Mechanisms and Advances in Anti-Ovarian Cancer with Natural Plants Component
Abstract
1. Cognitive Status of Ovarian Cancer
2. Current Treatments for Ovarian Cancer
3. Mechanisms with Natural Plants Compound in Ovarian Cancer Cells
3.1. Cytotoxic Effect
3.2. Inhibit Proliferation and Promote Apoptosis
3.3. Suppress Cell Migration and Invasion
3.4. Reactive Oxygen Species (ROS) Damage
3.5. Trigger Cell Cycle Arrest
3.6. Induce Autophagic Cell Death
3.7. Inhibit Angiogenesis
3.8. Interfere with RNA Expression
3.9. Promote DNA Damage Response
4. Mechanisms of Botanical Components Improve Drug Sensitivity
4.1. Modulate Immune Cell Responses
4.2. Regulate Immune Molecular Expression Level
4.3. Reverse Multiple-Drug Resistance (MDR)
5. Antitumor Effects and Chemical Structure Relationship in Natural Plants Compounds
6. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Webb, P.M.; Jordan, S.J. Epidemiology of epithelial ovarian cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 41, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Weidle, U.H.; Birzele, F.; Kollmorgen, G.; Rueger, R. Mechanisms and Targets Involved in Dissemination of Ovarian Cancer. Cancer Genom. Proteom. 2016, 13, 407–423. [Google Scholar] [CrossRef] [PubMed]
- Katabuchi, H.; Okamura, H. Cell biology of human ovarian surface epithelial cells and ovarian carcinogenesis. Med. Electron Microsc. 2003, 36, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Duska, L.R.; Kohn, E.C. The new classifications of ovarian, fallopian tube, and primary peritoneal cancer and their clinical implications. Ann. Oncol. 2017, 28. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, T.; Ledermann, J.A. Targeted Therapies for Ovarian Cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 41, 139–152. [Google Scholar] [CrossRef]
- Yoshida, K.; Miki, Y. Role of BRCA1 and BRCA2 as regulators of DNA repair, transcription, and cell cycle in response to DNA damage. Cancer Sci. 2004, 95, 866–871. [Google Scholar] [CrossRef]
- Bocchicchio, S.; Tesone, M.; Irusta, G. Convergence of Wnt and Notch signaling controls ovarian cancer cell survival. J. Cell Physiol. 2019, 234, 22130–22143. [Google Scholar] [CrossRef] [PubMed]
- Ediriweera, M.K.; Tennekoon, K.H.; Samarakoon, S.R. Role of the PI3K/AKT/mTOR signaling pathway in ovarian cancer: Biological and therapeutic significance. Semin. Cancer Biol. 2019, 59, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhu, Y.; Li, F.; Xie, Y. GATA1-regulated JAG1 promotes ovarian cancer progression by activating Notch signal pathway. Protoplasma 2020, 257, 901–910. [Google Scholar] [CrossRef]
- Chen, Y.; Bieber, M.M.; Teng, N.N.H. Hedgehog signaling regulates drug sensitivity by targeting ABC transporters ABCB1 and ABCG2 in epithelial ovarian cancer. Mol. Carcinog. 2014, 53, 625–634. [Google Scholar] [CrossRef]
- Dancey, J. Targeted therapies and clinical trials in ovarian cancer. Ann. Oncol. 2013, 24 (Suppl. 10), x59–x63. [Google Scholar] [CrossRef] [PubMed]
- Piccart, M.J.; Lamb, H.; Vermorken, J.B. Current and future potential roles of the platinum drugs in the treatment of ovarian cancer. Ann. Oncol. 2001, 12, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Lheureux, S.; Gourley, C.; Vergote, I.; Oza, A.M. Epithelial ovarian cancer. Lancet 2019, 393, 1240–1253. [Google Scholar] [CrossRef]
- Ottevanger, P.B. Ovarian cancer stem cells more questions than answers. Semin. Cancer Biol. 2017, 44, 67–71. [Google Scholar] [CrossRef]
- Coleman, R.L.; Monk, B.J.; Sood, A.K.; Herzog, T.J. Latest research and treatment of advanced-stage epithelial ovarian cancer. Nat. Rev. Clin. Oncol. 2013, 10, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, Q.Q.; Zhou, H.; Lu, Y.; Li, J.M.; Ma, Y.; Wang, L.; Fu, T.; Gong, X.; Weintraub, M.; et al. Selective tumor cell killing by triptolide in p53 wild-type and p53 mutant ovarian carcinomas. Med. Oncol. 2014, 31, 14. [Google Scholar] [CrossRef]
- Song, K.; Lv, T.; Chen, Y.; Diao, Y.; Yao, Q.; Wang, Y. Emodin inhibits TGF-β2 by activating the FOXD3/miR-199a axis in ovarian cancer cells In Vitro. Oncol. Rep. 2018, 39, 2063–2070. [Google Scholar] [CrossRef]
- Chen, W.; Lu, Y.; Chen, G.; Huang, S. Molecular evidence of cryptotanshinone for treatment and prevention of human cancer. Anticancer Agents Med. Chem. 2013, 13, 979–987. [Google Scholar] [CrossRef]
- Cullen, S.P.; Martin, S.J. Fas and TRAIL ‘death receptors’ as initiators of inflammation: Implications for cancer. Semin. Cell Dev. Biol. 2015, 39, 26–34. [Google Scholar] [CrossRef]
- Yuan, X.; Gajan, A.; Chu, Q.; Xiong, H.; Wu, K.; Wu, G.S. Developing TRAIL/TRAIL death receptor-based cancer therapies. Cancer Metastasis Rev. 2018, 37, 733–748. [Google Scholar] [CrossRef]
- Chang, C.-C.; Lai, J.-S.; Tsai, C.-S.; Ma, S.-W.; Lin, J.-Y.; Huang, L.-R.; Lu, C.-H.; Liao, E.-C.; Ho, T.-F. Proapoptotic and TRAIL-sensitizing constituents isolated from Salvia militiorrhiza (Danshen). J. Biosci. Bioeng. 2013, 116, 516–523. [Google Scholar] [CrossRef]
- Chang, C.-C.; Kuan, C.-P.; Lin, J.-Y.; Lai, J.-S.; Ho, T.-F. Tanshinone IIA Facilitates TRAIL Sensitization by up-regulating DR5 through the ROS-JNK-CHOP Signaling Axis in Human Ovarian Carcinoma Cell Lines. Chem. Res. Toxicol. 2015, 28, 1574–1583. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.; Kim, H.M.; Kim, H.J.; Choi, J.-H.; Jang, D.S. Kudsuphilactone B, a nortriterpenoid isolated from Schisandra chinensis fruit, induces caspase-dependent apoptosis in human ovarian cancer A2780 cells. Arch. Pharm. Res. 2017, 40, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.-H.; Shin, J.-W.; Pham, T.-H.; Choi, Y.-J.; Ryu, H.-W.; Oh, S.-R.; Oh, J.-W.; Yoon, D.-Y. Methyl lucidone induces apoptosis and G/M phase arrest the PI3K/Akt/NF-κB pathway in ovarian cancer cells. Pharm. Biol. 2020, 58, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Ferrell, J.E. Apoptosis propagates through the cytoplasm as trigger waves. Science 2018, 361, 607–612. [Google Scholar] [CrossRef]
- Zhang, L.; Jin, J.; Zhang, L.; Hu, R.; Gao, L.; Huo, X.; Liu, D.; Ma, X.; Wang, C.; Han, J.; et al. Quantitative analysis of differential protein expression in cervical carcinoma cells after zeylenone treatment by stable isotope labeling with amino acids in cell culture. J. Proteom. 2015, 126, 279–287. [Google Scholar] [CrossRef]
- Zhang, L.; Huo, X.; Liao, Y.; Yang, F.; Gao, L.; Cao, L. Zeylenone, a naturally occurring cyclohexene oxide, inhibits proliferation and induces apoptosis in cervical carcinoma cells via PI3K/AKT/mTOR and MAPK/ERK pathways. Sci. Rep. 2017, 7, 1669. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Shi, J.; Gao, H.; Li, Q. Zeylenone inhibits proliferation and promotes apoptosis in ovarian carcinoma cells via Janus kinase 2/signal transducers and activators of transcription 3 pathways. J. Obstet. Gynaecol. Res. 2018, 44, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jiao, Y.; Shi, C.; Song, X.; Chang, Y.; Ren, Y.; Shi, X. Berbamine suppresses cell proliferation and promotes apoptosis in ovarian cancer partially via the inhibition of Wnt/β-catenin signaling. Acta Biochim. Biophys. Sin. 2018, 50, 532–539. [Google Scholar] [CrossRef]
- Liu, J.; Bai, J.; Jiang, G.; Li, X.; Wang, J.; Wu, D.; Owusu, L.; Zhang, E.; Li, W. Anti-Tumor Effect of Pinus massoniana Bark Proanthocyanidins on Ovarian Cancer through Induction of Cell Apoptosis and Inhibition of Cell Migration. PLoS ONE 2015, 10, e0142157. [Google Scholar] [CrossRef]
- Lee, D.; Ko, H.; Kim, Y.-J.; Kim, S.-N.; Choi, K.-C.; Yamabe, N.; Kim, K.H.; Kang, K.S.; Kim, H.Y.; Shibamoto, T. Inhibition of A2780 Human Ovarian Carcinoma Cell Proliferation by a Rubus Component, Sanguiin H-6. J. Agric. Food Chem. 2016, 64, 801–805. [Google Scholar] [CrossRef]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef]
- Avila-Carrasco, L.; Majano, P.; Sánchez-Toméro, J.A.; Selgas, R.; López-Cabrera, M.; Aguilera, A.; González Mateo, G. Natural Plants Compounds as Modulators of Epithelial-to-Mesenchymal Transition. Front. Pharmacol. 2019, 10, 715. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ding, S.; Xia, L. Ligustrazine inhibits the proliferation and migration of ovarian cancer cells via regulating miR-211. Biosci. Rep. 2021, 41. [Google Scholar] [CrossRef]
- Yin, J.; Yu, C.; Yang, Z.; He, J.-L.; Chen, W.-J.; Liu, H.-Z.; Li, W.-M.; Liu, H.-T.; Wang, Y.-X. Tetramethylpyrazine inhibits migration of SKOV3 human ovarian carcinoma cells and decreases the expression of interleukin-8 via the ERK1/2, p38 and AP-1 signaling pathways. Oncol. Rep. 2011, 26, 671–679. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, Y.; Gao, S.; Zhu, J.; Zheng, Y.; Zhang, H.; Sun, H. Dihydroartemisinin induces apoptosis and inhibits proliferation, migration, and invasion in epithelial ovarian cancer via inhibition of the hedgehog signaling pathway. Cancer Med. 2018, 7, 5704–5715. [Google Scholar] [CrossRef]
- Dong, X.; Fu, J.; Yin, X.; Cao, S.; Li, X.; Lin, L.; Ni, J. Emodin: A Review of its Pharmacology, Toxicity and Pharmacokinetics. Phytother. Res. 2016, 30, 1207–1218. [Google Scholar] [CrossRef]
- Lu, J.; Xu, Y.; Wei, X.; Zhao, Z.; Xue, J.; Liu, P. Emodin Inhibits the Epithelial to Mesenchymal Transition of Epithelial Ovarian Cancer Cells via ILK/GSK-3/Slug Signaling Pathway. Biomed. Res. Int. 2016, 2016, 6253280. [Google Scholar] [CrossRef]
- Jiao, R.; Liu, Y.; Gao, H.; Xiao, J.; So, K.F. The Anti-Oxidant and Antitumor Properties of Plant Polysaccharides. Am. J. Chin. Med. 2016, 44, 463–488. [Google Scholar] [CrossRef]
- Kim, S.C.; Choi, B.; Kwon, Y. Thiol-reducing agents prevent sulforaphane-induced growth inhibition in ovarian cancer cells. Food Nutr. Res. 2017, 61, 1368321. [Google Scholar] [CrossRef]
- Sunil, C.; Xu, B. An insight into the health-promoting effects of taxifolin (dihydroquercetin). Phytochemistry 2019, 166, 112066. [Google Scholar] [CrossRef]
- Yi, L.; Zongyuan, Y.; Cheng, G.; Lingyun, Z.; Guilian, Y.; Wei, G. Quercetin enhances apoptotic effect of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in ovarian cancer cells through reactive oxygen species (ROS) mediated CCAAT enhancer-binding protein homologous protein (CHOP)-death receptor 5 pathway. Cancer Sci. 2014, 105, 520–527. [Google Scholar] [CrossRef]
- Park, S.; Bazer, F.W.; Lim, W.; Song, G. The O-methylated isoflavone, formononetin, inhibits human ovarian cancer cell proliferation by sub G0/G1 cell phase arrest through PI3K/AKT and ERK1/2 inactivation. J. Cell Biochem. 2018, 119, 7377–7387. [Google Scholar] [CrossRef]
- Evan, G.I.; Vousden, K.H. Proliferation, cell cycle and apoptosis in cancer. Nature 2001, 411, 342–348. [Google Scholar] [CrossRef]
- Jia, S.; Shen, M.; Zhang, F.; Xie, J. Recent Advances in Momordica charantia: Functional Components and Biological Activities. Int. J. Mol. Sci. 2017, 18, 2555. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, X.; Ma, W.; Kou, W.; Li, C.; Zhao, J. Anticancer activity of cucurbitacin-A in ovarian cancer cell line SKOV3 involves cell cycle arrest, apoptosis and inhibition of mTOR/PI3K/Akt signaling pathway. J. BUON 2018, 23, 124–128. [Google Scholar] [PubMed]
- Ren, L.; Cao, Q.-X.; Zhai, F.-R.; Yang, S.-Q.; Zhang, H.-X. Asiatic acid exerts anticancer potential in human ovarian cancer cells via suppression of PI3K/Akt/mTOR signalling. Pharm. Biol. 2016, 54, 2377–2382. [Google Scholar] [CrossRef]
- Yu, S.; Yan, H.; Zhang, L.; Shan, M.; Chen, P.; Ding, A.; Li, S.F.Y. A Review on the Phytochemistry, Pharmacology, and Pharmacokinetics of Amentoflavone, a Naturally-Occurring Biflavonoid. Molecules 2017, 22, 299. [Google Scholar] [CrossRef]
- Liu, H.; Yue, Q.; He, S. Amentoflavone suppresses tumor growth in ovarian cancer by modulating Skp2. Life Sci. 2017, 189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, S.; Wei, C.; Rankin, G.O.; Ye, X.; Chen, Y.C. Dietary compound proanthocyanidins from Chinese bayberry (Myrica rubra Sieb. et Zucc.) leaves attenuate chemotherapy-resistant ovarian cancer stem cell traits via targeting the Wnt/β-catenin signaling pathway and inducing G1 cell cycle arrest. Food Funct. 2018, 9, 525–533. [Google Scholar] [CrossRef]
- Kavandi, L.; Lee, L.R.; Bokhari, A.A.; Pirog, J.E.; Jiang, Y.; Ahmad, K.A.; Syed, V. The Chinese herbs Scutellaria baicalensis and Fritillaria cirrhosa target NFκB to inhibit proliferation of ovarian and endometrial cancer cells. Mol. Carcinog. 2015, 54, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Baehrecke, E.H. Autophagy, cell death, and cancer. Mol. Cell Oncol 2015, 2, e985913. [Google Scholar] [CrossRef]
- Zhou, J.; Jiang, Y.-Y.; Chen, H.; Wu, Y.-C.; Zhang, L. Tanshinone I attenuates the malignant biological properties of ovarian cancer by inducing apoptosis and autophagy via the inactivation of PI3K/AKT/mTOR pathway. Cell Prolif. 2020, 53, e12739. [Google Scholar] [CrossRef]
- Che, X.; Yan, H.; Sun, H.; Dongol, S.; Wang, Y.; Lv, Q.; Jiang, J. Grifolin induces autophagic cell death by inhibiting the Akt/mTOR/S6K pathway in human ovarian cancer cells. Oncol. Rep. 2016, 36, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, C.; Russo, G.L.; Orhan, I.E.; Habtemariam, S.; Daglia, M.; Sureda, A.; Nabavi, S.F.; Devi, K.P.; Loizzo, M.R.; Tundis, R.; et al. Genistein and cancer: Current status, challenges, and future directions. Adv. Nutr. 2015, 6, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Gossner, G.; Choi, M.; Tan, L.; Fogoros, S.; Griffith, K.A.; Kuenker, M.; Liu, J.R. Genistein-induced apoptosis and autophagocytosis in ovarian cancer cells. Gynecol. Oncol. 2007, 105, 23–30. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Huang, T.-C.; Shieh, T.-M.; Wu, C.-H.; Lin, L.-C.; Hsia, S.-M. Isoliquiritigenin Induces Autophagy and Inhibits Ovarian Cancer Cell Growth. Int. J. Mol. Sci. 2017, 18, 2025. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, S.; Wei, C.; Rankin, G.O.; Rojanasakul, Y.; Ren, N.; Ye, X.; Chen, Y.C. Dietary Compound Proanthocyanidins from Chinese bayberry (Sieb. et Zucc.) leaves inhibit angiogenesis and regulate cell cycle of cisplatin-resistant ovarian cancer cells via targeting Akt pathway. J. Funct. Foods 2018, 40, 573–581. [Google Scholar] [CrossRef]
- Luo, H.; Jiang, B.-H.; King, S.M.; Chen, Y.C. Inhibition of cell growth and VEGF expression in ovarian cancer cells by flavonoids. Nutr. Cancer 2008, 60, 800–809. [Google Scholar] [CrossRef]
- Dinda, B.; Dinda, S.; DasSharma, S.; Banik, R.; Chakraborty, A.; Dinda, M. Therapeutic potentials of baicalin and its aglycone, baicalein against inflammatory disorders. Eur. J. Med. Chem. 2017, 131, 68–80. [Google Scholar] [CrossRef]
- Chen, J.; Li, Z.; Chen, A.Y.; Ye, X.; Luo, H.; Rankin, G.O.; Chen, Y.C. Inhibitory effect of baicalin and baicalein on ovarian cancer cells. Int. J. Mol. Sci. 2013, 14, 6012–6025. [Google Scholar] [CrossRef]
- Gao, J.; Zhu, H.; Wan, H.; Zou, X.; Ma, X.; Gao, G. Harmine suppresses the proliferation and migration of human ovarian cancer cells through inhibiting ERK/CREB pathway. Oncol. Rep. 2017, 38, 2927–2934. [Google Scholar] [CrossRef]
- Kim, K.K.; Singh, A.P.; Singh, R.K.; Demartino, A.; Brard, L.; Vorsa, N.; Lange, T.S.; Moore, R.G. Anti-angiogenic activity of cranberry proanthocyanidins and cytotoxic properties in ovarian cancer cells. Int. J. Oncol. 2012, 40, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, Z.; Wang, Z.; Liu, G.; Liu, Y.; Wang, H. Astragalus polysaccharides inhibit ovarian cancer cell growth via microRNA-27a/FBXW7 signaling pathway. Biosci. Rep. 2020, 40. [Google Scholar] [CrossRef]
- Zhou, J.; Jiang, Y.-Y.; Wang, X.-X.; Wang, H.-P.; Chen, H.; Wu, Y.-C.; Wang, L.; Pu, X.; Yue, G.-Z.; Zhang, L. Tanshinone IIA suppresses ovarian cancer growth through inhibiting malignant properties and angiogenesis. Ann. Transl. Med. 2020, 8, 1295. [Google Scholar] [CrossRef] [PubMed]
- Ai, Z.; Lu, Y.; Qiu, S.; Fan, Z. Overcoming cisplatin resistance of ovarian cancer cells by targeting HIF-1-regulated cancer metabolism. Cancer Lett. 2016, 373, 36–44. [Google Scholar] [CrossRef]
- Park, S.; Lim, W.; Jeong, W.; Bazer, F.W.; Lee, D.; Song, G. Sideroxylin (Callistemon lanceolatus) suppressed cell proliferation and increased apoptosis in ovarian cancer cells accompanied by mitochondrial dysfunction, the generation of reactive oxygen species, and an increase of lipid peroxidation. J. Cell Physiol. 2018, 233, 8597–8604. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Landen, C.N.; Li, Y.; Alvarez, R.D.; Tollefsbol, T.O. Epigallocatechin gallate and sulforaphane combination treatment induce apoptosis in paclitaxel-resistant ovarian cancer cells through hTERT and Bcl-2 down-regulation. Exp. Cell Res. 2013, 319, 697–706. [Google Scholar] [CrossRef]
- Hou, D.; Xu, G.; Zhang, C.; Li, B.; Qin, J.; Hao, X.; Liu, Q.; Zhang, X.; Liu, J.; Wei, J.; et al. Berberine induces oxidative DNA damage and impairs homologous recombination repair in ovarian cancer cells to confer increased sensitivity to PARP inhibition. Cell Death Dis. 2017, 8, e3070. [Google Scholar] [CrossRef]
- Tiper, I.V.; Temkin, S.M.; Spiegel, S.; Goldblum, S.E.; Giuntoli, R.L.; Oelke, M.; Schneck, J.P.; Webb, T.J. VEGF Potentiates GD3-Mediated Immunosuppression by Human Ovarian Cancer Cells. Clin. Cancer Res. 2016, 22, 4249–4258. [Google Scholar] [CrossRef]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef]
- Holzapfel, N.P.; Shokoohmand, A.; Wagner, F.; Landgraf, M.; Champ, S.; Holzapfel, B.M.; Clements, J.A.; Hutmacher, D.W.; Loessner, D. Lycopene reduces ovarian tumor growth and intraperitoneal metastatic load. Am. J. Cancer Res. 2017, 7, 1322–1336. [Google Scholar]
- Lee, K.; Ahn, J.-H.; Lee, K.-T.; Jang, D.S.; Choi, J.-H. Deoxyschizandrin, Isolated from Schisandra Berries, Induces Cell Cycle Arrest in Ovarian Cancer Cells and Inhibits the Protumoural Activation of Tumour-Associated Macrophages. Nutrients 2018, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.G.; Kunnumakkara, A.B.; Nair, A.; Merritt, W.M.; Han, L.Y.; Armaiz-Pena, G.N.; Kamat, A.A.; Spannuth, W.A.; Gershenson, D.M.; Lutgendorf, S.K.; et al. Curcumin inhibits tumor growth and angiogenesis in ovarian carcinoma by targeting the nuclear factor-kappaB pathway. Clin. Cancer Res. 2007, 13, 3423–3430. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.-x.; Sun, Y.-b.; Wang, S.-q.; Duan, L.; Huo, Q.-l.; Ren, F.; Li, G.-f. Grape seed procyanidin reversal of p-glycoprotein associated multi-drug resistance via down-regulation of NF-κB and MAPK/ERK mediated YB-1 activity in A2780/T cells. PLoS ONE 2013, 8, e71071. [Google Scholar] [CrossRef] [PubMed]
- Vinay, D.S.; Ryan, E.P.; Pawelec, G.; Talib, W.H.; Stagg, J.; Elkord, E.; Lichtor, T.; Decker, W.K.; Whelan, R.L.; Kumara, H.; et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin. Cancer Biol. 2015, 35, S185–S198. [Google Scholar] [CrossRef]
- Costa-Rodrigues, J.; Pinho, O.; Monteiro, P.R.R. Can lycopene be considered an effective protection against cardiovascular disease? Food Chem. 2018, 245, 1148–1153. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, S.; Wang, Z.; Wang, Y.; Liu, T.; Li, S.; Wang, C.; Wang, H.; Tu, P. Identification of metabolites of deoxyschizandrin in rats by UPLC-Q-TOF-MS/MS based on multiple mass defect filter data acquisition and multiple data processing techniques. J. Chromatogr. B 2014, 949–950, 115–126. [Google Scholar] [CrossRef]
- Vaidyanathan, A.; Sawers, L.; Gannon, A.-L.; Chakravarty, P.; Scott, A.L.; Bray, S.E.; Ferguson, M.J.; Smith, G. ABCB1 (MDR1) induction defines a common resistance mechanism in paclitaxel- and olaparib-resistant ovarian cancer cells. Br. J. Cancer 2016, 115, 431–441. [Google Scholar] [CrossRef]
- Anand, P.; Sundaram, C.; Jhurani, S.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin and cancer: An “old-age” disease with an “age-old” solution. Cancer Lett. 2008, 267, 133–164. [Google Scholar] [CrossRef]
- Zou, L.; Liu, X.; Li, J.; Li, W.; Zhang, L.; Li, J.; Zhang, J. Tetramethylpyrazine Enhances the Antitumor Effect of Paclitaxel by Inhibiting Angiogenesis and Inducing Apoptosis. Front. Pharmacol. 2019, 10, 707. [Google Scholar] [CrossRef]
- Yang, F.; Yu, X.H.; Qiao, F.; Cheng, L.H.; Chen, G.; Long, X.; Wang, X.R.; Li, X.L.; Liang, R.C.; Chen, Y.Z. Formulation and characterization of Brucea javanica oil microemulsion for improving safety. Drug Dev. Ind. Pharm. 2014, 40, 266–277. [Google Scholar] [CrossRef]
- Russo, E.B. Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011, 163, 1344–1364. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Cai, Y.-Z.; Zhang, Y. Natural phenolic compounds from medicinal herbs and dietary plants: Potential use for cancer prevention. Nutr. Cancer 2010, 62. [Google Scholar] [CrossRef] [PubMed]
- Kozłowska, A.; Szostak-Wegierek, D. Flavonoids—Food sources and health benefits. Rocz Panstw Zakl Hig 2014, 65, 79–85. [Google Scholar] [PubMed]
- Middleton, E.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar] [PubMed]
- Feng, J.; Zhang, X.-L.; Li, Y.-Y.; Cui, Y.-Y.; Chen, Y.-H. Pinus massoniana Bark Extract: Structure-Activity Relationship and Biomedical Potentials. Am. J. Chin. Med. 2016, 44, 1559–1577. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Himmeldirk, K.B.; Qian, Y.; Ren, Y.; Malki, A.; Chen, X. Biological and biomedical functions of Penta-O-galloyl-D-glucose and its derivatives. J. Nat. Med. 2014, 68, 465–472. [Google Scholar] [CrossRef]
- Wu, S.; Tian, L. Diverse Phytochemicals and Bioactivities in the Ancient Fruit and Modern Functional Food Pomegranate (Punica granatum). Molecules 2017, 22, 1606. [Google Scholar] [CrossRef]
- Hayakawa, T.; Yaguchi, T.; Kawakami, Y. Enhanced anti-tumor effects of the PD-1 blockade combined with a highly absorptive form of curcumin targeting STAT3. Cancer Sci. 2020, 111, 4326–4335. [Google Scholar] [CrossRef]
- Liao, L.; Liu, C.; Xie, X.; Zhou, J. Betulinic acid induces apoptosis and impairs migration and invasion in a mouse model of ovarian cancer. J. Food Biochem. 2020, 44, e13278. [Google Scholar] [CrossRef]
- Mitra, A.; Nan, A.; Line, B.R.; Ghandehari, H. Nanocarriers for nuclear imaging and radiotherapy of cancer. Curr. Pharm Des. 2006, 12, 4729–4749. [Google Scholar] [CrossRef]
- Dragojevic, S.; Ryu, J.S.; Raucher, D. Polymer-Based Prodrugs: Improving Tumor Targeting and the Solubility of Small Molecule Drugs in Cancer Therapy. Molecules 2015, 20, 21750–21769. [Google Scholar] [CrossRef]
- Su, Y.; Chen, L.; Yang, F.; Cheung, P.C.K. Beta-d-glucan-based drug delivery system and its potential application in targeting tumor associated macrophages. Carbohydr. Polym. 2021, 253, 117258. [Google Scholar] [CrossRef]
- Ye, H.; Liu, X.; Sun, J.; Zhu, S.; Zhu, Y.; Chang, S. Enhanced therapeutic efficacy of LHRHa-targeted brucea javanica oil liposomes for ovarian cancer. BMC Cancer 2016, 16, 831. [Google Scholar] [CrossRef]
- Hardwick, J.; Taylor, J.; Mehta, M.; Satija, S.; Paudel, K.R.; Hansbro, P.M.; Chellappan, D.K.; Bebawy, M.; Dua, K. Targeting Cancer using Curcumin Encapsulated Vesicular Drug Delivery Systems. Curr. Pharm. Des. 2021, 27. [Google Scholar] [CrossRef] [PubMed]
- Vinayak, M.; Maurya, A.K. Quercetin Loaded Nanoparticles in Targeting Cancer: Recent Development. Anticancer Agents Med. Chem. 2019, 19, 1560–1576. [Google Scholar] [CrossRef]
- Aqil, F.; Jeyabalan, J.; Agrawal, A.K.; Kyakulaga, A.-H.; Munagala, R.; Parker, L.; Gupta, R.C. Exosomal delivery of berry anthocyanidins for the management of ovarian cancer. Food Funct. 2017, 8, 4100–4107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hagan, C.T.; Min, Y.; Foley, H.; Tian, X.; Yang, F.; Mi, Y.; Au, K.M.; Medik, Y.; Roche, K.; et al. Nanoparticle co-delivery of wortmannin and cisplatin synergistically enhances chemoradiotherapy and reverses platinum resistance in ovarian cancer models. Biomaterials 2018, 169. [Google Scholar] [CrossRef] [PubMed]
- Budisan, L.; Gulei, D.; Zanoaga, O.M.; Irimie, A.I.; Sergiu, C.; Braicu, C.; Gherman, C.D.; Berindan-Neagoe, I. Dietary Intervention by Phytochemicals and Their Role in Modulating Coding and Non-Coding Genes in Cancer. Int. J. Mol. Sci. 2017, 18, 1178. [Google Scholar] [CrossRef] [PubMed]
- Halim, C.E.; Xinjing, S.L.; Fan, L.; Bailey Vitarbo, J.; Arfuso, F.; Tan, C.H.; Narula, A.S.; Kumar, A.P.; Sethi, G.; Ahn, K.S. Anti-cancer effects of oxymatrine are mediated through multiple molecular mechanism(s) in tumor models. Pharmacol. Res. 2019, 147, 104327. [Google Scholar] [CrossRef] [PubMed]
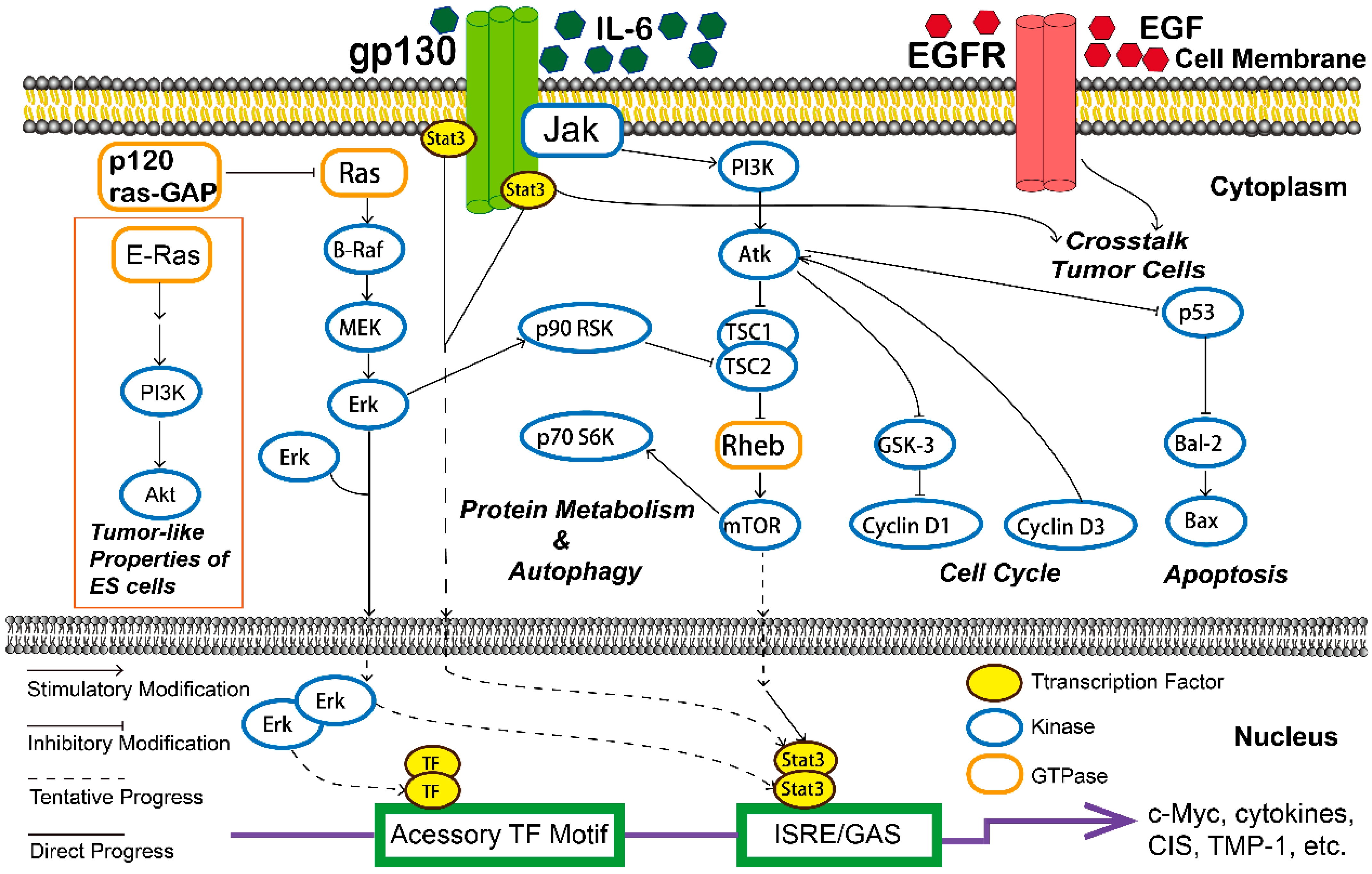
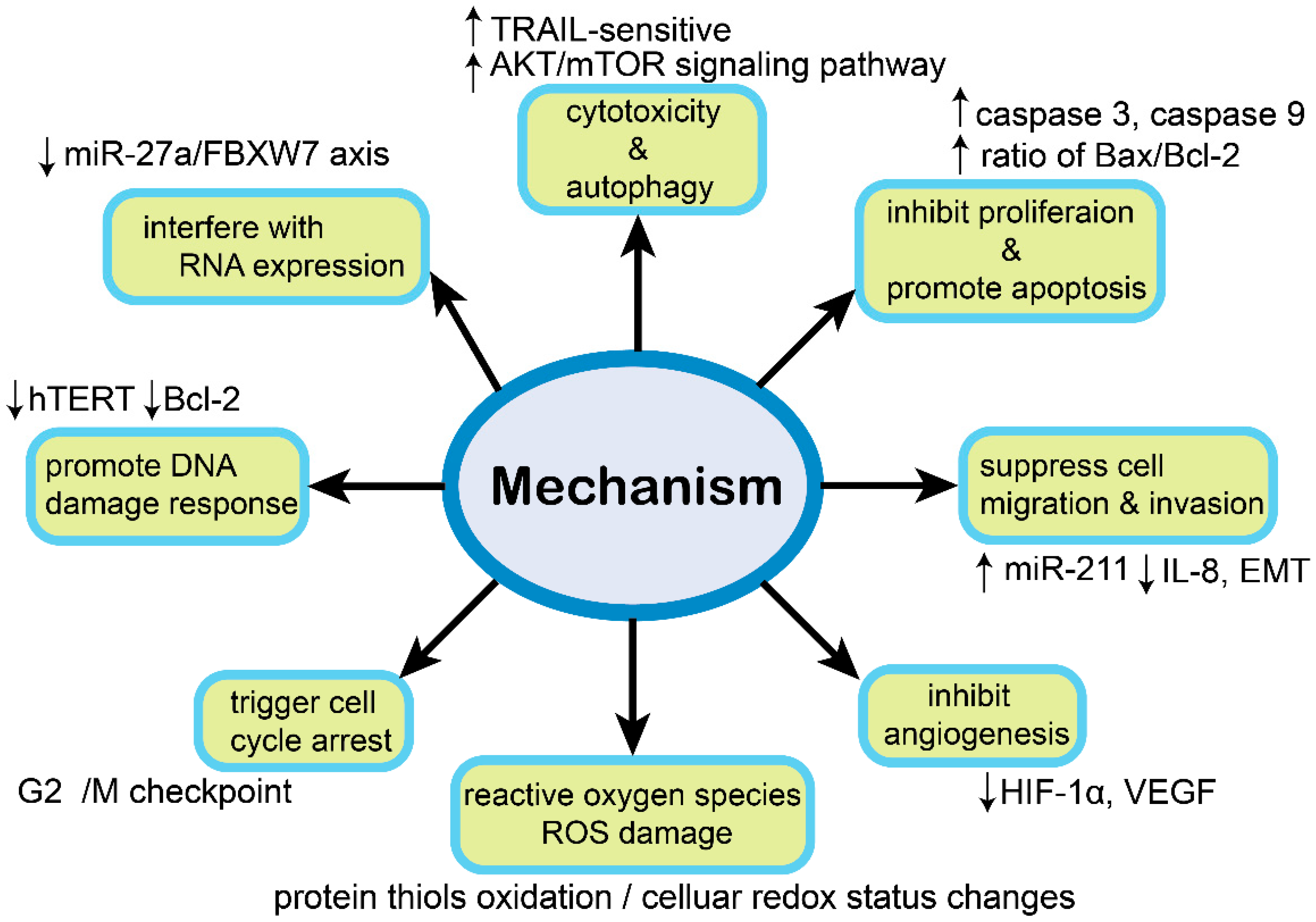
| Compound | Classification | Model | Effect | Mechanism | Reference |
|---|---|---|---|---|---|
| Tanshinones | Terpenoid/Abietane | A-549, TOV-21G | Cytotoxic action, ROS-JNK-CHOP, PI3K/AKT/mTOR signaling pathway | Reduce cell viability, inhibit colony formation capacity | [21,22,53] |
| Kadsuphilactone B | Terpenoid/Triterpene | A2780, Ishikawa cells | Cytotoxic action | Stimulate the activity of caspase-3/8/9 and MAPKs | [23] |
| Methyl lucidone (ML) | Oxo Steroid | OVCAR-8, SKOV-3 | Cytotoxic action | Induce cellular apoptosis, stimulate the cleavage of caspase-3/9 | [24] |
| Zeylenone (Zey) | Alicyclic | SKOV-3 | JAK2/STAT3 signaling pathway | Reduce p-JAK and p-STAT expression level | [28] |
| Berbamine (BBM) | Alkaloid/Benzylisoquinoline | SKOV-3 | Wnt/β-catenin signaling pathway | Increase activity of caspase-3/9, Bax and decrease Bcl-2 levels | [29] |
| Pinus massoniana bark proanthocyanidins (PMBP) | Phenol/Tannin | A-2780 | NF-κB signaling pathway | Activate mitochondria-associated apoptosis | [30] |
| Sanguiin H-6 (SH-6) | Phenol/Tannin | A-2780 | MAPK signaling pathway | Increase p15/BID level, activate MAPKs (p38) | [31] |
| Tetramethylpyrazine (TMP) | Alkaloid/Pyrazine | SKOV-3, OVCAR-3, A-2780 xenograft model | Suppress cell migration and invasion, reverse multiple-drug resistance (MDR) | Increase miR-211, diminish expression of proliferation and angiogenesis markers | [34,35,70] |
| Dihydroartemisinin (DHA) | Terpenoid/Artemisinin | HOSEPICs | Hedgehog signaling pathway | Inhibit cell malignant behaviors and augment apoptosis | [36] |
| Emodin | Quinone/Anthraquinones | A-2780, SKOV-3 | ILK/GSK- 3β/Slug signaling pathway | Diminish the levels of ILK, p-GSK-3β, β-catenin, and Slug, repress EMT | [38] |
| Sulforaphane (SFN) | Ester/Isothiocyanate | Xenograft model, SKOV3-ip1, SKOV3TR-ip2 | Promote DNA and ROS damage | Protein thiols oxidation, suppress cell viability, reduce hTERT and Bcl-2 levels | [40] |
| Quercetin | Flavonoid/Flavonol | SKOV-3, xenograft model | ROS-JNK-CHOP signaling pathway | Upregulate CHOP-induced DR5 expression following ROS damage | [42] |
| Formononetin (FMN) | Flavonoid/Isoflavone | ES2, OV-90 | ERK1/2 signaling pathway | Upregulate caspase 3/9 and bax/bcl-2, interfere with sub G0/G1 phase arrest, increase P38 phosphorylation | [43] |
| Cucurbitacin-A | Terpenoid | SKOV-3 | Trigger cell cycle arrest | DNA damage, ROS damage, MMP alterations, G2/M checkpoint | [46] |
| Asiatic acid | Terpenoid/Triterpene | SKOV-3, OVCAR-3 | PI3K/Akt/mTOR signaling pathway | Downregulate phosphorylation levels of PI3K, AKT, and mTOR | [47] |
| Amentoflavone (AF) | Flavonoid | Xenograft model | ROS/AMPK/mTOR signaling pathway | Downregulate the expression of Skp2 | [49] |
| Proanthocyanidins (BLPs) | Phenol/Tannin | A-2780, CP70, OVCAR-3 SP stem cell | Wnt/β-catenin, AKT/mTOR/p70S6K/4E-BP-1 signaling pathway | Reduce the expression of β-catenin, induce G1 cell cycle arrest, downregulate HIF-1α and VEGF | [50,58] |
| Scutellaria baicalensis (SB) and Fritillaria cirrhosa (FC) | Polysaccharides, Phenols, etc. | OVCA-420, OVCA-429 | NF-κB signaling pathway | Activation of caspase-3 along with downregulation of cyclins D1 and D3 | [51] |
| Grifolin | Terpenoid | A-2780, SKOV-3 | AKT/mTOR/S6K signaling pathway | Upregulate autophagy markers LC3B, Atg7, Beclin-1, downregulate P62 | [54] |
| Genistein | Terpenoid/sesquiterpenoid | OVCAR-3, SKOV-3 | Inhibit VEGF levels | Reduce GD3 levels and restore NKT-cell functions | [56,71] |
| Isoliquiritigenin (ISL) | Ketone | OVCAR5, ES-2 | Suppress cell viability | Increase cleaved PARP, caspase-3 and Bax/Bcl-2 ratio; trigger G2/M cell cycle phase | [57] |
| Flavonoids | Flavonoids | OVCAR-3 | Inhibit angiogenesis | Downregulate VEGF in dose-dependent manner | [59] |
| Baicalin and baicalein | Flavonoid | OVCAR-3, CP-70 | Inhibit angiogenesis | Suppress cancer promoting genes including VEGF, HIF-1α, cMy-c, and NF-kB | [61] |
| Harmine (HM) | Alkaloid | SKOV-3 | Inhibit cell proliferation | Inhibit both the basal and EGF-induced phosphorylation levels of ERK1/2 and CREB | [62] |
| Cranberry proanthocyanidin-1 (PAC-1) | Phenol/Tannin | Chemotherapy-resistant SKOV-3, human umbilical vein endothelial cells (HUVEC) | Increase ROS generation, induce apoptosis | Block G2/M phase cell cycle progression, interfere with the phosphorylation level of VEGF-stimulated receptor | [63] |
| Astragalus polysaccharide (APS) | Polysaccharide | OV-90, SKOV-3 | Inhibit proliferation and promote apoptosis | Upregulate miR-27a and FBXW7 expression levels | [64] |
| Sideroxylin | Flavonoid | ES2, OV-90 | ERK1/2 signaling pathway | DNA and ROS damage, depolarize mitochondrial membrane depolarization, increase lipid peroxidation levels | [67] |
| Epigallocatechin gallate (EGCG) | Flavonoid | SKOV3-ip1, SKOV3TR-ip2 | Promote DNA damage response | Suppress cell viability in time- and dose-dependent manner, reduce the expression of hTERT and Bcl-2 | [68] |
| Berberine | Alkaloid | A2780, HEY, HO8910 | Promote DNA damage response | Trigger oxidative DNA damages | [69] |
| Carotenoids | Terpenoid/Tetraterpenoid | Epidemiological statistics | Inhibit VEGF levels | Stimulate the activities of lymphocytes, macrophages, and cytotoxic T-cells | [72] |
| Lycopene | Terpenoid | OV-MZ-6 | Diminish tumor load | Decrease expression of MMP9, ILK and EMT biomarkers | [73] |
| Deoxyschizandrin | Phenol/Tannin | A-2780 | Increase ROS production | Inhibit macrophages, M2 phenotype markers CD163 and CD209 | [74] |
| Curcumin | Phenol | SKOV3-ip1, HeyA8 | Reduce mean tumor growth | Decrease microvessel density, increase tumor cell apoptosis | [75] |
| Procyanidin (GSP) | Phenol/Tannin | A-2780, SKOV-3 | NF-κB and MAPK/ERK pathway | Augment cytotoxicity of paclitaxel and adriamycin | [76] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Zhou, T.; Wang, Y.; Jiang, Y.; Wang, Y. Mechanisms and Advances in Anti-Ovarian Cancer with Natural Plants Component. Molecules 2021, 26, 5949. https://doi.org/10.3390/molecules26195949
Wu J, Zhou T, Wang Y, Jiang Y, Wang Y. Mechanisms and Advances in Anti-Ovarian Cancer with Natural Plants Component. Molecules. 2021; 26(19):5949. https://doi.org/10.3390/molecules26195949
Chicago/Turabian StyleWu, Jingyuan, Tuoyu Zhou, Yinxue Wang, Yanbiao Jiang, and Yiqing Wang. 2021. "Mechanisms and Advances in Anti-Ovarian Cancer with Natural Plants Component" Molecules 26, no. 19: 5949. https://doi.org/10.3390/molecules26195949
APA StyleWu, J., Zhou, T., Wang, Y., Jiang, Y., & Wang, Y. (2021). Mechanisms and Advances in Anti-Ovarian Cancer with Natural Plants Component. Molecules, 26(19), 5949. https://doi.org/10.3390/molecules26195949





