quenching is related to an activated photosystem I acceptor side. Biochim. Biophys. Acta Bioenerg. 2010, 1757, 787–797. [Google Scholar] [CrossRef] [Green Version]
- Tóth, S.Z.; Schansker, G.; Garab, G.; Strasser, R.J. Photosynthetic electron transport activity in heat-treated barley leaves: The role of internal alternative electron donors to photosystem II. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 295–305. [Google Scholar] [CrossRef] [Green Version]
- Tsimilli-Michael, M. Revisiting JIP-test: An educative review on concepts, assumptions, approximations, definitions and terminology. Photosynthetica 2020, 58, 275–292. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Lu, Y.; Goltsev, V.; Strasser, R.J.; Kalaji, H.M.; Wang, H.; Wang, X.; Chen, S.; Qiang, S. Comparative effect of tenuazonic acid, diuron, bentazone, dibromothymoquinone and methyl viologen on the kinetics of Chl a fluorescence rise OJIP and the MR820 signal. Plant Physiol. Biochem. 2020, 156, 39–48. [Google Scholar] [CrossRef]
- Schansker, G.; Srivastava, A.; Strasser, R.J. Characterization of the 820-nm transmission signal paralleling the chlorophyll a fluorescence rise (OJIP) in pea leaves. Funct. Plant Biol. 2003, 30, 785–796. [Google Scholar] [CrossRef] [Green Version]
- Schansker, G.; Tóth, S.Z.; Strasser, R.J. Methylviologen and dibromothymoquinone treatments of pea leaves reveal the role of photosystem I in the Chl a fluorescence rise OJIP. Biochim. Biophys. Acta Bioenerg. 2005, 1706, 250–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shikanai, T. Central role of cyclic electron transport around photosystem I in the regulation of photosynthesis. Curr. Opin. Biotechnol. 2014, 26, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Essemine, J.; Xiao, Y.; Qu, M.; Mi, H.; Zhu, X.-G. Cyclic electron flow may provide some protection against PSII photoinhibition in rice (Oryza sativa L.) leaves under heat stress. J. Plant Physiol. 2017, 211, 138–146. [Google Scholar] [CrossRef]
- Zhou, R.; Kan, X.; Chen, J.; Hua, H.; Li, Y.; Ren, J.; Feng, K.; Liu, H.; Deng, D.; Yin, Z. Drought-induced changes in photosynthetic electron transport in maize probed by prompt fluorescence, delayed fluorescence, P700 and cyclic electron flow signals. Environ. Exp. Bot. 2019, 158, 51–62. [Google Scholar] [CrossRef]
- Petrov, P.; Georgiev, G.; Momekova, D.; Momekov, G.; Tsvetanov, C.B. UV-assisted grafting of polymers: A method towards biocompatible carbon nanotubes. Polymer 2010, 51, 2465–2471. [Google Scholar] [CrossRef]
- Petrova, N.; Todinova, S.; Paunov, M.; Kovács, L.; Taneva, S.; Krumova, S. Thylakoid membrane unstacking increases LHCII thermal stability and lipid phase fluidity. J. Bioenerg. Biomembr. 2018, 50, 425–435. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).