A Study on the Behavior Patterns of Liquid Aerosols Using Disinfectant Chloromethylisothiazolinone/Methylisothiazolinone Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Scheme
2.2. Preparation of Working Standards and Solutions
2.3. Instrumental Setup for Analysis of MIT and CMIT
2.4. Experimental Approaches
2.4.1. Working Standards
2.4.2. CMIT/MIT Aerosol
3. Results and Discussion
3.1. Calibration and QA Data
3.2. MIT and CMIT Behavior Patterns with Different Sampling Volumes and Mass Concentrations of CMIT/MIT Aerosols (Exp Stage 1)
3.3. Relative Proportion Changes of MIT and CMIT at Different Aerosol Concentrations (Exp Stage 2)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kwon, S.-A.; Yoo, H.-J.; Song, E. Korean consumers’ recognition of risks depending on the provision of safety information for chemical products. Int. J. Environ. Res. Public Health 2020, 17, 1207. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Yoo, J.-H.; Cho, S. The role of local governments before and during chemical accidents, focusing on changes in hazardous chemical management systems. J. Environ. Policy Plan. 2020, 22, 328–342. [Google Scholar] [CrossRef]
- Buchmüller, K.; Bearth, A.; Siegrist, M. Consumers’ perceptions of chemical household products and the associated risks. Food Chem. Toxicol. 2020, 143, 111511. [Google Scholar] [CrossRef]
- Bearth, A.; Miesler, L.; Siegrist, M. Consumers’ risk perception of household cleaning and washing products. Risk Anal. 2017, 37, 647–660. [Google Scholar] [CrossRef] [PubMed]
- Tun, M.H.; Tun, H.M.; Mahoney, J.J.; Konya, T.B.; Guttman, D.S.; Becker, A.B.; Mandhane, P.J.; Turvey, S.E.; Subbarao, P.; Sears, M.R. Postnatal exposure to household disinfectants, infant gut microbiota and subsequent risk of overweight in children. CMAJ 2018, 190, E1097–E1107. [Google Scholar] [CrossRef] [PubMed]
- Bearth, A.; Siegrist, M. Situative and product-specific factors influencing consumers’ risk perception of household cleaning products. Saf. Sci. 2019, 113, 126–133. [Google Scholar] [CrossRef]
- Raslan, R.; Hassim, M.H.; Gopalakrishnan, N.; Chemmangattuvalappil, D.K.S.N.; Ten, J.Y. Design of Household Products Ingredients wiıth Minimum Safety and Health Risk. Pertanika J. Sci. Technol. 2020, 28, 137–149. [Google Scholar]
- Shetage, S.S.; Traynor, M.J.; Brown, M.B.; Chilcott, R.P. Sebomic identification of sex-and ethnicity-specific variations in residual skin surface components (RSSC) for bio-monitoring or forensic applications. Lipids Health Dis. 2018, 17, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, A.K.; Mishra, A.; Chattopadhyay, P. Screening of acute and sub-chronic dermal toxicity of Calendula officinalis L essential oil. Regul. Toxicol. Pharmacol. 2018, 98, 184–189. [Google Scholar] [CrossRef]
- Adeniran, J.A.; Yusuf, R.O.; Oke, E.O. Deposition and coagulation of aerosols from household spray products. Songklanakarin J. Sci. Technol. 2019, 41, 207–215. [Google Scholar]
- Shim, H.E.; Lee, J.Y.; Lee, C.H.; Mushtaq, S.; Song, H.Y.; Song, L.; Choi, S.-J.; Lee, K.; Jeon, J. Quantification of inhaled aerosol particles composed of toxic household disinfectant using radioanalytical method. Chemosphere 2018, 207, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Jo, S.; An, Y.-J.; Choi, S.-J.; Lee, K. Thermal desorption technique for analysis of aerosolized methylchloroisothiazolinone/methylisothiazolinone (CMIT/MIT) in inhalation toxicity testing. J. Chromatogr. Sci. 2020, 58, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Singer, B.C.; Delp, W.W. Response of consumer and research grade indoor air quality monitors to residential sources of fine particles. Indoor Air 2018, 28, 624–639. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Kim, K.-H. A simple methodological validation of the gas/particle fractionation of polycyclic aromatic hydrocarbons in ambient air. Sci. Rep. 2015, 5, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monn, C.; Becker, S. Cytotoxicity and induction of proinflammatory cytokines from human monocytes exposed to fine (PM2. 5) and coarse particles (PM10–2.5) in outdoor and indoor air. Toxicol. Appl. Pharmacol. 1999, 155, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Ma-Hock, L.; Brill, S.; Wohlleben, W.; Farias, P.M.; Chaves, C.R.; Tenório, D.P.; Fontes, A.; Santos, B.S.; Landsiedel, R.; Strauss, V. Short term inhalation toxicity of a liquid aerosol of CdS/Cd (OH) 2 core shell quantum dots in male Wistar rats. Toxicol. Lett. 2012, 208, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Turgeon, N.; Toulouse, M.-J.; Martel, B.; Moineau, S.; Duchaine, C. Comparison of five bacteriophages as models for viral aerosol studies. Appl. Environ. Microbiol. 2014, 80, 4242–4250. [Google Scholar] [CrossRef] [Green Version]
- Kuo, Y.-M.; Lin, C.-W.; Huang, S.-H.; Chang, K.-N.; Chen, C.-C. Effect of aerosol loading on breakthrough characteristics of activated charcoal cartridges. J. Aerosol Sci. 2013, 55, 57–65. [Google Scholar] [CrossRef]
- Wang, D.; Pakbin, P.; Saffari, A.; Shafer, M.M.; Schauer, J.J.; Sioutas, C. Development and evaluation of a high-volume aerosol-into-liquid collector for fine and ultrafine particulate matter. Aerosol Sci. Technol. 2013, 47, 1226–1238. [Google Scholar] [CrossRef] [Green Version]
- Olmedo, P.; Goessler, W.; Tanda, S.; Grau-Perez, M.; Jarmul, S.; Aherrera, A.; Chen, R.; Hilpert, M.; Cohen, J.E.; Navas-Acien, A. Metal concentrations in e-cigarette liquid and aerosol samples: The contribution of metallic coils. Environ. Health Perspect. 2018, 126, 027010. [Google Scholar] [CrossRef] [PubMed]
- Collier, P.; Ramsey, A.; Waigh, R.; Douglas, K.; Austin, P.; Gilbert, P. Chemical reactivity of some isothiazolone biocides. J. Appl. Bacteriol. 1990, 69, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Fewings, J.; Menné, T. An update of the risk assessment for methylchloroisothiazolinone/methylisothiazolinone (MCI/MI) with focus on rinse-off products. Contact Dermat. 1999, 41, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.M. The mechanism of action of isothiazolone biocides. Powerpl. Chem. 2007, 9, 14. [Google Scholar]
- Lundov, M.; Krongaard, T.; Menné, T.; Johansen, J. Methylisothiazolinone contact allergy: A review. Br. J. Dermatol. 2011, 165, 1178–1182. [Google Scholar] [CrossRef]
- Schnuch, A.; Lessmann, H.; Geier, J.; Uter, W. Contact allergy to preservatives. Analysis of IVDK data 1996–2009. Br. J. Dermatol. 2011, 164, 1316–1325. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kang, H.-J.; Seol, H.-S.; Kim, C.-K.; Yoon, S.-K.; Gwack, J.; Kim, Y.-H.; Kwon, J.-H. Refined exposure assessment for three active ingredients of humidifier disinfectants. Environ. Eng. Res. 2013, 18, 253–257. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, Y.-H.; Kwon, J.-H. Fatal misuse of humidifier disinfectants in Korea: Importance of screening risk assessment and implications for management of chemicals in consumer products. Environ. Sci. Technol. 2012, 46, 2498–2500. [Google Scholar] [CrossRef]
- Kim, Y.; Choi, J. Early life exposure of a biocide, CMIT/MIT causes metabolic toxicity via the O-GlcNAc transferase pathway in the nematode C. elegans. Toxicol. Appl. Pharmacol. 2019, 376, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.H.; Park, D.U.; Lee, E.; Park, S.; Lee, S.Y.; Jung, S.; Hong, S.B.; Park, J.; Hong, S.J. Humidifier disinfectant and use characteristics associated with lung injury in Korea. Indoor Air 2019, 29, 735–747. [Google Scholar] [CrossRef]
- Song, M.-K.; Kim, D.I.; Lee, K. Kathon induces fibrotic inflammation in lungs: The first animal study revealing a causal relationship between humidifier disinfectant exposure and eosinophil and Th2-mediated fibrosis induction. Molecules 2020, 25, 4684. [Google Scholar] [CrossRef]
- Park, S.-K.; Seol, H.-S.; Park, H.-J.; Kim, Y.-S.; Ryu, S.-H.; Kim, J.; Kim, S.; Lee, J.-H.; Kwon, J.-H. Experimental determination of indoor air concentration of 5-chloro-2-methylisothiazol-3(2H)-one/2-methylisothiazol-3(2H)-one (CMIT/MIT) emitted by the use of humidifier disinfectant. Environ. Anal. Health Toxicol. 2020, 35, e2020008. [Google Scholar] [CrossRef] [PubMed]
- Park, D.-U.; Park, S.-K.; Kim, J.; Park, J.; Ryu, S.-H.; Park, J.-H.; Lee, S.-Y.; Oh, H.-B.; Kim, S.; Zoh, K.E.; et al. Characteristics of exposure to chloromethylisothiazolinone (CMIT) and methylisothiazolinone (MIT) among humidifier disinfectant-associated lung injury (HDLI) patients in South Korea. Molecules 2020, 25, 5284. [Google Scholar] [CrossRef]
- EPA. Reregistration Eligibility Decision (RED) Methylisothiazolinone; EPA 738-R-98-012; EPA: Washington, DC, USA, 1998. [Google Scholar]
- Scientific Committee on Consumer Safety. Opinion on the Mixture of 5 Chloro-2-Methylisothiazolin-3(2H)-One and 2 Methylisothiazolin-3(2H)-One. COLIPA n° P56; Scientific Committee on Consumer Safety: Brussels, Belgium, 2009. [Google Scholar]
- Kim, Y.H.; Kim, K.H. Critical role of a pre-purge setup in the thermal desorption analysis of volatile organic compounds by gas chromatography with mass spectrometry. J. Sep. Sci. 2015, 38, 2455–2462. [Google Scholar] [CrossRef]
- Kim, K.-H.; Kim, Y.-H.; Brown, R.J. Conditions for the optimal analysis of volatile organic compounds in air with sorbent tube sampling and liquid standard calibration: Demonstration of solvent effect. Anal. Bioanal. Chem. 2013, 405, 8397–8408. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Kim, K.-H. Novel approach to test the relative recovery of liquid-phase standard in sorbent-tube analysis of gaseous volatile organic compounds. Anal. Chem. 2012, 84, 4126–4139. [Google Scholar] [CrossRef] [PubMed]

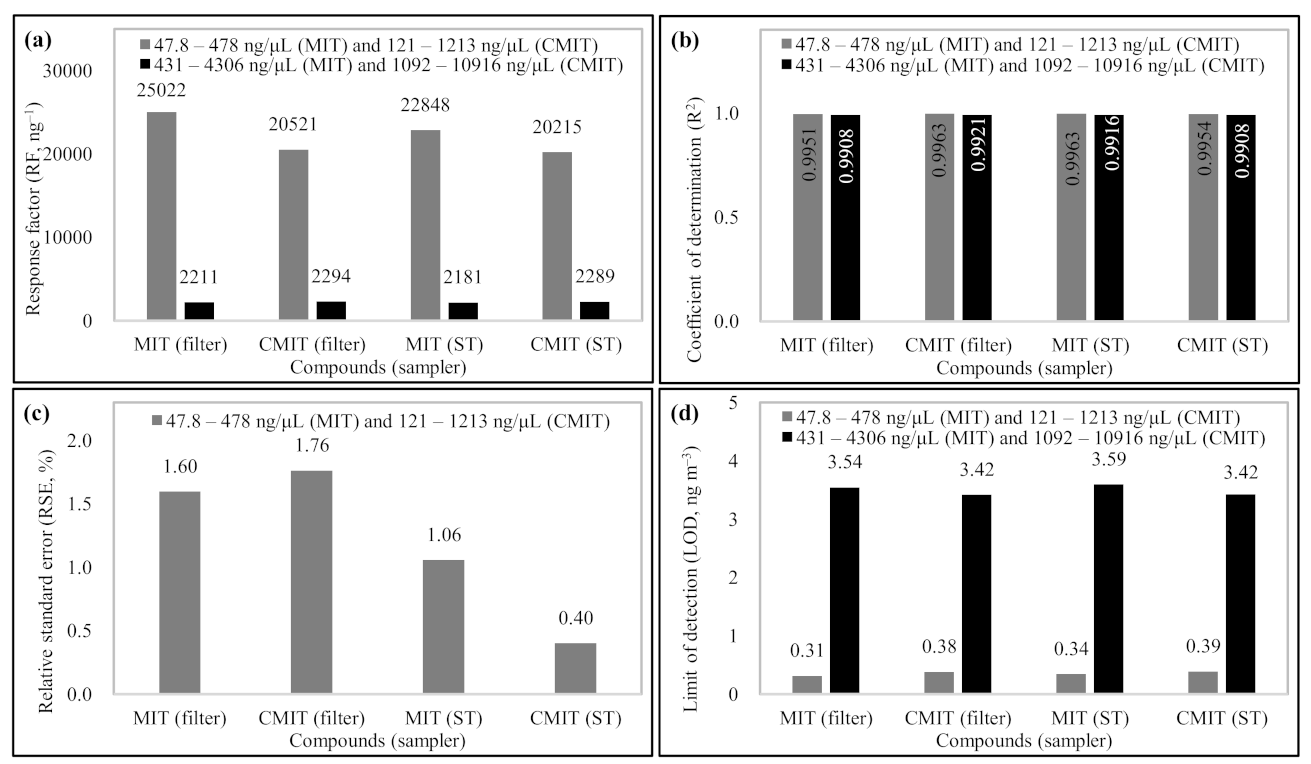
| Full name: | Methylisothiazolinone (2-Methyl-4-isothiazolin-3-one) | Chloromethylisothiazolinone (5-chloro-2-methyl-4-isothiazolin-3-one) |
| Short name: | MIT | CMIT |
| MW (g mol−1): | 115.1 | 149.59 |
| Density (g mL−3): | 1.293 | 1.02 |
| Boiling point (°C): | 182.8 | 200.2 |
| Formula: | C4H5NOS | C4H4ClNOS |
| CAS number: | 2682-20-4 | 26172-55-4 |
| Main spectra 1 (m/z): | 115 | 149 |
| Chemical structure: |  |  |
| A. Experimental Stage Information | |||
|---|---|---|---|
| Order | Exp Code | Contents | |
| 1 | Exp stage 1 | Filter breakthrough test of MIT and CMIT in the aerosols | |
| 2 | Exp stage 2 | Assessment of the relative proportions of MIT and CMIT in the aerosols with different aerosol mass concentrations | |
| B. Information of the Breakthrough Test of the Glass Fiber Filter Using the CMIT/MIT Aerosol Samples (Exp Stage 1) | |||
| Order | Preliminary test | Main test | |
| 1 | Sampling flow rate: | 0.5 L min−1 | 0.5 L min−1 |
| 2 | Sampling volume: | 65 L | 3, 6, 10, 15, and 30 L |
| 3 | Primary sampler: | Glass fiber filter 1 | Glass fiber filter 1 |
| 4 | Secondary sampler: | Sorbent tube 2 | Sorbent tube 2 |
| 5 | Tertiary sampler: | Sorbent tube 2 | − |
| 6 | Aerosol concentration 3: | 1.82 mg m−3 | 0.20–16.7 mg m−3 |
| 7 | Sample number: | 3 | 45 |
| C. Information of the Relative Proportion Test for MIT and CMIT in the Aerosol Samples (Exp Stage 2) | |||
| 1 | Sampling flow rate: | 0.5 L min−1 | |
| 2 | Primary sampler: | Glass fiber filter 1 | |
| 3 | Secondary sampler: | Sorbent tube 2 | |
| 4 | Aerosol concentration 3: | 0.9–60 mg m−3 | |
| 5 | Sample number: | 225 | |
| A. Pretreatment: Thermal Desorber (Model: Shimadzu, Kyoto, Japan) | |||
|---|---|---|---|
| a. Sampling Tube | |||
| 1. Trap tube: | Quartz (length: 90 mm, OD: 6.4 mm, and ID: 4.2 mm) | ||
| 2. Filter or Adsorbent: | Glass fiber filter or Carbopack × (200 mg) | ||
| 3. Desorption flow: | 100 mL min−1 (to cold-trap) | ||
| 4. Desorption time: | 5 min | ||
| 5. Desorption temp.: | 250 °C | ||
| b. Cold-Trap | |||
| 1. Trap tube: | Quartz coated by silcosteel (length: 100 mm, OD: 3.2 mm, and ID: 2 mm) | ||
| 2. Adsorbent: | Quartz wool and Tenax TA (volume ratio = 1:1) | ||
| 3. Adsorption temp.: | 0 °C (from sampling tube) | ||
| 4. Desorption temp.: | 280 °C (to GC) | ||
| 5. Desorption flow: | 23 mL min−1 | ||
| 6. Desorption time: | 5 min | ||
| c. Carrier Gas Setting | |||
| 1. Carrier gas: | Helium (> 99.999%) | ||
| 2. Initial gas flow: | 1 mL min−1 | (Constant gas flow) | |
| 3. Split flow: | 20 mL min−1 (Method A) and 200 mL min−1 (Method B) | ||
| d. Line and Interface Temp.: 280 °C | |||
| B. Separation: Gas Chromatography (Model: GC-2010, Shimadzu, Kyoto, Japan) | |||
| a. Column: | HP-5 (Agilent J&W, California, USA) | ||
| (length: 30 m, diameter: 0.32 mm, and film thickness: 0.25 µm) | |||
| b. Oven Setting: | 60 °C (1 min) → 10 °C/min → 140 °C (0 min) → 40 °C/min → 280 °C (3.5 min) | ||
| (Total program time = 16 min) | |||
| C. Detection: Mass Spectrometry (Model: GCMS-QP2010 ultra, Shimadzu, Kyoto, Japan) | |||
| a. Ionization Mode: | EI (70 ev) | d. TIC scan range: | 50~400 m/z |
| b. Ion source Temp.: | 280 °C | e. Scan speed: | 1250 |
| c. Interface Temp.: | 280 °C | ||
| A. ANOVA Test (One-Way): Total | ||||
|---|---|---|---|---|
| Group Variable | Compound | Grouping | p-Value | |
| Sampling volume | MIT | 3 6, 10, 15, and 30 L | 0.0149 * | |
| CMIT | 8.60 × 10−20 ** | |||
| Aerosol concentration | MIT | 0.2−2, 2−5, 5−10, 10−16.7 mg m−3 | 1.81 × 10−8 ** | |
| CMIT | 0.0616 | |||
| B. ANOVA Test (One-Way): Individual | ||||
| a. Sampling Volume (L) | ||||
| p-value | 3 | 6 | 10 | 15 |
| (a) MIT | ||||
| 6 | 0.8825 | |||
| 10 | 0.7989 | 0.9076 | ||
| 15 | 0.7756 | 0.612 | 0.5075 | |
| 30 | 0.0294 * | 0.0144 * | 0.0097 ** | 0.0255 * |
| (b) CMIT | ||||
| 6 | 0.0106 * | |||
| 10 | 5.99 × 10−7 ** | 3.79 × 10−5 ** | ||
| 15 | 5.03 × 10−11 ** | 1.94 × 10−9 ** | 6.04 × 10−3 ** | |
| 30 | 4.51 × 10−11 ** | 4.61 × 10−10 ** | 3.54 × 10−6 ** | 5.04 × 10−5 ** |
| b. Aerosol concentration (mg m−3) | ||||
| p-value | 0.2−2 | 2−5 | 5−10 | |
| (a) MIT | ||||
| 2−5 | 2.920 × 10−3 ** | |||
| 5−10 | 5.971 × 10−7 ** | 5.646 × 10−3 ** | ||
| 10−16.7 | 1.631 × 10−5 ** | 0.0343 * | 0.6702 | |
| (b) CMIT | ||||
| 2−5 | 0.7246 | |||
| 5−10 | 0.0256 * | 0.0427 * | ||
| 10–16.7 | 0.2442 | 0.3641 | 0.1912 | |
| * p-value < 0.05 | ||||
| ** p-value < 0.01 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-H.; Song, M.-K.; Lee, K. A Study on the Behavior Patterns of Liquid Aerosols Using Disinfectant Chloromethylisothiazolinone/Methylisothiazolinone Solution. Molecules 2021, 26, 5725. https://doi.org/10.3390/molecules26195725
Kim Y-H, Song M-K, Lee K. A Study on the Behavior Patterns of Liquid Aerosols Using Disinfectant Chloromethylisothiazolinone/Methylisothiazolinone Solution. Molecules. 2021; 26(19):5725. https://doi.org/10.3390/molecules26195725
Chicago/Turabian StyleKim, Yong-Hyun, Mi-Kyung Song, and Kyuhong Lee. 2021. "A Study on the Behavior Patterns of Liquid Aerosols Using Disinfectant Chloromethylisothiazolinone/Methylisothiazolinone Solution" Molecules 26, no. 19: 5725. https://doi.org/10.3390/molecules26195725
APA StyleKim, Y.-H., Song, M.-K., & Lee, K. (2021). A Study on the Behavior Patterns of Liquid Aerosols Using Disinfectant Chloromethylisothiazolinone/Methylisothiazolinone Solution. Molecules, 26(19), 5725. https://doi.org/10.3390/molecules26195725






