Therapeutic Efficacy of Sesquiterpene Farnesol in Treatment of Cutibacterium acnes-Induced Dermal Disorders
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Animals
2.2. Growth Curve of C. acnes
2.3. Antimicrobial Effect of Farnesol on C. acnes
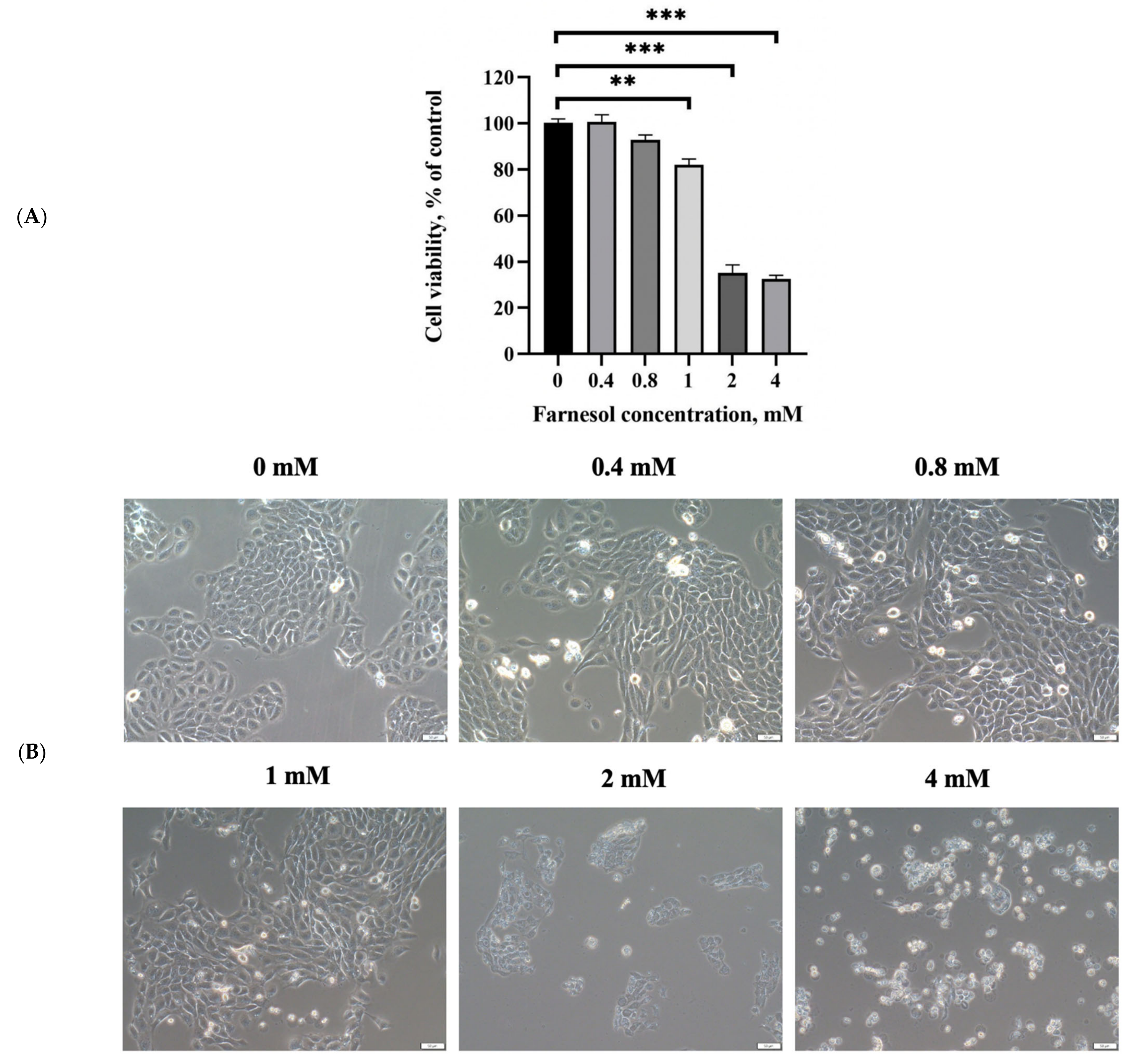
2.4. In Vitro Cell Cytotoxicity Assay for Farnesol
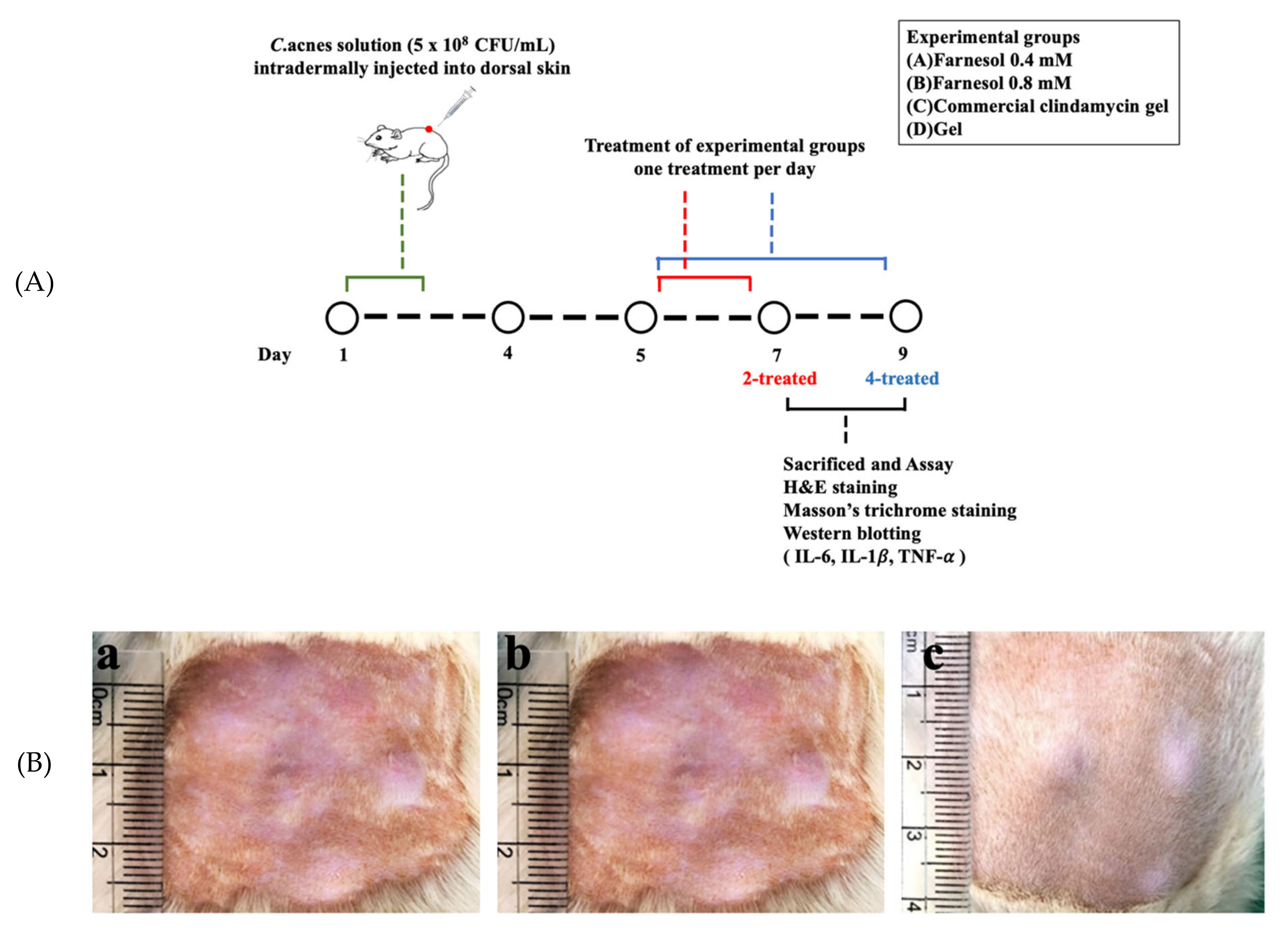
2.5. In Vivo Animal Experiments: Establishment of Skin Acne Model in a Rat
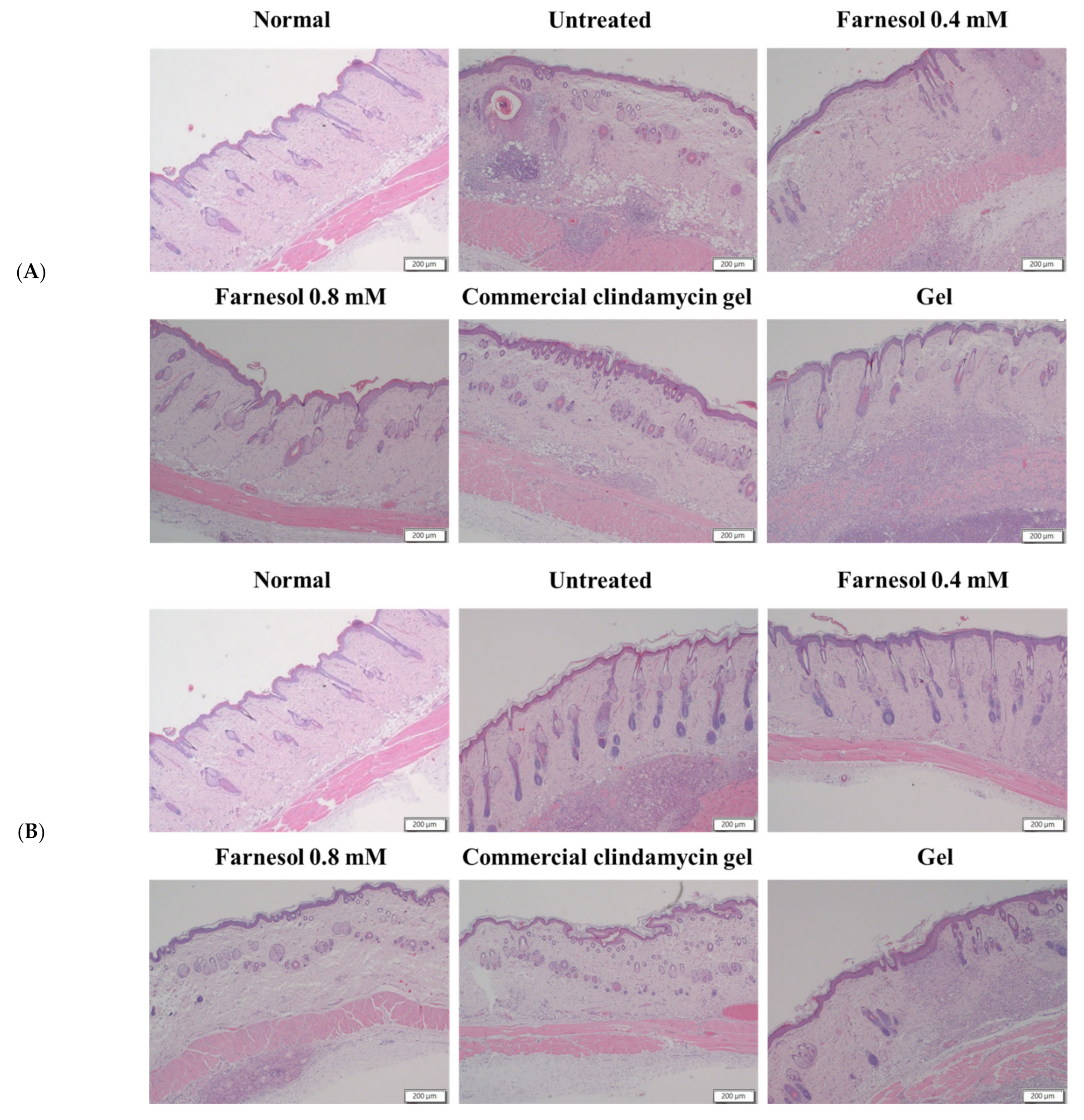
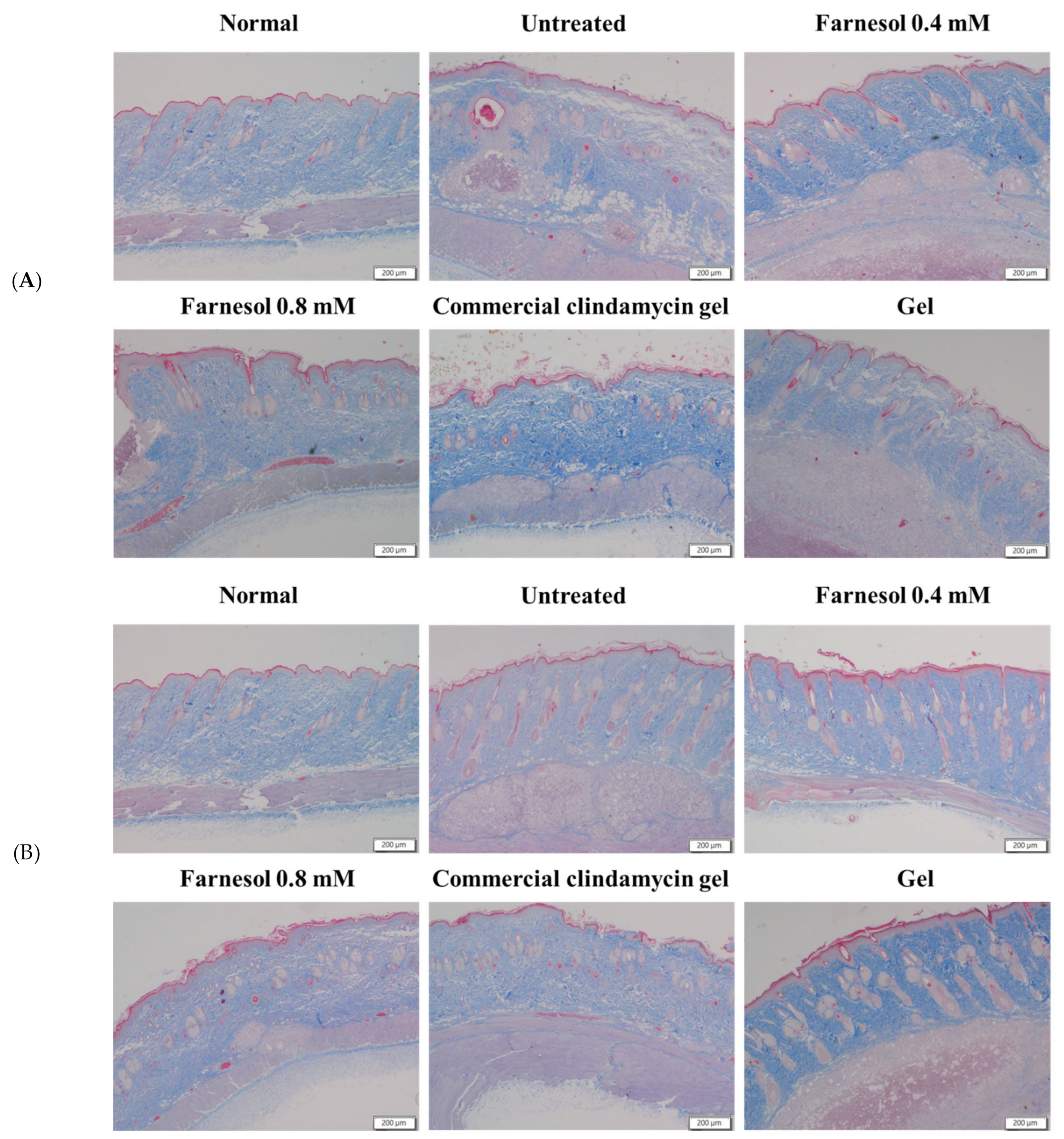
2.6. Histopathological Analyses
2.7. Histopathological Scoring of Skin Repair
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Growth of C. acnes and MIC Determination
3.2. In Vitro Viability of Keratinocytes after Farnesol Treatment
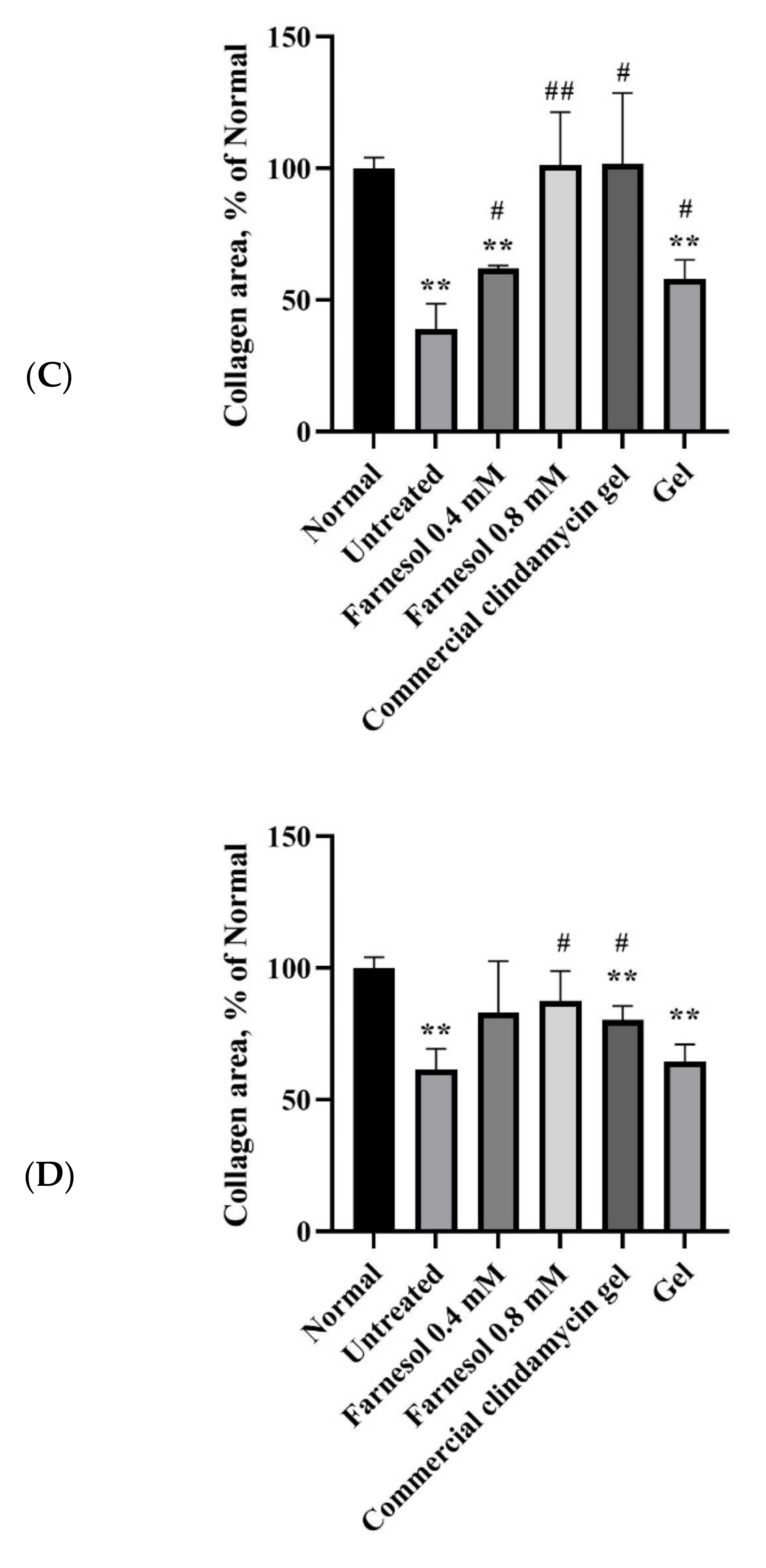
3.3. Histopathological Analysis
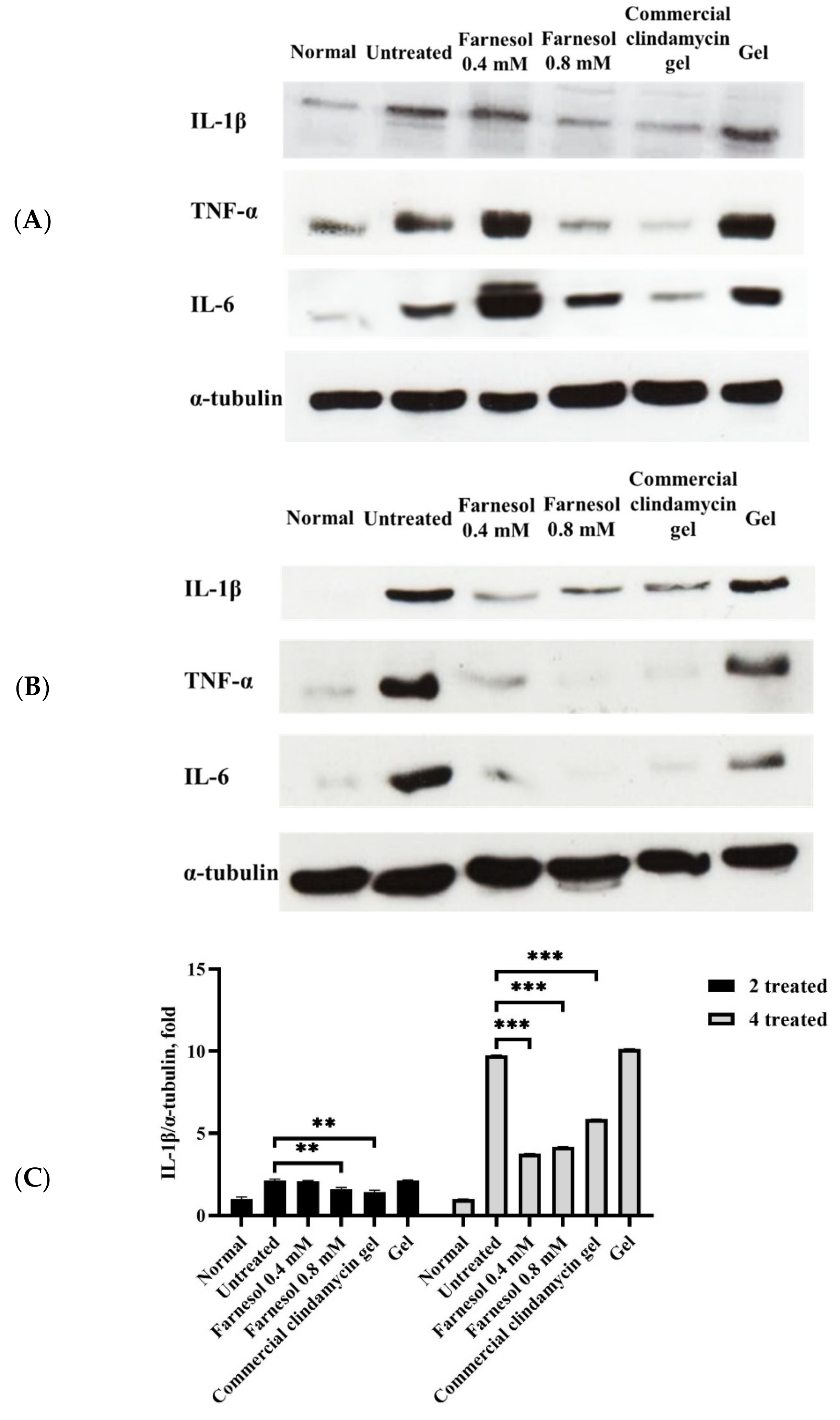
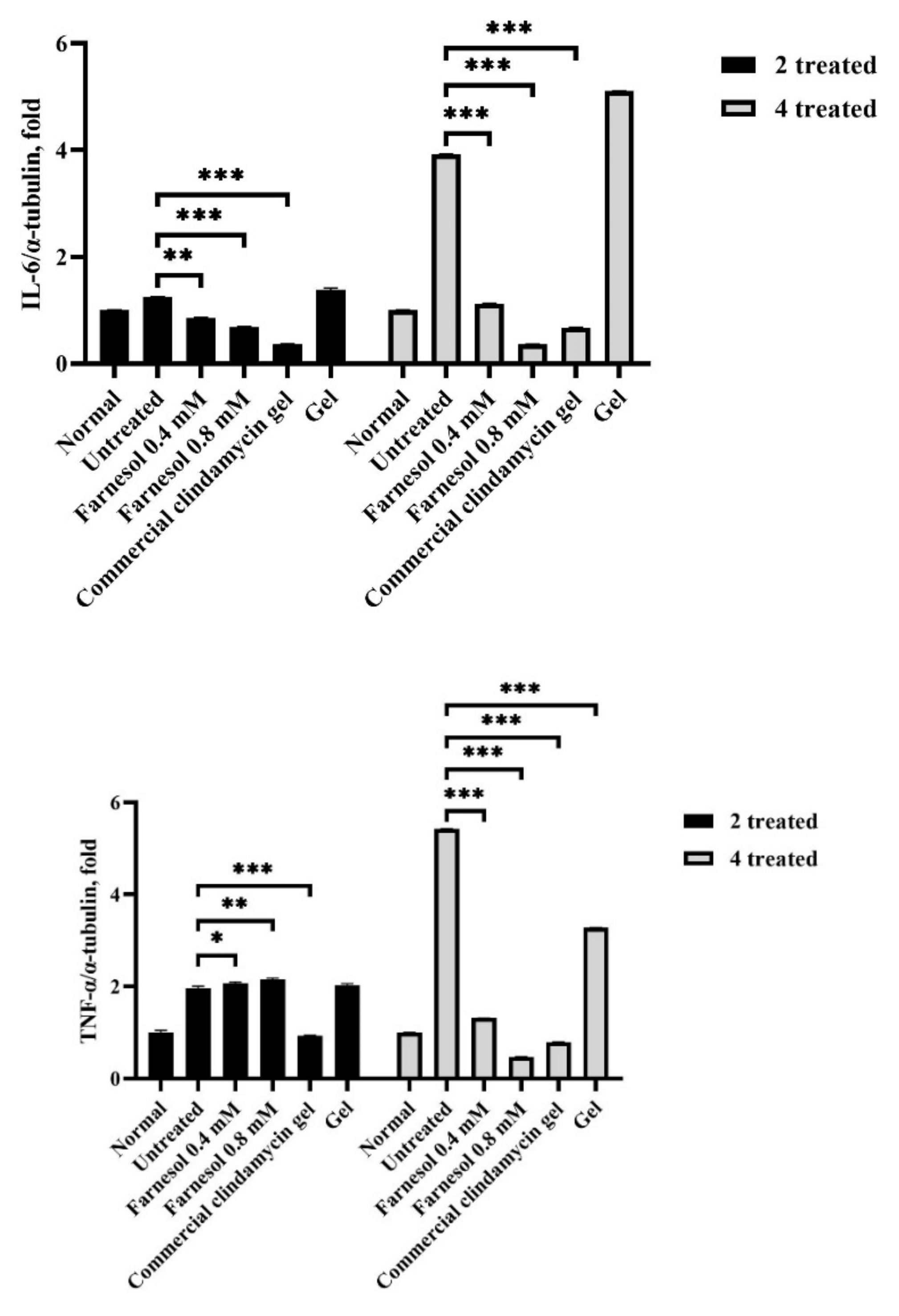
3.4. Western Blot Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Hammer, K.A. Treatment of acne with tea tree oil (melaleuca) products: A review of efficacy, tolerability and potential modes of action. Int. J. Antimicrob. Agents 2015, 45, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Vora, J.; Srivastava, A.; Modi, H. Antibacterial and antioxidant strategies for acne treatment through plant extracts. Inform. Med. Unlocked 2018, 13, 128–132. [Google Scholar] [CrossRef]
- Pektas, S.D.; Cinar, N.; Duman, D.D.; Kara, A.; Batu, J.; Karakas-Celik, S.; Aksoy, D.Y. The relationship among androgens, insulin resistance and ghrelin polymorphisms in post-adolescent male patients with severe acne vulgaris. Adv. Dermatol. Allergol. 2020, 37, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Peethambaran, B. Anti-Inflammatory and Anti-Microbial Properties of Achillea Millefolium in Acne Treatment; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 241–248. [Google Scholar]
- Tsai, T.-H.; Huang, W.-C.; Lien, T.-J.; Huang, Y.-H.; Chang, H.; Yu, C.-H.; Tsai, P.-J. Clove extract and eugenol suppress inflammatory responses elicited by Propionibacterium acnes in vitro and in vivo. Food Agric. Immunol. 2017, 28, 916–931. [Google Scholar] [CrossRef]
- Alibi, S.; Crespo, D.; Navas, J. Plant-Derivatives Small Molecules with Antibacterial Activity. Antibiotics 2021, 10, 231. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Shiraishi, A.; Hada, T.; Hirose, K.; Hamashima, H.; Shimada, J. The antibacterial effects of terpene alcohols on Staphylococcus aureus and their mode of action. FEMS Microbiol. Lett. 2004, 237, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Portillo, M.E.; Corvec, S.; Borens, O.; Trampuz, A. Propionibacterium acnes: An Underestimated Pathogen in Implant-Associated Infections. BioMed Res. Int. 2013, 2013, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.W.; Huang, H.H.; Wu, G.X.; Chang, H.R.; Wu, K.L.; Kuo, S.M. Antiaging and smoothness-improving properties of farnesol-based facial masks on rat skin exposed to ultraviolet B. J. Cosmet. Dermatol. 2019, 19, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Blencke, H.M.; Cheng, H.; Li, C. The antimicrobial effect of CEN1HC-Br against Propionibacterium acnes and its therapeutic and anti-inflammatory effects on acne vulgaris. Peptides 2018, 99, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Alves, F.R.F.; Neves, M.A.S.; Silva, M.G.; Rôças, I.N.; Siqueira, J.F., Jr. Antibiofilm and Antibacterial Activities of Farnesol and Xylitol as Potential Endodontic Irrigants. Braz. Dent. J. 2013, 24, 224–229. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Derengowski, L.S.; De-Souza-Silva, C.; Braz, S.V.; Mello-De-Sousa, T.M.; Báo, S.N.; Kyaw, C.M.; Silva-Pereira, I. Antimicrobial effect of farnesol, a Candida albicans quorum sensing molecule, on Paracoccidioides brasiliensis growth and morphogenesis. Ann. Clin. Microbiol. Antimicrob. 2009, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ganjali, A.; Sotoudeh, A.; Jahanshahi, A.; Takhtfooladi, M.A.; Bazzazan, A.; Roodbari, N.; Harati, M.P. Otostegia persica extraction on healing process of burn wounds. Acta Cir. Bras. 2013, 28, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-C.; Chen, W.-Y.; Chen, C.-Y.; Lee, S.; Wang, Y.-W.; Huang, H.-H.; Kuo, S.-M. Farnesol-Loaded Liposomes Protect the Epidermis and Dermis from PM2.5-Induced Cutaneous Injury. Int. J. Mol. Sci. 2021, 22, 6076. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.X.; Huang, H.H.; Chang, H.R.; Kuo, S.M. Evaluation of the UVB-screening capacity and restorative effects exerted by farnesol gel on UVB-caused sunburn. Environ. Toxicol. 2018, 33, 488–507. [Google Scholar] [CrossRef] [PubMed]
- Kistowska, M.; Gehrke, S.; Jankovic, D.; Kerl, K.; Fettelschoss-Gabriel, A.; Feldmeyer, L.; Fenini, G.; Kolios, A.; Navarini, A.; Ganceviciene, R.; et al. IL-1β Drives Inflammatory Responses to Propionibacterium acnes In Vitro and In Vivo. J. Investig. Dermatol. 2014, 134, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Ritsu, M.; Kanno, E.; Tanno, H.; Imai, Y.; Maruyama, R.; Tachi, M.; Kawakami, K.; Ishii, K. Critical role of tumor necrosis factor-α in the early process of wound healing in skin. J. Dermatol. Dermatol. Surg. 2017, 21, 14–19. [Google Scholar] [CrossRef]
- Parameswaran, N.; Patial, S. Tumor Necrosis Factor-α Signaling in Macrophages. Crit. Rev. Eukaryot. Gene Expr. 2010, 20, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Gabay, C. Interleukin-6 and chronic inflammation. Arthritis Res. Ther. 2006, 8, S3. [Google Scholar] [CrossRef] [PubMed]

| 1. Results for the two-time treatment category (at day 8 after acne induction). | |||||
| A | B | C | D | E | |
| Epithelialization | 0.67 ± 0.33 | 2.67 ± 0.33 ** | 2.67 ± 0.33 ** | 2.00 ± 0.33 | 1.67 ± 0.33 |
| Regeneration and reparation of pilosebaceous unit/epithelial cysts | 0.33 ± 0.33 | 3.33 ± 0.33 ***,# | 2.33 ± 0.33 * | 1.33 ± 0.33 | 1.67 ± 0.33 |
| Alleviation of inflammatory cell infiltration | 0.33 ± 0.33 | 3.67 ± 0.33 ***,### | 4.33 ± 0.33 ***,### | 3.33 ± 0.33 ***,### | 0.33 ± 0.33 |
| Improvement of abscesses and necrotic tissues | 0.33 ± 0.33 | 3.67 ± 0.33 ***,### | 4.33 ± 0.33 ***,### | 4.00 ± 0.00 ***,### | 0.33 ± 0.33 |
| Collagenization | 1.33 ± 0.33 | 4.33 ± 0.33 ***,### | 4.33 ± 0.33 ***,### | 4.00 ± 0.33 ***,## | 1.67 ± 0.33 |
| Total | 3.00 ± 0.58 | 17.67 ± 0.88 ***,### | 18.00 ± 0.58 ***,### | 14.67 ± 0.33 ***,### | 5.67 ± 0.33 |
| 2. Results for the four-time treatment category (at day 10 after acne induction). | |||||
| A | B | C | D | E | |
| Epithelialization | 2.67 ± 0.33 | 3.33 ± 0.33 # | 3.67 ± 0.33 # | 3.33 ± 0.33 # | 1.67 ± 0.33 |
| Regeneration and reparation of pilosebaceous unit/epithelial cysts | 2.67 ± 0.33 | 3.67 ± 0.33 # | 2.33 ± 0.33 | 2.33 ± 0.33 | 1.67 ± 0.33 |
| Alleviation of inflammatory cell infiltration | 1.67 ± 0.33 | 4.33 ± 0.33 ***,### | 4.67 ± 0.33 ***,### | 4.00 ± 0.00 **,### | 1.33 ± 0.33 |
| Improvement of abscesses and necrotic tissues | 1.67 ± 0.33 | 4.33 ± 0.33 **,### | 4.33 ± 0.33 **,### | 4.67 ± 0.33 ***,### | 1.33 ± 0.33 |
| Collagenization | 2.67 ± 0.33 | 4.33 ± 0.33 | 3.67 ± 0.33 | 3.67 ± 0.33 | 3.33 ± 0.33 |
| Total | 11.33 ± 0.33 | 20.00 ± 0.58 ***,### | 18.67 ± 0.67 ***,### | 18.00 ± 0.58 ***,### | 9.33 ± 0.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, G.-X.; Wang, Y.-W.; Wu, C.-S.; Lin, Y.-H.; Hung, C.-H.; Huang, H.-H.; Kuo, S.-M. Therapeutic Efficacy of Sesquiterpene Farnesol in Treatment of Cutibacterium acnes-Induced Dermal Disorders. Molecules 2021, 26, 5723. https://doi.org/10.3390/molecules26185723
Wu G-X, Wang Y-W, Wu C-S, Lin Y-H, Hung C-H, Huang H-H, Kuo S-M. Therapeutic Efficacy of Sesquiterpene Farnesol in Treatment of Cutibacterium acnes-Induced Dermal Disorders. Molecules. 2021; 26(18):5723. https://doi.org/10.3390/molecules26185723
Chicago/Turabian StyleWu, Guan-Xuan, Yu-Wen Wang, Chun-Shien Wu, Yen-Hung Lin, Chih-Hsin Hung, Han-Hsiang Huang, and Shyh-Ming Kuo. 2021. "Therapeutic Efficacy of Sesquiterpene Farnesol in Treatment of Cutibacterium acnes-Induced Dermal Disorders" Molecules 26, no. 18: 5723. https://doi.org/10.3390/molecules26185723
APA StyleWu, G.-X., Wang, Y.-W., Wu, C.-S., Lin, Y.-H., Hung, C.-H., Huang, H.-H., & Kuo, S.-M. (2021). Therapeutic Efficacy of Sesquiterpene Farnesol in Treatment of Cutibacterium acnes-Induced Dermal Disorders. Molecules, 26(18), 5723. https://doi.org/10.3390/molecules26185723





