Grape Extract Promoted α-MSH-Induced Melanogenesis in B16F10 Melanoma Cells, Which Was Inverse to Resveratrol
Abstract
:1. Introduction
2. Results
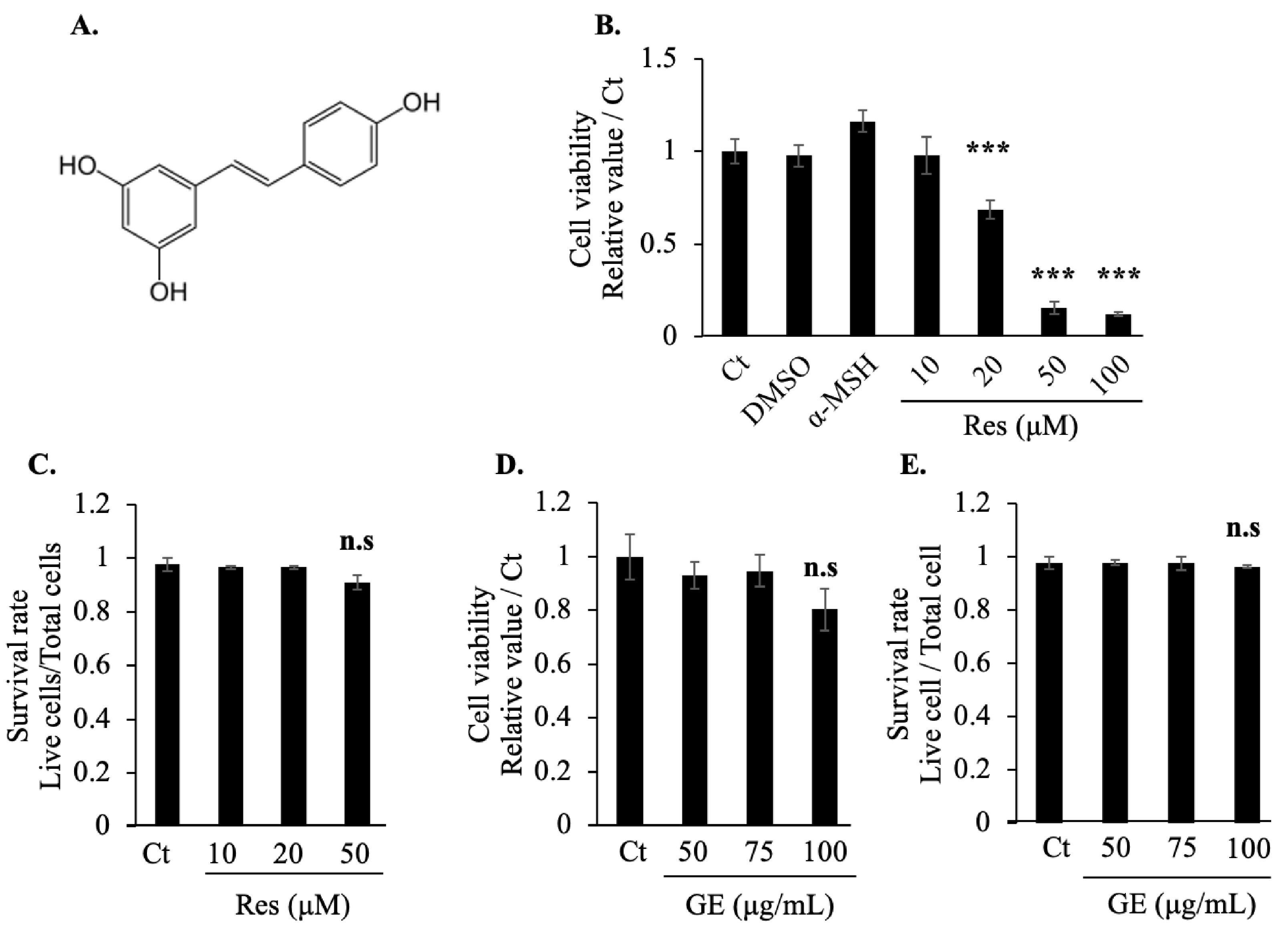
2.1. Effects of Grape Extract and Resveratrol on Cell Viability
2.2. Resveratrol and Grape Extract Have Inverse Effects on α-MSH-Induced Melanogenesis
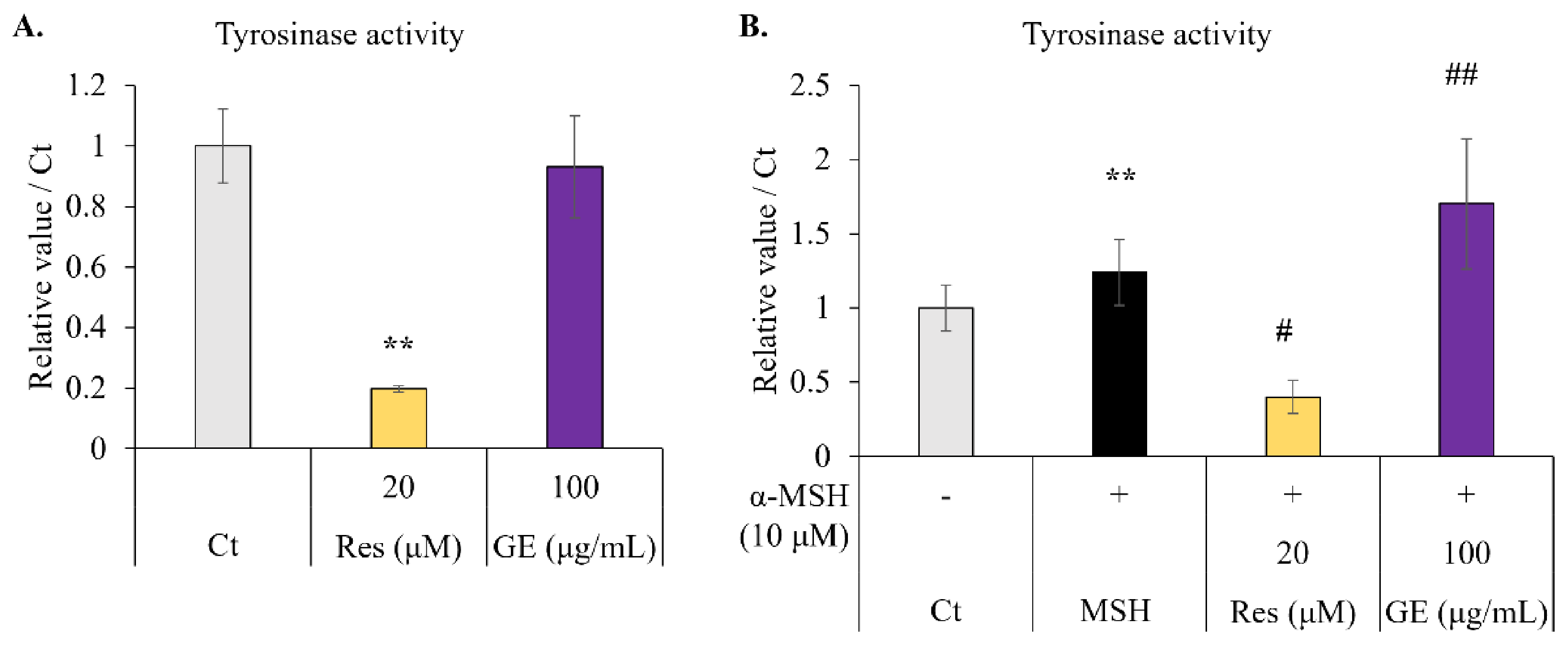
2.3. Effect of Resveratrol and Grape Extract on Intracellular Tyrosinase Activity
2.4. Effect of Resveratrol and Grape Extract on Melanogenesis-Related Gene Expression
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Grape Extract Sample Preparation
4.3. Cell Culture
4.4. MTT and Trypan Blue Assay
4.5. Melanin Content
4.6. Intracellular Tyrosinase Activity
4.7. Western Blot
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Moreiras, H.; Seabra, M.C.; Barral, D.C. Melanin Transfer in the Epidermis: The Pursuit of Skin Pigmentation Control Mechanisms. Int. J. Mol. Sci. 2021, 22, 4466. [Google Scholar] [CrossRef]
- Riley, P.A. Melanin. Int. J. Biochem. Cell Biol. 1997, 29, 1235–1239. [Google Scholar] [CrossRef]
- Cichorek, M.; Wachulska, M.; Stasiewicz, A.; Tymińska, A. Skin melanocytes: Biology and development. Postepy Dermatol. Alergol. 2013, 30, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Marmol, V.; Beermann, F. Tyrosinase and related proteins in mammalian pigmentation. FEBS Lett. 1996, 381, 165–168. [Google Scholar] [CrossRef]
- Kobayashi, T.; Urabe, K.; Winder, A.; Tsukamoto, K.; Brewington, T.; Imokawa, G.; Potterf, B.; Hearing, V.J. DHICA oxidase activity of TRP1 and interactions with other melanogenic enzymes. Pigment Cell Res. 1994, 7, 227–234. [Google Scholar] [CrossRef]
- Hearing, V.J. Determination of melanin synthetic pathways. J. Invest. Dermatol. 2011, 131, E8–E11. [Google Scholar] [CrossRef] [Green Version]
- Chao, H.C.; Najjaa, H.; Villareal, M.O.; Ksouri, R.; Han, J.; Neffati, M.; Isoda, H. Arthrophytum scoparium inhibits melanogenesis through the down-regulation of tyrosinase and melanogenic gene expressions in B16 melanoma cells. Exp. Dermatol. 2013, 22, 131–136. [Google Scholar] [CrossRef]
- Videira, I.F.; Moura, D.F.; Magina, S. Mechanisms regulating melanogenesis. An. Bras. Dermatol. 2013, 88, 76–83. [Google Scholar] [CrossRef] [Green Version]
- Nasti, T.H.; Timares, L. MC1R, Eumelanin and Pheomelanin: Their Role in Determining the Susceptibility to Skin Cancer. Photochem. Photobiol. 2015, 91, 188–200. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Mosby, N.; Yang, J.; Xu, A.; Abdel-Malek, Z.; Kadekaro, A.L. alpha-MSH activates immediate defense responses to UV-induced oxidative stress in human melanocytes. Pigment Cell Melanoma Res. 2009, 22, 809–818. [Google Scholar] [CrossRef]
- Lo, J.A.; Fisher, D.E. The melanoma revolution: From UV carcinogenesis to a new era in therapeutics. Science 2014, 346, 945–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jasiński, M.; Jasińska, L.; Ogrodowczyk, M. Resveratrol in prostate diseases—A short review. Cent. European J. Urol. 2013, 66, 144–149. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.H.; Seo, J.O.; Baek, S.H.; Kim, S.Y. Inhibitory effects of resveratrol on melanin synthesis in ultraviolet B-induced pigmentation in Guinea pig skin. Biomol. Ther. (Seoul) 2014, 22, 35–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catalgol, B.; Batirel, S.; Taga, Y.; Ozer, N.K. Resveratrol: French Paradox Revisited. Front. Pharmacol. 2012, 3, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, X.; Zhou, J.; Zhao, C.N.; Gan, R.Y.; Li, H.B. Health Benefits and Molecular Mechanisms of Resveratrol: A Narrative Review. Foods 2020, 9, 340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Athar, M.; Back, J.H.; Tang, X.; Kim, K.H.; Kopelovich, L.; Bickers, D.R.; Kim, A.L. Resveratrol: A review of preclinical studies for human cancer prevention. Toxicol. Appl. Pharmacol. 2007, 224, 274–283. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, D.M. More on antioxidant activity of resveratrol in red wine. Clin. Chem. 1996, 42, 113–114. [Google Scholar] [CrossRef]
- Gatson, J.W.; Liu, M.M.; Abdelfattah, K.; Wigginton, J.G.; Smith, S.; Wolf, S.; Minei, J.P. Resveratrol decreases inflammation in the brain of mice with mild traumatic brain injury. J. Trauma Acute Care Surg. 2013, 74, 470–474. [Google Scholar] [CrossRef]
- Marchal, J.; Pifferi, F.; Aujard, F. Resveratrol in mammals: Effects on aging biomarkers, age-related diseases, and life span. Ann. N Y Acad. Sci. 2013, 1290, 67–73. [Google Scholar] [CrossRef]
- Newton, R.A.; Cook, A.L.; Roberts, D.W.; Leonard, J.H.; Sturm, R.A. Post-transcriptional regulation of melanin biosynthetic enzymes by cAMP and resveratrol in human melanocytes. J. Invest. Dermatol. 2007, 127, 2216–2227. [Google Scholar] [CrossRef]
- Li, S.; Bouzar, C.; Cottet-Rousselle, C.; Zagotta, I.; Lamarche, F.; Wabitsch, M.; Tokarska-Schlattner, M.; Fischer-Posovszky, P.; Schlattner, U.; Rousseau, D. Resveratrol inhibits lipogenesis of 3T3-L1 and SGBS cells by inhibition of insulin signaling and mitochondrial mass increase. Biochim. Biophys. Acta. 2016, 1857, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Dugdale, H.F.; Hughes, D.C.; Allan, R.; Deane, C.S.; Coxon, C.R.; Morton, J.P.; Stewart, C.E.; Sharples, A.P. The role of resveratrol on skeletal muscle cell differentiation and myotube hypertrophy during glucose restriction. Mol. Cell Biochem. 2018, 444, 109–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arbo, B.D.; André-Miral, C.; Nasre-Nasser, R.G.; Schimith, L.E.; Santos, M.G.; Costa-Silva, D.; Muccillo-Baisch, A.L.; Hort, M.A. Resveratrol Derivatives as Potential Treatments for Alzheimer’s and Parkinson’s Disease. Front. Aging Neurosci. 2020, 12, 103. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.H.; Sethi, G.; Um, J.Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The Role of Resveratrol in Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef] [Green Version]
- D’Mello, S.A.N.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling Pathways in Melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef] [Green Version]
- Drira, R.; Sakamoto, K. Sakuranetin Induces Melanogenesis in B16BL6 Melanoma Cells through Inhibition of ERK and PI3K/AKT Signaling Pathways. Phytother. Res. 2016, 30, 997–1002. [Google Scholar] [CrossRef]
- Chung, Y.C.; Kim, S.; Kim, J.H.; Lee, G.S.; Lee, J.N.; Lee, N.H.; Hyun, C.G. Pratol, an O-Methylated Flavone, Induces Melanogenesis in B16F10 Melanoma Cells via p-p38 and p-JNK Upregulation. Molecules 2017, 22, 1704. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Jung, S.H. Recent development of signaling pathways inhibitors of melanogenesis. Cell Signal. 2017, 40, 99–115. [Google Scholar] [CrossRef]
- Huang, H.C.; Yen, H.; Lu, J.Y.; Chang, T.M.; Hii, C.H. Theophylline enhances melanogenesis in B16F10 murine melanoma cells through the activation of the MEK 1/2, and Wnt/β-catenin signaling pathways. Food Chem. Toxicol. 2020, 137, 111165. [Google Scholar] [CrossRef]
- Bellei, B.; Flori, E.; Izzo, E.; Maresca, V.; Picardo, M. GSK3β inhibition promotes melanogenesis in mouse B16 melanoma cells and normal human melanocytes. Cell Signal. 2008, 20, 1750–1761. [Google Scholar] [CrossRef]
- Ali, K.; Maltese, F.; Choi, Y.H.; Verpoorte, R. Metabolic constituents of grapevine and grape-derived products. Phytochem. Rev. 2010, 9, 357–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ando, H.; Hashimoto, A.; Masamoto, Y.; Ichihashi, M.; Mishima, Y. Inhibitory Effect of linoleic Acid on Melanogenesis. J. Soc. Cosmet. Chem. Jpn. 2010, 27, 415–423. [Google Scholar] [CrossRef]
- Su, T.R.; Lin, J.J.; Tsai, C.C.; Huang, T.K.; Yang, Z.Y.; Wu, M.O.; Zheng, Y.Q.; Su, C.C.; Wu, Y.J. Inhibition of Melanogenesis by Gallic Acid: Possible Involvement of the PI3K/Akt, MEK/ERK and Wnt/β-Catenin Signaling Pathways in B16F10 Cells. Int. J. Mol. Sci. 2013, 14, 20443–20458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, K.; Toriyama, M. Depigmenting Effect of Catechins. Molecules 2009, 14, 4425–4432. [Google Scholar] [CrossRef] [PubMed]
- Liu-Smith, F.; Meyskens, F.M. Molecular mechanisms of flavonoids in melanin synthesis and the potential for the prevention and treatment of melanoma. Mol. Nutr. Food Res. 2016, 60, 1264–1274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Mateos, A.; Cifuentes-Gomez, T.; Tabatabaee, S.; Lecras, C.; Spencer, J.P. Procyanidin, anthocyanin, and chlorogenic acid contents of highbush and lowbush blueberries. J. Agric. Food Chem. 2012, 60, 5772–5778. [Google Scholar] [CrossRef]
- Serafino, A.; Sinibaldi-Vallebona, P.; Lazzarino, G.; Tavazzi, B.; Rasi, G.; Pierimarchi, P.; Andreola, F.; Moroni, G.; Galvano, G.; Galvano, F.; et al. Differentiation of human melanoma cells induced by cyanidin-3-O-beta-glucopyranoside. FASEB J. 2004, 18, 1940–1942. [Google Scholar] [CrossRef]
- Choi, S.Y.; Kong, Y.H.; Lee, Y.; Baek, J.H.; Lee, S.H.; Lee, P. Inhibitory effects of Neo Muscat grape vine extracts on melanin biosynthesis. J. Korean Soc. Appl. Biol. Chem. 2010, 53, 566–569. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Riadh, D.; Sakamoto, K. Grape Extract Promoted α-MSH-Induced Melanogenesis in B16F10 Melanoma Cells, Which Was Inverse to Resveratrol. Molecules 2021, 26, 5959. https://doi.org/10.3390/molecules26195959
Zhou S, Riadh D, Sakamoto K. Grape Extract Promoted α-MSH-Induced Melanogenesis in B16F10 Melanoma Cells, Which Was Inverse to Resveratrol. Molecules. 2021; 26(19):5959. https://doi.org/10.3390/molecules26195959
Chicago/Turabian StyleZhou, Siqi, Drira Riadh, and Kazuichi Sakamoto. 2021. "Grape Extract Promoted α-MSH-Induced Melanogenesis in B16F10 Melanoma Cells, Which Was Inverse to Resveratrol" Molecules 26, no. 19: 5959. https://doi.org/10.3390/molecules26195959
APA StyleZhou, S., Riadh, D., & Sakamoto, K. (2021). Grape Extract Promoted α-MSH-Induced Melanogenesis in B16F10 Melanoma Cells, Which Was Inverse to Resveratrol. Molecules, 26(19), 5959. https://doi.org/10.3390/molecules26195959






