Effects of Fructose and Oligofructose Addition on Milk Fermentation Using Novel Lactobacillus Cultures to Obtain High-Quality Yogurt-like Products
Abstract
1. Introduction
2. Results
2.1. Lactobacillus Count Changes in Fermented Milk Samples during Storage
2.2. The pH Changes of Fermented Milk Samples during Storage
2.3. Viscosity, Firmness, Syneresis, and Color Changes in Fermented Milk Samples during Storage
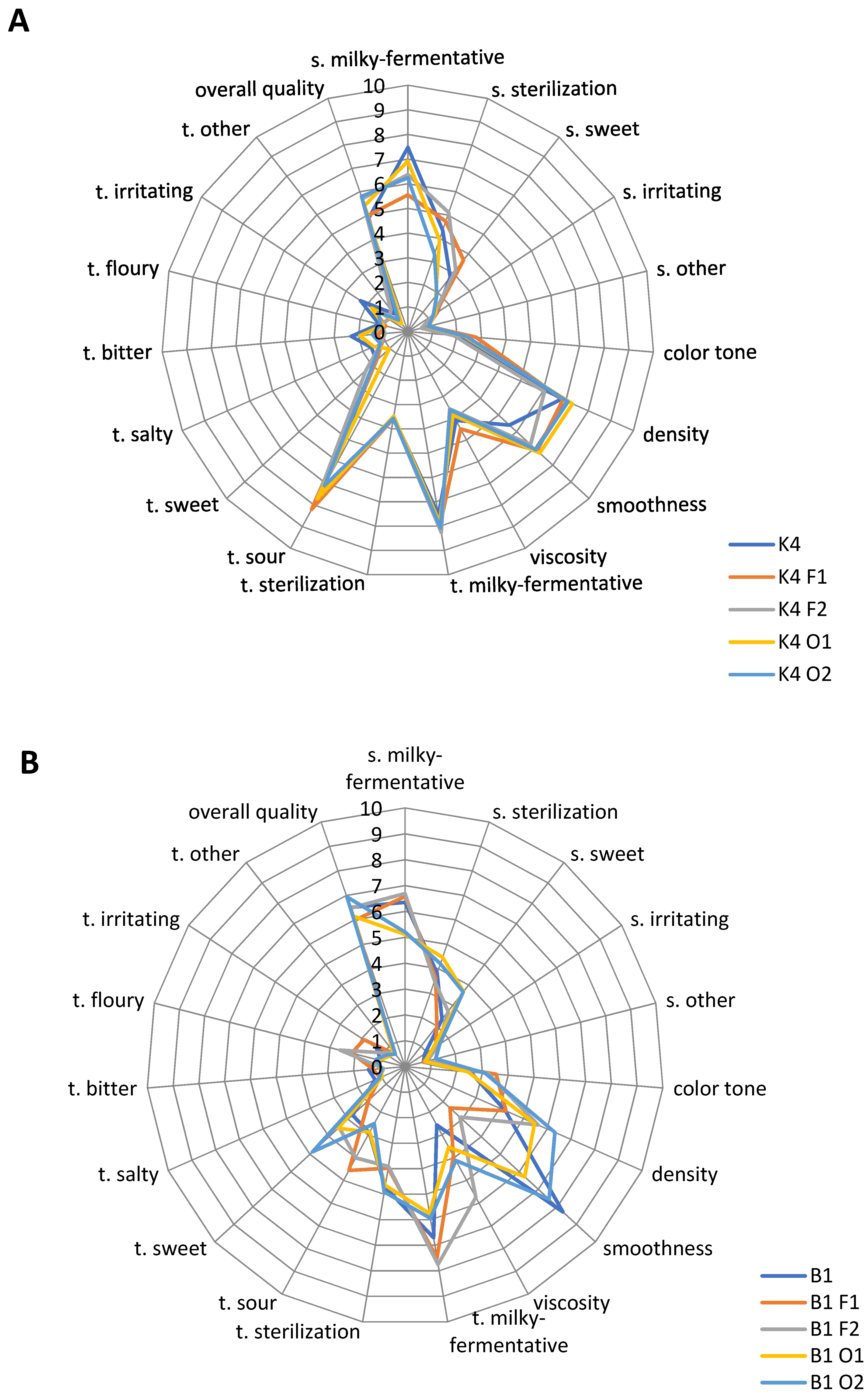
2.4. Sensory Characteristic
3. Discussion
4. Materials and Methods
4.1. Strains and Culture Conditions
4.2. Preparation of Saccharides and Fermented Milk Samples
4.3. Microbiological Analysis
4.4. Acidity Analysis (pH)
4.5. Texture Analysis
4.6. Color Measurement
4.7. Syneresis Measurement
4.8. Sensory Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Bintsis, T. Lactic acid bacteria as starter cultures: An update in their metabolism and genetics. AIMS Microbiol. 2018, 4, 665–684. [Google Scholar] [CrossRef]
- Rychen, G.; Aquilina, G.; Azimonti, G.; Bampidis, V.; Bastos, M.d.L.; Bories, G.; Chesson, A.; Cocconcelli, P.S.; Flachowsky, G.; Gropp, J.; et al. Guidance on the characterisation of microorganisms used as feed additives or as production organisms. EFSA J. 2018, 16, 3. [Google Scholar] [CrossRef]
- Freitas, M. The Benefits of Yogurt, Cultures, and Fermentation. In The Microbiota in Gastrointestinal Pathophysiology; Floch, M.H., Ringel, Y., Allan Walker, W., Eds.; Academic Press: Boston, MA, USA, 2017; pp. 209–223. [Google Scholar] [CrossRef]
- Melini, F.; Melini, V.; Luziatelli, F.; Ficca, A.G.; Ruzzi, M. Health-Promoting Components in Fermented Foods: An Up-to-Date Systematic Review. Nutrients 2019, 11, 1189. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Berni Canani, R.; Flint, H.J.; Salaminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastro. Hepat. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Guarner, F.; Perdigon, G.; Corthier, G.; Salminen, S.; Koletzko, B.; Morelli, L. Should yoghurt cultures be considered probiotic? Br. J. Nutr. 2005, 93, 783–786. [Google Scholar] [CrossRef]
- Patrignani, F.; D’Alessandro, M.; Vannini, L.; Lanciotti, R. Use of functional microbial starters and probiotics to improve functional compound availability in fermented dairy products and beverages. In Sustainability of the Food System; Noelia, B., Ester, B., Eds.; Academic Press Elsevier Inc.: Paterna, Spain, 2020; pp. 167–180. [Google Scholar]
- Khorshidian, N.; Yousefi, M.; Mortazavian, A.M. Fermented milk: The most popular probiotic food carrier. Adv. Food Nutr. Res. 2020, 94, 91–114. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, D.; Kolożyn-Krajewska, D. Food-origin lactic acid bacteria may exhibit probiotic properties. BioMed. Res. Int. 2018, 5063185. [Google Scholar] [CrossRef]
- Johansen, E. Use of Natural Selection and Evolution to Develop New Starter Cultures for Fermented Foods. Annu. Rev. Food Sci. Technol. 2018, 9, 411–428. [Google Scholar] [CrossRef] [PubMed]
- Caric, M.; Milanovic, S.; Iličić, M. Novel trends in fermented dairy technology. Zb. Matice Srp. Prir. Nauk. 2019, 136, 9–21. [Google Scholar] [CrossRef]
- Mishra, S.; Mishra, H.N. Technological aspects of probiotic functional food development. Nutrafoods 2012, 11, 117–130. [Google Scholar] [CrossRef]
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in Food Systems: Significance and Emerging Strategies Towards Improved Viability and Delivery of Enhanced Beneficial Value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef] [PubMed]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Srisuvor, N.; Chinprahast, N.; Prakitchaiwattana, C.; Subhimaros, S. Effects of inulin and polydextrose on physicochemical and sensory properties of low-fat set yoghurt with probiotic-cultured banana purée. LWT 2013, 51, 30–36. [Google Scholar] [CrossRef]
- Karimi, R.; Azizi, M.H.; Ghasemlou, M.; Vaziri, M. Application of inulin in cheese as prebiotic, fat replacer and texturizer: A review. Carbohydr. Polym. 2015, 119, 85–100. [Google Scholar] [CrossRef]
- Gourbeyre, P.; Denery, S.; Bodinier, M. Probiotics, prebiotics, and synbiotics: Impact on the gut immune system and allergic reactions. J. Leukoc. Biol. 2011, 89, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Roberfroid, M.B. Functional foods: Concepts and application to inulin and oligofructose. Br. J. Nutr. 2002, 87, 139–143. [Google Scholar] [CrossRef]
- Gibson, G.R. Dietary modulation of the human gut microflora using the prebiotics oligofructose and inulin. J. Nutr. 1999, 129, 1438S–1441S. [Google Scholar] [CrossRef]
- Cruz, A.G.; Cavalcanti, R.N.; Guerreiro, L.M.R.; Sant’Ana, A.S.; Nogueira, L.C.; Oliveira, C.A.F.; Deliza, R.; Cunha, R.L.; Faria, J.A.F.; Bolini, H.M.A. Developing a prebiotic yogurt: Rheological, physico-chemical and microbiological aspects and adequacy of survival analysis methodology. J. Food Eng. 2013, 114, 323–330. [Google Scholar] [CrossRef]
- Fornelli, A.; Bandiera, N.; Costa, M.; Souza, C.; Santana, E.; Sivieri, K.; Aragon-Alegro, L. Effect of inulin and oligofructose on the physicochemical, microbiological and sensory characteristics of symbiotic dairy beverages. Semin. Cienc. Agrar. 2014, 35, 3099. [Google Scholar] [CrossRef][Green Version]
- Castro, F.P.; Cunha, T.M.; Barreto, P.L.M.; Amboni, R.D.M.C.; Prudêncio, E.S. Effect of oligofructose incorporation on the properties of fermented probiotic lactic beverages. Int. J. Dairy Technol. 2009, 62, 68–74. [Google Scholar] [CrossRef]
- Oliveira, R.P.S.; Casazza, A.A.; Aliakbarian, B.; Perego, P.; Converti, A.; Oliveira, M.N. Influence of fructooligosaccharides on the fermentation profile and viable counts in a symbiotic low fat milk. Braz. J. Microbiol. 2013, 44, 431–434. [Google Scholar] [CrossRef]
- Kadrin, J.; Elias, P.; Roasto, M.; Tamme, T. Viability of Lactobacillus acidophilus in yoghurt containing inulin or oligofructose during refrigerated storage. Milchwissenschaft 2007, 62, 52–54. [Google Scholar]
- Gustaw, W.; Kordowska-Wiater, M.; Kozioł, J. The influence of selected prebiotics on the growth of lactic acid bacteria for bio-yoghurt production. Acta Sci. Pol. Technol. Aliment. 2011, 10, 455–466. [Google Scholar]
- Aghajani, A.; Pourahmad, R.; Mahdavi Adeli, H.R. Evaluation of physicochemical changes and survival of probiotic bacteria in synbiotic yoghurt. J. Food Biosci. Technol. 2012, 2, 13–22. [Google Scholar]
- Chand, P.; Kumar, M.D.; Singh, A.K.; Deshwal, G.K.; Rao, P.S.; Tomar, S.K.; Sharma, H. Low-calorie synbiotic yoghurt from indigenous probiotic culture and combination of inulin and oligofructose: Improved sensory, rheological and textural attributes. J. Food Process. Preserv. 2021, 45, e15322. [Google Scholar] [CrossRef]
- Kariyawasam, K.M.G.M.M.; Lee, N.; Paik, H. Synbiotic yoghurt supplemented with novel probiotic Lactobacillus brevis KU200019 and fructooligosaccharides. Food Biosci. 2021, 39, 100835. [Google Scholar] [CrossRef]
- Mohan, A.; Hadi, J.; Gutierrez-Maddox, N.; Li, Y.; Leung, I.K.H.; Gao, Y.; Shu, Q.; Quek, S.-Y. Sensory, Microbiological and Physicochemical Characterisation of Functional Manuka Honey Yogurts Containing Probiotic Lactobacillus reuteri DPC16. Foods 2020, 9, 106. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.N.; Mital, B.K.; Garg, S.K. Effect of casitone and fructose on the growth of Lactobacillus acidophilus and its survival during storage. Int. J. Food Microbiol. 1994, 21, 271–276. [Google Scholar] [CrossRef]
- Pangetika, W.; Al-Baarri, A.; Legowo, A. The Change in pH on Lactobacillus acidophillus Medium Containing D-fructose. JAFT 2018, 5, 37–38. [Google Scholar] [CrossRef]
- Popov-Raljić, J.V.; Lakić, N.S.; Laličić-Petronijević, J.G.; Barać, M.B.; Sikimić, V.M. Color changes of UHT milk during storage. Sensors 2008, 8, 5961–5974. [Google Scholar] [CrossRef] [PubMed]
- Franck, A. Technological functionality of inulin and oligofructose. Br. J. Nutr. 2002, 87, 287–291. [Google Scholar] [CrossRef]
- Waligora-Dupriet, A.J.; Campeotto, F.; Nicolis, I.; Bonet, A.; Soulaines, P.; Dupont, C.; Butel, M.J. Effect of oligofructose supplementation on gut microflora and well-being in young children atteding a day care center. Int. J. Food Microbiol. 2007, 113, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Gupta, A.K. Applications of inulin and oligofructose in health and nutrition. J. Biosci. 2002, 27, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Beatty, E.R.; Wang, X.; Cummings, J.H. Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology 1995, 108, 975–982. [Google Scholar] [CrossRef]
- Liu, F.; Li, P.; Chen, M.; Luo, Y.; Prabhakar, M.; Zheng, H.; He, Y.; Qi, Q.; Long, H.; Zhang, Y.; et al. Fructooligosaccharide (FOS) and Galactooligosaccharide (GOS) Increase Bifidobacterium but Reduce Butyrate Producing Bacteria with Adverse Glycemic Metabolism in healthy young population. Sci. Rep. 2017, 7, 11789. [Google Scholar] [CrossRef]
- Castro, F.P.; Cunha, T.M.; Ogliari, P.J.; Teófilo, R.F.; Ferreira, M.M.C.; Prudêncio, E.S. Influence of different content of cheese whey and oligofructose on the properties of fermented lactic beverages: Study using response surface methodology. LWT 2009, 42, 993–997. [Google Scholar] [CrossRef]
- Oliveira, R.P.S.; Florence, A.C.R.; Silva, R.C.; Perego, P.; Converti, A.; Gioielli, L.A.; Oliveira, M.N. Effect of different prebiotics on the fermentation kinetics, probiotic survival and fatty acids profiles in nonfat symbiotic fermented milk. Int. J. Food Microbiol. 2009, 128, 467–472. [Google Scholar] [CrossRef]
- Pereira, R.M.; Botezelli, J.D.; Da Cruz Rodrigues, K.C.; Mekary, R.A.; Cintra, D.E.; Pauli, J.R.; Da Silva, A.S.R.; Ropelle, E.R.; De Moura, L.P. Fructose Consumption in the Development of Obesity and the Effects of Different Protocols of Physical Exercise on the Hepatic Metabolism. Nutrients 2017, 9, 405. [Google Scholar] [CrossRef]
- Rizkalla, S.W. Health implications of fructose consumption: A review of recent data. Nutr. Metab. 2010, 7, 82. [Google Scholar] [CrossRef] [PubMed]
- Slačanac, V.; Hardi, J.; Lučan, M.; Kun, S.; Havas, P.; Krstanović, V. Effect of honey addition on fermentation activity of Lactobacillus casei Lc-01 in cow’s and goat’s milk: A kinetic study. Acta Aliment. 2011, 40, 270–281. [Google Scholar] [CrossRef]
- Önür, İ.; Misra, N.N.; Barba, F.J.; Putnik, P.; Lorenzo, J.M.; Gökmen, V.; Alpas, H. Effects of ultrasound and high pressure on physicochemical properties and HMF formation in Turkish honey types. J. Food Eng. 2018, 219, 129–136. [Google Scholar] [CrossRef]
- Mani-López, E.; Palou, E.; López-Malo, A. Probiotic viability and storage stability of yogurts and fermented milks prepared with several mixtures of lactic acid bacteria. J. Dairy Sci. 2014, 97, 2578–2590. [Google Scholar] [CrossRef] [PubMed]
- Donkor, O.N.; Nilmini, S.L.I.; Stolic, P.; Vasiljevic, T.; Shah, N.P. Survival and activity of selected probiotic organisms in set-type yoghurt during cold storage. Int. Dairy J. 2007, 17, 657–665. [Google Scholar] [CrossRef]
- Damin, M.R.; Minowa, E.; Alcântara, M.R.; Oliveira, M.N. Effect Of Cold Storage On Culture Viability And Some Rheological Properties Of Fermented Milk Prepared With Yogurt And Probiotic Bacteria. J. Texture Stud. 2008, 39, 40–55. [Google Scholar] [CrossRef]
- Oliveira, R.P.; Perego, P.; Oliveira, M.N.; Converti, A. Effect of inulin as prebiotic and synbiotic interactions between probiotics to improve fermented milk firmness. J. Food Eng. 2011, 107, 36–40. [Google Scholar] [CrossRef]
- Perrin, S.; Warchol, M.; Grill, J.P.; Schneider, F. Fermentations of fructo-oligosaccharides and their components by Bifidobacterium infantis ATCC 15697 on batch culture in semi-synthetic medium. J. Appl. Microbiol. 2001, 90, 859–865. [Google Scholar] [CrossRef]
- Barrangou, R.; Altermann, E.; Hutkins, R.; Cano, R.; Klaenhammer, T.R. Functional and comparative genomic analyses of an operon involved in fructooligosaccharide utilization by Lactobacillus acidophilus. PNAS 2003, 100, 8957–8962. [Google Scholar] [CrossRef]
- Bielecka, M.; Biedrzycka, E.; Majkowska, A. Selection of probiotics and prebiotics for synbiotics and confirmation of their in vivo effectiveness. Food Res. Int. 2002, 35, 125–131. [Google Scholar] [CrossRef]
- Saier, M.H.; Ye, J.J.; Klinke, S.; Nino, E. Identification of an anaerobically induced phosphoenolpyruvate-dependent fructose-specific phosphotransferase system and evidence for the Embden–Meyerhof glycolytic pathway in the heterofermented bacterium Lactobacillus brevis. J. Bacteriol. 1996, 178, 314–316. [Google Scholar] [CrossRef][Green Version]
- Zielińska, D.; Rzepkowska, A.; Radawska, A.; Zieliński, K. In Vitro Screening of Selected Probiotic Properties of Lactobacillus Strains Isolated from Traditional Fermented Cabbage and Cucumber. Curr. Microbiol. 2015, 70, 183–194. [Google Scholar] [CrossRef]
- Hartati, A.I.; Pramono, Y.; Legowo, A. Lactose and reduction sugar concentrations, pH and the sourness of date flavored yogurt drink as probiotic beverage. J. Appl. Food Technol. 2012, 1, 1–3. [Google Scholar]
- Glibowski, P.; Zielińska, E. Physicochemical and sensory properties of kefir containing inulin and oligofructose. Int. J. Dairy Technol. 2015, 68, 602–607. [Google Scholar] [CrossRef]
- Akalın, A.S.; Erişir, D. Effects of Inulin and Oligofructose on the Rheological Characteristics and Probiotic Culture Survival in Low-Fat Probiotic Ice Cream. J. Food Sci. 2008, 73, M184–M188. [Google Scholar] [CrossRef] [PubMed]
- Mercan, E.; Akın, N. Effect of different levels of pine honey addition on physicochemical, microbiological and sensory properties of set-type yoghurt. Int. J. Dairy Technol. 2016, 70, 245–252. [Google Scholar] [CrossRef]
- Amatayakul, T.; Sherkat, F.; Shah, N.P. Syneresis in set yogurt as affected by EPS starter cultures and levels of solids. Int. J. Dairy Technol. 2006, 59, 216–221. [Google Scholar] [CrossRef]
- González-Martínez, C.; Becerra, M.; Cháfer, M.; Albors, A.; Carot, J.M.; Chiralt, A. Influence of substituting milk powder for whey powder on yoghurt quality. Trends Food Sci. Technol. 2002, 13, 334–340. [Google Scholar] [CrossRef]
- Roberfroid, M.B. Inulin-type fructans: Functional food ingredients. J. Nutr. 2007, 137, 2493S–2502S. [Google Scholar] [CrossRef] [PubMed]
- Cardarelli, H.R.; Buriti, F.C.A.; Castro, I.A.; Saad, S.M.I. Inulin and oligofructose improve sensory quality and increase the probiotic viable count in potentially synbiotic petit-suisse cheese. LWT 2008, 41, 1037–1046. [Google Scholar] [CrossRef]
- Skryplonek, K. Production of yogurt-type fermented beverages. Mljekarstvo 2018, 68, 139–149. [Google Scholar] [CrossRef]
- Lee, S.-B.; Cosmas, B.; Park, H.-D. The Antimutagenic and Antioxidant Activity of Fermented Milk Supplemented with Cudrania tricuspidata Powder. Foods 2020, 9, 1762. [Google Scholar] [CrossRef]
- Xin, L.T.; Yue, S.J.; Fan, Y.C.; Wu, J.S.; Yan, D.; Guan, H.S.; Wang, C.Y. Cudrania tricuspidata: An updated review on ethnomedicine, phytochemistry and pharmacology. RSC Adv. 2017, 7, 31807–31832. [Google Scholar] [CrossRef]
- Rzepkowska, A.; Zielińska, D.; Ołdak, A.; Kołożyn-Krajewska, D. Safety assessment and antimicrobial properties of the lactic acid bacteria strains isolated from polish raw fermented meat products. Int. J. Food Prop. 2016, 20, 2736–2747. [Google Scholar] [CrossRef]
- Buffa, M.N.; Trujillo, A.J.; Pavia, M.; Guamis, B. Changes in textural, microstructural, and colour characteristics during ripening of cheeses made from raw, pasteurized or high-pressure-treated goats’ milk. Int. Dairy J. 2001, 11, 927–934. [Google Scholar] [CrossRef]
- Farnsworth, J.P.; Li, J.; Guo, M.R. Improved structure and consistency of probiotic goat’s milk yogurt. Aust. J. Dairy Technol. 2003, 58, 187. [Google Scholar]
- Keogh, M.K.; O’Kennedy, B.T. Rheology of Stirred Yogurt as Affected byAdded Milk Fat, Protein and Hydrocolloids. J. Food Sci. 1998, 63, 108–112. [Google Scholar] [CrossRef]
- Sensory Analysis—Methodology—General Guidance for Establishing a Sensory Profile. Available online: https://www.iso.org/standard/58042.html (accessed on 20 June 2021).

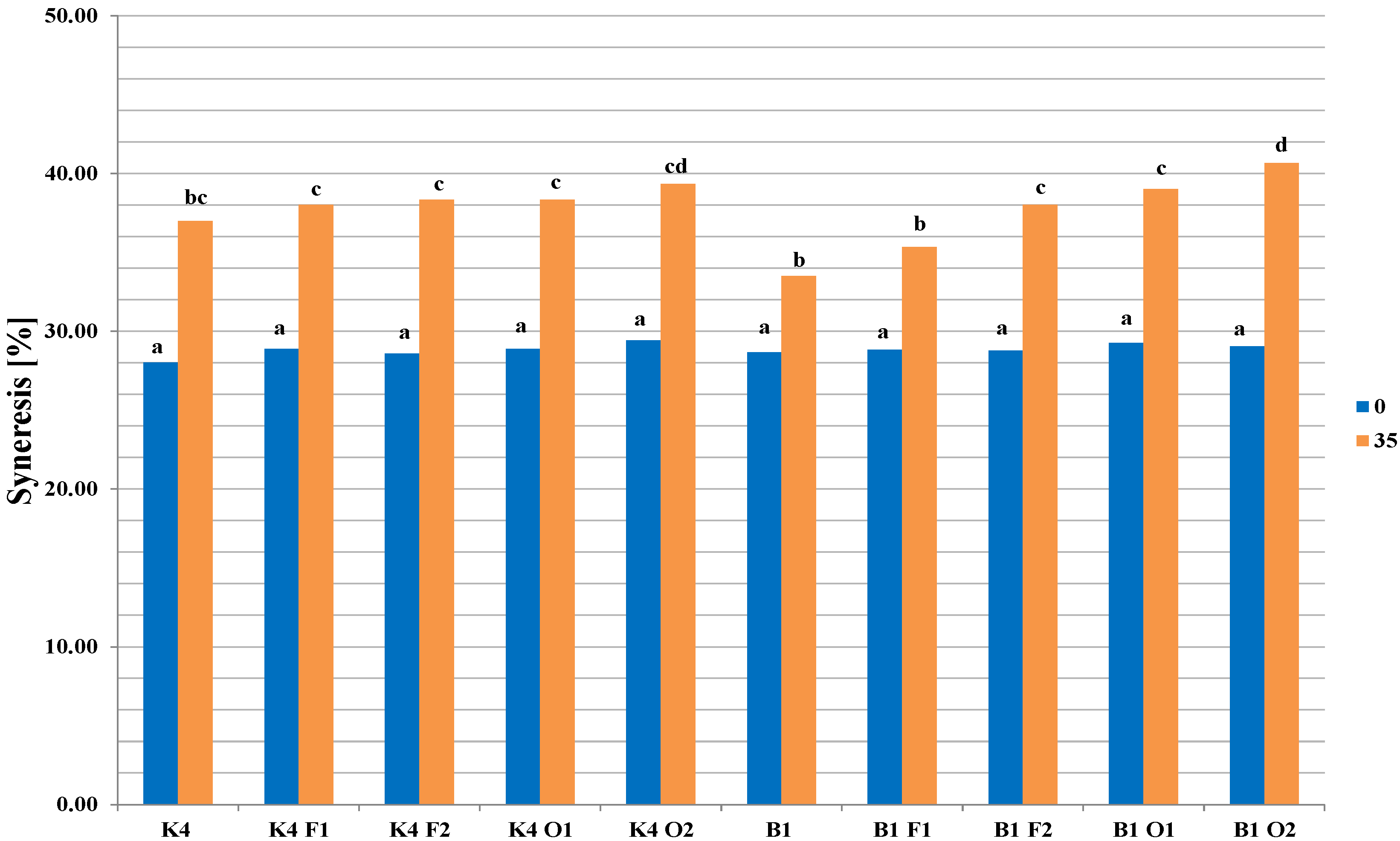

| Name of Sample | Time of Storage [Days] | |||||
|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | 28 | 35 | |
| K4 | 4.85 ± 0.17 a | 4.82 ± 0.15 a | 4.62 ± 0.17 bc | 4.39 ± 0.14 d | 4.32 ± 0.12 e | 4.28 ± 0.12 e |
| K4 F1 | 4.70 ± 0.05 a | 4.68 ± 0.05 a | 4.50 ± 0.04 c | 4.24 ± 0.08 d | 4.20 ± 0.10 ef | 4.15 ± 0.11 ef |
| K4 F2 | 4.83 ± 0.08 a | 4.80 ± 0.12 ab | 4.52 ± 0.03 c | 4.37 ± 0.28 e | 4.33 ± 0.30 ef | 4.26 ± 0.25 ef |
| K4 O1 | 4.69 ± 0.01 a | 4.66 ± 0.02 b | 4.42 ± 0.07 d | 4.25 ± 0.05 ef | 4.22 ± 0.06 f | 4.21 ± 0.07 f |
| K4 O2 | 4.68 ± 0.03 a | 4.66 ± 0.04 b | 4.41 ± 0.02 d | 4.20 ± 0.03 f | 4.22 ± 0.05 fg | 4.19 ± 0.06 g |
| B1 | 4.38 ± 0.03 d | 4.37 ± 0.02 d | 4.33 ± 0.01 e | 4.21 ± 0.01 f | 4.21 ± 0.02 fg | 4.15 ± 0.02 g |
| B1 F1 | 4.38 ± 0.12 d | 4.36 ± 0.11 de | 4.32 ± 0.10 e | 4.06 ± 0.11 fg | 4.06 ± 0.11 g | 4.02 ± 0.09 g |
| B1 F2 | 4.31 ± 0.12 d | 4.36 ± 0.11 e | 4.32 ± 0.10 e | 4.10 ± 0.12 g | 4.12 ± 0.12 g | 4.11 ± 0.10 g |
| B1 O1 | 4.31 ± 0.08 de | 4.32 ± 0.07 e | 4.32 ± 0.08 ef | 4.10 ± 0.09 g | 4.12 ± 0.08 g | 4.11 ± 0.07 g |
| B1 O2 | 4.32 ± 0.08 de | 4.33 ± 0.06 e | 4.33 ± 0.05 ef | 4.10 ± 0.05 g | 4.11 ± 0.04 g | 4.13 ± 0.04 g |
| Name of Sample | Time of Storage [Days] | |||
|---|---|---|---|---|
| 0 | 7 | 21 | 35 | |
| K4 | 39.56 ± 13.57 g | 61.81 ± 12.22 e | 45.45 ± 11.31 fg | 44.72 ± 5.37 fg |
| K4 F1 | 42.39 ± 7.34 e | 70.12 ± 15.40 d | 51.72 ± 9.78 f | 48.67 ± 9.70 f |
| K4 F2 | 39.33 ± 4.86 g | 69.59 ± 7.18 d | 58.25 ± 10.21 g | 52.76 ± 10.64 f |
| K4 O1 | 40.45 ± 12.56 g | 88.77 ± 17.89 bc | 61.51 ± 16.41 g | 57.52 ± 13.27 e |
| K4 O2 | 41.62 ± 2.65 g | 100.47 ± 7.95 b | 68.52 ± 8.55 e | 62.76 ± 5.44 g |
| B1 | 64.62 ± 5.09 ed | 84.43 ± 18.19 c | 75.05 ± 0.64 cd | 55.38 ± 18.97 ef |
| B1 F1 | 63.94 ± 10.57 e | 89.55 ± 9.36 bc | 79.89 ± 10.72 c | 60.75 ± 17.94 e |
| B1 F2 | 61.61 ± 15.71 e | 94.80 ± 18.22 b | 87.04 ± 16.19 bc | 69.37 ± 13.59 d |
| B1 O1 | 64.38 ± 18.59 ed | 114.15 ± 15.46 a | 90.90 ± 12.18 b | 78.71 ± 11.14 c |
| B1 O2 | 73.20 ± 7.41 cd | 120.88 ± 19.46 a | 100.43 ± 12.87 b | 84.48 ± 15.29 c |
| Name of Sample | Time of Storage [Days] | |||
|---|---|---|---|---|
| 0 | 7 | 21 | 35 | |
| K4 | 0.23 ± 0.02 b | 0.25 ± 0.05 a | 0.23 ± 0.05 b | 0.20 ± 0.00 c |
| K4 F1 | 0.22 ± 0.01 bc | 0.24 ± 0.02 ab | 0.23 ± 0.02 b | 0.21 ± 0.01 c |
| K4 F2 | 0.21 ± 0.01 c | 0.24 ± 0.01 ab | 0.22 ± 0.00 bc | 0.20 ± 0.01 c |
| K4 O1 | 0.23 ± 0.00 b | 0.26 ± 0.01 a | 0.22 ± 0.00 c | 0.20 ± 0.01 c |
| K4 O2 | 0.22 ± 0.01 bc | 0.24 ± 0.00 ab | 0.22 ± 0.00 bc | 0.20 ± 0.00 c |
| B1 | 0.23 ± 0.04 b | 0.26 ± 0.01 a | 0.23 ± 0.03 ab | 0.22 ± 0.02 bc |
| B1 F1 | 0.22 ± 0.00 bc | 0.24 ± 0.01 ab | 0.22 ± 0.01 bc | 0.21 ± 0.01 c |
| B1 F2 | 0.22 ± 0.01 bc | 0.25 ± 0.02 a | 0.23 ± 0.00 bc | 0.21 ± 0.01 c |
| B1 O1 | 0.23 ± 0.01 b | 0.25 ± 0.03 a | 0.23 ± 0.00 bc | 0.21 ± 0.00 c |
| B1 O2 | 0.22 ± 0.01 bc | 0.26 ± 0.03 a | 0.23 ± 0.00 b | 0.23 ± 0.01 b |
| Name of Sample | Time of Storage [Days] | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 7 | 21 | 35 | |||||||||
| L* | a* | b* | L* | a* | b* | L* | a* | b* | L* | a* | b* | |
| K4 | 83.77 ± 3.20 a | −1.34 ± 0.02 a | 7.52 ± 0.19 a | 90.01 ± 1.07 bc | −1.47 ± 0.01 b | 7.76 ± 0.20 b | 92.21 ± 2.04 c | −1.69 ± 0.07 c | 7.93 ± 0.07 bc | 84.65 ± 2.82 a | −1.50 ± 0.06 b | 7.65 ± 0.32 ab |
| K4 F1 | 83.06 ± 2.46 a | −1.31 ± 0.03 a | 7.54 ± 0.24 a | 86.51 ± 3.05 ab | −1.47 ± 0.12 b | 7.80 ± 0.27 b | 90.03 ± 3.04 bc | −1.65 ± 0.15 c | 7.97 ± 0.47 c | 84.45 ± 2.10 a | −1.45 ± 0.11b | 7.71 ± 0.26 ab |
| K4 F2 | 83.80 ± 2.44 a | −1.36 ± 0.03 a | 7.54 ± 0.09 a | 85.22 ± 1.91 a | −1.43 ± 0.07 b | 7.84 ± 0.16 b | 88.36 ± 1.62 b | −1.67 ± 0.05 c | 7.97 ± 0.10 c | 83.65 ± 2.12 a | −1.49 ± 0.06 b | 7.75 ± 0.05 b |
| K4 O1 | 83.49 ± 1.94 a | −1.33 ± 0.07 a | 7.53 ± 0.09 a | 89.49 ± 1.60 bc | −1.51 ± 0.02 b | 7.89 ± 0.23 bc | 91.52 ± 1.75 c | −1.69 ± 0.03 c | 8.02 ± 0.13 c | 85.50 ± 2.66 a | −1.52 ± 0.02 b | 7.83 ± 0.45 b |
| K4 O2 | 83.83 ± 4.10 a | −1.32 ± 0.02 a | 7.59 ± 0.022 a | 88.20 ± 2.21 b | −1.39 ± 0.03 b | 7.94 ± 0.17 bc | 90.99 ± 0.56 c | −1.65 ± 0.02 c | 8.09 ± 0.41 c | 84.67 ± 0.50 a | −1.55 ± 0.01 bc | 7.90 ± 0.05 bc |
| B1 | 85.33 ± 2.47 a | −1.31 ± 0.04 a | 7.53 ± 0.009 a | 89.02 ± 2.67 b | −1.41 ± 0.05 ab | 7.65 ± 0.27 ab | 92.48 ± 4.23 c | −1.65 ± 0.07 c | 7.82 ± 0.15 b | 84.85 ± 1.99 a | −1.52 ± 0.07 b | 7.70 ± 0.02 ab |
| B1 F1 | 85.49 ± 3.04a | −1.27 ± 0.05 a | 7.56 ± 0.23 a | 86.63 ± 2.30 ab | −1.38 ± 0.04 ab | 7.70 ± 0.21ab | 88.26 ± 1.78 b | −1.60 ± 0.16 c | 7.86 ± 0.40 b | 84.06 ± 0.27 a | −1.43 ± 0.03 b | 7.80 ± 0.10 b |
| B1 F2 | 85.36 ± 1.86 a | −1.31 ± 0.02 a | 7.57 ± 0.16 a | 86.47 ± 2.68 ab | −1.44 ± 0.13 b | 7.80 ± 0.07 b | 87.70 ± 1.48 b | −1.57 ± 0.09 bc | 7.87 ± 0.97 b | 83.87 ± 0.57 a | −1.53 ± 0.12 bc | 7.84 ± 0.26 b |
| B1 O1 | 85.17 ± 3.81 a | −1.26 ± 0.08 a | 7.53 ± 0.17 a | 88.67 ± 3.15 b | −1.45 ± 0.03 b | 7.89 ± 0.34 b | 90.95 ± 0.26 c | −1.70 ± 0.03 c | 7.95 ± 0.14 c | 84.50 ± 1.59 a | −1.49 ± 0.08 b | 7.87 ± 0.13 bc |
| B1 O2 | 85.04 ± 6.55 a | −1.26 ± 0.10 a | 7.58 ± 0.45 a | 87.26 ± 3.08 b | −1.40 ± 0.06 ab | 7.95 ± 0.28 c | 90.48 ± 2.59 c | −1.64 ± 0.12 c | 8.03 ± 0.08 c | 83.72 ± 0.48 a | −1.45 ± 0.03 b | 7.97 ± 0.18 c |
| Strain Inoculation | Saccharide Addition | ||||
|---|---|---|---|---|---|
| Control | Fructose (F) | Oligofructose (O) | |||
| 0% | 1% | 2% | 1% | 2% | |
| L. brevis B1 | B1 | B1 F1 | B1 F2 | B1 O1 | B1 O2 |
| L. johnsonii K4 | K4 | K4 F1 | K4 F2 | K4 O1 | K4 O2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zielińska, D.; Marciniak-Lukasiak, K.; Karbowiak, M.; Lukasiak, P. Effects of Fructose and Oligofructose Addition on Milk Fermentation Using Novel Lactobacillus Cultures to Obtain High-Quality Yogurt-like Products. Molecules 2021, 26, 5730. https://doi.org/10.3390/molecules26195730
Zielińska D, Marciniak-Lukasiak K, Karbowiak M, Lukasiak P. Effects of Fructose and Oligofructose Addition on Milk Fermentation Using Novel Lactobacillus Cultures to Obtain High-Quality Yogurt-like Products. Molecules. 2021; 26(19):5730. https://doi.org/10.3390/molecules26195730
Chicago/Turabian StyleZielińska, Dorota, Katarzyna Marciniak-Lukasiak, Marcelina Karbowiak, and Piotr Lukasiak. 2021. "Effects of Fructose and Oligofructose Addition on Milk Fermentation Using Novel Lactobacillus Cultures to Obtain High-Quality Yogurt-like Products" Molecules 26, no. 19: 5730. https://doi.org/10.3390/molecules26195730
APA StyleZielińska, D., Marciniak-Lukasiak, K., Karbowiak, M., & Lukasiak, P. (2021). Effects of Fructose and Oligofructose Addition on Milk Fermentation Using Novel Lactobacillus Cultures to Obtain High-Quality Yogurt-like Products. Molecules, 26(19), 5730. https://doi.org/10.3390/molecules26195730








